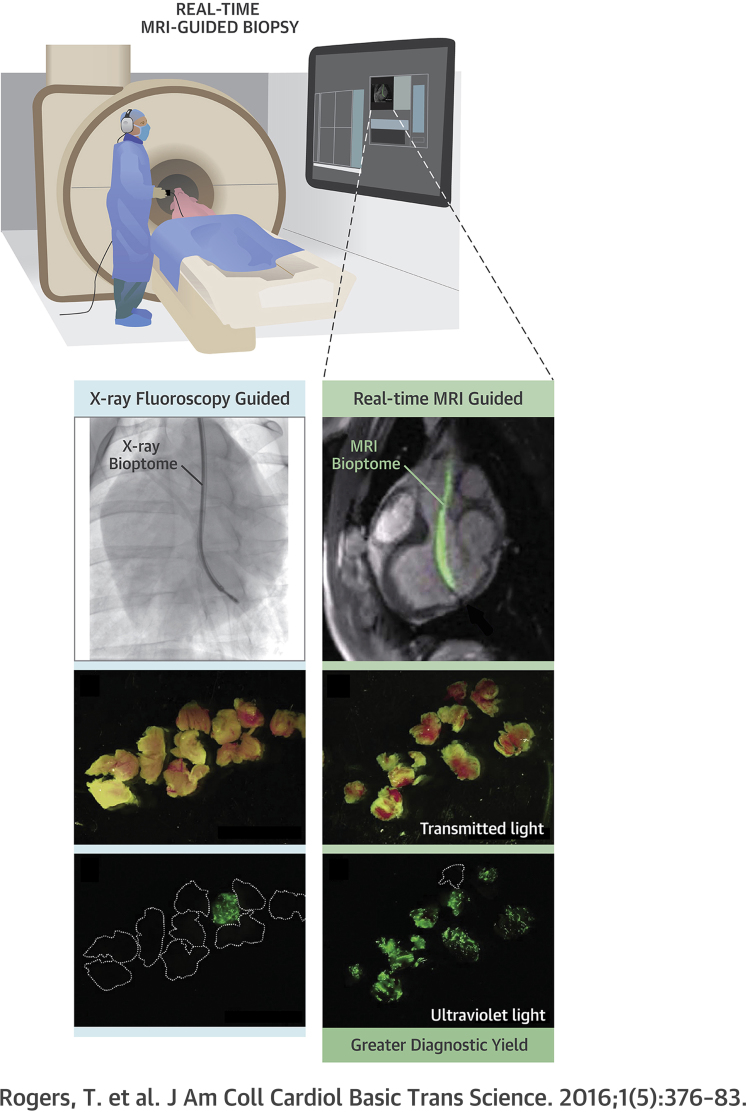
Summary
Diagnostic yield of endomyocardial biopsy is low, particularly in disease that affects the myocardium in a nonuniform distribution. The authors hypothesized that real-time MRI guidance could improve the yield through targeted biopsy of focal myocardial pathology. They tested this hypothesis in an animal model of focal myocardial pathology using intracoronary ethanol and microspheres. The authors compared real-time MRI-guided endomyocardial biopsy in swine using a custom actively visualized MRI bioptome against x-ray–guided biopsy using a commercial bioptome by skilled operators. Real-time MRI guidance significantly increased the diagnostic yield of endomyocardial biopsy.
Key Words: endomyocardial biopsy, heart failure, interventional cardiovascular magnetic resonance imaging, myocarditis
Abbreviations and Acronyms: MRI, magnetic resonance imaging
Visual Abstract
Highlights
-
•
The diagnostic yield of endomyocardial biopsy is low, particularly in diseases that affect the myocardium in a nonuniform distribution.
-
•
It is feasible to perform real-time MRI-guided endomyocardial biopsy using a dedicated active-visualization bioptome.
-
•
In an animal model of focal myocardial disease, real-time MRI guidance improved the diagnostic yield compared with x-ray fluoroscopy.
In contemporary practice, endomyocardial biopsy is still performed with the limited visual guidance afforded by x-ray fluoroscopy or by ultrasound. Technique has changed little since its inception in Japan in 1962 (1) and introduction to the United States in 1973 (2). Regardless of the suspected diagnosis, many operators perform only right ventricular biopsy, and yet the diagnostic yield varies dramatically depending on the distribution of disease (3). Endomyocardial biopsy has fallen somewhat out of favor in recent years, which in part may reflect the low diagnostic yield in patients without transplanted hearts, and the complexity of cardiac histopathology analysis (4). When positive, there is no question that endomyocardial biopsy aids diagnosis and directs therapy in patients with unexplained heart failure (5). In skilled hands, procedural risk of endomyocardial biopsy is low but complications such as cardiac perforation can be life threatening (6). Consequently, guidelines attempt to balance these risks with the potential diagnostic benefit. As a result, the only “strong recommendation” for endomyocardial biopsy in current guidelines is for new-onset heart failure associated with hemodynamic compromise or ventricular arrhythmias (7).
With better technology, it is conceivable that the diagnostic yield of endomyocardial biopsy could be significantly improved. Developments in real-time magnetic resonance imaging (MRI) pulse sequences enable excellent tissue characterization and visualization of custom-built MRI-conditional devices, while avoiding ionizing radiation (8). We hypothesized that MRI guidance could augment the diagnostic yield of endomyocardial biopsy of focal myocardial pathology. To test this hypothesis, we created an animal model of focal myocardial pathology and compared the yield of x-ray–guided endomyocardial biopsy using a commercial bioptome versus real-time MRI-guided endomyocardial biopsy using a novel MRI-conditional bioptome.
Methods
The institutional animal care and use committee approved all procedures, which were performed according to contemporary National Institutes of Health guidelines. Five Yorkshire swine with bodyweight 51 (47 to 56) kg were anesthetized with ketamine (25 mg/kg), midazolam (15 mg/kg), and glycopyrrolate (0.01 mg/kg), and maintained on isoflurane (2%) with mechanical ventilation.
Animal model of focal myocardial pathology
Animals were pre-treated with amiodarone and heparin, and underwent x-ray–guided selective left coronary artery catheterization from a transfemoral approach using 6-F guide catheters. A ≥2-mm obtuse marginal branch was wired with a 0.014-inch guidewire to position an over-the-wire balloon sized to occlude flow. Three milliliters of fluorescent microspheres (NuFlow Hydrocoat, 15-μm diameter, 5 million spheres/ml) was infused through the balloon guidewire lumen, followed by 2 ml of 100% ethanol to cause infarction within a controlled basal posterolateral distribution. The fluorescent microspheres ensured that tissue within the lesion was tagged for easy identification under ultraviolet light. There was no mortality from this procedure. Animals were survived for 21 (14 to 22) days before undergoing endomyocardial biopsy. Each animal underwent both MRI and x-ray–guided endomyocardial biopsy performed by different experienced operators.
Real-time MRI-guided endomyocardial biopsy
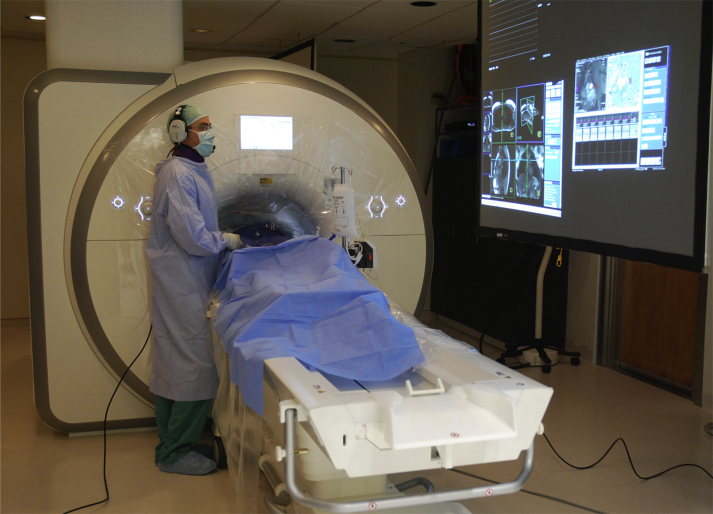
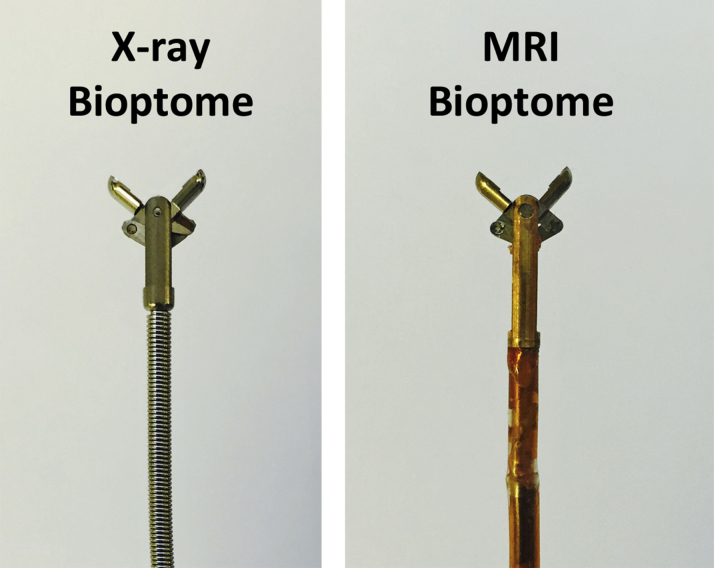
MRI-guided endomyocardial biopsy was performed in a MRI catheterization suite, equipped with a 1.5-T standard diagnostic MRI scanner (Aera, Siemens, Erlangen, Germany), noise-cancelling communication headsets, and video projectors to display hemodynamics and real-time MRI images at the bedside (Figure 1) (9). The 6.5-F MRI-conditional bioptome comprised hinged jaws fabricated from titanium alloy in a copper-beryllium housing with a Kevlar actuation cable and a shapeable distal tip (MRI Interventions, Irvine, California) (Figure 2). The catheter housing incorporated a dipole antenna and coaxial cable. The device was visualized by a combination of an active (“loopless”) dipole antenna receiver to depict the distal 25-cm shaft and tip, and forceps materials that do not create a large MRI susceptibility artifact. The bioptome included radiofrequency chokes and decoupling circuitry to minimize heating during MRI, and matching and tuning circuitry to connect to the MRI scanner for active visualization (Figure 3).
Figure 1.
Components of an Interventional MRI Suite
The operator wears a noise-cancelling headset for communication. Real-time magnetic resonance images and hemodynamics are displayed in the room. MRI = magnetic resonance imaging.
Figure 2.
Bioptome Design
Close up images of the jaws of conventional X-ray bioptome (Cordis) and the MRI-conditional bioptome (MRI Interventions). MRI = magnetic resonance imaging.
Figure 3.
Real-Time MRI-Guided Endomyocardial Biopsy
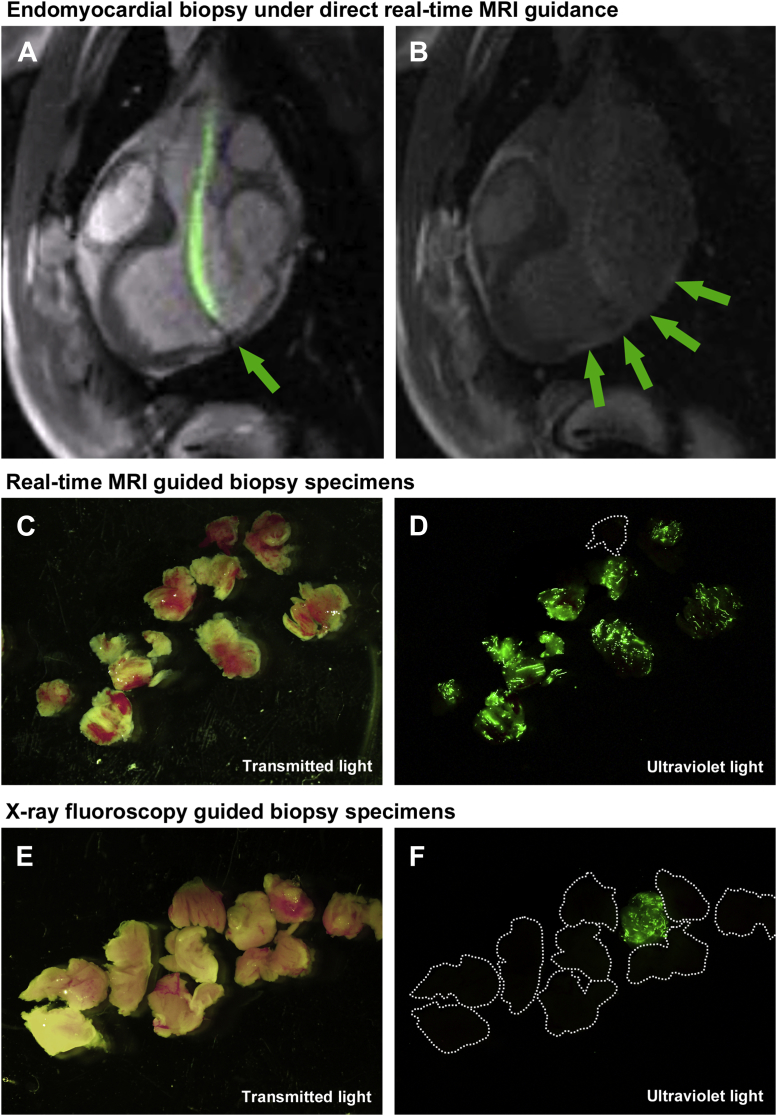
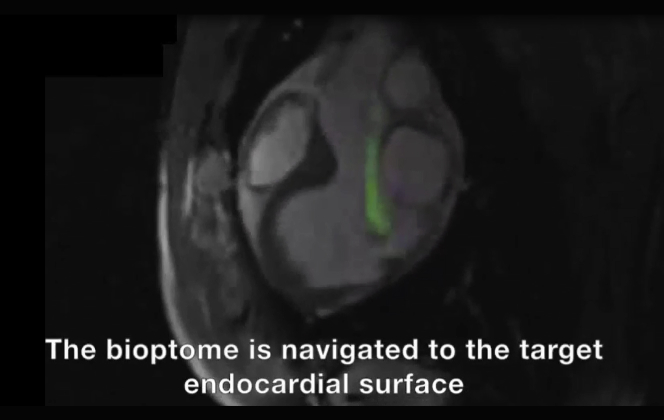
(A) Rapid frame-rate real-time magnetic resonance imaging (MRI) during navigation of the active visualization MRI bioptome within the left ventricle to the target endocardial surface. The jaws appear as a passive artifact (arrow). (B) After systemic gadolinium contrast administration, the lesion is visible using inversion-recovery real-time MRI (arrows). Real-time MRI-guided biopsy specimens viewed under (C) transmitted light and (D) ultraviolet light. X-ray fluoroscopy–guided biopsy specimens viewed under (E) transmitted light and (F) ultraviolet light. Also see Video 1.
A demonstration of real-time MRI guided endomyocardial biopsy in an animal model.
To aid visualization of the target infarcted myocardium, 0.2 mmol/kg gadopentetate dimeglumine (Bayer Healthcare, Wayne, New Jersey) was administered intravenously. After 10 min, the lesion was clearly visible using phase-sensitive inversion recovery late gadolinium enhancement imaging (Figure 4) (10). Under real-time MRI guidance, a 7-F SL0, SL1, or SL2 sheath was introduced into the left ventricle over a 0.035-inch nitinol guidewire (Nitrex, Covidien, Minneapolis, Minnesota) via a transarterial retrograde approach. The MRI bioptome was advanced to the tip of the sheath and navigated to the lesion using inversion recovery real-time imaging (Figure 4). Typical imaging parameters were TI 417 ms, TR/TE 2.54/1.27 ms, flip angle 45°, field of view 300 mm, slice thickness 6 mm, matrix 128 × 128, GRAPPA factor 2, and frame rate 2 frames/s.
Figure 4.
Magnetic Resonance Lesion Imaging
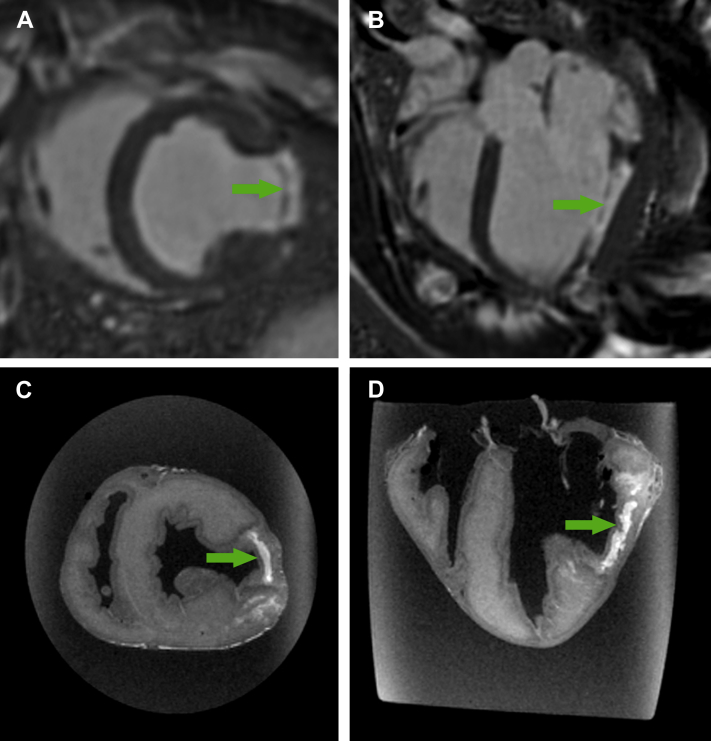
Phase-sensitive inversion recovery late gadolinium enhancement of lesion in (A) short axis and (B) long axis. Ex vivo high-resolution MRI of myocardial lesion in (C) short axis and (D) long axis. Arrows denote lesion. MRI = magnetic resonance imaging.
X-ray fluoroscopy–guided endomyocardial biopsy
X-ray–guided endomyocardial biopsy was performed using a single-plane x-ray fluoroscopy system (9). The operator was permitted to review the MRI study, including the late gadolinium enhancement images, before performing the biopsy. A commercial 7-F bioptome (Cordis, Fremont, California) was delivered to the left ventricle via transarterial retrograde approach through a 7-F SL0, SL1, or SL2 sheath.
Biopsy specimen analysis
Biopsy specimens were examined using a Leica MZFIII dissecting microscope under transmitted light and ultraviolet light with a 400- to 480-nm band pass filter. Each specimen was classified as “on-target” if it contained ≥1 fluorescent particles, or “miss” if it contained none.
Statistics
Data were analyzed using SPSS (version 19.0, IBM, Armonk, New York). Diagnostic yield is presented as percentage of on-target specimens. All other data are presented as median (first and third quartile). We tested the significance of x-ray versus MRI biopsy using a Poisson regression model given that multiple specimens were obtained from each animal and therefore were not independent. A p value <0.05 was considered significant.
Results
Animal model of focal myocardial pathology
An endomyocardial lesion was successfully created in all animals, without sustained ventricular arrhythmia, hemodynamic compromise or mortality. After 21 (14 to 22) days, the lesion was clearly visible with late gadolinium enhancement imaging (Figure 4). Left ventricular function was depressed with ejection fraction 38% (34% to 42%). Phase-sensitive inversion recovery and ex vivo MRI confirmed the lesion was transmural and focal (Figure 2).
Real-time MRI procedural guidance
Retrograde left ventricular access was achieved easily in all animals using real-time MRI guidance and a nitinol guidewire. The active-visualization MRI-conditional bioptome was clearly visible, and the bioptome jaws were distinguishable from the shaft of the device by a passive artifact (Figure 3, Video 1). Using the distal 10-cm shapeable tip of the bioptome and the angle of the sheath tip, the bioptome was steered to the target endocardial surface avoiding chordal entrapment (Video 1). From each animal, 13 (10 to 20) biopsy specimens were obtained under MRI guidance. Fifteen (12 to 22) specimens were obtained under x-ray guidance by a different skilled operator for comparison.
Endomyocardial biopsy yield
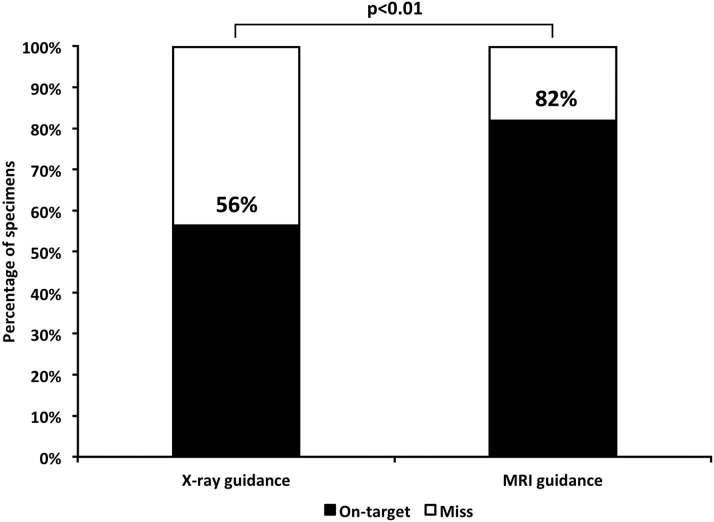
A total of 87 specimens were obtained using x-ray guidance and 77 using real-time MRI guidance. Specimens obtained with the 6.5-F MRI-conditional bioptome were smaller compared with the commercial 7-F x-ray bioptome (Figure 3). Specimens were classified as on-target or miss depending on whether they contained fluorescent particles (Figure 3). Real-time MRI guidance significantly increased the diagnostic yield of endomyocardial biopsy (63 of 77 [82%] vs. 49 of 87 [56%] on-target biopsy specimens with real-time MRI vs. x-ray guidance, p < 0.001, odds ratio: 1.43 [95% confidence interval: 1.19 to 1.74]) (Figure 5).
Figure 5.
Diagnostic Yield of Real-Time MRI Versus X-Ray–Guided Endomyocardial Biopsy
Specimens were classified as on-target or miss by the presence or absence of fluorescent particles under ultraviolet light. MRI = magnetic resonance imaging.
Discussion
In this study, we demonstrate for the first time, to our knowledge, the feasibility of real-time MRI-guided endomyocardial biopsy in vivo using an active visualization MRI-conditional bioptome. In an animal model of focal myocardial pathology, we demonstrate diagnostic superiority of MRI-guided biopsy in a direct head-to-head comparison with the current standard of care, x-ray fluoroscopy–guided biopsy.
The excellent soft tissue visualization of MRI is well suited to this application. Endomyocardial biopsy is usually performed with x-ray fluoroscopic guidance. However, although x-ray enables clear visualization of the bioptome, it cannot visualize the myocardium. One strategy described in the literature is to use electroanatomic mapping to identify abnormal myocardium to biopsy (11). Although this approach overcomes some of the limitations of x-ray–guided biopsy, surface voltage mapping is not a reliable surrogate for pathological substrate in nonischemic cardiomyopathies (12), especially when subendocardial. Pre-procedural MRI, with or without late gadolinium enhancement, can inform the operator of the location and extent of pathology. However, there is no way to confirm that the bioptome is delivered to the correct anatomic location until after the specimens have been analyzed. By contrast, performing the biopsy under direct real-time MRI guidance ensures accurate targeting of the bioptome to the pathology. In this experiment, we created a lesion that was visible using late gadolinium enhancement MRI. However, other MRI advanced tissue characterization techniques such as T1 mapping or edema imaging could be used in clinical practice to identify abnormal myocardium for biopsy. These techniques could in theory permit identification of abnormal myocardium without the need for systemic gadolinium contrast administration. Clinical studies in patients with a variety of diagnoses are required to confirm the hypothesis that taking biopsy samples from areas of late gadolinium enhancement or alternative pulse sequences for tissue characterization can improve diagnostic yield.
The MRI-conditional bioptome we used required considerable materials and electrical engineering for device visualization and MRI safety. The nonferrous forceps material needed to be MRI visible but not create a large MRI susceptibility artifact that would obscure nearby myocardium, and needed to be sufficiently strong and sharp to obtain diagnostic specimens. The catheter system was designed to serve both mechanically as a bioptome and electrically as a MRI receiver coil (13).
Repeat biopsies are often required to diagnose diseases that affect the myocardial in a nonuniform distribution (14). Targeted MRI-guided biopsy could enable the operator to reduce the number of procedures and the number of specimens needed for diagnosis. MRI-guided biopsy could also ensure diagnostic yield with smaller volume specimens, allowing bioptome caliber to be reduced, especially valuable for pediatric applications. Fewer and smaller specimens could reduce the risk of myocardial perforation from endomyocardial biopsy. Left ventricular biopsy is often discouraged because the procedural risk may be higher than right ventricular biopsy. However, the risk of cardiac perforation and tamponade is probably higher with right ventricular biopsy because the free wall is usually thin and more susceptible to injury (3), and the cumulative risk of biopsy-induced tricuspid valve injury is noteworthy especially when applied to allograft surveillance (15). Furthermore, the low risk of tamponade observed in transplant patients undergoing biopsy may not translate to patients with cardiomyopathy and a naive pericardium. The pericardium is usually adhered to the heart in transplant patients, which may contain a right ventricular free wall perforation.
In processes that affect the heart diffusely, for example infiltrative disease or transplant rejection, right ventricle biopsy is usually sufficient to reach diagnosis. However, if pathology is focal and confined to the left ventricle, in particular myocarditis, then the diagnostic yield of right ventricular biopsy will be understandably low (16). Myocarditis can also affect the epicardial surface preferentially. If this is the case, then MRI guidance may not increase diagnostic yield of biopsy, whether taken from the left or right ventricle. MRI guidance may offer the operator more confidence to perform left ventricular biopsy because of the ability to visualize in real time how the bioptome interacts with anatomic structures such as the mitral valve apparatus, and rapidly identify complications such as pericardial perforation and tamponade. Finally, MRI-guided biopsy avoids ionizing radiation, which is of particular benefit to pediatric patients, but also to the operator and catheterization laboratory personnel.
Study limitations
The specimens obtained with the 6.5-F MRI-conditional bioptome were smaller than those obtained using the 7-F commercial x-ray bioptome, but did not affect the diagnostic yield in this feasibility study. Different operators performed MRI- and x-ray–guided biopsies, but the study was not double blinded. Pre-procedural MRI could direct the x-ray operator to the correct endocardial surface. In order to minimize bias, the x-ray operator was permitted to review the MRI study, including late gadolinium enhancement images, beforehand. In this early feasibility study, we did not test whether results would vary for different regions of the left ventricle.
In 1 animal, the yield was low with both MRI and x-ray guidance. On necropsy, it was apparent that the lesion was “hidden” behind a papillary muscle. Although it was clearly visible with real-time MRI, it was difficult to navigate the bioptome to the lesion. The prototype MRI-conditional bioptome we tested had a shapeable tip, but was not deflectable. Future iterations of the MRI bioptome now in development will incorporate an active deflection mechanism, which should enable the operator to reach every part of the endocardium, including the recesses behind the papillary muscles. Steerable guiding catheters could also improve the performance of x-ray bioptomes.
We delivered the 7-F sheath to the left ventricle using a commercially available nitinol guidewire. Though nitinol guidewires are nonferromagnetic and do not cause imaging artifacts, they are susceptible to MRI radiofrequency-induced heating. For this reason, nitinol guidewires cannot be used safely in humans at present. In the future, low-energy MRI pulse sequences may reduce radiofrequency-induced heating sufficiently to enable safe use (17). Dedicated MRI-conditional guidewires are currently under development, incorporating either active or passive visualization technologies 18, 19. The sheath we used is intrinsically MRI safe because it does not contain any metallic components. However, it was not MRI conspicuous. A dedicated sheath with a passive MRI marker at the tip would be useful for clinical translation.
Conclusions
Targeted endomyocardial biopsy is feasible in a large animal model using real-time MRI guidance. Compared with conventional x-ray fluoroscopy guidance, MRI significantly improves the diagnostic yield despite smaller specimen volumes. MRI-guided endomyocardial biopsy could be of particular value in disease processes that affect the myocardium in a nonuniform distribution.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE 1: The diagnostic yield of endomyocardial biopsy is low in disease processes that affect the myocardium in a non-uniform distribution because it is difficult to selectively sample abnormal tissue using current techniques.
COMPETENCY IN MEDICAL KNOWLEDGE 2: MRI guidance can improve the diagnostic yield of endomyocardial biopsy by enabling real-time visualization and targeting of abnormal myocardium with a novel MRI-conditional bioptome.
TRANSLATIONAL OUTLOOK: The utility of MRI-conditional myocardial bioptome should be tested in adult and pediatric patients with myocarditis and after heart transplantation.
Acknowledgments
The authors thank Katherine Lucas and Joni Taylor from the NHLBI Animal Surgery and Resources Core, and Daniela Malide from the NHLBI Light Microscopy Core Lab.
For supplemental videos and their legends, please see the online version of this article.
Footnotes
Supported by the Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health (Z01-HL005062). Dr. Karmarkar was an employee of MRI Interventions Inc., which supplied the MRI-conditional bioptomes. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Dr. Karmarkar is currently affiliated with the Russell H. Morgan Department of Radiology and Radiological Sciences, Division of MRI Research, John’s Hopkins University, Baltimore, Maryland.
References
- 1.Sakakibara S., Konno S. Endomyocardial biopsy. Jpn Heart J. 1962;3:537–543. doi: 10.1536/ihj.3.537. [DOI] [PubMed] [Google Scholar]
- 2.Caves P.K., Stinson E.B., Graham A.F., Billingham M.E., Grehl T.M., Shumway N.E. Percutaneous transvenous endomyocardial biopsy. JAMA. 1973;225:288–291. [PubMed] [Google Scholar]
- 3.Chimenti C., Frustaci A. Contribution and risks of left ventricular endomyocardial biopsy in patients with cardiomyopathies: a retrospective study over a 28-year period. Circulation. 2013;128:1531–1541. doi: 10.1161/CIRCULATIONAHA.13.001414. [DOI] [PubMed] [Google Scholar]
- 4.Leone O., Veinot J.P., Angelini A. 2011 consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc Pathol. 2012;21:245–274. doi: 10.1016/j.carpath.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Bennett M.K., Gilotra N.A., Harrington C. Evaluation of the role of endomyocardial biopsy in 851 patients with unexplained heart failure from 2000–2009. Circ Heart Fail. 2013;6:676–684. doi: 10.1161/CIRCHEARTFAILURE.112.000087. [DOI] [PubMed] [Google Scholar]
- 6.Holzmann M., Nicko A., Kuhl U. Complication rate of right ventricular endomyocardial biopsy via the femoral approach: a retrospective and prospective study analyzing 3048 diagnostic procedures over an 11-year period. Circulation. 2008;118:1722–1728. doi: 10.1161/CIRCULATIONAHA.107.743427. [DOI] [PubMed] [Google Scholar]
- 7.Cooper L.T., Baughman K.L., Feldman A.M. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007;116:2216–2233. doi: 10.1161/CIRCULATIONAHA.107.186093. [DOI] [PubMed] [Google Scholar]
- 8.Campbell-Washburn A.E., Faranesh A.Z., Lederman R.J., Hansen M.S. Magnetic resonance sequences and rapid acquisition for MR-guided interventions. Magn Reson Imaging Clin N Am. 2015;23:669–679. doi: 10.1016/j.mric.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ratnayaka K., Faranesh A.Z., Hansen M.S. Real-time MRI-guided right heart catheterization in adults using passive catheters. Eur Heart J. 2013;34:380–389. doi: 10.1093/eurheartj/ehs189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kellman P., Arai A., McVeigh E., Aletras A. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med. 2002;47:372–383. doi: 10.1002/mrm.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casella M., Pizzamiglio F., Dello Russo A. Feasibility of combined unipolar and bipolar voltage maps to improve sensitivity of endomyocardial biopsy. Circ Arrhythm Electrophysiol. 2015;8:625–632. doi: 10.1161/CIRCEP.114.002216. [DOI] [PubMed] [Google Scholar]
- 12.Cano O., Hutchinson M., Lin D. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol. 2009;54:799–808. doi: 10.1016/j.jacc.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 13.Lederman R.J. Cardiovascular interventional magnetic resonance imaging. Circulation. 2005;112:3009–3017. doi: 10.1161/CIRCULATIONAHA.104.531368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kandolin R., Lehtonen J., Salmenkivi K., Raisanen-Sokolowski A., Lommi J., Kupari M. Diagnosis, treatment, and outcome of giant-cell myocarditis in the era of combined immunosuppression. Circ Heart Fail. 2013;6:15–22. doi: 10.1161/CIRCHEARTFAILURE.112.969261. [DOI] [PubMed] [Google Scholar]
- 15.Dweck M.R., Joshi S., Murigu T. Midwall fibrosis is an independent predictor of mortality in patients with aortic stenosis. J Am Coll Cardiol. 2011;58:1271–1279. doi: 10.1016/j.jacc.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 16.Yilmaz A., Kindermann I., Kindermann M. Comparative evaluation of left and right ventricular endomyocardial biopsy: differences in complication rate and diagnostic performance. Circulation. 2010;122:900–909. doi: 10.1161/CIRCULATIONAHA.109.924167. [DOI] [PubMed] [Google Scholar]
- 17.Campbell-Washburn A., Rogers T., Ratnayaka K. Spiral imaging with off-resonance reconstruction for MRI-guided cardiovascular catheterizations using commercial off-the-shelf nitinol guidewires. J Cardiovasc Magn Reson. 2015;17(Suppl 1):Q15. [Google Scholar]
- 18.Sonmez M., Saikus C.E., Bell J.A. MRI active guidewire with an embedded temperature probe and providing a distinct tip signal to enhance clinical safety. J Cardiovasc Magn Reson. 2012;14:38. doi: 10.1186/1532-429X-14-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Basar B., Rogers T., Ratnayaka K. Segmented nitinol guidewires with stiffness-matched connectors for cardiovascular magnetic resonance catheterization: preserved mechanical performance and freedom from heating. J Cardiovasc Magn Reson. 2015;17:105. doi: 10.1186/s12968-015-0210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]