Abstract
Complement is well appreciated as critical arm of innate immunity. It is required for the removal of invading pathogens and functions by direct pathogen destruction and through the activation of innate and adaptive immune cells. However, complement activation and function is not confined to the extracellular space but also occurs within cells. Recent work indicates that complement activation regulates key metabolic pathways and thus can impact fundamental processes of the cell, such as survival, proliferation, and autophagy. Novel identified functions of complement include a key role in shaping metabolic reprogramming, which underlies T cell effector differentiation, and a role as a nexus for interactions with other effector systems, in particular the inflammasome and Notch transcription factor networks. This review focuses on the contributions of complement to basic processes of the cell, in particular the integration of complement with cellular metabolism, and the potential implications in infection and other disease settings.
Introduction
The immune system continuously orchestrates many effector pathways to ensure protection against invading pathogens. Innate detection of microbes is mediated by a range of different pattern recognition receptors (PRRs) expressed on, or secreted by, immune cells that recognize microbe-derived molecules, known as pathogen-associated molecular patterns (PAMPs). Members of the PRR system family include the toll-like receptors (TLRs), the Nod-like receptors (NLRs) that are critical to the formation of the inflammasomes, the retinoic acid inducible gene 1 (RIG-I) receptors, and proteins belonging to the complement system (Creagh and O’Neill, 2006; Guo et al., 2015; Kolev et al., 2014). Activation of PRRs leads to immune cell activation, with induction of appropriate protective effector responses – specific for the cells that receive these signals – and, ultimately, clearance of the invading pathogen.
All PRR systems had initially been discovered as sensor and effector systems fighting exogenous threats in form of pathogenic microbes. However, it is now established that they also play central roles in detecting and removing noxious self-derived molecules, so-called danger-associated molecular patterns (DAMPs), commonly generated during cell (hyper)activity, stress responses and cell death (Latz et al., 2013; Wen et al., 2013). Moreover, it is becoming increasingly clear that the ability of the TLR network and the NLRP3 inflammasome to sense imbalances in normal metabolic processes of the cell – and to subsequently direct appropriate reactive responses – is of critical importance to cellular homeostasis (Coll et al., 2016; Konner and Bruning, 2011). The realization that drivers of the ‘classic’ innate immune response are also vital to basic physiological pathways helped explaining why dysregulation in the TLR and/or inflammasome systems not only affects pathogen sensing, but is also strongly associated with inflammatory, and specifically metabolic, diseases (Coll et al., 2016).
As complement was historically viewed as a liver-derived and serum-effective system, a prominent role for complement in the regulation of cell physiology has previously not been broadly considered. However, recent studies demonstrate that complement activation and function is not confined to the extracellular space but also occurs within cells (Liszewski et al., 2013), and that complement further plays a central role in the induction of key metabolic pathways (Kolev et al., 2015), as well as in regulating cell death (Lalli et al., 2008; Strainic et al., 2008). Moreover, studies on intracellular complement activity led to the discovery of a crosstalk between complement and intracellular sensor, and effector, pathways that had been overlooked due to their spatial separation (Arbore et al., 2016). These paradigm shifts in the field create new anchor points to delineate mechanisms underlying the wide-reaching effects of complement functions in immunity and beyond. In this review, we develop a conceptual framework (based on evolutionary and functional data) that places complement within a network of effector systems closely interlinking with basic processes of the cell, including cellular metabolism – thus regulating homeostasis and effector functions of T cells. We also discuss briefly the potential implications of this emerging complement–metabolism axis in infection and further disease settings.
Evolutionary Aspects in the Regulation of Life and Death by Metabolism and Innate Immunity
Metabolism is the root of life. Using stored energy and molecular building blocks generated in catabolic reactions, metabolism underpins cellular housekeeping functions – repairing or spatially organizing organelles, transporting substances across membranes, etc. – while anabolic pathways are engaged to build biomass (proteins, lipids, and DNA) in activated and proliferating cells. Together these metabolic reactions generate and maintain the order (i.e. cellular organization) that fundamentally defines life.
Ancient eukaryotic organisms emerged in an oxygen-poor atmosphere and likely used glycolysis as main form of energy production. To this day, glycolysis remains a key metabolic pathway in all cells, braking down glucose to pyruvate, yielding ATP and important intermediary metabolites for anabolic reactions (Lunt and Vander Heiden, 2011). Driven by increasingly abundant photosynthetic organisms that started to evolve approximately 2.7 billion years ago, an oxygen-rich atmosphere emerged. The ability to use the large amount of energy stored in oxygen – via oxidation of glucose in newly acquired mitochondria (oxidative phosphorylation - OXPHOS) – has greatly increased the bioenergetic efficiency of cells (Hsia et al., 2013). While essential to the prevailing aerobic life forms, oxygen is actually a toxic gas and inevitably linked with oxidative molecular damage brought by reactive oxygen species (ROS). This has placed organisms under significant evolutionary pressure to adapt to oxidative stress. Notably, adaptation to high oxygen abundance was very efficient and, in fact, ROS now play indispensible roles as signaling molecules in various redox-sensitive pathways – also in the modern adaptive immune system (Sena et al., 2013).
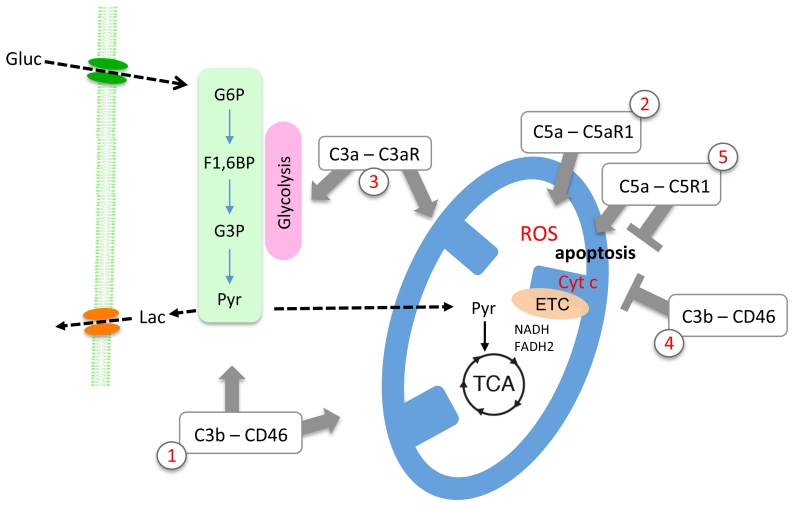
The evolution of multicellular animals from single-cell ancestors about 600 million years ago made the development of mechanisms coordinating cell division, cell and tissue specialization, recognition of non-self, and (controlled) cell death mandatory. With cellular metabolism essentially defining life it does not come as surprise that cell death, and prevention thereof, is likewise intimately linked to metabolism. Apoptosis is triggered by mitochondrial alterations resulting in dysfunction of this organelle with the release of cytochrome c and increased ROS production (Gottlieb et al., 2002; Zamzami et al., 1995). Glycolysis is tightly interlinked with mitochondria-driven apoptosis via the pro-apoptotic B-cell lymphoma 2 (Bcl-2) family member Bcl-2-associated death promotor (Bad), which is located on mitochondria where it interacts with glucokinase (Danial et al., 2003). Glucose deprivation results in Bad dephosphorylation and apoptosis. Independently, also the phosphatidylinositol 3 kinase (PI(3)K–Akt) pathway links glycolysis, mitochondria and apoptosis versus survival, as activation of the PI(3)K–Akt pathway induces glycolysis and inhibits apoptosis (Kalaany and Sabatini, 2009). Mechanistically, Akt increases mitochondria-associated hexokinase and thereby mediates its anti-apoptotic activity. Thus both, the PI(3)K–Akt pathway and the Bad-pathway converge on hexokinase to integrate metabolic sufficiency and cell survival. ROS forms another crucial link between cellular metabolism and apoptosis (Zamzami et al., 1995), as a close interaction between glucose metabolism, mitochondrial function and the handling of ROS exists. An important mechanism connecting glucose metabolism and ROS is diversion of glucose-6-P into the oxidative arm of the pentose phosphate pathway (PPP). The PPP generates reducing equivalents in the form of NADPH that drive the conversion of oxidized glutathione to its reduced form. Reduced glutathione is a major antioxidant in cells, and its depletion causes an increase in ROS, oxidative damage and apoptosis (Fico et al., 2004). From an evolutionary perspective, key metabolic pathways thus also balance cell death versus survival. In Figure 1, key cellular metabolic pathways are depicted together with the emerging links to the complement system discussed in this review.
Figure 1. Key metabolic pathways and their link with the complement system.
Glycolysis, braking down glucose to pyruvate, is yielding low amounts of ATP, yet provides important intermediary metabolites for anabolic reactions and shapes T cell immune functioning. In mitochondria, full oxidation of pyruvate greatly increases the bioenergetic efficiency of cells. C3b–CD46-medited signaling enhances both glycolysis and mitochondrial respiration (1). Oxygen is toxic and inevitably linked with oxidative molecular damage brought by reactive oxygen species (ROS). However, evolution has incorporated ROS as indispensible signaling molecules, and triggering intracellular C5aR1 via C5a during T cell activation impacts on oxygen metabolism and mediates increased production of ROS (2). Mitochondria are furthermore key organelles in orchestrating programmed cell death, which is triggered by the release of cytochrome c and increased ROS production. In T cell homeostasis, complement drives pro-survival pathways. Specifically, C3a generated intracellularly binds it receptor, C3aR, expressed on lysosomes, thereby maintaining constant low-level activation of the mechanistic target of rapamycin (mTOR), which in turn controls glycolysis and mitochondrial function and biogenesis (3). Yet, context specifically, complement also plays critical roles in the regulation of apoptosis: While C3b–CD46-mediated signals counteract apoptosis in stimulated cells via Bcl-2 expression induction (4), local generation of C5a can activate the same anti-apoptotic Bcl-2 pathway but also promote apoptosis via autocrine C5aR1 signaling via the extracellular signal regulated kinase (ERK) pathway (5). Of note, the role(s) of the alternative C5aR2 in these pathways is currently least well understood. Gluc=glucose; G6P=glucose-6-phosphate; F1,6BP=fructose,1,6,-bisphosphate; G3P=glyceraldehyde-3-phosphate; Pyr=pyruvate; Lac=lactate; TCA=tricarboxylic acid cycle; ETC=electron transport chain.
Comparing the immune systems of extant vertebrate and invertebrate species gives important clues to how recognition of PAMPS and DAMPS evolved and how links between cell effector systems have been established. Marine sponges (Demospongiae: Porifera) are filter-feeders, have no intestinal, neuronal or circulatory systems or other organs, and are classified as Parazoa (a subkingdom of animals). However, their phylogenetic status as Eumetazoa, a sister group of Metazoa (true animals), has ideally placed them to inform about the origin and (co)evolution of metabolism and innate immunity as traits shared between sponges and other animals indicate that the latter evolved from sponge-like ancestors (Philippe et al., 2009). Aligning with this notion, analysis of the genome of the demosponge Amphimedon queenslandica, revealed that it is remarkably similar in content and organization to the genome of ‘modern’ animals: It contains key gene groups representing hallmark pathways of animal multi-cellularity including those for cell cycling and growth (comprising key genes involved in glycolysis, OXPHOS, ROS management etc.), regulation of apoptosis, and innate immunity (Srivastava et al., 2010). In regards to the PRR systems, A. queenslandica and other sponges express several TLR proteins and the respective downstream adaptor, signaling and effector molecules (Srivastava et al., 2010; Wiens et al., 2007; Yuen et al., 2014). Similarly, key components of the complement system also appear very early in Cnidaria, at the same time as Porifera evolved (Le Friec and Kemper, 2009). Thus, core pathways regulating cell physiology and innate immunity ‘met’ early in evolution, already in the lineage that that led to our common metazoan ancestor(s).
The early evolutionary appearance of PRRs and their extensive involvement in normal cell physiology (see below) fuels into an emerging discussion surrounding the idea that the PRRs may not have evolved primarily to protect against infection, but rather as sensors of metabolic changes or imbalances (Coll et al., 2016). In support of this notion, dysregulation in these innate immune components is strongly associated with inflammatory and/or metabolic disease states (Coll et al., 2016; Konner and Bruning, 2011; Phieler et al., 2013a). Actually, although immunology and metabolism may be viewed as being distinct, at a fundamental level both systems fulfill similar purposes. Whereas the body responds to infections and injuries by activation of the immune system, which aims to restore homeostasis (Medzhitov, 2010), metabolic stress likewise drives corrective measures in metabolic pathway usage and induces autophagy or even apoptosis – with the very same goal. Given the metabolic stress imposed by infection and injury alike (hypoxia, tissue disruption, competition for nutrients, etc.), separating the cellular metabolic response from the immune response seems almost artificial.
Complement Functions – Systemic, Autocrine and Intracellular
Jules Bordet discovered the complement system over a century ago, as ‘system of serum-circulating proteins able to complement the antibody-mediated and cell-mediated immune responses’ (Bordet, 1909). Complement is a key component of innate immunity and central to the detection and removal of invading pathogens (Kolev et al., 2014; Ricklin et al., 2010). The system consists of >50 blood and lymph circulating (mostly liver-derived), as well as membrane-bound proteins. The fluid phase complement proteins comprise the PRR components; the effector molecules circulate in inactive pro-forms and become rapidly activated when the system is triggered. Membrane-expressed proteins are receptors and/or regulators of complement activation fragments that can also transmit signals into cells. Three activation pathways, the classical, the lectin, and the alternative pathway induce complement activation, which leads to induction of a general inflammatory reaction and removal of pathogens via their direct lysis and/or phagocytic uptake by scavenger cells (Figure 2). In addition, complement receptors expressed on adaptive immune cells also provide key signals for B cell maturation and induction of T effector cell functions. For two recent reviews covering these ‘classic’ complement functions see (Kolev et al., 2014; Ricklin et al., 2010).
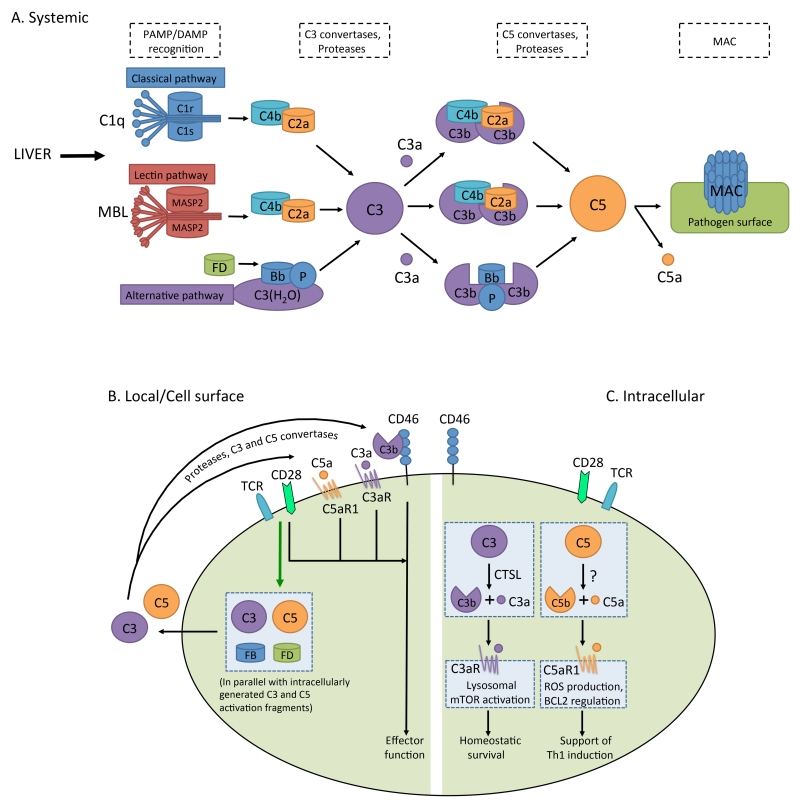
Figure 2. Distinct locations of complement activation.
A. Liver-derived, systemically circulating complement can be activated through the classical, lectin or alternative pathway. Via the formation of C3 convertases (C4bC2a for the classical and lectin pathways and C3bBb for the alternative pathway), these pathways lead to generation of C3b and C3a. Upon subsequent generation of C5 convertase (C4bC2aC3b for the classical and lectin pathways, C3bBbC3b for the alternative pathway), C5b and C5a are produced, with surface-bound C5b initiating the formation and insertion of the MAC on pathogens (or other target membranes). B. Local complement activation is triggered when activating signals (here, TCR stimulation or TLR activation on APCs (not shown)) initiate generation and secretion of C3, C5, Factor B, and Factor D, leading to C3 and C5 convertase formation in the extracellular space and on the cell surface – and ultimately the generation of the complement activation fragments C3a, C3b, C5b and C5a (C3 and C5 can also be cleaved by proteases in the extracellular space). These complement fragments bind to their respective receptors on the T cell surface and induce cellular responses. Note that such autocrine activation is also supported by preformed C3 and C5 activation fragments that are generated intracellularly (C), and rapidly transported to the cell surface to mediate autocrine signaling from that location. C. Intracellular complement activation in CD4+ T cells (and possibly other cells) occurs through the action of the C3-cleaving protease CTSL for C3, while the protease activating C5 is currently undefined. The resulting C3a and C5a fragments engage intracellular C3aR and C5aR1, respectively, and mediate effector function. Intracellular C3aR signaling occurs on lysosomes, while the intracellular compartment(s) expressing C5aR1 is, respectively are not yet defined. Self-tissue is protected from inappropriate complement deposition through fluid phase and cell-bound regulators (not shown in this schematic). C3b and C4b are inactivated by serine protease factor I, which requires one of several cofactor proteins (cell-bound CD46 and CR1 (CD35), or fluid phase factors H and C4bp). The C3 and C5 convertases are regulated through disassembly by regulators that have decay accelerating activity (DAA) (membrane-bound CD55 and CR1 (CD35), factor H and C4bp). The formation of the MAC is controlled by CD59 and protein S (vitronectin). CTSL, cathepsin L; MASP2, mannose-binding lectin serine protease 2; TCR, T cell receptor.
For many years, the prevailing opinion was that the key complement functions were in principle defined. However, exciting discoveries, such as those connecting complement activity with normal neuronal development, or with regulation of basic cellular processes (see below), renewed the interest in complement research. Further, the recent success of the humanized anti-C5 antibody, Eculizumab, as treatment for paroxysmal nocturnal hemoglobinuria and atypical haemolytic uremic syndrome points at complement as an underexplored therapeutic target (Keating, 2013). Contributing to the re-awakened attention towards complement are two unexpected observations of novel ‘modes and locations’ of complement activity: Firstly, the effects of complement on T cells and APCs are independent of serum-derived complement, but are driven by C3 and C5 activation fragments produced by the T cells and APCs themselves – directly engaging cell-surface complement receptors in an autocrine fashion (Figure 2) (Cardone et al., 2010; Lalli et al., 2008; Strainic et al., 2008). Secondly, complement activation is not confined to the extracellular space but also occurs intracellularly (Figure 2) (Arbore et al., 2016; Liszewski et al., 2013). Importantly, engaging intracellular complement receptors – including both anaphylatoxin receptors – induces signaling pathways distinct from those triggered by the same receptors when expressed on the cell surface (Arbore et al., 2016; Kolev et al., 2014; Liszewski et al., 2013). Specifically, in human CD4+ T cells, activation of C3 occurs intracellularly via cathepsin L-mediated cleavage, and C3a generated via this pathway stimulates intracellular C3aR signaling on lysosomes, thereby sustaining homeostatic T cell survival. T cells also continuously generate intracellular C5a through an as yet undefined mechanism. Upon TCR activation, intracellular C5a engages the intracellular C5aR1 and induces mitochondrial ROS, while C3a and C3b generated intracellularly rapidly translocate to the cell surface, where they engage cell surface C3aR and CD46, respectively –together driving the secretion of IFN-γ. The biologic role of intracellular complement activation is currently best studied in human CD4+ T cells, yet occurred in all cells analyzed so far – and hence is likely to be of broad relevance (Liszewski et al., 2013). Thus, serum-circulating complement is critical for its sentinel function in the recognition and removal of pathogens breaching host barriers; autocrine functioning complement directs adaptive T cell and APC responses, and intracellular complement (the ‘complosome’ (Kolev et al., 2014)) emerges as critical regulator of normal cell physiology.
Roles for Complement in Regulation of T Cell Effector Function and Contraction
The metabolic repertoire of immune cells encompasses the metabolic enzymes and/or pathways of the cell (basal signature), the available nutrient sensors and metabolic checkpoint kinases (adaptation response), and the epigenetic programming of metabolic genes (transcriptional memory). Polarized differentiation of immune cells, following stimulation by distinct activation, stress or danger signals, drives cell-specific remodeling of metabolic pathways.
Over the past years significant progress has been made in understanding how various facets of immune cell functioning are enabled and driven by cell-intrinsic adaptation of the metabolic machinery (Buck et al., 2015). In T cells, cognate antigen and co-stimulation of metabolically quiescent naïve and memory CD4+ and CD8+ T cells, in combination with specific of environmental cues, induces proliferation and differentiation into distinct helper subsets, regulatory T (Treg) cells, and cytotoxic cells – each with distinct metabolic profiles (MacIver et al., 2013; Zhou et al., 2009). A hallmark of the metabolic remodeling observed in in activated T cells is upregulation of aerobic glycolysis (Warburg effect), which provides the metabolites essential for biomolecular synthesis in proliferating cells (Lunt and Vander Heiden, 2011). T cell activation also enhances mitochondrial biogenesis and oxidative phosphorylation (OXPHOS), and uptake of amino acids (AAs) (van der Windt et al., 2012; Wang et al., 2011). The metabolic-checkpoint kinase mechanistic target of rapamycin (mTOR) senses and integrates environmental signals to regulate metabolic adaptation in cells. Activation of mTOR triggers glycolysis, OXPHOS and lipid synthesis, thus supporting proliferation and differentiation of resting T cells into effector cells (Cunningham et al., 2007; Delgoffe et al., 2009; Zheng et al., 2007).
The finding that induction of Th1 responses in humans fails in absence of autocrine C3 and C5 activity (Ghannam et al., 2014; Liszewski et al., 2013) indicates that engagement of receptors for C3 and C5 activation fragments may be critical in the regulation of cellular metabolism. Indeed, we recently determined that TCR triggering, coupled with CD46 co-stimulation, enhances OXPHOS and glycolysis, and increases glucose and AA uptake in CD4+ T cells (Figure 3). How complement signaling impacts fatty acid uptake, oxidation, or genesis in T cells, or other cells, remains largely unexplored. CD46 stimulation is required to drive the expression of the glucose transporter, GLUT1, and the L-type AA transporter, LAT1, and blockade of both glycolysis and AA uptake resulted in decreased IFN-γ production. mTOR complex 1 (mTORC1) is a known regulator of LAT1 expression, and mTORC1 activity was decreased in CD46-deficient patients. Late Endosomal-Lysosomal Adaptor, MAPK and MTOR Activator 5 (LAMTOR5), a component of the Ragulator complex, is essential for AA sensing by mTORC1. Upon activation, CD4+ T cells from CD46-deficient patients display severely decreased LAMTOR5 expression, and in healthy donor T cells CD46 co-stimulation is required to drive mTOR and LAMTOR co-localization – which is critically linked to their ability to produce IFN-γ (Figure 3) (Kolev et al., 2015). Importantly, pathologically increased intracellular C3 activation (through cathepsin L), and thus hyperactive CD46 signaling, occurs in T cells in the inflamed joints of patients with juvenile idiopathic arthritis. This contributes to exaggerated mTOR activity and IFN-γ secretion in these patients’ T cells, a process that can be normalized with a cell-permeable inhibitor of cathepsin L (Liszewski et al., 2013). These studies provide a molecular framework linking the inflammatory mediator and danger signal C3b with immune-metabolic reprogramming of human T cells, and demonstrate that dysregulation in this crosstalk contributes to human immunodeficiency and autoimmune disease.
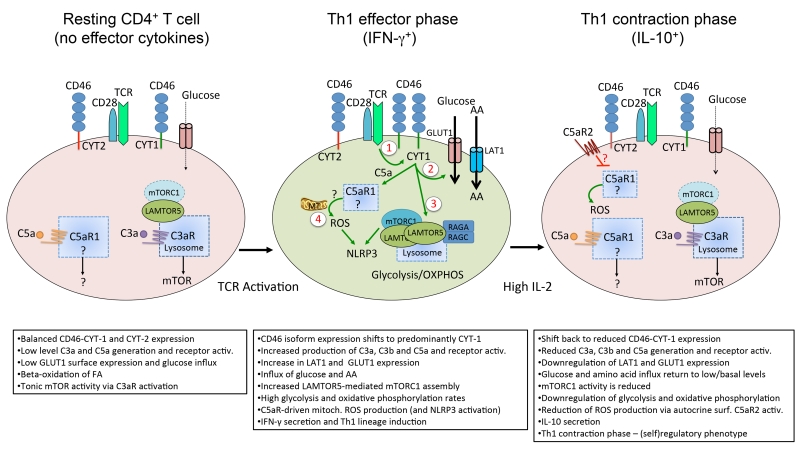
Figure 3. Model of the role of complement in Th1 cell homeostasis and effector function.
In resting T cells, the ‘tonic’ generation of intracellular C3a via cathepsin L sustains C3aR stimulation expressed on lysosomes and the low level activation of mTOR required for T cell survival. Resting T cells also generate low levels of C5a, and although tonic C5a activity is also required for T cell survival, the mechanism underlying this function is unclear. T cell receptor activation and CD28 co-stimulation of resting T cells induces the local generation of the CD46 ligand C3b and increased expression of CD46 isoforms bearing CYT-1 (1). Autocrine CD46 CYT-1-driven signals then lead to up-regulation of the glucose transporter, GLUT1, and the amino acid (AA) channel, LAT1, allowing for increased influx of glucose and AAs into the cell (2). In parallel, CD46 CYT-1-mediated signals induce increased expression of LAMTOR5, and via this assembly of the lysosome-based machinery enabling amino acid sensing via mTORC1, which then leads to the induction of glycolysis and OXPHOS required for IFN-γ secretion (3). CD46-mediated signals also trigger increased intracellular C5a generation, which supports mitochondrial metabolic activity and ROS production critical to normal Th1 induction (4). Of note, complement-driven ROS production (via intracellular C5aR1 activation) and mTORC1 activity also activate the NLRP3 inflammasome in TCR-stimulated T cells, a process that supports Th1 expansion via IL-1β functioning in an autocrine fashion (not shown). During Th1 contraction and induction of IL-10 co-expression, CD46 isoform expression reverts to a CYT-2 predominant pattern (through a mechanism that is currently unknown) and this is accompanied by reduced expression of GLUT1 and LAT1, down-regulation of glycolysis and OXPHOS and re-instatement of C3a-driven low level mTOR activity. Furthermore, autocrine engagement of the surface-expressed C5aR2 via C5a-C5adesArg secreted upon T cell activation contributes to negative regulation of mitochondrial activity and reduction of ROS (through inhibition of intracellular C5aR1 activity and/or a yet undefined mechanism). (Adapted from Kolev et al., Immunity 2015).
T cells also harbor an intracellular C5 activation system, and activity of this system – and hence generation of C5a and C5b – is also regulated by TCR triggering and autocrine CD46 engagement: TCR and CD46 signals amplify low-level steady-state intracellular C5a generation (Arbore et al., 2016). C5a, as well as the ‘des-Arginized’ form of C5a (C5a-desArg) generated by carboxypeptidases, can bind two distinct GPCRs, C5aR1 (CD88) and C5aR2 (GPR77, C5L2) (Sarma and Ward, 2012; Scola et al., 2009). Binding of C5a to C5aR1 preferentially mediates pro-inflammatory responses. The function of C5aR2 varies with cell type, and C5aR2 can function as a non-signaling decoy receptor antagonizing C5aR1, or as an active transducer of pro- or anti-inflammatory signals (Croker et al., 2016; Gerard et al., 2005; Scola et al., 2009). Human CD4+ T cells, both resting and activated, express the C5aR1 only intracellularly, while C5aR2 is expressed within the cell and on the cell surface. Activation of intracellular C5aR1 during T cell stimulation impacts on oxygen metabolism and generates a (as yet undefined) signal driving increased production of mitochondrial ROS, needed for the generation of Th1 responses (Sena et al., 2013) (Figure 3). The intracellular C5 system also induces expression of genes coding for NLRP3 and IL1B and the subsequent assembly of an NLRP3 inflammasome in T cells. Activation of the NLRP3 inflammasome leads to caspase-1 mediated activation and secretion of IL-1β by T cells, which further supports Th1 induction in an autocrine fashion (Arbore et al., 2016). Thus, the complosome engages a key metabolic checkpoint kinase (mTOR) as well as mitochondrial ROS, to modulate IFN-γ secretion and Th1 induction in human CD4+ T cells. Mitochondria are evolutionary derived from bacteria and considered the powerhouse(s) of the cell (Gray et al., 1999). It thus makes sense that structural damage to the powerhouse and recognition of bacterial-, respectively mitochondrial products go hand in hand. In addition, however (as stated above), evolution has integrated mitochondrial ROS in actively regulating key processes also of T cells (Sena et al., 2013). Of note, C5aR1 activation has traditionally been connected to ROS production in innate immune cells, including macrophages, monocytes and neutrophils (Samstad et al., 2014; Sarma and Ward, 2012). Supporting the notion that C5 plays also a critical role in regulating T cell effector maturation, absence of anaphylatoxin receptors in mouse CD4+ T cells, and inhibition of C5aR1 signaling in human CD4+ T cells, prevents effector functions and induces generation of forkhead box P3 (Foxp3)+ Treg cells (Kwan et al., 2013; Le Friec and Kemper, 2009; Strainic et al., 2013). It should be mentioned, though, that the expression(pattern) of C5aR1 and C5aR2 in mouse T cells remains a matter of debate, with some reports suggesting induction of C5aR1 upon stimulation (Lalli et al., 2008; Strainic et al., 2008) while others failed to detect expression in resting or activated T cells (Dunkelberger et al., 2012; Karsten et al., 2015).
Recent work defining the functional connection between cellular metabolism and immune cell function demonstrated that threshold aspects and distinct metabolic programs during T cell activation affect polarization and/or differentiation of Th1, Th17 and Treg cell subsets (MacIver et al., 2013; Zhou et al., 2009). Congruent with this, decreased CD46 expression on CD4+ T cells impacts proportionally on mTORC1 activity and Th1 cell induction, whereas Th2 cell and Foxp3+ Treg cell numbers, and responses, are induced normally. Furthermore, only in complete absence of CD46, T cells fail to become Th17 cells, suggesting that complement indeed interlinks with metabolic threshold differences underlying T cell polarization (Kolev et al., 2015). Further, proliferation of T cells from CD46-deficient patients is surprisingly normal, indicating that non-bioenergetic aspects of metabolic reprogramming relate to the observed differences in subset differentiation. Aligning with this observation is the finding that pyruvate dehydrogenase kinase 1 (PDK1) is selectively expressed in Th17, but not Th1, cells (Gerriets et al., 2015). Upon clearance of a given pathogen, successful contraction of Th1 responses is critically important to health, and uncontrolled Th1 activity contributes to a range of auto-inflammatory diseases (Stummvoll et al., 2008). Quiescent cells generally maintain a status of low glycolytic activity, using OXPHOS as ‘homeostatic’ energy source, while activated cells increase glycolysis and OXPHOS, again shutting down glycolysis and returning to a state of low-level OXPHOS upon contraction (MacIver et al., 2013). Interestingly, CD46 not only drives Th1 induction, but it also coordinates the co-production of IL-10 in Th1 cells – and thereby their transition towards a (self)suppressive contraction phase (Figure 3) (Cardone et al., 2010). In CD4+ T cells, CD46 is expressed in distinct isoforms (differential splicing of a single gene) differing with regard to their cytoplasmic tails, CYT-1 and CYT-2, respectively. Both cytoplasmic domains transduce distinct signals (Liszewski et al., 2013; Yamamoto et al., 2013). Resting T cells predominantly express CD46-CYT-2. Upon TCR activation, CYT-1-bearing CD46 isoforms are upregulated and engaged via autocrine C3b production, and specifically induce the metabolic changes required for IFN-γ production described above (Kolev et al., 2015). During IL-10 induction, the switch from high to low glycolysis and OXPHOS is mediated by CD46 isoforms expressing CYT-2, which again become the predominant CD46 isoforms in contracting T cells. In addition, signals mediated by autocrine activation of C5aR2 expressed on the surface of expanded Th1 cells, inhibit mitochondrial activity and reduce C5aR1-driven ROS production (Figure 3). Currently, work performed on the newly identified roles of CD46 and the complosome in regulating cell metabolism mostly uses CD4+ T cells. However, these pathways are likely operative also in other cells. Future studies should, for example, determine whether the complosome–metabolism axis also regulates effector function of CD8+ T cells, natural killer cells, T follicular helper cells and innate lymphoid cells, and whether this system also regulates the metabolic events tipping the balance between M1 and M2 macrophage development.
Another critical question is whether complement is required for the induction of normal T cell memory. In B cells, Cr2 and Cr1-deficient animal models clearly demonstrated that complement-mediated signals are required for optimal induction of memory, although the exact molecular mechanisms remain undefined (Carroll and Isenman, 2012). The role of complement signaling in T cell memory development and function has not been explored. However qualitative differences in response to activation signals between naïve and memory CD8+ T cells are intricately linked to disparities in their metabolic repertoires (Gubser et al., 2013). In CD8+ T cells, for example, histone remodeling is impacted by glycolytic flux, and the rapid and stable increase of glycolysis following TCR and CD28 stimulation is a defining feature of the early recall function of memory CD8+ T cells (Gubser et al., 2013). Inversely, inhibition of mTOR activity by the AMP-activated protein kinase (AMPK) results in downregulation of basal glycolysis and enhancement of OXPHOS, spare respiratory capacity, and glycolytic capacity, all indicators of the effector to memory transition in CD8+ T cells (Pearce et al., 2009). As complement ‘dabbles’ with these key pathways, one can envision that the complosome activation state, or possibly a specific complosome signature (for example, a distinct CD46-CYT-1 versus CYT-2 expression profile in naïve and memory cells), emerges as a critical component of T cell memory-induction and -function.
Roles for Complement in Regulation of Cell Homeostasis: Survival, Proliferation and Death
Cells can die many deaths, including necrosis, apoptosis, autophagic cell death, and cornification (for details see (Kroemer et al., 2009)). Of note, morphologic criteria of the various modalities of self-destruction remain better defined than their precise biochemical mechanisms (Shi, 2002). Necrosis, which is thought to be an uncontrolled process of cell death (although necrosis-regulative pathways are now being discovered (Kroemer et al., 2009)), is closely tied in with immunity, as it is accompanied by immune responses and inflammation. By contrast, apoptosis and autophagic cell death are grouped into the programmed cell death (PCD) pathways, commonly considered to be operating without inducing an inflammatory response.
Complement activity can contribute to both inflammatory and non-inflammatory cell death pathways. Activation of the complement cascade with formation of the membrane attack complex (MAC) on target cells induces cytoplasmic swelling and rupture of cell membranes, representing classic features of necrosis (Ziporen et al., 2009). Inversely, the complement system also aids in the detection and non-inflammatory removal of apoptotic cells on an organismal level (Trouw et al., 2008). With increasing understanding of the signaling pathways triggered by complement receptors engaged by immune cells, also on a single-cell level complement emerges to regulate cell survival and PCD.
In T cell homeostasis, complement drives pro-survival pathways. Specifically, C3a generated intracellularly binds it receptor, C3aR, expressed on lysosomes, thereby maintaining constant low-level activation of mTOR (Figure 2) – which is required for homeostatic survival of human CD4+ T cells (Liszewski et al., 2013). Interestingly, T cells from patients with serum C3-deficiency are unable to generate Th1 responses, but survive normally both in vitro and in vivo. We observed that immune cells from patients with systemic C3-deficiency generate sufficient intracellular C3a from the mutated C3 protein to survive, yet C3 and activation fragments fail to be secreted. Thus, homeostatic pro-survival signals are generated, whereas autocrine activation of complement receptor pathways required for T cell effector responses cannot be induced (Liszewski et al., 2013). Similarly, our unpublished data from patients with serum C5-deficiency demonstrate that T cells and monocytes from these individuals in fact generate intracellular C5a and engage intracellular C5aR1 pathways – but cannot secrete C5 and its activation products (Lappegard, Mollnes, Niyonzima, Espevik and Kemper, unpublished data). In line with an important pro-survival function also of the intracellular C5 system, studies performed using C5ar1−/− mice showed that circulating T cells in non-challenged animals have a decreased lifespan (Strainic et al., 2008). In essence this phenotype is unsurprising, given that anaphylatoxin receptors trigger the PI(3)K–Akt–mTOR pathway, which activates glycolysis and anabolic pathways, and inhibits apoptosis (Bosmann et al., 2012; Chang et al., 2014; Hao et al., 2012; Kalaany and Sabatini, 2009; Li et al., 2012; Strainic et al., 2008). C3 and C5 serum deficiency is associated with recurrent infections (Katz et al., 1994; Schejbel et al., 2013). However, since C5a and C3a-driven signals emerge as important control elements of the PI(3)K–Akt–mTOR axis, it is conceivable that complete functional deficiency of C3 and C5 activity may in fact be incompatible with life – thus not occurring in humans.
Intracellular complement activity also counteracts apoptotic pathways: CD46 co-engagement during T cell activation leads to high expression of the BCL2 gene (Kolev et al., 2015) and a similar role for the C5ar1 has been described in activated mouse CD4+ T cells, were C5a increases Bcl-2 expression and prevents Fas up-regulation (Lalli et al., 2008). Notably, on oligodendrocytes, sublytic insertion of the MAC also inhibits apoptosis (Rus et al., 2001; Triantafilou et al., 2016) by down-regulating BAD expression in a PI(3)K-dependent manner (Rus et al., 2001), or via inhibition of caspase-8 (Cudrici et al., 2006). Aligning with these data, sublytic MAC insertion also protects oligodendrocytes from tumor necrosis factor (TNF)-induced apoptosis via inhibition of caspase-3 activity (Soane et al., 1999).
Complement-driven pathways likewise promote cell cycle progression in a range of cells: C5aR1-mediated signals in human endothelial cells mediate progression into the G2 and M phase, together with DNA synthesis and cell proliferation (Kurihara et al., 2010); sublytic MAC stimulates oligodendrocytes to enter the cell cycle by induction of c-Jun through activation of the c-Jun NH2-terminal kinase pathway (Fishelson et al., 2001; Rus et al., 2001), and complement receptors 3 and/or 4 (CR3, CD11b-CD18; CR4, CD11c-CD18) promote cell cycling and proliferation in macrophages by supporting the G1 to S phase transition (Luo et al., 2005).
Upon clearance of a given pathogen, tightly controlled contraction, i.e. apoptosis, is mandatory to minimize immune pathology. Given the increasingly recognized role complement plays in orchestrating ‘shut down and repair’ pathways (Kolev et al., 2014), it is not surprising that complement also regulates the induction of apoptosis. For example, local generation of C5a by ischemic neurons promotes neuronal apoptosis via autocrine C5aR1 signaling (Pavlovski et al., 2012). Furthermore, complement activation products, either directly or indirectly, contribute to increased apoptosis observed in a mouse model of lupus cerebritis (Alexander et al., 2005).
In all, a scheme emerges were complement activation, and complement-dependent signaling pathways, actively regulate cell survival and cell death, both in homeostatic and inflammatory conditions. To possibly harness these new pathways therapeutically, it will be important to understand the cellular and environmental context that tips the balance in either direction.
Against the above background, complement – not unexpectedly – also interlinks with autophagy and possibly also with pyroptosis (Takeshige et al., 1992; Tsujimoto and Shimizu, 2005). Autophagy is referred to as a ‘self-eating’ form of PCD, as opposed to the ‘self-killing’ term used in conjunction with classic apoptosis (Maiuri et al., 2007). Autophagy describes the controlled degradation and re-utilization of cellular components, were cytoplasmic constituents are isolated from the rest of the cell within autophagosomes, degraded upon fusion with lysosomes – and eventually recycled. Autophagy is also a key defense mechanism against cell-invading pathogens (Campoy and Colombo, 2009), and while autophagy and apoptosis can be initiated concurrently or exclusively, their effector machineries share common pathways. Of note, activation via both PAMPS and DAMPs can induce autophagy, yet the exact range and composition of upstream signals integrating towards this cellular program are not well defined. However, CD46 activation has been connected with the induction of autophagy of epithelial cells in the context of infection. CD46 is used by several important pathogens as cellular entry receptor (Cattaneo, 2004). Upon binding of both measles virus (MV) and group A Streptococci to CD46, the CYT-1 isoform of this receptor induces the autophagosome formation-complex Beclin 1-VPS34 (Joubert et al., 2009). Thus, upon recognition of microorganisms, CD46 can directly trigger autophagy, a critical step to control infection. In addition to its role in controlling autophagy in infection, complement also contributes to (dys)regulated autophagy in inflammatory disease. For example, C5aR1-mediated signals funnel via ATG5 (a protein required for autophagosome formation) into the regulation of autophagy in alveolar macrophages. Pathologic levels of C5a generated during acute lung injury drive autophagic cell death in alveolar macrophages, thereby disrupting pulmonary homeostasis and contributing to the development of disease (Hu et al., 2014). Inversely, autophagy induced by sublytic MAC formation on mouse podocytes protects these cells from injury (Lv et al., 2016) and, accordingly, rapamycin, which targets mTORC1 and promotes autophagy, reduces cellular pathology. This is of particular interest since, aside from tightly regulating the metabolic checkpoint-kinase mTOR, controlled induction of autophagy during CD8+ T cell responses is a further critical determinant of normal T cell memory formation (Xu et al., 2014). It will be fascinating to explore whether complement indeed intersects at those pathways in CD8+ T cells. Finally, the recent observations that complement regulates NLRP3 inflammasome activation, caspase-1 activation and IL-1β production in epithelial cells, monocytes and T cells (Arbore et al., 2016; Asgari et al., 2013; Laudisi et al., 2013; Samstad et al., 2014; Triantafilou et al., 2013) suggests that complement is also involved in pyroptosis, an inflammatory form of PCD crucial for controlling certain microbial infections that requires caspase-1 activity and intracellular release of IL-1β (de Gassart and Martinon, 2015).
Thus, complement, metabolism and PCD seem to be intimately intertwined, and although this notion is currently somewhat pieced together based on data derived from a range of cells and model systems, we are confident that future research will demonstrate just that.
Intersection of Complement with other Key Effector Networks
Activation of complement, and engagement of complement receptors does not occur in isolation. Indeed, functional intersections between complement and the TLRs, as well as the coagulation system, have long been recognized (Amara et al., 2008; Kohl, 2006; Song, 2012). In addition, during the induction of immune responses, complement receptors also engage in a crosstalk with Fc and carbohydrate receptors, with growth factor (cytokine) receptors, and with the Notch system and NLRP3 inflammasome (for a recent review see (Kolev et al., 2014)). In this section we will focus on the emerging interrelations between metabolism, complement, and further effector systems, in regulating basic cellular processes.
In T cells, CD46-mediated signals regulate expression of the receptors for the key survival and or growth factors IL-7 (CD127-CD132) and IL-2 (CD25-CD132-CD122) (Le Friec et al., 2012; Liao et al., 2013). IL-7 receptor (IL-7R) engagement provides an important T cell survival signal through STAT5-mediated activation of Akt, which enhances GLUT1 cell surface expression and glucose uptake. Inversely, deletion of IL-7R reduces cellular levels of anti-apoptotic BCL-2 (Jacobs et al., 2010; Wofford et al., 2008). Since engaging intracellular C3aR – mediating tonic mTOR activity – is also required for T cell survival (see above), the crosstalk between complement C3, cytokine receptors and cellular metabolism emerges to be important in sustaining T cell pools in the periphery. Generation of intracellular C3a occurs in many cell types beyond lymphocytes, and indeed also dendritic cells lacking expression of C3aR have reduces survival rates (Strainic et al., 2008). In CD46-deficient individuals, expression of the IL-7R is normal on quiescent cells – in line with their unaltered peripheral T cell counts. However, upon activation of T cells from these patients, CD127 fails to down-regulate, which leads to disproportionate surface levels of CD25, CD122, CD123 and CD127, and results in reduced assembly of IL-2R (Le Friec et al., 2012). Since IL-2R mediated signals are needed for Th1 induction (Liao et al., 2013), CD46-deficient patients fail to produce IFN-γ (a) because of faulty IL-2R signaling and (b) due to their inability to induce metabolic reprograming and normal activation of mTORC1 (Kolev et al., 2015; Le Friec et al., 2012). Importantly, CD46 also contributes to the Th1 shutdown program, integrating cues indicative of a successfully expanded Th1 response (‘high environmental IL-2’). This aligns well with the recent observation that, in Th1 cells, high levels of IL-2 drive expression of BCL6, encoding a repressor of several genes required for glycolysis – which induces cell contraction (Oestreich et al., 2014). Interestingly, BCL-6 expression is dysregulated in T cells from CD46-deficient patients (unpublished data), further underpinning the role for CD46 as key constituent of a sensory network integrating signals in a dynamic immune-environment.
CD46 not only regulates the expression of receptors and ligands of the Notch system, CD46 also directly interacts with Jagged1 on resting T cells, thereby preventing interaction between Notch1 and Jagged1. TCR activation induces shedding of CD46 from the surface, thus enabling activation of Notch1 by Jagged1, which is also required for IFN-γ production (Le Friec et al., 2012). Further, the CD46 tails, similar to the intracellular domain of Notch, translocate to the nucleus, which is needed for Th1 induction (Kolev et al., 2015; Le Friec et al., 2012). Thus, a spatially and temporally regulated crosstalk between complement and Notch is critical to normal human Th1 biology. An intriguing (but not formally proven) hypothesis could state that in mice, Notch may play similar roles during T cell activation as CD46 does in humans: The ‘Notch system’, like complement, is evolutionary old, is conserved among species, and is central to normal organ and tissue development (Fortini, 2009). Signaling events mediated by Notch receptors (Notch1-4) and ligands (Jagged1 and −2 and delta-like 1, 3 and 4) likewise play a crucial role in the induction of Th1 and Th2 lineage differentiation (Amsen et al., 2015), and also in the co-induction of IL-10 in Th1 cells (Rutz et al., 2008). The possibility for a ‘CD46-like’ role assigned to Notch in the mouse system is further supported by the fact that Notch is critically involved in regulating the same metabolic pathways as CD46 does in humans (Ciofani and Zuniga-Pflucker, 2005; Landor et al., 2011; Maekawa et al., 2015; Xu et al., 2015). Moreover, a recent study has identified Glut1, and other key genes involved in the regulation of glycolysis and the tricarboxylic acid cycle, as direct transcriptional targets of Notch1 in mice (Slaninova et al., 2016). It is not clear why rodents ‘avoided’ CD46 expression during evolution. However, as CD46 is used by several important pathogens as cellular entry receptor (Cattaneo, 2004), lacking broad expression may have simply reduced their risk of infection.
Recent studies suggest a tight functional connection (with activating and inhibitory circuits) between complement and intracellular PAMP-DAMP sensors. For example, upon infection, C1q enhances IFN-α production via the RIG-I myeloid differentiation-associated protein 5 (MDA5) pathway (Wang et al., 2012), while virus-induced expression of the C1q receptor, gC1qR, on mitochondria, inhibits RIG-I function (Xu et al., 2009). Also, optimal IL-1β production in human monocytes and macrophages relies on TLR-mediated signals and concurrent activation of C3aR and C5aR1 (Asgari et al., 2013; Niyonzima et al., 2015; Samstad et al., 2014). Further, sublytic MAC deposition can induce high levels of IL-1β and IL-18 in a NLRP3 inflammasome-dependent manner in mouse dendritic cells and lung epithelial cells (Laudisi et al., 2013; Triantafilou et al., 2013). Defining the precise molecular events underlying the crosstalk between complement and inflammasome formation during infection is an area of intense research. It is, however, already becoming apparent that regulating and/or sensing changes in the metabolic balance of the cell represents a key intersection between complement and the inflammasomes. Indeed, the existence of such a complement–metabolism–inflammasome axis has already been demonstrated in human T cells: optimal Th1 induction requires synergy between CD46-driven assembly of mTORC1, glycolysis and activation of the NLRP3 inflammasome (Moon et al., 2015), as well as C5aR1-mediated ROS production – also activating the NLRP3 inflammasome (Figure 3) (Arbore et al., 2016). As a general concept this likely extends to a wide range of cells. For example, sublytic MAC induces changes in the mitochondrial membrane potential of epithelial cells, which triggers NLRP3 inflammasome activity (Triantafilou et al., 2013). Further, upon viral infection, the receptor for the globular heads of C1q, gC1qR, translocates to the mitochondrial matrix and regulates OXPHOS activity, ROS generation and NLRP3 inflammasome activation (West et al., 2011). Although the functional significance of the crosstalk between the complosome and other effector systems is evident for the protection against infection, these newly identified roles of complement in the regulation of basic cellular processes suggests to re-evaluate also a range of autoimmune and chronic inflammatory diseases (see below) for dysregulation of pathways and networks discussed here.
We only begin to uncover how distinct homeostatic and inflammatory microenvironments impact on T cell and general immune cell metabolism, and hence function. Based on the above discussion we propose a model that emphasizes the link between cellular metabolism, complement, and further effector systems, in both immune homeostasis and adaptive immunity (Figure 4).
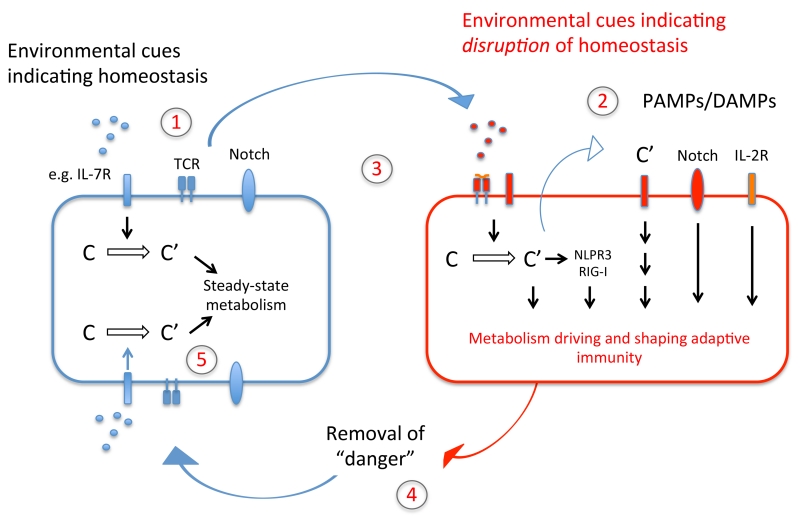
Figure 4. ‘Feedback model’ of the complement–metabolism system in T cells.
During organismal homeostasis, intracellularly generated complement fragments sustain T cell self-regulation through the fine-tuning of basal metabolic activity, and this quiescent state is supported by complement driven assembly of the IL-7R and restraining of Notch activity (1). Together with cognate T cell activation, signals indicative of disrupted homeostasis (2), initiate complement-driven metabolic reprogramming, IL-2R assembly, Notch and NLRP3 inflammasome activation and context-specific activation of the cell (3). This enables an efficient immune response, which in turn closes a feedback-loop to the sensing system by modifying the input on the sensor-module – namely removal of cognate pathogen and thus the inflammatory trigger (4). Metabolic reprogramming back towards steady-state metabolism thus occurs (5). The same basic principle of a complement-dependent ‘sensing–response–feedback system’ would also apply to other immune cells (for example, antigen-presenting cells, where TLR activation may induce complement-mediated metabolic programming) and in cell-autonomous models, e.g. when intracellular sensing of C3 fragments deposited onto cell-invading pathogens triggers a mitochondrial antiviral signaling protein-driven immune response, leading to degradation of the pathogens by the proteasome. Of note, we have predominantly integrated into this schematic the effector systems networking with complement that have a defined metabolic role in immune cell activation.
New Aspects of Complement in Human Disease
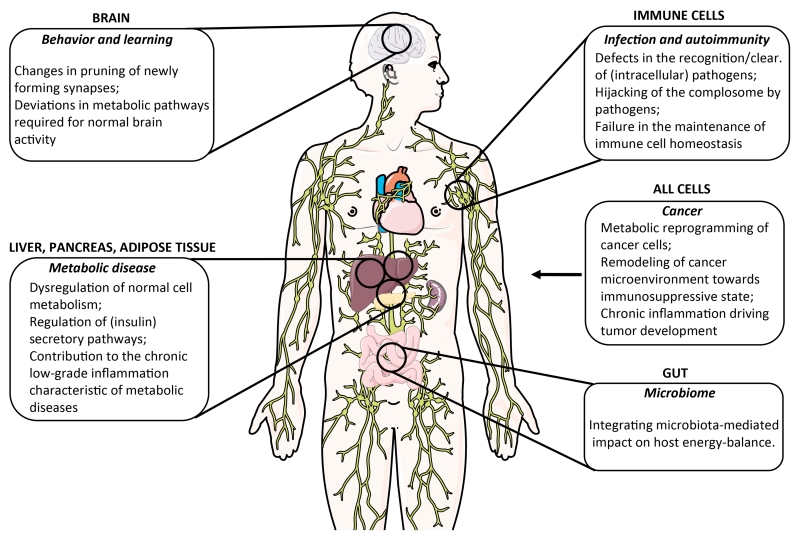
Lack of normal serum complement activity causes severe and recurrent infections, and dysregulation of complement activation and/or control contributes to a range of autoimmune diseases (Sullivan, 1998). These clear and longstanding observations contrast the, with few exceptions, limited success of medical interventions targeting serum complement activity. In hindsight, at least a partial explanation for this clinical reality comes with the insight that human Th1 (and Th17) responses are regulated by autocrine and intracellular C3 and C5 activation. Engagement of intracellular complement receptors and pathologically increased generation of intracellular C3 activation fragments or C5aR1-mediated downstream signals, is a critical component of diseases characterized by Th1 hyperactivity – such as RA, gout and possibly colitis (Arbore et al., 2016; Cumpelik et al., 2015; Liszewski et al., 2013). Controlling intracellular, but not extracellular, C3 activation normalizes Th1 responses in experimental systems – suggesting that the design of next generation complement-targeted therapeutics may have to take this new location of complement activity into consideration. Similarly, failure to opsonize invading encapsulated bacteria, due to systemic complement deficiency, is only partially covering the complex relation between complement and infectious diseases. For example, the unexpected observation that intracellular pathogens trigger mitochondrial antiviral signaling (MAVS) responses in a C3-dependent manner (Tam et al., 2014) strongly suggests that deviations in complosome function may also alter the course of infections. Further, intracellular C3a-mediated signals in APCs regulate trafficking of apoptotic cargo (Baudino et al., 2014), and since this pathway likely also ‘handles’ pathogen-derived antigens, its aberrant function will presumably also impact on infection. Conversely, it seems likely that infectious microbes have evolved to abuse or divert these newly described complement activities to their benefit. For example, we originally proposed that CD46-binding microbes abuse the receptors’ activity to induce immunosuppressive IL-10 (Price et al., 2005). However, adenoviruses (which bind CD46) induce glycolysis in epithelial cells, which in turn is needed for successful viral replication (Thai et al., 2014). Furthermore, although autophagy triggered upon infection is mostly considered a host protective measure, in the case of MV infection, CD46-mediated signals lead to sustained autophagy and ultimately increased virion formation (Richetta et al., 2013). This indicates that some viruses may have hijacked this complement co-controlled cellular program needed for normal cell function. The interplay between CD46 and CD46-binding pathogens – or complement and pathogens in general – may therefore have a previously unappreciated ‘metabolic–cell biologic dimension‘ (Figure 5).
Figure 5. Potential novel contributions of complement dysregulation to human disease.
Novel areas and aspects of human cell, tissue and organ function, where deviations from normal complement activity contribute to disease induction, pathogenesis, and/or failure of disease resolution. We have focused on disease settings where the new emerging roles of complement in cell metabolism and death are likely critical drivers.
Analogous to immune cells, complement activation in metabolic tissues may also tip the balance, which could sustain or exacerbate metabolic diseases via detrimental feedback loops. In fact, complement research has pioneered the concept linking nutrition and inflammation. Already in the late ‘80s it was reported that increased C3 activation promotes triglyceride formation, insulin resistance and altered energy expenditure in a range of cells (Cianflone et al., 1988; Rosen et al., 1989). Around the same time, connections between serum complement activation and lipid metabolism have been made (Cianflone et al., 1990; Cui et al., 2007). Today, we appreciate that complement plays an important role in the pancreas, liver and in adipose tissue also to maintain healthy systemic metabolism (Phieler et al., 2013b): Similar to the role of intracellular C3a in homeostatic survival of T cells, tonic levels of complement, i.e. complosome activation, contribute to the normal function of these tissues via C3a, C3a-desArg and C5a, that directly regulate adipocyte energy regulation (Faraj et al., 2004; Roy et al., 2013) and control insulin secretion by β-cells (Phieler et al., 2013b). This idea is supported by the observation that lack of intracellular CD59 also reduces insulin secretion in β-cell (Krus et al., 2014). Many lines of evidence now indicate that over-nutrition- and obesity-associated stress, resp. danger signals, lead to a chronic activation of innate immunity, in particular via activation of the NLPR3 inflammasome, and subsequent development of metabolic diseases such as type 2 diabetes (Donath and Shoelson, 2011). Glucotoxicity and lipotoxicity induce oxidative stress, inflammasome activation and cytokine production by macrophages (Mathis, 2013) and macrophages accumulate in great numbers in the visceral adipose tissues of obese humans and mice (Weisberg et al., 2003). These macrophages typically display an M1 phenotype, are the primary source of inflammatory cytokines like IL-1β, IL-18, TNF-α, and IL-6 in obese adipose tissue (Lumeng et al., 2007) and likely help driving insulin resistance. T cells play an important role in shaping the pathogenesis of metabolic stress (Mathis, 2013; Nishimura et al., 2009). While Treg cells and Th2 cells, typically present in lean adipose tissue and exerting an anti-inflammatory effect on the surrounding tissue (Feuerer et al., 2009; Winer et al., 2009), CD8+, Th1 and Th17 T cells support remodeling in obese adipose tissue, at least partially by mediating macrophage M1 proliferation (Feuerer et al., 2009; Nishimura et al., 2009; Zuniga et al., 2010). Given the new roles for complement in the regulation of key metabolic pathways and innate sensor systems in a range of cells, complement undisputedly contributes to this chronic inflammatory remodeling at various levels, and studying the role of the complosome in metabolic disease will likely add new insights into disease mechanisms (Figure 5) (Richardson et al., 2013).
Otto Warburg originally observed that many cancer cells are highly glycolytic despite normal oxygen abundance. He postulated this to be a cancer-defining feature (Warburg hypothesis) (Warburg, 1956). We now appreciate that Warburg metabolism, accompanied by activation of the PI(3)-Akt–mTOR axis, is a metabolic program supporting anabolic metabolism and cell proliferation also in non-malignant cells. However, the links between glucose metabolism and newly recognized intracellular complement functions make the metabolism–complement axis an obvious area to (re-)explore in oncology. Indeed, complement activation plays a declared role in cancer biology and can be protective or detrimental. For example, increased complement activation observed in most cancer patients (Macor and Tedesco, 2007) contributes to tumor control via classical pathway-mediated cytotoxicity (Okroj et al., 2013), but C5a generated in the tumor environment supports tumor growth through recruitment of myeloid-derived suppressor cells (Markiewski et al., 2008). Further, sustained complement activation supports chronic inflammation, which drives tumor development and/or survival (Bonavita et al., 2015). Thus, we predict that the interplay between malignancies and complement also impacts cancer metabolism and, indeed, a recent study demonstrates that autocrine C5aR and C3aR activation sustains PI3K–Akt pathway activation and cell proliferation in tumor cells from patients with ovarian or lung cancer (Cho et al., 2014).
The contributions of complement to neuronal disease are usually considered driven by complement-mediated injury to cells of the nervous system during brain injury or inflammation. However, it is now acknowledged that activated brain-resident cells, including astrocytes, microglia and neurons, can release complement upon activation. Thus, (unwanted) complement activation in the brain does not necessarily require breach of the blood brain barrier (BBB) as previously thought (Leinhase et al., 2006; Orsini et al., 2014). Formation of the MAC on brain cells contributes to ischemic injury after stroke (Wang et al., 2010), and C5aR1 activation, at least in the acute phase of neurotrauma, promotes neuronal death, demyelination and further dysfunction of the BBB (Ingersoll et al., 2010; Pavlovski et al., 2012). Although an area of divergent opinion, there are data indicating that complement may contribute to the pathophysiology of Alzheimer’s disease. Indeed, a recent report suggests that excessive C1q and C3-dependent pruning of newly forming synapses is involved in the pathogenesis of Alzheimer’s disease (Heppner et al., 2015). The pathophysiologic involvement of complement in behavioral disorders, such as Schizophrenia, is plausible yet remains to be demonstrated (Bloomfield et al., 2016). However, glycolysis is the major energy source in the brain and controlled autophagy is also a prerequisite for normal brain function (Nixon, 2013). Indeed, mutations in glycolysis and autophagy regulating genes have been connected with both neurodegenerative disease and schizophrenia (Nixon, 2013). Interestingly, C3-deficient mice have reduced learning ability (Perez-Alcazar et al., 2014), and polymorphisms in CD46 and SPAK, an intracellular CD46-interacting protein, were identified as risk factors for schizophrenia and autism in humans (Chehoud et al., 2013; Ramoz et al., 2008). It may thus be worthy assessing a potential connection between complement-regulated metabolic and basic cell physiology pathways in normal and aberrant learning and behavior (Figure 5).
Future Directions and Challenges
The recent advances and – sometimes surprising – novel mechanistic insights into complement’s diverse activities in immunity and beyond suggest that the regulated collaboration between complement, specifically the complosome, other intracellular danger sensors and cellular metabolic pathways is critical to normal cell physiology. Among the key questions that need now to be answered are: How is the complosome regulated? How does intracellular and autocrine complement intersect with the extracellular, i.e. the serum system? Are systemic and local metabolic changes integrated by complement-dependent mechanisms at the cellular level? Does complement, systemic and at a cellular level, intersect with the microbiome (Chehoud et al., 2013)? Notably, in the intestine the microbiota is critical for maintaining the host energy balance via regulation of dietary fat absorption by gut epithelial cells (Velagapudi et al., 2010). We would predict that complement also partakes in this basic process (Figure 5). Given the significant differences between the murine and human complement system, a major challenge will be to rigorously link human in vitro observations to their in vivo relevance.
ACKNOWLEDGMENTS
Work in the Hess and Kemper laboratories is supported by the MRC Centre grant MR/J006742/1, an EU-funded Innovative Medicines Initiative BTCURE (C.K.), a Wellcome Trust Investigator Award (C.K.), and the King’s Bioscience Institute at King’s College London (G.A.), the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London, and by the Division of Intramural Research, National Heart, Lung, and Blood Institute, NIH and the intramural research program of NIAID, NIH, as well as the Swiss National Science Foundation (310030_154059 and CRSII_160766) (C.H.), the Gebert-Rüf Foundation (GER-058/14) and the Swiss Cancer League (KFS-3773-08-2015).
Footnotes
AUTHOR CONTRIBUTION
Conceptualization, C.H. and C.K.; Writing – Original draft, C.H. and C.K.; Writing – Review & Editing, C.H. and C.K.
REFERENCES
- Alexander JJ, Jacob A, Bao L, Macdonald RL, Quigg RJ. Complement-dependent apoptosis and inflammatory gene changes in murine lupus cerebritis. J Immunol. 2005;175:8312–8319. doi: 10.4049/jimmunol.175.12.8312. [DOI] [PubMed] [Google Scholar]
- Amara U, Rittirsch D, Flierl M, Bruckner U, Klos A, Gebhard F, Lambris JD, Huber-Lang M. Interaction between the coagulation and complement system. Adv Exp Med Biol. 2008;632:71–79. doi: 10.1007/978-0-387-78952-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsen D, Helbig C, Backer RA. Notch in T Cell Differentiation: All Things Considered. Trends Immunol. 2015;36:802–814. doi: 10.1016/j.it.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Arbore G, West EE, Spolski R, Robertson AA, Klos A, Rheinheimer C, Dutow P, Woodruff TM, Yu ZX, O’Neill LA, et al. T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4(+) T cells. Science. 2016;352:aad1210. doi: 10.1126/science.aad1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgari E, Le Friec G, Yamamoto H, Perucha E, Sacks SS, Kohl J, Cook HT, Kemper C. C3a modulates IL-1beta secretion in human monocytes by regulating ATP efflux and subsequent NLRP3 inflammasome activation. Blood. 2013;122:3473–3481. doi: 10.1182/blood-2013-05-502229. [DOI] [PubMed] [Google Scholar]
- Baudino L, Sardini A, Ruseva MM, Fossati-Jimack L, Cook HT, Scott D, Simpson E, Botto M. C3 opsonization regulates endocytic handling of apoptotic cells resulting in enhanced T-cell responses to cargo-derived antigens. Proc Natl Acad Sci U S A. 2014;111:1503–1508. doi: 10.1073/pnas.1316877111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR, Bloomfield MA, Bonoldi I, Kalk N, Turkheimer F, et al. Microglial Activity in People at Ultra High Risk of Psychosis and in Schizophrenia: An [(11)C]PBR28 PET Brain Imaging Study. Am J Psychiatry. 2016;173:44–52. doi: 10.1176/appi.ajp.2015.14101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonavita E, Gentile S, Rubino M, Maina V, Papait R, Kunderfranco P, Greco C, Feruglio F, Molgora M, Laface I, et al. PTX3 is an extrinsic oncosuppressor regulating complement-dependent inflammation in cancer. Cell. 2015;160:700–714. doi: 10.1016/j.cell.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Bordet J. In: Studies in Immunity - Collected and translated. Gay FP, editor. Wiley; New York: 1909. [Google Scholar]
- Bosmann M, Haggadone MD, Hemmila MR, Zetoune FS, Sarma JV, Ward PA. Complement activation product C5a is a selective suppressor of TLR4-induced, but not TLR3-induced, production of IL-27(p28) from macrophages. J Immunol. 2012;188:5086–5093. doi: 10.4049/jimmunol.1102914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck MD, O’Sullivan D, Pearce EL. T cell metabolism drives immunity. J Exp Med. 2015;212:1345–1360. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campoy E, Colombo MI. Autophagy in intracellular bacterial infection. Biochim Biophys Acta. 2009;1793:1465–1477. doi: 10.1016/j.bbamcr.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Cardone J, Le Friec G, Vantourout P, Roberts A, Fuchs A, Jackson I, Suddason T, Lord G, Atkinson JP, Cope A, et al. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat Immunol. 2010;11:862–871. doi: 10.1038/ni.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll MC, Isenman DE. Regulation of humoral immunity by complement. Immunity. 2012;37:199–207. doi: 10.1016/j.immuni.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R. Four viruses, two bacteria, and one receptor: membrane cofactor protein (CD46) as pathogens’ magnet. J Virol. 2004;78:4385–4388. doi: 10.1128/JVI.78.9.4385-4388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Graham PH, Hao J, Ni J, Bucci J, Cozzi PJ, Kearsley JH, Li Y. PI3K/Akt/mTOR pathway inhibitors enhance radiosensitivity in radioresistant prostate cancer cells through inducing apoptosis, reducing autophagy, suppressing NHEJ and HR repair pathways. Cell Death Dis. 2014;5:e1437. doi: 10.1038/cddis.2014.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehoud C, Rafail S, Tyldsley AS, Seykora JT, Lambris JD, Grice EA. Complement modulates the cutaneous microbiome and inflammatory milieu. Proc Natl Acad Sci U S A. 2013;110:15061–15066. doi: 10.1073/pnas.1307855110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MS, Vasquez HG, Rupaimoole R, Pradeep S, Wu S, Zand B, Han HD, Rodriguez-Aguayo C, Bottsford-Miller J, Huang J, et al. Autocrine effects of tumor-derived complement. Cell Rep. 2014;6:1085–1095. doi: 10.1016/j.celrep.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianflone K, Rodriguez MA, Walsh M, Vu H, Sniderman AD. The effect of a plasma protein fraction on lipid synthesis in cultured skin fibroblasts from normals and patients with hyperapobetalipoproteinemia. Clin Invest Med. 1988;11:99–107. [PubMed] [Google Scholar]
- Cianflone KM, Maslowska MH, Sniderman AD. Impaired response of fibroblasts from patients with hyperapobetalipoproteinemia to acylation-stimulating protein. J Clin Invest. 1990;85:722–730. doi: 10.1172/JCI114497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofani M, Zuniga-Pflucker JC. Notch promotes survival of pre-T cells at the beta-selection checkpoint by regulating cellular metabolism. Nat Immunol. 2005;6:881–888. doi: 10.1038/ni1234. [DOI] [PubMed] [Google Scholar]
- Coll RC, O’Neill LAJ, Schroder K. Questions and Controversies in innate immune research: What is the physiological role of NLRP3? Cell Death Discovery. 2016;2 doi: 10.1038/cddiscovery.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creagh EM, O’Neill LA. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 2006;27:352–357. doi: 10.1016/j.it.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Croker DE, Monk PN, Halai R, Kaeslin G, Schofield Z, Wu MC, Clark RJ, Blaskovich MA, Morikis D, Floudas CA, et al. Discovery of functionally selective C5aR2 ligands: novel modulators of C5a signaling. Immunol Cell Biol. 2016 doi: 10.1038/icb.2016.43. [DOI] [PubMed] [Google Scholar]
- Cudrici C, Niculescu F, Jensen T, Zafranskaia E, Fosbrink M, Rus V, Shin ML, Rus H. C5b-9 terminal complex protects oligodendrocytes from apoptotic cell death by inhibiting caspase-8 processing and up-regulating FLIP. J Immunol. 2006;176:3173–3180. doi: 10.4049/jimmunol.176.5.3173. [DOI] [PubMed] [Google Scholar]
- Cui W, Paglialunga S, Kalant D, Lu H, Roy C, Laplante M, Deshaies Y, Cianflone K. Acylation-stimulating protein/C5L2-neutralizing antibodies alter triglyceride metabolism in vitro and in vivo. Am J Physiol Endocrinol Metab. 2007;293:E1482–1491. doi: 10.1152/ajpendo.00565.2006. [DOI] [PubMed] [Google Scholar]
- Cumpelik A, Ankli B, Zecher D, Schifferli JA. Neutrophil microvesicles resolve gout by inhibiting C5a-mediated priming of the inflammasome. Ann Rheum Dis. 2015 doi: 10.1136/annrheumdis-2015-207338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–740. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, Datta SR, Greenberg ME, Licklider LJ, Lowell BB, et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424:952–956. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- de Gassart A, Martinon F. Pyroptosis: Caspase-11 Unlocks the Gates of Death. Immunity. 2015;43:835–837. doi: 10.1016/j.immuni.2015.10.024. [DOI] [PubMed] [Google Scholar]
- Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- Dunkelberger J, Zhou L, Miwa T, Song WC. C5aR expression in a novel GFP reporter gene knockin mouse: implications for the mechanism of action of C5aR signaling in T cell immunity. J Immunol. 2012;188:4032–4042. doi: 10.4049/jimmunol.1103141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraj M, Sniderman AD, Cianflone K. ASP enhances in situ lipoprotein lipase activity by increasing fatty acid trapping in adipocytes. J Lipid Res. 2004;45:657–666. doi: 10.1194/jlr.M300299-JLR200. [DOI] [PubMed] [Google Scholar]
- Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, Mathis D. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fico A, Paglialunga F, Cigliano L, Abrescia P, Verde P, Martini G, Iaccarino I, Filosa S. Glucose-6-phosphate dehydrogenase plays a crucial role in protection from redox-stress-induced apoptosis. Cell Death Differ. 2004;11:823–831. doi: 10.1038/sj.cdd.4401420. [DOI] [PubMed] [Google Scholar]
- Fishelson Z, Attali G, Mevorach D. Complement and apoptosis. Mol Immunol. 2001;38:207–219. doi: 10.1016/s0161-5890(01)00055-4. [DOI] [PubMed] [Google Scholar]
- Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell. 2009;16:633–647. doi: 10.1016/j.devcel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Gerard NP, Lu B, Liu P, Craig S, Fujiwara Y, Okinaga S, Gerard C. An anti-inflammatory function for the complement anaphylatoxin C5a-binding protein, C5L2. J Biol Chem. 2005;280:39677–39680. doi: 10.1074/jbc.C500287200. [DOI] [PubMed] [Google Scholar]
- Gerriets VA, Kishton RJ, Nichols AG, Macintyre AN, Inoue M, Ilkayeva O, Winter PS, Liu X, Priyadharshini B, Slawinska ME, et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Invest. 2015;125:194–207. doi: 10.1172/JCI76012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannam A, Fauquert JL, Thomas C, Kemper C, Drouet C. Human complement C3 deficiency: Th1 induction requires T cell-derived complement C3a and CD46 activation. Mol Immunol. 2014;58:98–107. doi: 10.1016/j.molimm.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Gottlieb E, Armour SM, Thompson CB. Mitochondrial respiratory control is lost during growth factor deprivation. Proc Natl Acad Sci U S A. 2002;99:12801–12806. doi: 10.1073/pnas.202477599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MW, Burger G, Lang BF. Mitochondrial evolution. Science. 1999;283:1476–1481. doi: 10.1126/science.283.5407.1476. [DOI] [PubMed] [Google Scholar]
- Gubser PM, Bantug GR, Razik L, Fischer M, Dimeloe S, Hoenger G, Durovic B, Jauch A, Hess C. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat Immunol. 2013;14:1064–1072. doi: 10.1038/ni.2687. [DOI] [PubMed] [Google Scholar]
- Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Meng LQ, Xu PC, Chen M, Zhao MH. p38MAPK, ERK and PI3K signaling pathways are involved in C5a-primed neutrophils for ANCA-mediated activation. PLoS One. 2012;7:e38317. doi: 10.1371/journal.pone.0038317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- Hsia CC, Schmitz A, Lambertz M, Perry SF, Maina JN. Evolution of air breathing: oxygen homeostasis and the transitions from water to land and sky. Compr Physiol. 2013;3:849–915. doi: 10.1002/cphy.c120003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, Chen ZF, Yan J, Li QF, Huang Y, Xu H, Zhang X, Jiang H. Complement C5a exacerbates acute lung injury induced through autophagy-mediated alveolar macrophage apoptosis. Cell Death Dis. 2014;5:e1330. doi: 10.1038/cddis.2014.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingersoll SA, Martin CB, Barnum SR, Martin BK. CNS-specific expression of C3a and C5a exacerbate demyelination severity in the cuprizone model. Mol Immunol. 2010;48:219–230. doi: 10.1016/j.molimm.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs SR, Michalek RD, Rathmell JC. IL-7 is essential for homeostatic control of T cell metabolism in vivo. J Immunol. 2010;184:3461–3469. doi: 10.4049/jimmunol.0902593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert PE, Meiffren G, Gregoire IP, Pontini G, Richetta C, Flacher M, Azocar O, Vidalain PO, Vidal M, Lotteau V, et al. Autophagy induction by the pathogen receptor CD46. Cell Host Microbe. 2009;6:354–366. doi: 10.1016/j.chom.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458:725–731. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten CM, Laumonnier Y, Eurich B, Ender F, Broker K, Roy S, Czabanska A, Vollbrandt T, Figge J, Kohl J. Monitoring and cell-specific deletion of C5aR1 using a novel floxed GFP-C5aR1 reporter knock-in mouse. J Immunol. 2015;194:1841–1855. doi: 10.4049/jimmunol.1401401. [DOI] [PubMed] [Google Scholar]
- Katz Y, Singer L, Wetsel RA, Schlesinger M, Fishelson Z. Inherited complement C3 deficiency: a defect in C3 secretion. Eur J Immunol. 1994;24:1517–1522. doi: 10.1002/eji.1830240709. [DOI] [PubMed] [Google Scholar]
- Keating GM. Eculizumab: a review of its use in atypical haemolytic uraemic syndrome. Drugs. 2013;73:2053–2066. doi: 10.1007/s40265-013-0147-7. [DOI] [PubMed] [Google Scholar]
- Kohl J. The role of complement in danger sensing and transmission. Immunol Res. 2006;34:157–176. doi: 10.1385/IR:34:2:157. [DOI] [PubMed] [Google Scholar]
- Kolev M, Dimeloe S, Le Friec G, Navarini A, Arbore G, Povoleri GA, Fischer M, Belle R, Loeliger J, Develioglu L, et al. Complement Regulates Nutrient Influx and Metabolic Reprogramming during Th1 Cell Responses. Immunity. 2015;42:1033–1047. doi: 10.1016/j.immuni.2015.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolev M, Le Friec G, Kemper C. Complement--tapping into new sites and effector systems. Nat Rev Immunol. 2014;14:811–820. doi: 10.1038/nri3761. [DOI] [PubMed] [Google Scholar]
- Konner AC, Bruning JC. Toll-like receptors: linking inflammation to metabolism. Trends Endocrinol Metab. 2011;22:16–23. doi: 10.1016/j.tem.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krus U, King BC, Nagaraj V, Gandasi NR, Sjolander J, Buda P, Garcia-Vaz E, Gomez MF, Ottosson-Laakso E, Storm P, et al. The complement inhibitor CD59 regulates insulin secretion by modulating exocytotic events. Cell Metab. 2014;19:883–890. doi: 10.1016/j.cmet.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Kurihara R, Yamaoka K, Sawamukai N, Shimajiri S, Oshita K, Yukawa S, Tokunaga M, Iwata S, Saito K, Chiba K, Tanaka Y. C5a promotes migration, proliferation, and vessel formation in endothelial cells. Inflamm Res. 2010;59:659–666. doi: 10.1007/s00011-010-0178-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan WH, van der Touw W, Paz-Artal E, Li MO, Heeger PS. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J Exp Med. 2013;210:257–268. doi: 10.1084/jem.20121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalli PN, Strainic MG, Yang M, Lin F, Medof ME, Heeger PS. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood. 2008;112:1759–1766. doi: 10.1182/blood-2008-04-151068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landor SK, Mutvei AP, Mamaeva V, Jin S, Busk M, Borra R, Gronroos TJ, Kronqvist P, Lendahl U, Sahlgren CM. Hypo- and hyperactivated Notch signaling induce a glycolytic switch through distinct mechanisms. Proc Natl Acad Sci U S A. 2011;108:18814–18819. doi: 10.1073/pnas.1104943108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudisi F, Spreafico R, Evrard M, Hughes TR, Mandriani B, Kandasamy M, Morgan BP, Sivasankar B, Mortellaro A. Cutting edge: the NLRP3 inflammasome links complement-mediated inflammation and IL-1beta release. J Immunol. 2013;191:1006–1010. doi: 10.4049/jimmunol.1300489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Friec G, Kemper C. Complement: coming full circle. Arch Immunol Ther Exp (Warsz) 2009;57:393–407. doi: 10.1007/s00005-009-0047-4. [DOI] [PubMed] [Google Scholar]
- Le Friec G, Sheppard D, Whiteman P, Karsten CM, Shamoun SA, Laing A, Bugeon L, Dallman MJ, Melchionna T, Chillakuri C, et al. The CD46-Jagged1 interaction is critical for human TH1 immunity. Nat Immunol. 2012;13:1213–1221. doi: 10.1038/ni.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinhase I, Holers VM, Thurman JM, Harhausen D, Schmidt OI, Pietzcker M, Taha ME, Rittirsch D, Huber-Lang M, Smith WR, et al. Reduced neuronal cell death after experimental brain injury in mice lacking a functional alternative pathway of complement activation. BMC Neurosci. 2006;7:55. doi: 10.1186/1471-2202-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Fazekasova H, Wang N, Peng Q, Sacks SH, Lombardi G, Zhou W. Functional modulation of human monocytes derived DCs by anaphylatoxins C3a and C5a. Immunobiology. 2012;217:65–73. doi: 10.1016/j.imbio.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38:13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liszewski MK, Kolev M, Le Friec G, Leung M, Bertram PG, Fara AF, Subias M, Pickering MC, Drouet C, Meri S, et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity. 2013;39:1143–1157. doi: 10.1016/j.immuni.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- Luo Y, Tucker SC, Casadevall A. Fc- and complement-receptor activation stimulates cell cycle progression of macrophage cells from G1 to S. J Immunol. 2005;174:7226–7233. doi: 10.4049/jimmunol.174.11.7226. [DOI] [PubMed] [Google Scholar]
- Lv Q, Yang F, Chen K, Zhang Y. Autophagy protects podocytes from sublytic complement induced injury. Exp Cell Res. 2016;341:132–138. doi: 10.1016/j.yexcr.2016.02.009. [DOI] [PubMed] [Google Scholar]
- MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macor P, Tedesco F. Complement as effector system in cancer immunotherapy. Immunol Lett. 2007;111:6–13. doi: 10.1016/j.imlet.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Maekawa Y, Ishifune C, Tsukumo S, Hozumi K, Yagita H, Yasutomo K. Notch controls the survival of memory CD4+ T cells by regulating glucose uptake. Nat Med. 2015;21:55–61. doi: 10.1038/nm.3758. [DOI] [PubMed] [Google Scholar]
- Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, Coukos G, Lambris JD. Modulation of the antitumor immune response by complement. Nat Immunol. 2008;9:1225–1235. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metab. 2013;17:851–859. doi: 10.1016/j.cmet.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Moon JS, Hisata S, Park MA, DeNicola GM, Ryter SW, Nakahira K, Choi AM. mTORC1-Induced HK1-Dependent Glycolysis Regulates NLRP3 Inflammasome Activation. Cell Rep. 2015;12:102–115. doi: 10.1016/j.celrep.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. 2013;19:983–997. doi: 10.1038/nm.3232. [DOI] [PubMed] [Google Scholar]
- Niyonzima N, Samstad EO, Aune MH, Ryan L, Bakke SS, Rokstad AM, Wright SD, Damas JK, Mollnes TE, Latz E, Espevik T. Reconstituted High-Density Lipoprotein Attenuates Cholesterol Crystal-Induced Inflammatory Responses by Reducing Complement Activation. J Immunol. 2015;195:257–264. doi: 10.4049/jimmunol.1403044. [DOI] [PubMed] [Google Scholar]
- Oestreich KJ, Read KA, Gilbertson SE, Hough KP, McDonald PW, Krishnamoorthy V, Weinmann AS. Bcl-6 directly represses the gene program of the glycolysis pathway. Nat Immunol. 2014;15:957–964. doi: 10.1038/ni.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okroj M, Osterborg A, Blom AM. Effector mechanisms of anti-CD20 monoclonal antibodies in B cell malignancies. Cancer Treat Rev. 2013;39:632–639. doi: 10.1016/j.ctrv.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Orsini F, De Blasio D, Zangari R, Zanier ER, De Simoni MG. Versatility of the complement system in neuroinflammation, neurodegeneration and brain homeostasis. Front Cell Neurosci. 2014;8:380. doi: 10.3389/fncel.2014.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovski D, Thundyil J, Monk PN, Wetsel RA, Taylor SM, Woodruff TM. Generation of complement component C5a by ischemic neurons promotes neuronal apoptosis. FASEB J. 2012;26:3680–3690. doi: 10.1096/fj.11-202382. [DOI] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Alcazar M, Daborg J, Stokowska A, Wasling P, Bjorefeldt A, Kalm M, Zetterberg H, Carlstrom KE, Blomgren K, Ekdahl CT, et al. Altered cognitive performance and synaptic function in the hippocampus of mice lacking C3. Exp Neurol. 2014;253:154–164. doi: 10.1016/j.expneurol.2013.12.013. [DOI] [PubMed] [Google Scholar]
- Phieler J, Chung KJ, Chatzigeorgiou A, Klotzsche-von Ameln A, Garcia-Martin R, Sprott D, Moisidou M, Tzanavari T, Ludwig B, Baraban E, et al. The complement anaphylatoxin C5a receptor contributes to obese adipose tissue inflammation and insulin resistance. J Immunol. 2013a;191:4367–4374. doi: 10.4049/jimmunol.1300038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phieler J, Garcia-Martin R, Lambris JD, Chavakis T. The role of the complement system in metabolic organs and metabolic diseases. Semin Immunol. 2013b;25:47–53. doi: 10.1016/j.smim.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe H, Derelle R, Lopez P, Pick K, Borchiellini C, Boury-Esnault N, Vacelet J, Renard E, Houliston E, Queinnec E, et al. Phylogenomics revives traditional views on deep animal relationships. Curr Biol. 2009;19:706–712. doi: 10.1016/j.cub.2009.02.052. [DOI] [PubMed] [Google Scholar]
- Price JD, Schaumburg J, Sandin C, Atkinson JP, Lindahl G, Kemper C. Induction of a regulatory phenotype in human CD4+ T cells by streptococcal M protein. J Immunol. 2005;175:677–684. doi: 10.4049/jimmunol.175.2.677. [DOI] [PubMed] [Google Scholar]
- Ramoz N, Cai G, Reichert JG, Silverman JM, Buxbaum JD. An analysis of candidate autism loci on chromosome 2q24-q33: evidence for association to the STK39 gene. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1152–1158. doi: 10.1002/ajmg.b.30739. [DOI] [PubMed] [Google Scholar]
- Richardson VR, Smith KA, Carter AM. Adipose tissue inflammation: feeding the development of type 2 diabetes mellitus. Immunobiology. 2013;218:1497–1504. doi: 10.1016/j.imbio.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Richetta C, Gregoire IP, Verlhac P, Azocar O, Baguet J, Flacher M, Tangy F, Rabourdin-Combe C, Faure M. Sustained autophagy contributes to measles virus infectivity. PLoS Pathog. 2013;9:e1003599. doi: 10.1371/journal.ppat.1003599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen BS, Cook KS, Yaglom J, Groves DL, Volanakis JE, Damm D, White T, Spiegelman BM. Adipsin and complement factor D activity: an immune-related defect in obesity. Science. 1989;244:1483–1487. doi: 10.1126/science.2734615. [DOI] [PubMed] [Google Scholar]
- Roy C, Paglialunga S, Schaart G, Moonen-Kornips E, Meex RC, Phielix E, Hoeks J, Hesselink MK, Cianflone K, Schrauwen P. Relationship of C5L2 receptor to skeletal muscle substrate utilization. PLoS One. 2013;8:e57494. doi: 10.1371/journal.pone.0057494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rus HG, Niculescu FI, Shin ML. Role of the C5b-9 complement complex in cell cycle and apoptosis. Immunol Rev. 2001;180:49–55. doi: 10.1034/j.1600-065x.2001.1800104.x. [DOI] [PubMed] [Google Scholar]
- Rutz S, Janke M, Kassner N, Hohnstein T, Krueger M, Scheffold A. Notch regulates IL-10 production by T helper 1 cells. Proc Natl Acad Sci U S A. 2008;105:3497–3502. doi: 10.1073/pnas.0712102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samstad EO, Niyonzima N, Nymo S, Aune MH, Ryan L, Bakke SS, Lappegard KT, Brekke OL, Lambris JD, Damas JK, et al. Cholesterol crystals induce complement-dependent inflammasome activation and cytokine release. J Immunol. 2014;192:2837–2845. doi: 10.4049/jimmunol.1302484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma JV, Ward PA. New developments in C5a receptor signaling. Cell Health Cytoskelet. 2012;4:73–82. doi: 10.2147/CHC.S27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schejbel L, Fadnes D, Permin H, Lappegard KT, Garred P, Mollnes TE. Primary complement C5 deficiencies - molecular characterization and clinical review of two families. Immunobiology. 2013;218:1304–1310. doi: 10.1016/j.imbio.2013.04.021. [DOI] [PubMed] [Google Scholar]
- Scola AM, Johswich KO, Morgan BP, Klos A, Monk PN. The human complement fragment receptor, C5L2, is a recycling decoy receptor. Mol Immunol. 2009;46:1149–1162. doi: 10.1016/j.molimm.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman H, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38:225–236. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell. 2002;9:459–470. doi: 10.1016/s1097-2765(02)00482-3. [DOI] [PubMed] [Google Scholar]
- Slaninova V, Krafcikova M, Perez-Gomez R, Steffal P, Trantirek L, Bray SJ, Krejci A. Notch stimulates growth by direct regulation of genes involved in the control of glycolysis and the tricarboxylic acid cycle. Open Biol. 2016;6 doi: 10.1098/rsob.150155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soane L, Rus H, Niculescu F, Shin ML. Inhibition of oligodendrocyte apoptosis by sublytic C5b-9 is associated with enhanced synthesis of bcl-2 and mediated by inhibition of caspase-3 activation. J Immunol. 1999;163:6132–6138. [PubMed] [Google Scholar]
- Song WC. Crosstalk between complement and toll-like receptors. Toxicol Pathol. 2012;40:174–182. doi: 10.1177/0192623311428478. [DOI] [PubMed] [Google Scholar]
- Srivastava M, Simakov O, Chapman J, Fahey B, Gauthier ME, Mitros T, Richards GS, Conaco C, Dacre M, Hellsten U, et al. The Amphimedon queenslandica genome and the evolution of animal complexity. Nature. 2010;466:720–726. doi: 10.1038/nature09201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strainic MG, Liu J, Huang D, An F, Lalli PN, Muqim N, Shapiro VS, Dubyak GR, Heeger PS, Medof ME. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity. 2008;28:425–435. doi: 10.1016/j.immuni.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strainic MG, Shevach EM, An F, Lin F, Medof ME. Absence of signaling into CD4(+) cells via C3aR and C5aR enables autoinductive TGF-beta1 signaling and induction of Foxp3(+) regulatory T cells. Nat Immunol. 2013;14:162–171. doi: 10.1038/ni.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stummvoll GH, DiPaolo RJ, Huter EN, Davidson TS, Glass D, Ward JM, Shevach EM. Th1, Th2, and Th17 effector T cell-induced autoimmune gastritis differs in pathological pattern and in susceptibility to suppression by regulatory T cells. J Immunol. 2008;181:1908–1916. doi: 10.4049/jimmunol.181.3.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KE. Complement deficiency and autoimmunity. Curr Opin Pediatr. 1998;10:600–606. doi: 10.1097/00008480-199810060-00011. [DOI] [PubMed] [Google Scholar]
- Takeshige K, Baba M, Tsuboi S, Noda T, Ohsumi Y. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J Cell Biol. 1992;119:301–311. doi: 10.1083/jcb.119.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam JC, Bidgood SR, McEwan WA, James LC. Intracellular sensing of complement C3 activates cell autonomous immunity. Science. 2014;345:1256070. doi: 10.1126/science.1256070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai M, Graham NA, Braas D, Nehil M, Komisopoulou E, Kurdistani SK, McCormick F, Graeber TG, Christofk HR. Adenovirus E4ORF1-induced MYC activation promotes host cell anabolic glucose metabolism and virus replication. Cell Metab. 2014;19:694–701. doi: 10.1016/j.cmet.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafilou K, Hughes TR, Triantafilou M, Morgan BP. The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. J Cell Sci. 2013;126:2903–2913. doi: 10.1242/jcs.124388. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Hughes TR, Morgan BP, Triantafilou K. Complementing the inflammasome. Immunology. 2016;147:152–164. doi: 10.1111/imm.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouw LA, Blom AM, Gasque P. Role of complement and complement regulators in the removal of apoptotic cells. Mol Immunol. 2008;45:1199–1207. doi: 10.1016/j.molimm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Shimizu S. Another way to die: autophagic programmed cell death. Cell Death Differ. 2005;12(Suppl 2):1528–1534. doi: 10.1038/sj.cdd.4401777. [DOI] [PubMed] [Google Scholar]
- van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velagapudi VR, Hezaveh R, Reigstad CS, Gopalacharyulu P, Yetukuri L, Islam S, Felin J, Perkins R, Boren J, Oresic M, Backhed F. The gut microbiota modulates host energy and lipid metabolism in mice. J Lipid Res. 2010;51:1101–1112. doi: 10.1194/jlr.M002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger J, Green DR. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Li Y, Dalle Lucca SL, Simovic M, Tsokos GC, Dalle Lucca JJ. Decay accelerating factor (CD55) protects neuronal cells from chemical hypoxia-induced injury. J Neuroinflammation. 2010;7:24. doi: 10.1186/1742-2094-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Tong X, Zhang J, Ye X. The complement C1qA enhances retinoic acid-inducible gene-I-mediated immune signalling. Immunology. 2012;136:78–85. doi: 10.1111/j.1365-2567.2012.03561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, Miao EA, Ting JP. Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity. 2013;39:432–441. doi: 10.1016/j.immuni.2013.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AP, Shadel GS, Ghosh S. Mitochondria in innate immune responses. Nat Rev Immunol. 2011;11:389–402. doi: 10.1038/nri2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens M, Korzhev M, Perovic-Ottstadt S, Luthringer B, Brandt D, Klein S, Muller WE. Toll-like receptors are part of the innate immune defense system of sponges (demospongiae: Porifera) Mol Biol Evol. 2007;24:792–804. doi: 10.1093/molbev/msl208. [DOI] [PubMed] [Google Scholar]
- Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wofford JA, Wieman HL, Jacobs SR, Zhao Y, Rathmell JC. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood. 2008;111:2101–2111. doi: 10.1182/blood-2007-06-096297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Chi F, Tsukamoto H. Notch, metabolism and macrophages. Oncotarget. 2015;6:19942–19943. doi: 10.18632/oncotarget.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Xiao N, Liu F, Ren H, Gu J. Inhibition of RIG-I and MDA5-dependent antiviral response by gC1qR at mitochondria. Proc Natl Acad Sci U S A. 2009;106:1530–1535. doi: 10.1073/pnas.0811029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Araki K, Li S, Han JH, Ye L, Tan WG, Konieczny BT, Bruinsma MW, Martinez J, Pearce EL, et al. Autophagy is essential for effector CD8(+) T cell survival and memory formation. Nat Immunol. 2014;15:1152–1161. doi: 10.1038/ni.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Fara AF, Dasgupta P, Kemper C. CD46: the ‘multitasker’ of complement proteins. Int J Biochem Cell Biol. 2013;45:2808–2820. doi: 10.1016/j.biocel.2013.09.016. [DOI] [PubMed] [Google Scholar]
- Yuen B, Bayes JM, Degnan SM. The characterization of sponge NLRs provides insight into the origin and evolution of this innate immune gene family in animals. Mol Biol Evol. 2014;31:106–120. doi: 10.1093/molbev/mst174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin SA, Petit PX, Mignotte B, Kroemer G. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med. 1995;182:367–377. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Collins SL, Lutz MA, Allen AN, Kole TP, Zarek PE, Powell JD. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J Immunol. 2007;178:2163–2170. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]
- Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Ziporen L, Donin N, Shmushkovich T, Gross A, Fishelson Z. Programmed necrotic cell death induced by complement involves a Bid-dependent pathway. J Immunol. 2009;182:515–521. doi: 10.4049/jimmunol.182.1.515. [DOI] [PubMed] [Google Scholar]
- Zuniga LA, Shen WJ, Joyce-Shaikh B, Pyatnova EA, Richards AG, Thom C, Andrade SM, Cua DJ, Kraemer FB, Butcher EC. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185:6947–6959. doi: 10.4049/jimmunol.1001269. [DOI] [PMC free article] [PubMed] [Google Scholar]