Abstract
Background
Hemichorea–hemiballism (HCHB) is a hyperkinetic movement disorder with features of both chorea and ballism occurring on the same side.
Case report
We present a case of HCHB due to nonketotic hyperglycemia (NKH) that was the initial presentation of diabetes and was irreversible clinically even after 6 months of optimal blood sugar control.
Discussion
Although HCHB due to hyperglycemia is a potentially reversible condition in the majority of patients, prolonged uncontrolled hyperglycemia may cause ischemic insult and persistent symptoms. Hyperglycemia should always be kept in the list of differentials while dealing with patients who are newly diagnosed with HCHB.
Keywords: Hemichorea–hemiballism, nonketotic hyperglycemia, magnetic resonance spectroscopy
Introduction
Hemichorea–hemiballism (HCHB) is a hyperkinetic movement disorder that includes features of both chorea and ballism and is characterized by unilateral, proximal, and/or distal movements that are continuous or intermittent, abrupt, jerky, and involuntary.1 The basal ganglia (BG) influences body movement via thalamocortical tracts, and dysfunction in this structure is associated with a myriad of different movement disorders including ballism (predominantly proximal, higher amplitude movements) and chorea (relatively lower amplitude, both proximal and distal movements). HCHB is diagnosed when chorea and ballism occur together clinically on one side of the body. This condition is more common among elderly, and females are slightly more affected than males (1.76:1).1
Although various structural lesions have been associated with HCHB, the most common cause is cerebral vascular disease either in the form of an infarct or hemorrhage of the BG, thalamus, and subthalamic nucleus followed by nonketotic hyperglycemia (NKH).2 Notably, HCHB due to NKH occurs unilaterally in contrast to other metabolic causes that result in bilateral chorea.3 Furthermore, HCHB due to NKH resolves after correction of hyperglycemia in the majority of cases; however, the time frame for improvement varies from case to case and may be as long as 6 months.4 Other identified causes of bilateral chorea/ballism that are initially more unilateral include chorea associated with infections, sequela of rheumatic fever, thyrotoxicosis, systemic lupus erythematosus, systemic vasculitis (inflammatory), autoimmune etiologies, hypoxic encephalopathy, and drug-induced or substance abuse. Rarely, chorea associated with neuroacanthocytosis and Huntington disease may also start unilaterally.1,3,5 Magnetic resonance imaging (MRI) of brain is the most important investigation for delineating these lesions, but it does not provide definitive guidance regarding prognosis and long-term outcome. Developing tools to assess the likelihood of reversibility and predict outcome remains an important goal.
Case Report
A 52-year-old male from a rural background who was not known to be diabetic presented to the outpatient department with a 5-week history of generalized fatigue and shoulder and back pain. For the previous month he had developed involuntary, arrhythmic, and irregular abnormal movements of his left side including the face. These initially occurred in an intermittent fashion but had been nearly continuous for the last 2 days. He denied any specific relieving or aggravating factors or diurnal variation; however, the movements disappeared during sleep. He was a known hypertensive patient on regular medications and did not report a history of diabetes mellitus or stroke. He also denied recent drug intake including antipsychotics and any addiction. None of his family members had a history of similar illness. The hyperkinetic movements were quite disabling and interfered with volitional left-sided movements so that he could not perform activities of daily living. Neurologic examination revealed mildly increased tone of the left arm and leg along with mild weakness of the left proximal upper and lower limbs. Other notable findings were distal and proximal choreoathetoid movements of the left upper and lower limbs (upper more than lower), intermittent dyskinesia of the tongue and face, and infrequent proximal ballistic swings of moderate amplitude that involved the left arm (Video 1).
Video 1. Initial Presentation. The patient had a distal and proximal choreoathetoid movements of the left upper and lower limbs (upper more than lower) and intermittent dyskinesia of the tongue and face along with infrequent proximal ballistic swings involving the left arm.
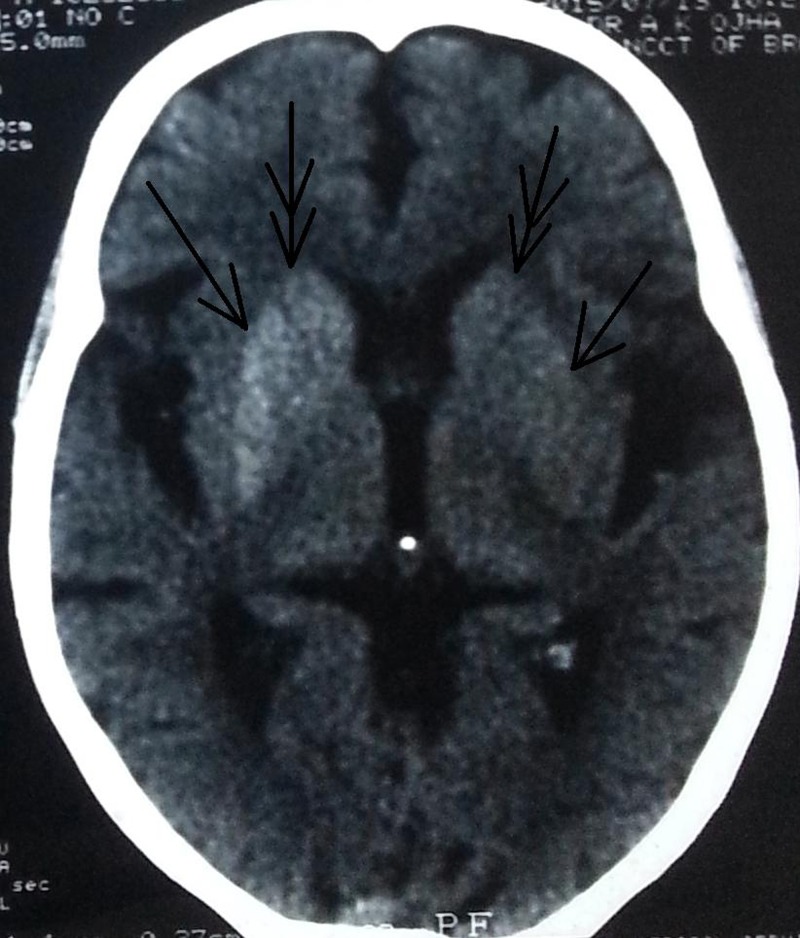
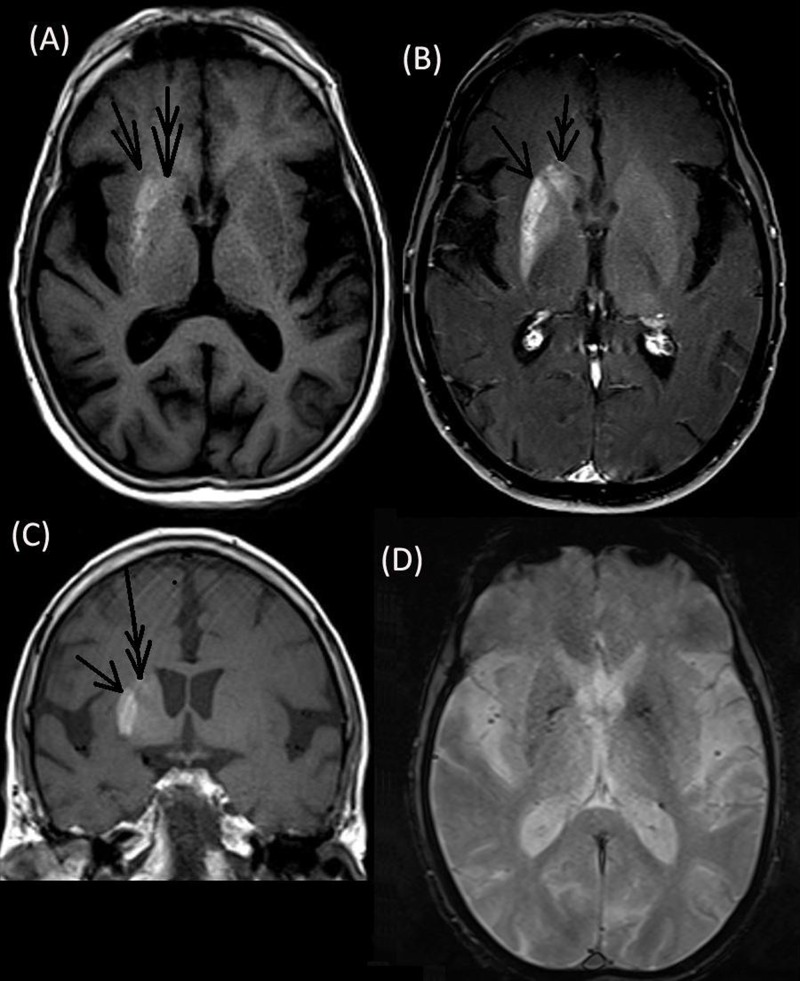
Computed tomography (CT) was performed to rule out vascular causes and showed hyperdensity of the putamen and head of the caudate nucleus, which was more pronounced on the right side (Figure 1). Subsequent brain MRI revealed T1 hyperintensity of corresponding regions that enhanced mildly on contrast but were not bright on diffusion imaging and had no bloom on gradient echo (GRE) (Figure 2). Magnetic resonance spectroscopy (MRS) showed the N-acetyl aspartate (NAA)/creatine (Cr) ratio as 1.01 (normal side 1.82), choline (Cho)/Cr as 1.29 (normal side 1.01) and NAA/Cho ratio as 0.78 (normal side 1.80) along with a lactate (Lac) peak. Magnetic resonance angiography (MRA) and electroencephalogram (EEG) were normal. The patient’s random blood sugar was recorded as 356 mg/dL (normal <126 mg/dL) and glycated hemoglobin A1C (HbA1C) was 16.2 (normal <5.7). However, his urine was negative for ketone bodies, and serum osmolality level was 291 mOsm/kg (normal range, 270–290 mOsm/kg). Serum calcium level was recorded as 8.7 mg/dL (normal range – 8.5–10.2 mg/dl), serum phosphate was 3 mg/dL (normal range 2.5–4.5 mg/dL, intact parathyroid hormone level was 30 ng/L (normal range 10–65 ng/L), and thyroid-stimulating hormone was 4 mIU/L (normal range 0.4–4.2). Other parameters like renal and liver function, antinuclear antibodies, antineutrophil cytoplasmic antibodies, and serum ceruloplasmin levels were within normal limits.
Figure 1. Brain Computed Tomography. The scan revealed hyperdensity of the lentiform and caudate nuclei that was more prominent on the right side.
Figure 2. Brain Magnetic Resonance Imaging. Axial T1 (A) and coronal (C) sequences showing hyperintensity of the right putamen (single arrow) and caudate nucleus (double arrow) that enhanced mildly on contrast (B) with a normal gradient echo sequence (D).
The patient was prescribed long-acting insulin along with short acting premeal insulin, and lifestyle interventions like calorie restriction, low fat intake, and weight loss were advised. Haloperidol was added at an initial dose of 0.25 ,g/day and titrated slowly to a dose of 3.0 mg/day over 12 weeks. Despite this drug regimen and adequate blood sugar control, his choreiform movements did not decrease. Subsequently tetrabenazine was also added and titrated up to 50 mg/day. Movements did not decrease even at 6 months after therapy initiation despite resolution of findings on brain MRI (Video 2).
Video 2. Presentation 6 Months After Therapy Initiation: The movements of the patient persisted even at 6 months after initiation of therapy.
Discussion
Classical HCHB caused by a focal vascular lesion can be a life-threatening situation that may complicate with inexorable progression to death within weeks or months; therefore, early recognition is important. This is in contrast to HCHB caused by hyperglycemia, which is a treatable disorder with a good prognosis.3,5,6 Imaging is useful in planning a treatment approach for HCHB.
There are few causes of asymmetric/unilateral BG hyperdensity on noncontrast head CT and T1 hyperintensity on MRI, such as early subacute hemorrhage/blood products and asymmetric calcification/mineralization including those associated with underlying lesions such as developmental venous anomalies.7,8 In the current case, brain MRI revealed T1 hyperintensity of the putamen and head of the caudate nucleus, which mildly enhanced on contrast but was neither bright on diffusion imaging nor black on GRE (which could suggest vascular pathology). Thus, we suspected diabetes mellitus with NKH as the cause of HCHB in this patient. His random blood sugar was subsequently recorded as 356 mg/dL, and HbA1C was 16.2. Furthermore, venous anomaly of brain was ruled out by normal MRA findings. CT revealed bilateral hyperdensity of the putamen and head of the caudate nucleus that was more pronounced on the right side. Among various regions of BG, the putamen is almost always involved in HCHB.1 Although isolated involvement of the globus pallidus or caudate nucleus has not been reported, both structures can be involved in conjunction with other areas as in the present case.1 Findings on susceptibility-weighted imaging (SWI) or GRE have been mixed.8–10 In general, lesion contrast enhancement is not observed.8
Several researchers have hypothesized that T1 hyperintensity may be due to the protein hydration layer in the cytoplasm of swollen, reactive astrocytes (gemistocytes).8–10 Others have claimed localized Wallerian degeneration as a consequence of transient ischemic changes or desiccation as the main pathogenic mechanism.1,10 The signal abnormality has also been proposed to represent putaminal petechial hemorrhage,11 but the hyperintensity respects neuroanatomic boundaries, there is no evolution of methemoglobin in T2-weighted sequence, and neither GRE nor SWI show any evidence of blood.12 Demyelination has also been proposed as a plausible explanation responsible for the characteristic lesions, similar to those seen in diabetic peripheral neuropathy that lead to exchange of myelin-bound and axonal water with resultant T1 shortening.10 HCHB in NKH is due to the shift of cerebral metabolism to the anaerobic pathway, abandoning the Krebs cycle and thereby increasing metabolization of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) into succinic acid. Subsequently, GABA and acetate are rapidly depleted and not readily resynthesized, ultimately leading to reductions of both GABA and acetylcholine in the BG. Coupled with metabolic acidosis and a lack of energy production, these events can cause HCHB.1 However, the reason for unilateral symptoms and BG involvement remains unclear.
Neuroimaging findings of NKH are usually reversible with appropriate treatment.1 Our patient had only a partially reversible syndrome; imaging abnormalities resolved, but HCHB persisted even 6 months after therapy initiation and achieving blood sugar control. Wu et al reported a case of irreversible HCHB and showed that EEG appearance of periodic lateralized epileptiform discharges (PLEDs) could indicate an irreversible outcome.13 They concluded that cerebral events caused by hyperglycemia can be permanent due to a prolonged and untreated course and may lead to PLEDs. However, the present patient’s EEG was normal. Similarly, other researchers have reported cases of HCHB with irreversible clinical and/or imaging findings.4,14,15
Tung et al hypothesized that hyperglycemia can result in an ischemic penumbra and reversible clinical syndrome/neuroimaging abnormalities in patients with HCHB; however, prolonged hyperglycemia may result in true infarction with an irreversible clinical syndrome.4 Lin et al studied stroke patients and postulated that decreased NAA/Cr and NAA/Cho ratios, as well as an increased Lac/Cr ratio often indicate irreversible infarction, and the monitoring of the NAA peak may be considered as an indicator for evaluating the effectiveness of treatment for cerebral infarction.16 In the current case there was both decreased NAA/Cr and NAA/Cho ratios (NAA/creatine (Cr) ratio = 1.01 and NAA/Cho ratio = 0.78 as compared to other side) along with a Lac peak prompting towards an infarction. Thus, we postulate that because the patient had prolonged hyperglycemia that was not controlled, it resulted in “true infarction” leading to irreversible/only partially reversible clinical findings. Lai et al performed MRS in eight patients of HCHB due to NKH and showed the mean NAA/Cr ratio to be 1.45 in the HCHB side compared to 1.82 on the contralateral side (P = 0.01). The corresponding Cho/Cr ratios were 1.3 and 1.11, respectively (P = 0.005). They concluded that low NAA/Cr suggested neuronal loss or damage, high Cho/Cr indicated gliosis, and the presence of Lac could suggest mild ischemia due to acute vascular events during hyperglycemia and underlying chronic focal cerebrovascular diseases in diabetes.17 Most of the patients in their cohort had good prognoses; however, the mean NAA/Cr ratio in their series was 1.45, and the lowest value was 1.15. For comparison, our patient’s ratio was 1.01. NAA/Ch ratios were not assessed in their study.
Our case is atypical in two aspects. Firstly, HCHB is rarely the initial presentation of diabetes,6 and it can usually be reversed within 6 months. To summarize, HCHB due to NKH is a reversible condition in most patients, especially if hyperglycemia is corrected early in the disease course. All patients with this clinical presentation should be screened for diabetes as HCHB can be the initial manifestation of NKH associated with diabetes.
Footnotes
Funding: None.
Financial Disclosures: None.
Conflict of Interest: The authors report no conflict of interest.
Ethics Statement: All patients that appear on video have provided written informed consent; authorization for the videotaping and for publication of the videotape was provided.
References
- 1.Oh SH, Lee KY, Im JH, Lee MS. Chorea associated with non-ketotic hyperglycemia and hyperintensity basal ganglia lesion on T1-weighted brain MRI study: a meta-analysis of 53 cases including four present cases. J Neurol Sci. 2002;200:57–62. doi: 10.1016/s0022-510x(02)00133-8. doi: 10.1016/S0022-510X(02)00133-8. [DOI] [PubMed] [Google Scholar]
- 2.Ohara S. Diabetic hemichorea-hemiballism. Austin J Clin Neurol. 2015;2((4)):1037. [Google Scholar]
- 3.Lai PH, Tien RD, Chang MH, et al. Chorea-ballismus with nonketotic hyperglycemia in primary diabetes mellitus. AJNR Am J Neuroradiol. 1996;17:1057–1064. [PMC free article] [PubMed] [Google Scholar]
- 4.Tung CS, Guo YC, Lai CL, Liou LM. Irreversible striatal neuroimaging abnormalities secondary to prolonged, uncontrolled diabetes mellitus in the setting of progressive focal neurological symptoms. Neurol Sci. 2010;31((1)):57–60. doi: 10.1007/s10072-009-0127-6. doi: 10.1007/s10072-009-0127-6. [DOI] [PubMed] [Google Scholar]
- 5.Piccolo I, Defanti CA, Soliveri P, Volontè MA, Cislaghi G, Girotti F. Cause and course in a series of patients with sporadic chorea. J Neurol. 2003;250((4)):429–435. doi: 10.1007/s00415-003-1010-7. doi: 10.1007/s00415-003-1010-7. [DOI] [PubMed] [Google Scholar]
- 6.Ray S, Howlader S, Chakraborty S, Chakraborty PP, Ghosh S. Hemichorea-hemiballism as the first presentation of type 2 diabetes. Clin Diabetes. 2015;33((2)):87–89. doi: 10.2337/diaclin.33.2.87. doi: 10.2337/diaclin.33.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai PH, Chen C, Liang HL, Pan HB. Hyperintense basal ganglia on T1-weighted MR imaging. AJR Am J Roentgenol. 1999;172((4)):1109–1115. doi: 10.2214/ajr.172.4.10587157. doi: 10.2214/ajr.172.4.10587157. [DOI] [PubMed] [Google Scholar]
- 8.Lee EJ, Choi JY, Lee SH, Song SY, Lee YS. Hemichorea-hemiballism in primary diabetic patients: MR correlation. J Comput Assist Tomogr. 2002;26((6)):905–911. doi: 10.1097/00004728-200211000-00009. doi: 10.1097/00004728-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Cherian A, Thomas B, Baheti NN, Chemmanam T, Kesavadas C. Concepts and controversies in nonketotic hyperglycemia-induced hemichorea: further evidence from susceptibility-weighted MR imaging. J Magn Reson Imaging. 2009;29:699–703. doi: 10.1002/jmri.21672. doi: 10.1002/jmri.21672. [DOI] [PubMed] [Google Scholar]
- 10.Wintermark M, Fischbein NJ, Mukherjee P, Yuh EL, Dillon WP. Unilateral putaminal CT, MR, and diffusion abnormalities secondary to nonketotic hyperglycemia in the setting of acute neurologic symptoms mimicking stroke. AJNR Am J Neuroradiol. 2004;25:975–976. [PMC free article] [PubMed] [Google Scholar]
- 11.Shan DE, Ho DM, Chang C, et al. Hemichorea-hemiballism: an explanation for MR signal changes. AJNR Am J Neuroradiol. 1998;19:863–870. [PMC free article] [PubMed] [Google Scholar]
- 12.Nagai C, Kato T, Katagiri T, Sasaki H. Hyperintense putamen on T1-weighted MR images in a case of chorea with hyperglycemia. AJNR Am J Neuroradiol. 1995;16:1243–1246. [PMC free article] [PubMed] [Google Scholar]
- 13.Wu MN, Ruge D, Tsai CL, Hsu CY, Lai CL, Liou LM. Periodic lateralized epileptiform discharges associated with irreversible hyperglycemic hemichorea-hemiballism. Clin EEG Neurosci. 2014;45((4)):315–317. doi: 10.1177/1550059413508555. doi: 10.1177/1550059413508555. [DOI] [PubMed] [Google Scholar]
- 14.Ahlskog JE, Nishino H, Evidente VG, et al. Persistent chorea triggered by hyperglycemic crisis in diabetics. Mov Disord. 2001;16((5)):890–898. doi: 10.1002/mds.1171. doi: 10.1002/mds.1171. [DOI] [PubMed] [Google Scholar]
- 15.Chung SJ, Lee JH, Lee SA, No YJ, Im JH, Lee MC. Co-occurrence of seizure and chorea in a patient with nonketotic hyperglycemia. Eur Neurol. 2005;54((4)):230–232. doi: 10.1159/000090717. doi: 10.1159/000090717. [DOI] [PubMed] [Google Scholar]
- 16.Lin A-Q, Shou J-X, Li X-Y, Ma L, Zhu X-H. Metabolic changes in acute cerebral infarction: findings from proton magnetic resonance spectroscopic imaging. Exp Ther Med. 2014;7((2)):451–455. doi: 10.3892/etm.2013.1418. doi: 10.3892/etm.2013.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai PH, Chen PC, Chang MH, et al. In vivo proton MR spectroscopy of chorea-ballismus in diabetes mellitus. Neuroradiology. 2001;43:525–531. doi: 10.1007/s002340100538. doi: 10.1007/s002340100538. [DOI] [PubMed] [Google Scholar]