Abstract
Purpose
Recent studies propose TH2-mediated inflammation in patients with asthma–chronic obstructive pulmonary disease (COPD) overlap syndrome (ACOS). However, little is known about whether fractional exhaled nitric oxide (FeNO) differs between patients with ACOS and those with COPD alone. To address this knowledge gap, a nationally representative sample was analyzed to determine the difference in FeNO levels between patients with ACOS and those with COPD alone in the US population.
Patients and methods
This is a cross-sectional analysis of the National Health and Nutrition Examination Survey from 2007 through 2012. All subjects aged ≥40 years with COPD were identified. ACOS was defined as self-reported wheezing in past 12 months plus bronchodilator response (forced expiratory volume increase of >200 mL and >12%) or self-reported physician diagnosis of asthma.
Results
A total of 197 subjects with COPD were identified in the National Health and Nutrition Examination Survey. Of these, 23% met the criteria of ACOS. The FeNO level was higher in subjects with ACOS compared with those with COPD alone in both unadjusted (mean 21.2 ppb vs 13.0 ppb; difference, 8.2 [95% CI, 0.2 to 16.2]; P=0.045) and adjusted (difference, 8.2 [95% CI, 0.9 to 15.5]; P=0.03) analyses. Although there was no significant difference among current smokers, the FeNO level was significantly higher in non-current smokers with ACOS than nonsmokers with COPD alone (mean 31.9 ppb vs 20.3 ppb; adjusted difference, 20.5 [95% CI, 4.4 to 36.6]; P=0.02). In a sensitivity analysis using an alternative definition of ACOS, the results did not change materially. The diagnostic value of FeNO to discriminate ACOS from COPD alone was not sufficient, with the area under the curve of 0.63 (95% CI, 0.54 to 0.72).
Conclusion
By using nationally representative US data, it was found that 23% of COPD subjects met the ACOS criteria and also that the FeNO level was higher in subjects with ACOS compared with those with COPD alone, particularly in non-current smokers.
Keywords: COPD, asthma–COPD overlap syndrome, fractional exhaled nitric oxide
Background
Asthma–chronic obstructive pulmonary disease (COPD) overlap syndrome (ACOS) has been recognized as a phenotype of COPD that has clinical features of both asthma and COPD.1 Patients with ACOS account for 15%–20% of COPD2 with greater disease morbidity compared with those with COPD alone.3,4 Although the underlying pathobiology of ACOS is largely unclear, recent genomic studies have demonstrated a potential role of TH2 inflammation5 and eosinophil activation6 in ACOS.
Fractional exhaled nitric oxide (FeNO) has been used as a marker for TH2-mediated airway inflammation in asthma.7 FeNO gas is produced in the epithelial cells of the bronchial wall in response to interleukin-4 and -13,8 which are produced locally by the TH2 cells, mast cells, and eosinophils. Therefore, exhaled FeNO indicates the pro-inflammatory cytokine mechanisms in the pathophysiology of eosinophilic airway inflammation. Although studies have shown that the eosinophilic airway inflammation is a feature of ACOS,5,6 little is known about the FeNO levels in patients with ACOS in comparison with those with COPD alone.
To address this knowledge gap, a nationally representative sample was analyzed to determine the difference in FeNO levels between patients with ACOS and those with COPD alone in the US population. It was hypothesized that FeNO level is significantly higher in subjects with ACOS compared with those with COPD alone.
Methods
This is a cross-sectional analysis of the National Health and Nutrition Examination Survey (NHANES) from 2007 through 2012. Details of the study design, setting, and analysis may be found in the Supplementary material. The NHANES is a national survey that collects biologic samples on a representative sample of the US population, provided by the National Center for Health Statistics at the Centers for Disease Control and Prevention.9 NHANES participants were sampled through a complex, multistage sampling methodology to ensure that the sample is nationally representative. Because the NHANES survey is a single-point interview, follow-up data are not available.
Participants were interviewed for demographics including age, sex, race/ethnicity, primary health insurance, smoking history, medical history, comorbidities, and health care utilization. In addition, participants underwent medical and physiologic examinations as well as laboratory testing. During the household interview survey, participants were asked whether they had taken medications in the past month for which they needed a prescription. Those who answered “yes” were asked to show the medication containers of all the products used to the interviewer. If no container was available, the interviewer asked the participant to verbally report the name of the medication. Written informed consent was obtained from all the participants, and the National Center for Health Statistics Research Ethics Review Board approved the protocol. The institutional review board of Massachusetts General Hospital approved this analysis.
All subjects aged ≥40 years with COPD, defined as a post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) <0.70 with a history of smoking ≥100 cigarettes total in their life and with at least one of following symptoms, cough, sputum production, wheezing, and shortness of breath on exertion, were identified.10,11 In the NHANES, spirometry was conducted according to the American Thoracic Society recommendations.12 Participants who met the following criteria were excluded from spirometry for safety reasons: current chest pain or pain with forceful expiration; current use of daytime supplemental oxygen; had recent surgery of the eye, chest, or abdomen; or had a recent heart attack, stroke, tuberculosis exposure, hemoptysis, a history of detached retina, or pneumothorax. Participants who underwent a “baseline” (pre-bronchodilator) spirometry were selected for follow-up “post-bronchodilator” spirometry if their baseline spirometry values indicated possible airflow obstruction.10,13,14 The Global initiative of chronic Obstructive Lung Disease (GOLD) stage was defined on the basis of the post-bronchodilator spirometry parameters (ie, FEV1/FVC and FEV1 percent of predicted).10 Predicted FEV1 was calculated on the basis of sex, age, and height according to a previous literature.14
According to the literature, ACOS was defined as self-reported wheezing in past 12 months plus bronchodilator response (FVC increase of >200 mL and >12%) or self-reported physician diagnosis of asthma.15 In sensitivity analysis, an alternative definition of ACOS was also used – defined by the presence of one major criterion or two minor criteria.16 Major criteria were 1) history of asthma and 2) bronchodilator response of ≥15% and 400 mL; minor criteria were 1) history of hay fever, 2) bronchodilator responses to salbutamol of ≥12% and 200 mL, and 3) blood eosinophils ≥5%.
The primary outcome was FeNO level (ppb). The unadjusted and multivariable linear regression models were fitted. Multivariable model adjusted for up to six confounders (age, sex, race/ethnicity, steroid use, medications for wheezing, and current smoking status) as the number of subjects with ACOS was relatively small. Given the known effects of smoking on FeNO levels, the models were stratified by current smoking status (yes/no). These analyses were repeated with the use of alternative definition of ACOS. The distinction between active-smokers and non-current smokers was ascertained through the NHANES question asking, “are you smoking currently?”
The receiver operating characteristic curves were drawn to evaluate the diagnostic value of FeNO for discriminating ACOS from COPD alone. To maximize the sum of sensitivity and specificity for all the possible values of the cutoff point, the Youden index method was applied. Sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio were calculated and given with 95% confidence intervals. Statistical analyses were performed using Stata version 13.1 (StataCorp, College Station, TX, USA), accounting for the complex survey design.
Results
We identified 197 subjects with COPD in the NHANES. Overall, the mean age was 58 years and 69% were male. Of these, 23% met the primary criteria of ACOS while 28% met the alternative criteria of ACOS. The mean age of asthma diagnosis in ACOS group was 22 years. The body mass index and the prevalence of wheezing were higher in subjects with ACOS group compared with those with COPD alone. Between subjects with ACOS and those with COPD alone, most of demographic characteristics, medical history, pulmonary function test, or GOLD stage did not significantly differ (P>0.05), regardless of ACOS definition (Tables 1 and S1).
Table 1.
Characteristics of subjects with asthma–COPD overlap syndrome and those with COPD alone
| Characteristicsa | ACOS | COPD alone | P-value |
|---|---|---|---|
| Unweighted sample, n | 48 | 149 | – |
| Weighted estimate, n | 662,424 | 2,165,367 | – |
| Demographics | |||
| Age (year), mean (SE) | 56 (1.4) | 58 (1.1) | 0.23 |
| Male sex | 75 (53–88) | 67 (58–75) | 0.47 |
| Race/ethnicity | 0.74 | ||
| Non-Hispanic white | 88 (78–94) | 91 (85–94) | |
| Non-Hispanic black | 8 (4–18) | 5 (3–9) | |
| Hispanics | 1 (0–3) | 1 (0–3) | |
| Others | 3 (1–7) | 3 (1–8) | |
| Primary health insurance | 0.80 | ||
| Medicare | 10 (5–21) | 12 (8–19) | |
| Medicaid | 3 (1–12) | 2 (1–4) | |
| Private | 73 (53–86) | 68 (59–76) | |
| No insurance | 2 (0–10) | 5 (3–10) | |
| Others | 12 (4–32) | 12 (7–20) | |
| Medical history | |||
| Body mass index (kg/m2), mean (SE) | 30.1 (1.1) | 27.2 (0.5) | 0.03 |
| Smoking status | |||
| Current smoker | 48 (33–64) | 59 (47–70) | 0.34 |
| Pack-years, mean (SE) | 32 (3.3) | 36 (2.2) | 0.30 |
| ≥10 pack-years | 79 (63–89) | 83 (74–90) | 0.58 |
| Symptoms | |||
| Cough | 33 (17–53) | 37 (29–45) | 0.72 |
| Sputum | 34 (19–53) | 31 (22–42) | 0.78 |
| Wheezing | 72 (48–88) | 35 (27–45) | 0.01 |
| Shortness of breath on exertion | 60 (42–75) | 70 (61–78) | 0.34 |
| Comorbidities | |||
| Coronary heart diseasesb | 10 (3–30) | 15 (9–24) | 0.43 |
| Diabetes | 11 (4–25) | 11 (7–18) | 0.92 |
| Hypertension | 36 (22–53) | 41 (31–52) | 0.62 |
| Renal failure | 9 (2–37) | 2 (1–6) | 0.38 |
| Health care utilization | |||
| Having a routine place for health care | 91 (79–97) | 94 (90–97) | 0.55 |
| Number of health care visits in the past year, mean (SE) | 2 (0.2) | 2 (0.2) | 0.51 |
| Number of health care visits for wheezing in the past year, mean (SE) | 1 (0.5) | 1 (0.3) | 0.64 |
| Overnight hospital stay in the past year | 9 (3–23) | 14 (9–23) | 0.37 |
| Medications for wheezing | 58 (37–77) | 32 (19–49) | 0.01 |
| Oral corticosteroids | 3 (1–21) | 1 (0–5) | 0.47 |
| Inhaled corticosteroids | 5 (1–19) | 0 (0–0) | 0.18 |
| Laboratory tests | |||
| White blood cell count (cells/μL), mean (SE) | |||
| Total | 7,349 (297) | 7,972 (178) | 0.09 |
| Neutrophils | 4,538 (256) | 4,909 (148) | 0.25 |
| Lymphocytes | 1,951 (87) | 2,194 (79) | 0.04 |
| Eosinophils | 272 (44) | 236 (13) | 0.45 |
| Spirometry, mean (SE) | |||
| Baseline FVC (L) | 3.9 (0.2) | 4.0 (0.1) | 0.85 |
| Baseline FEV1 (L) | 2.4 (0.1) | 2.4 (0.1) | 0.97 |
| Baseline PEF (L/min) | 383 (25) | 377 (9) | 0.81 |
| Baseline FEV1 % predicted | 58 (53–62) | 61 (57–64) | 0.32 |
| Baseline FEV1/FVC% | 60 (58–62) | 59 (57–61) | 0.54 |
| Post-bronchodilator FVC (L) | 4.2 (0.2) | 4.0 (0.1) | 0.50 |
| Post-bronchodilator FEV1 (L) | 2.6 (0.1) | 2.5 (0.1) | 0.40 |
| Post-bronchodilator PEF (L/min) | 430 (27) | 402 (10) | 0.33 |
| Post-bronchodilator FEV1 % predicted | 63 (59–67) | 63 (60–67) | 0.92 |
| Post-bronchodilator FEV1/FVC% | 62 (59–65) | 61 (59–63) | 0.50 |
| GOLD stage | 0.07 | ||
| I | 2 (0–16) | 17 (10–25) | |
| II | 84 (73–91) | 63 (50–74) | |
| III | 14 (6–27) | 19 (12–30) | |
| IV | 0 (0–0) | 1 (0–6) | |
Notes:
Data are expressed as % (95% confidence interval) unless otherwise indicated.
Coronary heart diseases include ischemic heart disease, angina, myocardial infarction, and heart failure.
Abbreviations: ACOS, asthma–COPD overlap syndrome; COPD, chronic obstructive pulmonary disease; GOLD, the Global initiative of chronic Obstructive Lung Disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; PEF, peak flow; SE, standard error.
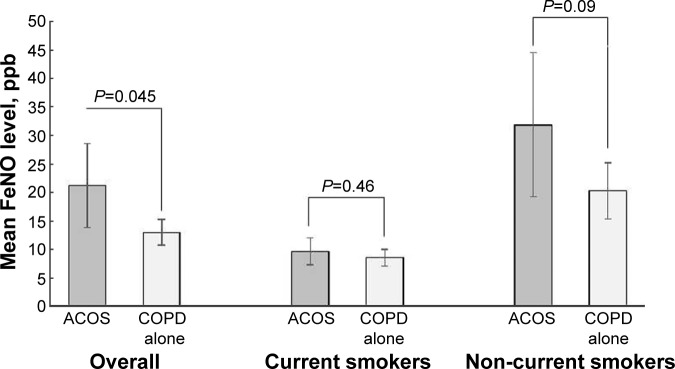
In contrast, the FeNO level was higher in subjects with ACOS compared to those with COPD alone (mean 21.2 ppb vs 13.0 ppb; difference, 8.2 [95% CI, 0.2 to 16.2]; P=0.045; Table 2 and Figure 1). After adjusting for potential confounders, the significant difference persisted (adjusted difference, 8.2 [95% CI, 0.9 to 15.5]; P=0.03). In the stratified analysis with a limited statistical power, among current smokers, there was no significant difference in FeNO levels between the two groups (mean 9.6 ppb vs 8.5 ppb; adjusted difference, 3.2 [95% CI, −0.1 to 6.6]; P=0.06). By contrast, among non-current smokers, subjects with ACOS had a significantly higher FeNO level (mean 31.9 ppb vs 20.3 ppb; adjusted difference, 20.5 [95% CI, 4.4 to 36.6]; P=0.02). These results did not change materially with the use of alternative definition of ACOS (Figure S1 and Table 3).
Table 2.
Unadjusted and adjusted differences in fractional exhaled nitric oxide level between subjects with asthma–COPD overlap syndrome and those with COPD alone
| Models | Primary analysis | Stratified analysis
|
||||
|---|---|---|---|---|---|---|
|
|
Current smokers n=112 |
Non-current smokers n=85 |
||||
| β coefficient (95% CI) | P-value | β coefficient (95% CI) | P-value | β coefficient (95% CI) | P-value | |
| Unadjusted model | ||||||
| ACOS (vs COPD alone) | 8.2 (0.2 to 16.2)a | 0.045 | 1.1 (−1.9 to 4.2)b | 0.46 | 11.6 (−1.7 to 24.9)c | 0.09 |
| Multivariable model | ||||||
| ACOS (vs COPD alone) | 8.2 (0.9 to 15.5) | 0.03 | 3.2 (−0.1 to 6.6) | 0.06 | 20.5 (4.4 to 36.6) | 0.02 |
| Age | 0.2 (−0.2 to 0.6) | 0.38 | −0.04 (−0.2 to 0.1) | 0.52 | 0.7 (−0.1 to 1.5) | 0.08 |
| Female sex | 3.2 (−13.5 to 19.9) | 0.70 | 1.1 (−3.6 to 5.8) | 0.63 | 5.4 (−18.1 to 28.9) | 0.64 |
| Race/ethnicity | ||||||
| Non-Hispanic white | 1 (reference) | 1 (reference) | 1 (reference) | |||
| Non-Hispanic black | −3.0 (−12.4 to 6.4) | 0.52 | 5.3 (−2.0 to 12.6) | 0.15 | −25.6 (−45.4 to −5.8) | 0.01 |
| Mexican–American | 2.1 (−5.1 to 9.4) | 0.56 | 2.6 (−1.3 to 6.5) | 0.18 | 11.8 (−0.8 to 24.3) | 0.07 |
| Other race | −1.6 (−11.8 to 8.6) | 0.75 | 2.3 (−4.0 to 8.6) | 0.46 | −7.5 (−17.3 to 2.2) | 0.12 |
| Corticosteroid used | −1.9 (−14.7 to 11.0) | 0.77 | 8.2 (5.7 to 10.8) | <0.001 | −20.6 (−48.2 to 7.0) | 0.14 |
| Medications for wheezing | −4.8 (−12.9 to 3.2) | 0.23 | −0.5 (−3.2 to 2.2) | 0.72 | −10.4 (−22.8 to 2.0) | 0.10 |
| Current smoking | −15.8 (−23.9 to −7.8) | <0.001 | ||||
Notes:
Mean 21.2 ppb in ACOS vs 13.0 ppb in COPD alone.
Mean 9.6 ppb in ACOS vs 8.5 ppb in COPD alone.
Mean 31.9 ppb in ACOS vs 20.3 ppb in COPD alone.
Including oral corticosteroids and inhaled corticosteroids.
Abbreviations: ACOS, asthma–COPD overlap syndrome; CI, confidence interval; COPD, chronic obstructive pulmonary disease.
Figure 1.
Unadjusted differences in mean fractional exhaled nitric oxide level between subjects with asthma–COPD overlap syndrome and those with COPD alone.
Abbreviations: ACOS, asthma–COPD overlap syndrome; COPD, chronic obstructive pulmonary disease.
Table 3.
Unadjusted and adjusted differences in fractional exhaled nitric oxide level between subjects with asthma–COPD overlap syndrome (alternative definition) and those with COPD alone
| Models | Primary analysis | Stratified analysis
|
||||
|---|---|---|---|---|---|---|
|
|
Current smokers n=112 |
Non-current smokers n=85 |
||||
| β coefficient (95% CI) | P-value | β coefficient (95% CI) | P-value | β coefficient (95% CI) | P-value | |
| Unadjusted model | ||||||
| ACOS (vs COPD alone) | 6.7 (−0.2 to 13.8)a | 0.06 | 1.6 (−1.4 to 4.7)b | 0.29 | 7.1 (−4.0 to 18.2)c | 0.20 |
| Multivariable model | ||||||
| ACOS (vs COPD alone) | 7.2 (0.8 to 13.5) | 0.03 | 2.9 (−0.4 to 6.2) | 0.08 | 19.0 (3.8 to 34.1) | 0.02 |
| Age | 0.2 (−0.2 to 0.6) | 0.37 | −0.03 (−0.2 to 0.1) | 0.63 | 0.7 (−0.2 to 1.5) | 0.11 |
| Female sex | 1.6 (−16.7 to 20.0) | 0.86 | 1.0 (−4.0 to 5.9) | 0.69 | −0.04 (−28.3 to 28.3) | 0.99 |
| Race/ethnicity | ||||||
| Non-Hispanic white | 1 (reference) | 1 (reference) | 1 (reference) | |||
| Non-Hispanic black | −2.7 (−11.9 to 6.6) | 0.57 | 5.3 (−2.0 to 12.6) | 0.15 | −24.9 (−43.9 to −5.8) | 0.01 |
| Mexican–American | 3.3 (−2.9 to 9.6) | 0.29 | 3.1 (0.3 to 6.0) | 0.03 | 13.1 (−1.3 to 27.4) | 0.07 |
| Other race | −3.8 (−14.2 to 6.6) | 0.47 | 0.2 (−7.1 to 7.6) | 0.95 | −8.5 (−19.5 to 2.6) | 0.13 |
| Corticosteroid used | −1.2 (−12.9 to 10.5) | 0.84 | 8.0 (5.5 to 10.5) | <0.001 | −17.0 (−43.4 to 9.4) | 0.20 |
| Medications for wheezing | −5.7 (−14.7 to 3.3) | 0.21 | −0.5 (−3.2 to 2.3) | 0.73 | −14.1 (−29.9 to 1.7) | 0.08 |
| Current smoking | −15.8 (−24.3 to −7.2) | 0.001 | – | – | – | – |
Notes:
Mean 19.8 ppb in ACOS vs 13.1 ppb in COPD alone.
Mean 10.0 ppb in ACOS vs 8.3 ppb in COPD alone.
Mean 28.3 ppb in ACOS vs 21.2 ppb in COPD alone.
Including oral corticosteroids and inhaled corticosteroids.
Abbreviations: ACOS, asthma–COPD overlap syndrome; CI, confidence interval; COPD, chronic obstructive pulmonary disease.
The diagnostic value of FeNO to discriminate ACOS from COPD alone was not sufficient, with the area under the curve (AUC) of 0.63 (95% CI, 0.54 to 0.72; Figure 2). By using Youden index, the FeNO level ≥8.5 ppb was the best cutoff value for the maximum potential effectiveness of FeNO (sensitivity, 80.9%; specificity, 44.5%; positive likelihood ratio [LR+], 1.45; and negative likelihood ratio [LR−], 0.43). To maximize the diagnostic value for discrimination with a clinically meaningful value, the FeNO level ≥36 ppb was another cutoff value (sensitivity, 12.8%; specificity, 95.2%; LR+, 2.66; and LR−, 0.92). These diagnostic values did not change materially in the sensitivity analysis with the use of alternative definition of ACOS. The AUC was 0.63 (95% CI, 0.54–0.72; Figure S2), and the cutoff value using Youden index was the FeNO level ≥8.0 ppb (sensitivity, 80.8%; specificity, 51.8%; LR+, 1.37; LR−, 0.47). To maximize the diagnostic value for discrimination with a clinically meaningful value, the FeNO level ≥36 ppb was another cutoff value (sensitivity, 11.5%; specificity, 95.4%; LR+, 2.3; LR−, 0.93).
Figure 2.
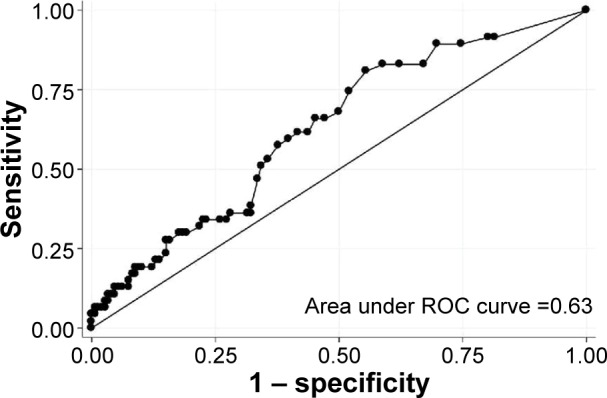
Receiver operating characteristic curve for fractional exhaled nitric oxide level to discriminate asthma–COPD overlap syndrome from COPD alone.
Abbreviations: COPD, chronic obstructive pulmonary disease; ROC, receiver operating characteristic.
Discussion
Although inflammation in COPD is thought to be driven primarily by neutrophilic inflammation, recent studies suggest the presence of TH2-mediated airway inflammation in subsets of patients – for example, those with ACOS. Within the limited literature, Christenson et al demonstrated that, in patient with COPD, airway epithelial gene signatures of TH2-mediated inflammation are associated with “asthma-like” features, favorable corticosteroid response, and increased disease severity.5 Similarly, using a genome-wide association approach, Hardin et al reported an association between GPR65 (a gene involved in eosinophil activation) and COPD with physician-diagnosed asthma.6 Along with these genomic studies, Donohue et al, in a two-center cross-sectional study, found higher FeNO levels in patients with COPD with an International Classification of Diseases-9 diagnosis code of asthma compared with those with COPD alone.17 In addition, a small study in Japan reported that FeNO >35 ppb and evidence of atopy predicted improvement in FEV1 with inhaled corticosteroids and long-acting beta agonist on airflow limitation in COPD.18 Our results corroborate on these studies and extend them by demonstrating the difference in FeNO levels between patients with ACOS and those with COPD alone using nationally representative data with comprehensive clinical, physiological, and biological assessment.
Our study has several potential limitations. First, there is no universally accepted definition of ACOS. Nevertheless, with the use of two different, previously applied definitions of ACOS,15,16 consistent findings were observed. Second, there might be a misclassification and underreporting of subjects’ medications (eg, systemic or inhaled corticosteroids), although the self-reported use of prescription medications was verified with prescription bottles. However, assuming that the frequency of misclassifications does not vary significantly between the two groups, this nondifferential misclassification would have biased our inferences toward the null. Third, some of the elderly subjects, particularly individuals ≥70 years, were not eligible for spirometry testing based on the NHANES protocol. Thus, our findings may have less generalizability to the elderly patients with COPD. In addition, the underrepresentation of subjects with severe GOLD stages might have overestimated the prevalence of ACOS in this study, although our estimate of the prevalence of ACOS was consistent with the previous literature (15%–20%).2 Finally, as with any observational study, the significant difference in FeNO between the groups might be explained, at least partly, by unmeasured confounders.
Conclusion
In sum, these nationally representative data demonstrated that approximately one in four COPD subjects had ACOS, indicating the substantial public health burden of ACOS in the US. It was also found that FeNO level was significantly higher in subjects with ACOS compared with those with COPD alone, while the diagnostic value of FeNO to discriminate ACOS from COPD alone was not sufficient. Although the clinical role of FeNO in the identification of patients with ACOS requires further study, our findings should encourage researchers to investigate pathobiology of ACOS (eg, TH2-mediated airway inflammation) and develop targeted therapeutic strategies (eg, anti-TH2 therapies) for this important subtype of COPD.
Acknowledgments
This study was supported by the grant R01 HS023305 from the Agency for Healthcare Research and Quality (Rockville, MD, USA). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. Dr Goto was supported by a grant from St Luke’s Life Science Institute and Uehara Kinen Memorial Foundation. The abstract of this paper was presented at the AJRCCM Conference on May 17, 2016, as a poster presentation.
Footnotes
Disclosure
Dr Camargo has provided asthma- and COPD-related consultation for GlaxoSmithKline and Teva. Dr Hasegawa has received research support from Teva. The authors have no other conflicts of interest to report.
References
- 1.Postma DS, Rabe KF. The asthma-COPD overlap syndrome. N Engl J Med. 2015;373(13):1241–1249. doi: 10.1056/NEJMra1411863. [DOI] [PubMed] [Google Scholar]
- 2.Barrecheguren M, Esquinas C, Miravitlles M. The asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): opportunities and challenges. Curr Opin Pulm Med. 2015;21(1):74–79. doi: 10.1097/MCP.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 3.Gibson PG, Simpson JL. The overlap syndrome of asthma and COPD: what are its features and how important is it? Thorax. 2009;64(8):728–735. doi: 10.1136/thx.2008.108027. [DOI] [PubMed] [Google Scholar]
- 4.Kauppi P, Kupiainen H, Lindqvist A, et al. Overlap syndrome of asthma and COPD predicts low quality of life. J Asthma. 2011;48(3):279–285. doi: 10.3109/02770903.2011.555576. [DOI] [PubMed] [Google Scholar]
- 5.Christenson SA, Steiling K, van den Berge M, et al. Asthma-COPD overlap. Clinical relevance of genomic signatures of type 2 inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191(7):758–766. doi: 10.1164/rccm.201408-1458OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardin M, Cho M, McDonald ML, et al. The clinical and genetic features of COPD-asthma overlap syndrome. Eur Respir J. 2014;44(2):341–350. doi: 10.1183/09031936.00216013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FeNO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo FH, Comhair SA, Zheng S, et al. Molecular mechanisms of increased nitric oxide (NO) in asthma: evidence for transcriptional and post-translational regulation of NO synthesis. J Immunol. 2000;164(11):5970–5980. doi: 10.4049/jimmunol.164.11.5970. [DOI] [PubMed] [Google Scholar]
- 9.National Health and Nutrition Examination Survey Centers for Disease Control and Prevention. [Accessed June 10, 2016]. Available from: http://www.cdc.gov/nchs/nhanes.htm.
- 10.Global Strategy for Diagnosis, Management, Prevention of COPD. 2016. [Accessed June 10, 2016]. Available from: http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/
- 11.Hnizdo E, Sullivan PA, Bang KM, Wagner G. Association between chronic obstructive pulmonary disease and employment by industry and occupation in the US population: a study of data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2002;156(8):738–746. doi: 10.1093/aje/kwf105. [DOI] [PubMed] [Google Scholar]
- 12.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 13.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 14.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 15.Menezes AM, Montes de Oca M, Perez-Padilla R, et al. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest. 2014;145(2):297–304. doi: 10.1378/chest.13-0622. [DOI] [PubMed] [Google Scholar]
- 16.Cosio BG, Soriano JB, Lopez-Campos JL, et al. Defining the asthma-COPD overlap syndrome in a COPD cohort. Chest. 2016;149(1):45–52. doi: 10.1378/chest.15-1055. [DOI] [PubMed] [Google Scholar]
- 17.Donohue JF, Herje N, Crater G, Rickard K. Characterization of airway inflammation in patients with COPD using fractional exhaled nitric oxide levels: a pilot study. Int J Chron Obstruct Pulmon Dis. 2014;9:745–751. doi: 10.2147/COPD.S44552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akamatsu K, Matsunaga K, Sugiura H, et al. Improvement of airflow limitation by fluticasone propionate/salmeterol in chronic obstructive pulmonary disease: what is the specific marker? Front Pharmacol. 2011;2:36. doi: 10.3389/fphar.2011.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]