Abstract
Familial adenomatous polyposis is characterized by the development of multiple (>100) colorectal adenomas throughout the colorectum. This disorder can be caused by a germline mutation in the adenomatous polyposis coli gene and can be diagnosed either clinically or genetically. After diagnosis with the condition, patients should undergo prophylactic proctocolectomy with a neoreservoir, usually an ileoanal pouch, at an appropriate time. Individuals with a family history of this disease who have not been diagnosed should be advised to attend genetic counseling and to enroll in appropriate clinical and genetic surveillance programs. Recent progress in endoscopic technology, including high-resolution endoscopy, capsule endoscopy, and double-balloon endoscopy, has made possible more detailed and wide-ranging investigation of the gastrointestinal tract. Although there has been limited evidence, further studies on these new endoscopic technologies might alter the surveillance strategies for familial adenomatous polyposis.
Keywords: adenomatous polyposis coli gene, attenuated familial adenomatous polyposis, familial adenomatous polyposis, genetic testing, human MutY homolog-associated polyposis
Introduction
Familial adenomatous polyposis (FAP) is an autosomal dominant disorder [1] that can be caused by a germline mutation in the adenomatous polyposis coli (APC) gene [2–4]. FAP is characterized by the development of multiple (> 100) colorectal adenomas throughout the colorectum. This disorder affects both sexes equally and is estimated to occur in 1/8300–1/14 025 live births [5]. Approximately 50% of FAP patients develop adenomas by the age of 15 years, which increases to 95% by the age of 35 years. The lifetime risk of colorectal cancer (CRC) approaches 100% if patients are not treated by prophylactic colectomy [6]. Patients with FAP can also have a variety of extraintestinal disorders, which include adenomas of the duodenum, papilla [7], small intestine, and stomach; gastric fundic gland polyps [8]; desmoid tumors [9]; osteomas [10]; skin lesions (lipoma, fibroma, and epidermoid cysts) [10]; dental abnormalities [11]; congenital hypertrophy of the retinal pigment epithelium (CHRPE) [12]; hepatoblastoma [13]; and cancers in the thyroid gland, biliary system, pancreas [14], and brain [15]. On the basis of these factors, patients with FAP should be enrolled in a lifetime surveillance program to detect these disorders. In this article, we have reviewed the significant scientific results for current FAP diagnostic, surveillance, and treatment strategies. We have focused on gastrointestinal disorders and investigated their trends in accordance with recent advances in endoscopic technology.
Clinical diagnosis of familial adenomatous polyposis
The diagnosis of FAP relies primarily on clinical findings on the number and history of colorectal adenomatous polyps. Individuals with 100 or more polyps, or with fewer than 100 polyps but with a family history of FAP, are clinically diagnosed with FAP (Fig. 1). Attenuated FAP (AFAP) is a recognized variant of FAP and is characterized by a later onset of disease compared with classical FAP and fewer adenomatous polyps, typically between 10 and 99 [16,17]. These adenomatous polyps are more prone to occur in the right colon and phenotypic expression is often variable within families. The presence and incidence of extraintestinal disorders such as gastric polyps and thyroid and duodenal cancers are similar to those of classical FAP and are potentially helpful in the clinical diagnosis of AFAP.
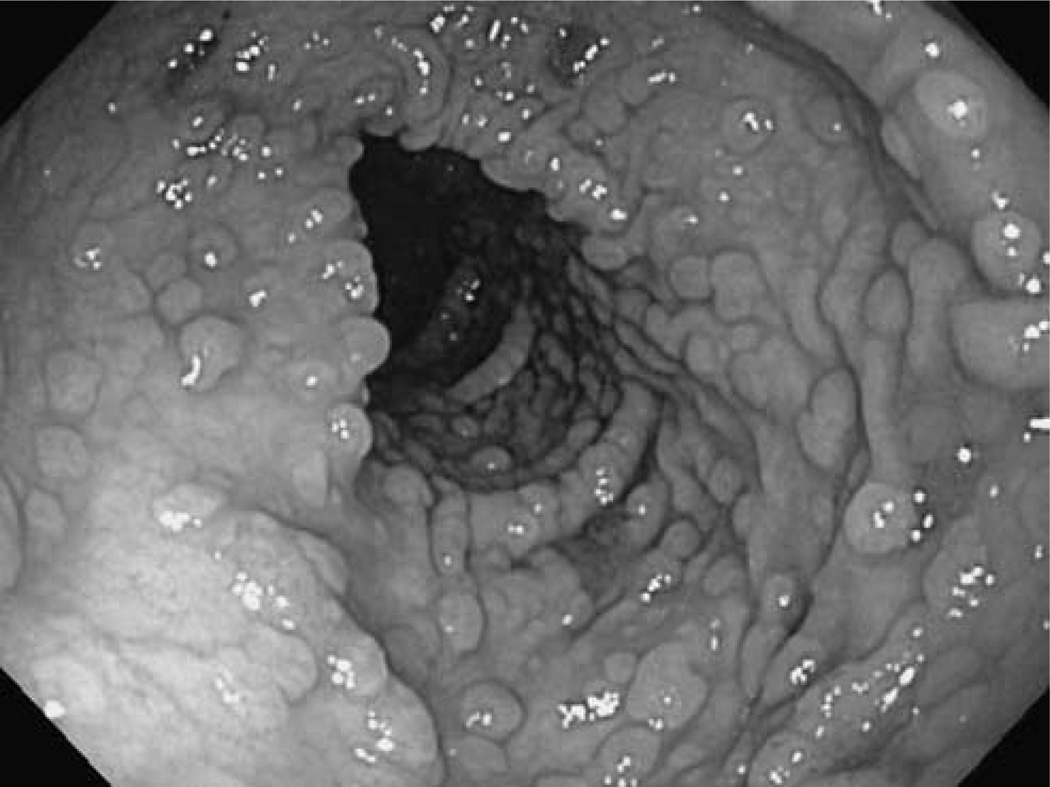
Fig. 1.
Colonoscopic findings of familial adenomatous polyposis.
Genetic diagnosis of familial adenomatous polyposis
Genetic defect
Germline mutations in the APC gene on chromosome 5q21 account for almost all cases of FAP [2–4]. The APC gene has 15 exons and encodes a gene product of 2843 amino acids with a molecular weight of ~309 000 Da. APC is a tumor suppressor gene or ‘gatekeeping’ gene [18]. APC suppresses canonical Wnt signaling, which is essential for tumorigenesis and development and homeostasis of a variety of cell types, such as epithelial and lymphoid cells [19]. In addition, APC plays roles in several other fundamental cellular processes. These include cell adhesion and migration, organization of the actin and microtubule networks, spindle formation, and chromosome segregation. Deregulation of these processes caused by mutations in APC is implicated in the initiation and expansion of colon cancer [20]. Approximately 33% of APC mutations occur between amino acids 1061 and 1309 [21], which lead to a high number of colonic adenomas at a younger age [22,23]. Moreover, the location of the mutation is related to the average age at onset. Patients with a mutation at codon 1309 have an average age at onset of 20 years; a mutation between codons 168 and 1580 (excluding 1309) results in an average age at onset of 30 years; mutations at the 5′ end of codon 168 and 3′ end of codon 1580 result in an average at onset of 52 years [22]. APC gene mutations between codons 169 and 1393 result in FAP, whereas more 3′ and 5′ mutations and somatic mosaicism for APC mutations predispose to AFAP [24–27]. Other correlations between the mutation site and the phenotype include profuse colorectal polyposis on mutations between codons 1250 and 1464 [28]; desmoid tumors, osteomas, and epidermoid cysts on mutations in codons 1395–1493 [29]; desmoid tumors on mutations distal to codon 1444 [30]; and CHRPE on mutations between codons 311 and 1444 [30]. Although there may be an average correlation between a specific genotype and phenotype, there is vast heterogeneity in expression, even between patients with identical mutations.
Recently, it was clarified that loss of APC causes upregulation of a DNA demethylase system and the concomitant hypomethylation of key intestinal cell fating genes. APC seems to control intestinal cell fating through a switch in DNA methylation dynamics. Wild-type APC and retinoic acid downregulate demethylase components, thereby promoting DNA methylation of key genes and helping progenitors commit to differentiation [31].
Molecular genetic testing
Genetic testing should be performed for certain indications including confirmation of the diagnosis of FAP and presymptomatic diagnosis of individuals 10 years of age or older who are at risk for FAP [32]. The likelihood of detecting an APC mutation is highly related to the severity of polyposis and the family history. Patients with an FAP phenotype are significantly more likely to have an APC mutation than patients with an AFAP phenotype [33,34]. Fewer than 30% of individuals with attenuated phenotypes are expected to have an identifiable APC mutation [35].
Several methods for APC gene testing are currently available. Full gene sequencing of all APC exons and intron–exon boundaries shows the highest sensitivity in detecting APC mutations but is labor intensive and is not cost effective [36]. Alternatively, the protein truncation test has the advantage of being cost effective when compared with full gene sequencing, despite having a lower detection rate for APC gene mutations of ~80% [37]. Southern blot analysis can be used to detect partial and whole-gene deletions or other large rearrangements, although partial or whole APC gene deletion has been identified in only ~8–12% of individuals with FAP [38]. Linkage studies can be carried out to provide an accurate diagnosis of APC-associated polyposis in affected family members and should be performed with the consent of the family members to be tested [39]. These studies should be carried out on families with more than one affected family member belonging to different generations to detect individuals with the disease-related mutation who cannot be identified by any other gene-testing method. The markers used for linkage studies are very tightly linked to the APC locus, and this method has 98% accuracy in detecting APC mutations in more than 95% of families with an APC-associated polyposis condition [40].
Surveillance
Colorectal advanced adenoma and cancer
Genetic testing for the APC gene mutation is one of the screening strategies for FAP. Individuals with a family history of FAP (first-degree relatives of FAP patients) should undergo genetic counseling and screening for FAP between the ages of 10 and 12 years to identify carriers of the APC gene mutation [41,42]. Surveillance and treatment strategies should be determined on the basis of each patient’s personal history. (i) Patients with a personal history of classical FAP should undergo prophylactic proctocolectomy or colectomy at the appropriate time (details in the Surgical options section). (ii) Unaffected patients with a family history of mutation and with a known APC disease-causing mutation should be recommended for flexible sigmoidoscopy or colonoscopy every 12 months beginning at 10–15 years of age. (iii) Unaffected patients with family history of mutation with negative molecular genetic testing results should be recommended for the same surveillance schedule as that for patients with average risk. (iv) Unaffected patients with a family history of mutation who have not undergone molecular genetic testing should be recommended for the following surveillance strategy: colonoscopy screening should start at 10–15 years of age, and the screening frequency should reduce with each subsequent decade. After the age of 50 years, patients should be advised to follow the American Gastroenterology Association guidelines for screening average-risk patients [41].
Gastric neoplasia
Numerous fundic gland polyps, often numbering in the hundreds, are observed in 12.5–84% of patients with FAP (Fig. 2) [43]. The polyps may cover the entire surface of the acid-secreting epithelium and even coalesce to give the mucosal surface a ‘matted’ appearance [44]. Gastric cancer can arise from fundic gland polyps in FAP patients [45,46], although the lifetime risk of gastric cancer in FAP patients is estimated to be only 0.6% [47].
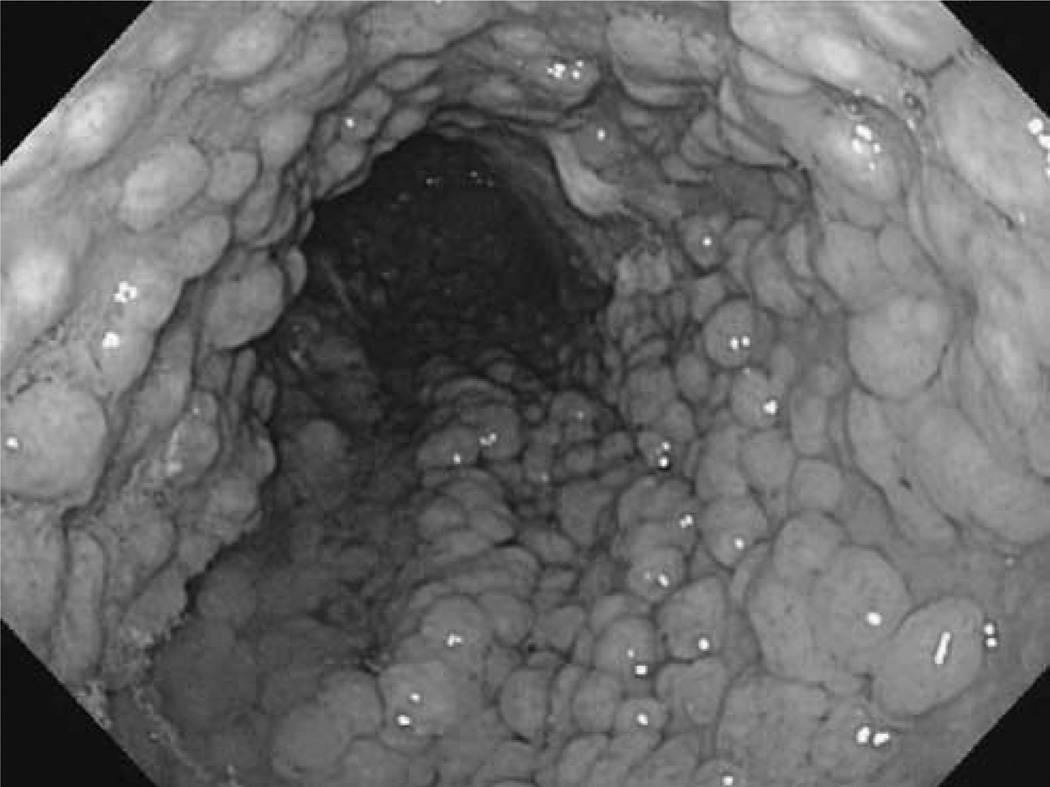
Fig. 2.
Gastric fundic gland polyps in a familial adenomatous polyposis patient.
Gastric adenomatous polyps can develop into gastric adenocarcinoma and are the second most prevalent gastric lesions in individuals with FAP. Gastric adenomatous polyps are usually detected within the gastric antrum [48]. Although the risk for gastric cancer among individuals with FAP living in Western countries is low, the risk among Japanese and Korean individuals with FAP may be 10-fold higher than that in the general population [8]. Esophagogastroduodenoscopy (EGD) should be advised for individuals with FAP either by the age of 25 years or before colectomy and EGD should be repeated every 1–3 years.
Neoplasia in the small intestine
Adenomatous polyps of the duodenum are observed in 50–90% of individuals with FAP and are commonly found in the second and third portions of the duodenum, and less frequently in the distal small bowel [49]. Duodenal adenomas are distributed throughout the duodenum with the majority being found in clusters around and distal to the ampulla of Vater. Adenomatous polyps of the periampullary region, which includes the duodenal papilla and ampulla of Vater, are seen in at least 50% of individuals with classic FAP. Polyps in this area can cause obstruction of the pancreatic duct, which results in pancreatitis or biliary obstruction, both of which occur at increased frequency in individuals with FAP. Periampullary carcinoma occurs in ~4% of individuals with FAP and is the leading cause of cancer death among FAP patients who have undergone a colectomy [50]. Very few adenomas are found proximal to the ampulla compared with the number found in the second part of the duodenum and they typically present as multiple discrete adenomas (1–10 mm in diameter) or as flat confluent plaques (Fig. 3) [51]. The average age at diagnosis of duodenal cancer is 50 years (range, 18–78 years) [49], which is ~10 years after the expected development of colonic cancer in untreated individuals with FAP [1]. The estimated relative risks for duodenal adenocarcinoma and ampullary carcinoma compared with the general population are 331 and 124, respectively [51].
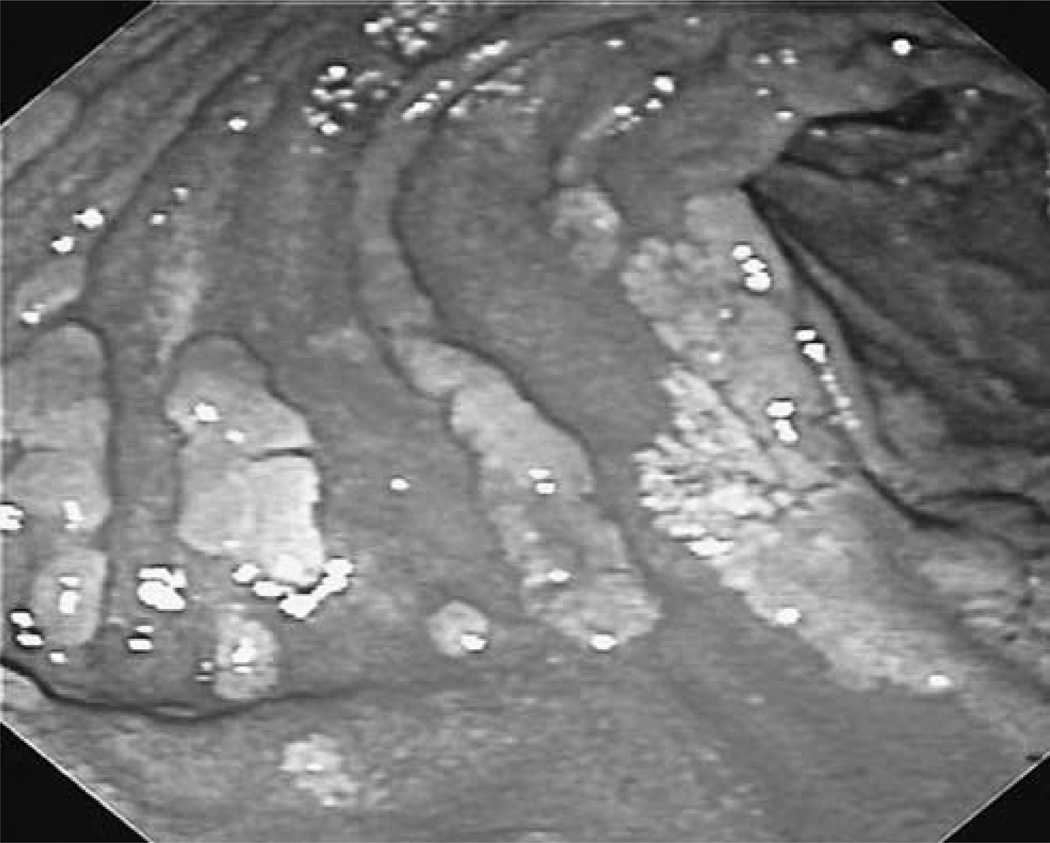
Fig. 3.
Endoscopic view of the duodenum in a familial adenomatous polyposis patient showing confluent adenomatous plaques.
The frequency of EGD depends on the severity of the duodenal adenoma, which can be determined using the Spigelman staging criteria (Table 1) [51]. This classification system comprises five stages (0–IV) depending on the number of points that have been accumulated for number, size, histology, and degree of dysplasia of the duodenal adenomas. In a previous 10-year follow-up case series, the stages of this classification system were correlated with the risk of developing duodenal cancer. Stage II, III, and IV disease were associated with a 2.3, 2.4, and 36% risk for duodenal cancer, respectively [51]. Thus, surveillance intervals can be adjusted or treatment initiated in patients with FAP and duodenal adenomatosis according to the Spigelman stage. A side-viewing endoscope should be used to visualize the duodenal papilla, and a tissue biopsy specimen should be dissected even if no polyps can be visualized but the papilla is enlarged. Endoscopic retrograde cholangiopancreatography may be necessary to evaluate for adenomas of the common bile duct.
Table 1.
Classification of the severity of duodenal polyposis [51]
| No. of points |
|||
|---|---|---|---|
| 1 | 2 | 3 | |
| No. of polyps | 1–4 | 5–20 | >20 |
| Polyp size (mm) | 1–4 | 5–10 | >10 |
| Histology | Tubulous | Tubulovillous | Villous |
| Dysplasia | Mild | Moderate | Severe |
Stage 0, 0 points; stage I, 1–4 points; stage II, 5–6 points; stage III, 7–8 points; stage IV, 9–1 2 points.
Small-bowel cancer distal to the duodenum occurs rarely, with only 17 cases of jejunal carcinoma and three cases of ileal carcinoma in individuals with FAP reported [52]; hence, the clinical relevance of small-bowel polyps beyond the duodenum appears to be limited. Push enteroscopy (PE) has been commonly used for endoscopic screening in FAP patients to identify high-risk individuals. However, PE commonly results in insufficient screening of the small intestine, and the best screening method for small-bowel polyps in FAP patients is yet to be established [53].
Human MutY homolog-associated polyposis
Human MutY homolog (MUTYH)-associated polyposis (MAP) is a recently described adenomatous polyposis syndrome related to mutations in the base excision repair (BER) gene, MUTYH (formerly known as MYH) [54]. Patients with MAP typically present with clinical manifestations similar to that of AFAP, and the risk for CRC is estimated to be as high as 80% for biallelic MUTYH mutation carriers [55]. The exact incidence of MAP is unknown, but mutations in MUTYH likely account for 28% of cases of adenomatous polyposis syndrome [56]. In addition, biallelic mutations in MUTYH are related to early onset CRC without polyposis or defective DNA mismatch repair (MMR) genes. Patients who exhibit features of (A)FAP and have a family history of adenomas or CRC compatible with a recessive pattern of inheritance are appropriate candidates for MUTYH mutation analysis [57]. Screening for APC and MUTYH gene mutations may be performed in parallel in some patients, such as in those with isolated cases of multiple adenomas [58].
Biallelic mutation carriers should be managed in a manner similar to FAP patients, but because of the later age of polyposis onset, commencement of surveillance with colonoscopy is recommended at 25 years, followed by repeat colonoscopies every 1–2 years. In monoallelic MUTYH mutation carriers, baseline colonoscopy is suggested at 25 years of age; however, in the absence of adenomatous polyps, repeat screening should be performed every 3–5 years [58].
Gardner syndrome
Gardner syndrome (GS) is characterized by inherited colonic adenomatous polyposis together with a number of extracolonic lesions [10]. This syndrome arises from a mutation in the APC gene, and the number of colonic polyps is related to the locus of the mutation in the APC gene [23]. The common extraintestinal manifestations including osteomas [59] and dental abnormalities [60], cutaneous lesions [61], desmoid tumors [62,63], CHRPE [64], adrenal adenomas [65], and nasal angiofibromas [66] have been described in ~20% of patients with FAP. However, more patients with FAP show these features if they undergo detailed physical and radiologic examinations [67]. Thus, the difference between FAP and GS is somewhat semantic, and GS is usually considered to be a subset of FAP. In contrast, the term GS continues to be commonly applied, particularly in families that exhibit frequent and obvious extraintestinal lesions.
Turcot syndrome
Turcot syndrome (TS) was originally described by Turcot in 1959 [68,69]. It is characterized by the development of primary tumors of the central nervous system, such as glioblastoma multiforme and medulloblastoma, along with numerous adenomatous colorectal polyps and colonic adenocarcinoma. TS type I is characterized by the presence of glial tumors, relatively few colonic polyps, and cancer. TS type II is characterized by thousands of colonic polyps and increased risk for medulloblastoma [70]. The association between brain tumors and multiple colorectal adenomas can result from two distinct types of germline defects: mutation of the APC gene or mutation of a mismatch-repair gene [71]. However, the term ‘TS’ is no longer clinically meaningful because with the definition of the genetics of the familial colon cancers it became clear that brain tumors were associated with both FAP and Lynch syndrome.
Surgical options
For patients with known classical FAP, prophylactic proctocolectomy or colectomy is recommended [43,72]. The timing of surgery in patients less than 18 years of age is not yet established. In those patients with mild polyposis without a family history of early cancer or severe genotype, the timing of surgery can be individualized. Generally, total abdominal colectomy with ileorectal anastomosis (IRA) is preferred for AFAP and total proctocolectomy with ileal pouch-anal anastomosis (TPC/ IPAA) is recommended for FAR.
Total abdominal colectomy with ileorectal anastomosis
This surgical approach is indicated when the polyps in the rectum are amenable to endoscopic surveillance and resection. This also has advantages such as a low complication rate, good functional outcome, and lower risks for sexual or bladder dysfunction.
Total proctocolectomy with ileal pouch-anal anastomosis
TPC/IPAA is usually indicated for classical FAP patients with severe disease in the colon and/or rectum and patients with an unstable rectum after total abdominal colectomy with IRA. Patients with low adherence to follow-up would be indicated for this surgical option as well.
Total proctocolectomy with end ileostomy
This option can be indicated when IPAA is not feasible because of either tumor location or lack of technical skill. This procedure will lead to permanent stoma; hence, its indication should be carefully determined.
Treatment options for the patients with AFAP should be determined according to patient age and severity of disease. Patients younger than 21 years with small adenoma burden (fewer than 20 adenomas, all less than 1 cm in diameter, and none with advanced histology) can be followed up by colonoscopy and polypectomy every 1–2 years. Patients who are 21 or older with small adenoma burden can be followed up under the same strategies as the younger patients; however, colectomy with IRA may be considered. Patients with significant polyposis that is not considered manageable with polypectomy should be referred for colectomy with IRA; however, TPC/IPAA could be also indicated on the basis of the burden of disease in the rectum.
Chemoprevention of familial adenomatous polyposis
Prostaglandin plays a key role in the adenoma–carcinoma sequence by altering cell adhesion, inhibiting apoptosis, and promoting angiogenesis [73,74]. NSAIDs inhibit cyclooxygenase (COX), a key enzyme in the conversion of arachidonic acid to prostaglandins. Many drugs have been studied as potential agents for chemoprevention in FAP patients. Treatment with 150 mg sulindac [75–77] (an NSAID) twice daily resulted in a statistically significant reduction in polyp count and diameter compared with treatment with placebo [78]. In FAP patients who had undergone colectomy with IRA, the use of sulindac significantly reduced rectal polyp number, as well as led to a higher grade of adenoma recurrence [79].
Celecoxib, a selective COX-2 inhibitor, also caused a reduction in the mean number of polyps and polyp burden at a high dose compared with placebo [80]. However, the use of a COX-2 inhibitor for chemoprevention in FAP patients is limited because of potential cardiovascular toxicity [81–83].
Aspirin has not only a favorable cardiovascular profile but is used as primary pharmacotherapy in patients with cardiovascular risk factors. A dose of 600 mg/day aspirin for a mean of 25 months substantially reduced cancer incidence after 55.7 months in carriers of hereditary CRC. However, further studies are needed to establish the optimum dose and duration of aspirin treatment [84].
Recently reported studies on the surveillance for familial adenomatous polyposis patients
Progress in endoscopic technology in the current decade has provided more detailed and broad-ranging information on the extent of comorbidities related to FAP.
Colorectal screening
Colorectal screening with high-resolution (HR) chromo-endoscopy for FAP patients was reported to result in significantly better adenomatous lesion detection compared with conventional white light endoscopy [85]. Colorectal screening with HR chromoendoscopy may facilitate earlier and more effective detection of disease development in patients with AFAP, which might lead to prompt decision making on salvage surgery, although further prospective study is needed.
Screening for gastric and duodenal adenomas
HR upper endoscopy combined with chromoendoscopy improves the detection of duodenal adenomas compared with conventional white light endoscopy [86,87]. In addition, a combination of forward-viewing HR endoscopy for the duodenal region and side-viewing endoscopy for the periampullary region is useful for surveillance of duodenal adenomatosis in FAP patients [87]. The use of narrow-band imaging did not improve the detection rate of gastric polyps, but resulted in detection of more duodenal adenomas. Although this resulted in upgrades of the Spigelman stage, it was not clinically relevant partly because of the limited number of cases. Further study is needed to validate these results [88].
Screening for small-bowel lesions
Visualization of the ampulla using capsule endoscopy (CE) is apparently not sufficient [89]; however, CE is reported to be useful for the surveillance of jejunal–ileal polyps in high-risk patients with FAP [90–95]. Compared with PE or ileoscopy, CE results in a higher completion rate for small-bowel screening with less invasiveness [96]. The use of double-balloon enteroscopy-assisted chromoendoscopy of the small bowel also improves the detection rate of small intestine polyps in patients with FAP [97].
Although adenomas in the small intestine are reported to be rare, these two modalities should be performed complementarily to each other to provide the best outcome.
Conclusion
We reviewed the literature on the strategies for the diagnosis, surveillance, and treatment of FAP. Recent progress in endoscopic technology, including HR endoscopy, capsule endoscopy, and double-balloon endoscopy, has facilitated more detailed and wide-ranging investigation of the gastrointestinal tract. Further study will elucidate the potential of these new endoscopic technologies to enhance surveillance strategies for FAP.
Biography

Nitin Kumar
Harvard Medical School
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bussey H. Familial polyposis coli. Baltimore: Johns Hopkins University Press; 1975. [Google Scholar]
- 2.Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, et al. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 3.Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 4.Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 5.Wennstrom J, Pierce ER, McKusick VA. Hereditary benign and malignant lesions of the large bowel. Cancer. 1974;34:850. doi: 10.1002/1097-0142(197409)34:3+<850::aid-cncr2820340711>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 6.Petersen GM, Slack J, Nakamura Y. Screening guidelines and premorbid diagnosis of familial adenomatous polyposis using linkage. Gastroenterology. 1990;100:1658–1664. doi: 10.1016/0016-5085(91)90666-9. [DOI] [PubMed] [Google Scholar]
- 7.Brosens LA, Keller JJ, Offerhaus GJ, Goggins M, Giardiello FM. Prevention and management of duodenal polyps in familial adenomatous polyposis. Gut. 2005;54:1034–1043. doi: 10.1136/gut.2004.053843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Offerhaus GJ, Giardiello FM, Krush AJ, Booker SV, Tersmette AC, Kelley NC, Hamilton SR. The risk of upper gastrointestinal cancer in familial adenomatous polyposis. Gastroenterology. 1992;102:1980–1982. doi: 10.1016/0016-5085(92)90322-p. [DOI] [PubMed] [Google Scholar]
- 9.Gurbuz AK, Giardiello FM, Petersen GM, Krush AJ, Offerhaus GJ, Booker SV, et al. Desmoid tumors in familial adenomatous polyposis. Gut. 1993;35:377–381. doi: 10.1136/gut.35.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner EJ. Follow-up study of a group exhibiting dominant inheritance for a syndrome including intestinal polyps, osteomas, fibromas, and epidermal cysts. Am J Hum Genet. 1962;14:376–390. [PMC free article] [PubMed] [Google Scholar]
- 11.Søndergaard JO, Bülow S, Järvinen H, Wolf J, Witt IN, Tetens G. Dental anomalies in familial adenomatous polyposis coli. Acta Odontol Scand. 1987;45:61–63. doi: 10.3109/00016358709094355. [DOI] [PubMed] [Google Scholar]
- 12.Traboulsi EI, Murphy S, Dela ZC, Maumenee IH, Green WR. A clinicopathological study of the eyes in familial adenomatous polyposis with extracolonic manifestations (Gardner syndrome) Am J Ophthalmol. 1990;110:550–561. doi: 10.1016/s0002-9394(14)77880-8. [DOI] [PubMed] [Google Scholar]
- 13.Giardiello FM, Offerhaus GJA, Krush AJ, Booker SV, Tersmette AC, Mulder JW, et al. The risk of hepatoblastoma in familial adenomatous polyposis. J Pediatrics. 1991;119:766–768. doi: 10.1016/s0022-3476(05)80297-5. [DOI] [PubMed] [Google Scholar]
- 14.Giardiello FM, Offerhaus GJA, Lee DH, Krush AJ, Tersmette AC, Booker SV, et al. Increased risk of thyroid and pancreatic carcinoma in familial adenomatous polyposis. Gut. 1993;34:1394–1396. doi: 10.1136/gut.34.10.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton SR, Liu B, Parsons RE, Papadopoulos N, Jen J, Powell SM, et al. The molecular basis of Turcot syndrome. N Engl J Med. 1995;332:839–847. doi: 10.1056/NEJM199503303321302. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen M, Hes FJ, Nagengast FM, Weiss MM, Mathus-Vliegen EM, Morreau H, et al. Germline mutations in APC and MUTYH are responsible for the majority of families with attenuated familial adenomatous polyposis. Clin Genet. 2007;71:427–433. doi: 10.1111/j.1399-0004.2007.00766.x. [DOI] [PubMed] [Google Scholar]
- 17.Knudsen AL, Bülow S, Tomlinson I, Möslein G, Heinimann K, Christensen IJ AFAP Study Group. Attenuated familial adenomatous polyposis: results from an international collaborative study. Colorectal Dis. 2010;12:e243–e249. doi: 10.1111/j.1463-1318.2010.02218.x. [DOI] [PubMed] [Google Scholar]
- 18.Kinzler KW, Vogelstein B. Colorectal tumors. The genetic basis of human cancer. New York: McGraw-Hill; 1998. pp. 269–270. [Google Scholar]
- 19.Van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 20.Aoki K, Taketo MM. Adenomatous polyposis coli (APC): a multi-functional tumor suppressor gene. J Cell Sci. 2007;120:3327–3335. doi: 10.1242/jcs.03485. [DOI] [PubMed] [Google Scholar]
- 21.Friedl W, Aretz S. Familial adenomatous polyposis: experience from a study of 1164 unrelated German polyposis patients. Hered Cancer Clin Pract. 2005;3:95–114. doi: 10.1186/1897-4287-3-3-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedl W, Caspari R, Sengteller M, Uhlhaas S, Lamberti C, Jungck M, et al. Can APC mutation analysis contribute to therapeutic decisions in familial adenomatous polyposis? Experience from 680 FAP families. Gut. 2001;48:515–521. doi: 10.1136/gut.48.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bertario L, Russo A, Sala P, Varesco L, Giarola M, Mondini P, et al. Hereditary Colorectal Tumor Registry. Multiple approach to the exploration of genotype-phenotype correlations in familial adenomatous polyposis. J Clin Oncol. 2003;21:1698–1707. doi: 10.1200/JCO.2003.09.118. [DOI] [PubMed] [Google Scholar]
- 24.Spirio L, Olschwang S, Groden J, Robertson M, Samowitz W, Joslyn G, et al. Alleles of the APC gene: an attenuated form of familial polyposis. Cell. 1993;75:951–957. doi: 10.1016/0092-8674(93)90538-2. [DOI] [PubMed] [Google Scholar]
- 25.Giardiello FM, Brensinger JD, Luce MC, Petersen GM, Cayouette MC, Krush AJ, et al. Phenotypic expression in adenomatous polyposis families with mutation in the 5′ region of the adenomatous polyposis coli gene. Ann Intern Med. 1997;126:514–519. doi: 10.7326/0003-4819-126-7-199704010-00003. [DOI] [PubMed] [Google Scholar]
- 26.Brensinger JD, Laken SJ, Luce MC, Powell SM, Vance GH, Ahnen DJ, et al. Variable phenotype of familial adenomatous polyposis in pedigrees with 3′ mutations in the APC gene. Gut. 1998;43:548–552. doi: 10.1136/gut.43.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hes FJ, Nielsen M, Bik EC, Konvalinka D, Wijnen JT, Bakker E, et al. Somatic APC mosaicism: an underestimated cause of polyposis coli. Gut. 2007;57:71–76. doi: 10.1136/gut.2006.117796. [DOI] [PubMed] [Google Scholar]
- 28.Nagase H, Miyoshi Y, Horii A, Aoki T, Ogawa M, Utsunomiya J, et al. Correlation between the location of germ-line mutations in the APC gene and the number of colorectal polyps in familial adenomatous polyposis patients. Cancer Res. 1992;52:4055–4057. [PubMed] [Google Scholar]
- 29.Wallis YL, Morton DG, McKeown CM, Macdonald F. Molecular analysis of the APC gene in 205 families: extended genotype-phenotype correlations in FAP and evidence for the role of APC amino acid changes in colorectal cancer predisposition. J Med Genet. 1999;36:14–20. [PMC free article] [PubMed] [Google Scholar]
- 30.Nieuwenhuis MH, Vasen HF. Correlations between mutation site in APC and phenotype of familial adenomatous polyposis (FAP): a review of the literature. Crit Rev Oncol Hematol. 2007;61:153–161. doi: 10.1016/j.critrevonc.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Rai K, Sarkar S, Broadbent TJ, Voas M, Grossmann KF, Nadauld LD, et al. DNA demethylase activity maintains intestinal cells in an undifferentiated state following loss of APC. Cell. 2010;142:930–942. doi: 10.1016/j.cell.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Comprehensive Cancer Network. National Comprehensive Cancer Network NCCN colorectal cancer screening practice guidelines. Oncology. 1999;13:152–179. [PubMed] [Google Scholar]
- 33.Sieber OM, Lamlum H, Crabtree MD, Rowan AJ, Barclay E, Lipton L, et al. Whole-gene APC deletions cause classical familial adenomatous polyposis, but not attenuated polyposis or “multiple” colorectal adenomas. Proc Natl Acad Sci USA. 2002;99:2954–2958. doi: 10.1073/pnas.042699199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aretz S, Stienen D, Uhlhaas S, Pagenstecher C, Mangold E, Caspari R, et al. Large submicroscopic genomic APC deletions are a common cause of typical familial adenomatous polyposis. J Med Genet. 2005;42:185–192. doi: 10.1136/jmg.2004.022822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyoshi Y, Ando H, Nagase H, Nishisho I, Horii A, Miki Y, et al. Germ-line mutations of the APC gene in 53 familial adenomatous polyposis patients. Proc Natl Acad Sci USA. 1992;89:4452–4456. doi: 10.1073/pnas.89.10.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powell SM, Petersen GM, Krush AJ, Booker S, Jen J, Giardiello FM, et al. Molecular diagnosis of familial adenomatous polyposis. N Engl J Med. 1993;329:1982–1987. doi: 10.1056/NEJM199312303292702. [DOI] [PubMed] [Google Scholar]
- 37.Bunyan DJ, Eccles DM, Sillibourne J, Wilkins E, Thomas NS, Shea-Simonds J, et al. Dosage analysis of cancer predisposition genes by multiplex ligation-dependent probe amplification. Br J Cancer. 2004;91:1155–1159. doi: 10.1038/sj.bjc.6602121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michils G, Tejpar S, Thoelen R, van Cutsem E, Vermeesch JR, Fryns JP, et al. Large deletions of the APC gene in 15% of mutation-negative patients with classical polyposis (FAP): a Belgian study. Hum Mutat. 2005;25:125–134. doi: 10.1002/humu.20122. [DOI] [PubMed] [Google Scholar]
- 39.Smith-Ravin J, Pack K, Hodgson S, Tay SK, Phillips R, Bodmer W. APC mutation associated with late onset of familial adenomatous polyposis. J Med Genet. 1994;31:888–90. doi: 10.1136/jmg.31.11.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petersen GM, Slack J, Nakamura Y. Screening guidelines and premorbid diagnosis of familial adenomatous polyposis using linkage. Gastroenterology. 1991;100:1658–1664. doi: 10.1016/0016-5085(91)90666-9. [DOI] [PubMed] [Google Scholar]
- 41.Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, et al. Colorectal cancer screening and surveillance: clinical guidelines, evidence, and rationale. Gastroenterology. 1997;112:594–642. doi: 10.1053/gast.1997.v112.agast970594. [DOI] [PubMed] [Google Scholar]
- 42.Burt RW, Cannon JA, David S, Ford JM, Giardiello FM, Halvers AL, et al. NCCN Clinical Practice Guidelines in Oncology; Colorectal Cancer Screening. doi: 10.6004/jnccn.2010.0003. [Online, 4 Aug 2013]. www.nccn.org. [DOI] [PubMed]
- 43.Abraham SC, Nobukawa B, Giardiello FM, Hamilton SR, Wu TT. Sporadic fundic gland polyps: common gastric polyps arising through activating mutations in the beta-catenin gene. Am J Pathol. 2001;158:1005–1010. doi: 10.1016/s0002-9440(10)64047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burt RW. Gastric fundic gland polyps. Gastroenterology. 2003;125:1462–1469. doi: 10.1016/j.gastro.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 45.Zwick A, Munir M, Ryan CK, Gian J, Burt RW, Leppert M, et al. Gastric adenocarcinoma and dysplasia in fundic gland polyps of a patient with attenuated adenomatous polyposis coli. Gastroenterology. 1997;113:659–663. doi: 10.1053/gast.1997.v113.pm9247488. [DOI] [PubMed] [Google Scholar]
- 46.Hofgartner WT, Thorp M, Ramus MW, Delorefice G, Chey WY, Ryan CK, et al. Gastric adenocarcinoma associated with fundic gland polyps in a patient with attenuated familial adenomatous polyposis. Am J Gastroenterol. 1999;94:2275–2281. doi: 10.1111/j.1572-0241.1999.01312.x. [DOI] [PubMed] [Google Scholar]
- 47.Wallace MH, Phillips RK. Upper gastrointestinal disease in patients with familial adenomatous polyposis. Br J Surg. 1998;85:742–750. doi: 10.1046/j.1365-2168.1998.00776.x. [DOI] [PubMed] [Google Scholar]
- 48.Spigelman AD, Williams CB, Talbot IC, Domizio P, Phillips RK. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet. 1989;2:783–785. doi: 10.1016/s0140-6736(89)90840-4. [DOI] [PubMed] [Google Scholar]
- 49.Iida M, Yao T, Itoh H, Watanabe H, Matsui T, Iwashita A, Fujishima M. Natural history of duodenal lesions in Japanese patients with familial adenomatosis coli (Gardner’s syndrome) Gastroenterology. 1989;96:1301–1306. doi: 10.1016/s0016-5085(89)80017-4. [DOI] [PubMed] [Google Scholar]
- 50.Iida M, Aoyagi K, Fujimura Y, Matsumoto T, Hizawa K, Nakamura S. Nonpolypoid adenomas of the duodenum in patients with familial adenomatous polyposis (Gardner’s syndrome) Gastrointest Endosc. 1996;44:305–308. doi: 10.1016/s0016-5107(96)70169-4. [DOI] [PubMed] [Google Scholar]
- 51.Groves CJ, Saunders BP, Spigelman AD, Phillips RK. Duodenal cancer in patients with familial adenomatous polyposis (FAP): results of a 10 year prospective study. Gut. 2002;50:636–641. doi: 10.1136/gut.50.5.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruys AT, Alderlieste YA, Gouma DJ, Dekker E, Mathus-Vliegen EM. Jejunal cancer in patients with familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2010;8:731–733. doi: 10.1016/j.cgh.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 53.Sidhu R, Sanders DS, Morris AJ, McAlindon ME. Guidelines on small bowel enteroscopy and capsule endoscopy in adults. Gut. 2008;57:125–136. doi: 10.1136/gut.2007.129999. [DOI] [PubMed] [Google Scholar]
- 54.Claes K, Dahan K, Tejpar S, De Paepe A, Bonduelle M, Abramowicz M, et al. The genetics of familial adenomatous polyposis (FAP) and MutYH-associated polyposis (MAP) Acta Gastroenterol Belg. 2011;74:421–426. [PubMed] [Google Scholar]
- 55.Jenkins MA, Croitoru ME, Monga N, Cleary SP, Cotterchio M, Hopper JL, Gallinger S. Risk of colorectal cancer in monoallelic and biallelic carriers of MYH mutations: a population-based case-family study. Cancer Epidemiol Biomarkers Prev. 2006;15:312–314. doi: 10.1158/1055-9965.EPI-05-0793. [DOI] [PubMed] [Google Scholar]
- 56.Zorcolo L, Fantola G, Balestrino L, Restivo A, Vivanet C, Spina F, et al. MUTYH-associated colon disease: adenomatous polyposis is only one of the possible phenotypes. A family report and literature review. Tumori. 2011;97:676–680. doi: 10.1177/030089161109700523. [DOI] [PubMed] [Google Scholar]
- 57.Lefevre JH, Rodrigue CM, Mourra N, Bennis M, Flejou JF, Parc R, et al. Implication of MYH in colorectal polyposis. Ann Surg. 2006;244:874–879. doi: 10.1097/01.sla.0000246937.54435.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jasperson KW. Genetic testing by cancer site: colon (polyposis syndromes) Cancer J. 2012;18:328–333. doi: 10.1097/PPO.0b013e3182609300. [DOI] [PubMed] [Google Scholar]
- 59.Bilkay U, Erdem O, Ozek C, Helvaci E, Kilic K, Ertan Y, Gurler T. Benign osteoma with Gardner syndrome: review of the literature and report of a case. J Craniofac Surg. 2004;15:506–509. doi: 10.1097/00001665-200405000-00032. [DOI] [PubMed] [Google Scholar]
- 60.Bussey HJ, Veale AM, Morson BC. Genetics of gastrointestinal polyposis. Gastroenterology. 1978;74:1325–1330. [PubMed] [Google Scholar]
- 61.Sturt NJ, Clark SK. Current ideas in desmoid tumours. Fam Cancer. 2006;5:275–285. doi: 10.1007/s10689-005-5675-1. [DOI] [PubMed] [Google Scholar]
- 62.Clark SK, Neale KF, Landgrebe JC, Phillips RK. Desmoid tumours complicating familial adenomatous polyposis. Br J Surg. 1999;86:1185–1189. doi: 10.1046/j.1365-2168.1999.01222.x. [DOI] [PubMed] [Google Scholar]
- 63.Nieuwenhuis MH, De Vos Tot Nederveen Cappel W, Botma A, Nagengast FM, Kleibeuker JH, Mathus-Vliegen EM, et al. Desmoid tumors in a Dutch cohort of patients with familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2008;6:215–219. doi: 10.1016/j.cgh.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 64.Touriño R, Conde-Freire R, Cabezas-Agrícola JM, Rodríguez-Aves T, López-Valladares MJ, Otero-Cepeda JL, Capeans C. Value of the congenital hypertrophy of the retinal pigment epithelium in the diagnosis of familial adenomatous polyposis. Int Ophthalmol. 2004;25:101–112. doi: 10.1023/b:inte.0000031739.62559.ac. [DOI] [PubMed] [Google Scholar]
- 65.Bläker H, Sutter C, Kadmon M, Otto HF, Von Knebel-Doeberitz M, Gebert J, Helmke BM. Analysis of somatic APC mutations in rare extracolonic tumors of patients with familial adenomatous polyposis coli. Genes Chromosomes Cancer. 2004;41:93–98. doi: 10.1002/gcc.20071. [DOI] [PubMed] [Google Scholar]
- 66.Giardiello FM, Hamilton SR, Krush AJ, Offerhaus JA, Booker SV, Petersen GM. Nasopharyngeal angiofibroma in patients with familial adenomatous polyposis. Gastroenterology. 1993;105:1550–1552. doi: 10.1016/0016-5085(93)90164-8. [DOI] [PubMed] [Google Scholar]
- 67.Bisgaard ML, Bülow S. Familial adenomatous polyposis (FAP): genotype correlation to FAP phenotype with osteomas and sebaceous cysts. Am J Med Genet A. 2006;140:200–204. doi: 10.1002/ajmg.a.31010. [DOI] [PubMed] [Google Scholar]
- 68.Turcot J, Despres JP, Pierre F. Malignant tumors of the central nervous system associated with familial. Dis Colon Rectum. 1959;2:465–468. doi: 10.1007/BF02616938. [DOI] [PubMed] [Google Scholar]
- 69.Tops CMJ, Vasen HFA, van Berge Henegouwen G, Simoons PP, van de Klift HM, van Leeuwen SJ, et al. Genetic evidence that Turcot syndrome is not allelic to familial adenomatous polyposis. Am J Med Genet. 1992;43:888–893. doi: 10.1002/ajmg.1320430528. [DOI] [PubMed] [Google Scholar]
- 70.Paraf F, Jothy S, Van Meir EG. Brain tumor-polyposis syndrome: two genetic diseases? J Clin Oncol. 1997;15:2744–2758. doi: 10.1200/JCO.1997.15.7.2744. [DOI] [PubMed] [Google Scholar]
- 71.Hamilton SR, Liu B, Parsons RE, Papadopoulos N, Jen J, Powell SM, et al. The molecular basis of Turcot’s syndrome. N Engl J Med. 1995;332:839. doi: 10.1056/NEJM199503303321302. [DOI] [PubMed] [Google Scholar]
- 72.Kastrinos F, Syngal S. Inherited colorectal cancer syndromes. Cancer J. 2011;17:405–415. doi: 10.1097/PPO.0b013e318237e408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 74.Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, Dubois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 75.Giardiello FM, Offerhaus GJ, DuBois RN. The role of nonsteroidal anti-inflammatory drugs in colorectal cancer prevention. Eur J Cancer. 1995;31A:1071–1076. doi: 10.1016/0959-8049(95)00137-8. [DOI] [PubMed] [Google Scholar]
- 76.Tonelli F, Valanzano R, Messerini L, Ficari F. Long-term treatment with sulindac in familial adenomatous polyposis: is there an actual efficacy in prevention of rectal cancer? J Surg Oncol. 2000;74:15–20. doi: 10.1002/1096-9098(200005)74:1<15::aid-jso4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 77.Matsumoto T, Nakarmura S, Esaki M, Yao T, Iida M. Effect of the non-steroidal anti-inflammatory drug sulindac on colorectal adenomas of uncolectomized familial adenomatous polyposis. J Gastroenterol Hepatol. 2006;21:251–257. doi: 10.1111/j.1440-1746.2006.04181.x. [DOI] [PubMed] [Google Scholar]
- 78.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 79.Cruz-Correa M, Hylind LM, Romans KE, Booker SV, Giardiello FM. Long-term treatment with sulindac in familial adenomatous polyposis: a prospective cohort study. Gastroenterology. 2002;122:641–645. doi: 10.1053/gast.2002.31890. [DOI] [PubMed] [Google Scholar]
- 80.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 81.Solomon SD, McMurray JJV, Pfeffer MA, Wittes J, Fowler R, Finn P, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 82.Baron JA, Sandler RS, Bresalier RS, Lanas A, Morton DG, Riddell R, et al. Cardiovascular events associated with rofecoxib: final analysis of the APPROVe trial. Lancet. 2008;372:1756–1764. doi: 10.1016/S0140-6736(08)61490-7. [DOI] [PubMed] [Google Scholar]
- 83.Solomon SD, Wittes J, Finn PV, Fowler R, Viner J, Bertagnolli MM. Cardiovascular risk of celecoxib in 6 randomized placebo-controlled trials: the cross trial safety analysis. Circulation. 2008;117:2104–2113. doi: 10.1161/CIRCULATIONAHA.108.764530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Burn J, Gerdes AM, Macrae F, Mecklin JP, Moeslein G, Olschwang S, et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378:2081–2087. doi: 10.1016/S0140-6736(11)61049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Matsumoto T, Esaki M, Fujisawa R, Nakamura S, Yao T, Iida M. Chromoendoscopy, narrow-band imaging colonoscopy, and autofluorescence colonoscopy for detection of diminutive colorectal neoplasia in familial adenomatous polyposis. Dis Colon Rectum. 2009;52:1160–1165. doi: 10.1007/DCR.0b013e31819ef6fe. [DOI] [PubMed] [Google Scholar]
- 86.Picasso M, Filiberti R, Blanchi S, Conio M. The role of chromoendoscopy in the surveillance of the duodenum of patients with familial adenomatous polyposis. Dig Dis Sci. 2007;52:1906–1909. doi: 10.1007/s10620-006-9653-8. [DOI] [PubMed] [Google Scholar]
- 87.Dekker E, Boparai KS, Poley JW, Mathus-Vliegen EM, Offerhaus GJ, Kuipers EJ, et al. High resolution endoscopy and the additional value of chromoendoscopy in the evaluation of duodenal adenomatosis in patients with familial adenomatous polyposis. Endoscopy. 2009;41:666–669. doi: 10.1055/s-0029-1214980. [DOI] [PubMed] [Google Scholar]
- 88.Lopez-Ceron M, van den Broek FJ, Mathus-Vliegen EM, Boparai KS, van Eeden S, Fockens P, Dekker E. The role of high-resolution endoscopy and narrow-band imaging in the evaluation of upper GI neoplasia in familial adenomatous polyposis. Gastrointest Endosc. 2013;77:542–550. doi: 10.1016/j.gie.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 89.Iaquinto G, Fornasarig M, Quaia M, Giardullo N, D’Onofrio V, Iaquinto S, et al. Capsule endoscopy is useful and safe for small-bowel surveillance in familial adenomatous polyposis. Gastrointest Endosc. 2008;67:61–67. doi: 10.1016/j.gie.2007.07.048. [DOI] [PubMed] [Google Scholar]
- 90.Wong RF, Tuteja AK, Haslem DS, Pappas L, Szabo A, Ogara MM, DiSario JA. Video capsule endoscopy compared with standard endoscopy for the evaluation of small-bowel polyps in persons with familial adenomatous polyposis (with video) Gastrointest Endosc. 2006;64:530–537. doi: 10.1016/j.gie.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 91.Tescher P, Macrae FA, Speer T, Stella D, Gibson R, Tye-Din JA, et al. Surveillance of FAP: a prospective blinded comparison of capsule endoscopy and other GI imaging to detect small bowel polyps. Hered Cancer Clin Pract. 2010;8:3. doi: 10.1186/1897-4287-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schulmann K, Hollerbach S, Kraus K, Willert J, Vogel T, Möslein G, et al. Feasibility and diagnostic utility of video capsule endoscopy for the detection of small bowel polyps in patients with hereditary polyposis syndromes. Am J Gastroenterol. 2005;100:27–37. doi: 10.1111/j.1572-0241.2005.40102.x. [DOI] [PubMed] [Google Scholar]
- 93.Katsinelos P, Kountouras J, Chatzimavroudis G, Zavos C, Pilpilidis I, Fasoulas K, et al. Wireless capsule endoscopy in detecting small-intestinal polyps in familial adenomatous polyposis. World J Gastroenterol. 2009;15:6075–6079. doi: 10.3748/wjg.15.6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Günther U, Bojarski C, Buhr HJ, Zeitz M, Heller F. Capsule endoscopy in small-bowel surveillance of patients with hereditary polyposis syndromes. Int J Colorectal Dis. 2010;25:1377–1382. doi: 10.1007/s00384-010-0982-x. [DOI] [PubMed] [Google Scholar]
- 95.Yamada A, Watabe H, Iwama T, Obi S, Omata M, Koike K. The prevalence of small intestinal polyps in patients with familial adenomatous polyposis: a prospective capsule endoscopy study. Fam Cancer. 2013 doi: 10.1007/s10689-013-9668-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 96.Plum N, May A, Manner H, Ell C. Small-bowel diagnosis in patients with familial adenomatous polyposis: comparison of push enteroscopy, capsule endoscopy, ileoscopy, and enteroclysis. Z Gastroenterol. 2009;47:339–346. doi: 10.1055/s-2008-1027984. [DOI] [PubMed] [Google Scholar]
- 97.Mönkemüller K, Fry LC, Ebert M, Bellutti M, Venerito M, Knippig C, et al. Feasibility of double-balloon enteroscopy-assisted chromoendoscopy of the small bowel in patients with familial adenomatous polyposis. Endoscopy. 2007;39:52–57. doi: 10.1055/s-2006-945116. [DOI] [PubMed] [Google Scholar]