Abstract
Background:
Prolonged pulmonary air leaks (PALs) are associated with increased morbidity and extended hospital stay. We sought to investigate the role of bronchoscopic placement of 1-way valves in treating this condition.
Methods:
We queried a prospectively maintained database of patients with PAL lasting more than 7 days at a tertiary medical center. Main outcome measures included duration of chest tube placement and hospital stay before and after valve deployment.
Results:
Sixteen patients were eligible to be enrolled from September 2012 through December 2014. One patient refused to give consent, and in 4 patients, the source of air leak could not be identified with bronchoscopic balloon occlusion. Eleven patients (9 men; mean age, 65 ± 15 years) underwent bronchoscopic valve deployment. Eight patients had postoperative PAL and 3 had a secondary spontaneous pneumothorax. The mean duration of air leak before valve deployment was 16 ± 12 days, and the mean number of implanted valves was 1.9 (median, 2). Mean duration of hospital stay before and after valve deployment was 18 and 9 days, respectively (P = .03). Patients who had more than a 50% decrease in air leak on digital monitoring had the thoracostomy tube removed within 3–6 days. There were no procedural complications related to deployment or removal of the valves.
Conclusions:
Bronchoscopic placement of 1-way valves is a safe procedure that could help manage patients with prolonged PAL. A prospective randomized trial with cost-efficiency analysis is necessary to better define the role of this bronchoscopic intervention and demonstrate its effect on air leak duration.
Keywords: Endoscopic, Lobectomy, Wedge, Air leak, Valve
INTRODUCTION
Pulmonary air leaks (PAL) result from abnormal communication between the alveoli or bronchi and the pleural space. Prolonged PAL are defined as an air leak lasting more than 7 days, despite adequate drainage, and can lead to increased morbidity, hospital stay and health care costs.1 Although rare in cases of primary spontaneous pneumothorax, persistent air leaks are substantially more frequent (∼20%) in the setting of underlying COPD.2 Other etiologies of prolonged PAL include trauma, necrotizing infections and procedural interventions (biopsy, CPR). More commonly, lung resections carry significant risks of persistent air leaks, with an incidence ranging from 8% after a sublobar resection to 45% after lung volume reduction surgery.3,4
Current treatment options of prolonged PAL include continued observation with thoracostomy tube drainage, with or without a Heimlich valve. In cases of persistent air leaks with good apposition of the lung against the chest wall, a bedside pleurodesis can be performed with a variable success rate, using autologous blood or other agents.5 Surgical repair, space obliteration, or both can also be attempted, but carries the risk of exacerbating the ongoing problem by creating additional lung injury.6
Placement of endobronchial one-way valves is a minimally invasive procedure that was initially developed to treat patients with heterogeneous emphysema by means of occluding the airway while allowing drainage of distal secretions and trapped air.7,8 An example of these valves is the Spiration IBV (intrabronchial valve) system (Olympus Respiratory, Redmond, Washington, USA), which in 2006 received a humanitarian use device designation by the U.S. Food and Drug Administration for the treatment of postsurgical prolonged PAL. Being less invasive than traditional surgical intervention, this treatment modality appears particularly attractive in patients with overall poor health and those who have exhausted the usual approaches. By reducing the forward air flow in the segmental bronchi leading to the injured lung parenchyma, the valves can expedite the healing and allow the fistula to close.
In this study, we sought to investigate the role of bronchoscopic 1-way valve placement in managing patients with prolonged PAL after a surgical intervention or in the setting of secondary spontaneous pneumothorax.
MATERIALS AND METHODS
All adult patients (age >18) with prolonged PAL, defined as lasting more than 7 days from clinical presentation or procedural intervention, were eligible to enroll in the study from September 2012 through December 2014. Institutional review board approval was obtained. The data were collected and maintained in a prospective fashion and included demographic and medical characteristics, indication for valve placement and thoracostomy tube duration before and after IBV placement. Main outcomes measures were length of hospital stay and air leak duration before and after valve placement. We used the thoracostomy tube duration after placement as a surrogate marker for overall resolution of air leak. Successful valve deployment was defined as resolution of air leak without need for further intervention or death. Only 1 thoracic surgeon had privileges for valve placement at our 734-bed tertiary referral center.
Valve Placement
Valve deployment occurred in the operating room under general anesthesia, with the thoracostomy tube being on standard 20-cm H2O suction. Flexible endoscopy is performed through the endotracheal tube with a 2.8-mm working channel bronchoscope, followed by sequential occlusion of the bronchi and subsegmental bronchi to identify the source of the air leak. We used the manufacturer's prepackaged balloon for that purpose and made sure to wait for at least 5 breathing cycles after each balloon occlusion to determine whether the decrease in air leak was significant. We used digital monitoring for the last 4 cases and measured the air leak using a TSI thermal mass flow meter (TSI Incorporated, Shoreview, Minnesota, USA), connected to the thoracostomy drainage system and a computer-based data-acquisition system (Dataq Instruments, Akron, Ohio, USA). In cases of air leak cessation or significant reduction with the balloon occlusion, the corresponding bronchial lumen was measured with the manufacturer's sizing device. The IBV was then deployed accordingly through the flexible bronchoscope and under direct vision. Additional balloon occlusion and valve deployment are performed to account for collateral ventilation and in cases of significantly persistent air leak. We did not use fluoroscopy in any of the cases.
Postreplacement Management
After valve implantation, patients were permitted to recover from anesthesia in accordance with the standard protocol. Air leaks were clinically monitored by the treating physician, and chest radiography performed daily to evaluate the status of lung inflation. The decision of thoracostomy tube removal was left to the clinical discretion of the attending physicians. The intervention was deemed effective if the patient's chest tube was successfully removed without the need for additional procedures. All the patients were seen in clinic for follow-up and for close air leak monitoring if they were discharged home with a Heimlich valve. Valve removal was performed as an outpatient procedure with flexible bronchoscopy under monitored anesthesia care.
Statistical Analysis
Continuous data are summarized as means ± SD and categorical data by frequencies and percentages. Wilcoxon signed-rank test was used for continuous variables. We considered P < .05 to indicate significance.
RESULTS
Sixteen patients were eligible for enrollment during the study period. One patient refused to give consent for the valve placement and died 6 days later of respiratory failure and progression of interstitial lung disease. The source of air leak could not be identified with bronchoscopic balloon occlusion in 4 other patients. In total, 11 patients underwent bronchoscopic valve deployment. Eight patients had postoperative PAL and 3 had a secondary spontaneous pneumothorax. The mean age was 65 ± 15 years (range, 33–83), and 9 patients were men. In the surgical resection group, an air leak sealant was sprayed during surgery (Progel; Davol Bard Inc., Warwick, Rhode Island, USA) in 1 patient only. Clinical characteristics and demographic variables are presented in Table 1.
Table 1.
Clinical Characteristics of the Study Population
| Characteristics (n = 11) | Data |
|---|---|
| Age (years) | 65 ± 15 |
| Male (n) | 9 |
| Smoking history (n) | 9 |
| BMI (kg/m2) | 26 ± 6 |
| Pulmonary function test results | |
| FEV1 (L) | 1.9 ± 0.7 (L) |
| FEV1/FVC ratio (%) | 52 ± 23 (%) |
| DLCO (%) | 51 ± 20 (%) |
| Home O2 use before presentation (n) | 2 |
| Comorbidities (n) | |
| Lung cancer | 5 |
| Mesothelioma | 1 |
| COPD | 6 |
| Coccidiomycosis | 1 |
| Interstitial lung disease | 2 |
Data are presented as means ± SD or absolute values.
The clinical indications, hospital length of stay, and air leak duration before and after valve placement for each patient are presented in Table 2. The mean number of implanted valves was 1.9 (mode, 2; median, 2). There was no statistically significant difference in the mean air leak duration before and after valve placement (16 ± 12 vs 13 ± 11; P = .21), but the mean hospital stay was significantly lower after the procedure (18 ± 11 vs 9 ± 8; P = .03; median 12 vs 7 days). The mean air leak duration before and after valve placement was 10.9 vs 11.3 days in the surgical patients (n = 8) and 29 vs 17.7 in the medical patients with a secondary spontaneous pneumothorax (n = 3).
Table 2.
Clinical Indications and Outcomes of Valve Placement
| Patients | Cause of Prolonged PAL | Pre-Valve Hospital Duration | Pre-Valve Air Leak Duration | Lobe Involved | Valves (n) | Post-valve Hospital Duration | Post-Valve Air Leak Duration |
|---|---|---|---|---|---|---|---|
| 1 | Right upper lobectomy | 7 | 7 | RLL | 2 | 23 | 7 |
| 2 | Right upper lobectomy | 23 | 23 | RUL | 1 | 1 | 12 |
| 3 | Left lower lobectomy | 11 | 11 | LUL | 2 | 4 | 3 |
| 4 | Right upper lobectomy | 11 | 11 | RLL | 1 | 12 | 10 |
| 5 | Wedge resection RLL superior segment | 13 | 8 | RLL | 1 | 10 | 19 |
| 6 | Wedge resection RML | 28 | 8 | RML | 1 | 4 | 3 |
| 7 | Blebectomy | 10 | 8 | LUL | 2 | 0 | 6 |
| 8 | Pleurectomy | 11 | 11 | RUL | 4 | 3 | 30 |
| 9 | Spontaneous pneumothorax | 12 | 12 | RUL and RML | 2 | 7 | 6 |
| 10 | Spontaneous pneumothorax | 39 | 39 | RUL | 3 | 20 | 34 |
| 11 | Spontaneous pneumothorax | 36 | 36 | LUL | 2 | 19 | 13 |
RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe. Durations are in days.
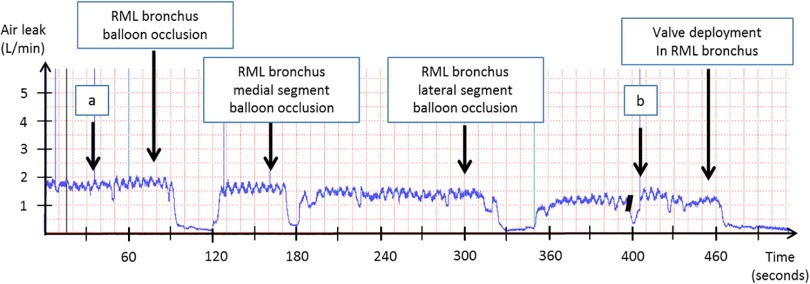
Digital monitoring was used in the last 4 patients and demonstrated a mean air leak drop of 1.2 ± 0.7 l/min. The 3 patients with more than a 50% decrease in the air leak after valve placement had their thoracostomy tube removed within 3–6 days (Table 3). A digital monitoring graph during valve deployment is presented in Figure 1.
Table 3.
Mean Air Leak Drop on Digital Monitoring and Post–Valve Air Leak Duration
| Patient | Etiology Air Leak | Mean Drop in Air Leak (%) (L/min) | Air Leak Duration After Valve (days) |
|---|---|---|---|
| 6 | Wedge resection | 1.8 (90%) | 3 |
| 7 | Blebectomy | 1.0 (70%) | 6 |
| 9 | Spontaneous pneumothorax | 1.7 (50%) | 6 |
| 8 | Pleurectomy/decortication | 0.4 (18%) | 30 |
Figure 1.
Digital air leak monitoring guiding the placement of a single endobronchial valve in a patient with interstitial lung disease after a thoracoscopic wedge resection of an adenocarcinoma of the right middle lobe. (a) Balloon occlusion of right upper lobe bronchus. (b) Balloon occlusion of right lower lobe bronchus.
All the procedures were performed in 1 session, and there was no need for any additional valve placement. All the patients were extubated after surgery, and none of them experienced postobstructive pneumonia, valve dislodgment or migration during the follow-up period. Four patients required a temporary stay in the intensive care unit for respiratory support, including 2 who were originally there before the procedure. Five of 11 patients (45%) were discharged home with a Heimlich valve, including 1 with 2 thoracostomy tubes. One patient required placement of a pigtail catheter for recurrence of pneumothorax after a coughing spell, 4 days after air leak resolution. The pigtail was removed 5 days later.
There were no complications during deployment or removal of the valves, which was performed in 8 of 11 patients (73%) at a median of 60 days (mean, 70 ± 42). Among the patients in whom we left the valves in place, 1 died of lung angiosarcoma and COPD progression 7 months later. There was no 30- or 90-day mortality in the study population.
DISCUSSION
In this study, we present a single tertiary center experience with endobronchial placement of 1-way (IBV) valves in patients with prolonged PAL. Bronchoscopic management of persistent air leak has been described, with different techniques used in the past. These include endoscopic placement of fibrin glue9 or even instillation of alcohol.10 In addition to airway stents, a few endobronchial devices have also been described in treating patients with pulmonary fistulas, such as the Watanabe Spigot (Novatech, Grasse, France), which consist of a silicone plug that comes in different sizes.11 Similarly, the EBV Zephyr valve (Emphasys Medical, Redwood City, California, USA) is a silicone-based, 1-way valve mounted in a self-expanding nitinol retainer, and was reported in a multicenter study to have overall success rate of 92% in patients with persistent PAL.12
In our hospital, we use the IBV system from Spiration (Olympus Respiratory). Valves were placed under compassionate regulations (off label) in patients with a secondary spontaneous pneumothorax, and under humanitarian-use device regulations in surgical patients. All the valves deployments were performed by 1 surgeon, according to the same protocol, therefore eliminating any risk of procedural variation; furthermore, none of the authors has any financial relationship with the manufacturing company.
We found that endobronchial valve placement is safe and feasible and may shorten the hospital stay, making it a potentially good adjunct in the treatment of prolonged PAL in patients with multiple underlying comorbidities. This observation occurred despite some of the patients staying in the hospital beyond resolution of their air leak; for instance, patient 1 had evidence of pulmonary embolism and severe heparin-induced thrombocytopenia, which delayed her discharge, even though the thoracostomy tube had been removed.
However, the mean air leak and thoracostomy tube duration before and after valve placement were 16 and 13 days, respectively, showing no obvious statistical difference. One explanation could be that the valve deployment reduces the air flow across the alveolopleural fistula, enough to allow placement of the thoracostomy tube on water seal, facilitating the patient's disposition and shortening the hospital stay. For instance, patient 7 was discharged home the same day after the valve placement, as he became able to tolerate a Heimlich valve. Interestingly enough, a clear or statistically significant difference in air leak duration has not been consistently demonstrated by multiple other endobronchial valve series, and our study is no exception.12–14 Many other factors can explain this lack of difference in the air leak duration before and after the valve deployment. First, the sample size of the study is small, even if comparable to many other series in the literature.15,16 Second, prolonged PALs often occur in the setting of severe underlying lung disease, which naturally has a substantial effect on the rate of parenchymal healing.17 Decreasing the air flow across the fistula may not be the most important parameter in a patient with poor lung compliance and propensity to develop an air space for example. Third, we were conservative overall in the number of valves placed in any single procedure (median 2, maximum of 4), especially when compared with other series.12,13,15 We also aborted the procedure in 4 out of 15 eligible patients (27%), where the source of air leak could not be well identified with balloon occlusion, probably because of underlying collateral ventilation.
Considering that IBV placement requires anesthesia in patients with significant comorbidities, the lack of a clear cut reduction in air leak duration is a definite shortcoming, especially give that some patients may not even undergo valve deployment. One would expect a multicenter study with higher patient accrual to shed further light on that issue, but a recent retrospective review from 8 institutions reported a median air leak duration of 16 days after valve implantation, compared to 9 days before it. Naturally, the authors admitted the lack of a standard protocol for air leak management between the centers to be a significant limitation of their study.18 In that regard, we stress the importance of a set protocol and a team approach involving thoracic surgery, pulmonary medicine, and interventional pulmonology, to optimize the management of prolonged PAL.
We also believe that patient selection is paramount to improving the success rate of IBV placement. We therefore sought to use digital monitoring to better quantify the change in air leak flow and help guide the valve placement while we were accruing experience. Digital monitoring has in fact been shown to be helpful in the management of air leaks in a few other reports, using the Thopaz (Medela, Switzerland) and Digivent (Millicore, Sweden) draining systems.16, 19, 20 More recently, a multicenter international randomized study showed that patients who were managed with digital drainage systems after lung resections experienced a shorter duration of chest tube placement, shorter hospital stays, and a higher satisfaction rate, compared with those managed with traditional devices.21 In our experience, we were able to remove the thoracostomy tube within 3–6 days in the 3 patients who had more than a 50% drop in air leak flow after valve deployment. The fourth patient had a much less significant decrease (18%), suggesting in retrospect that we should not have placed any valve, with the air leak persisting for 30 d. Comparatively, Firlinger and colleagues16 suggested that a 50% decline in air flow is significant and used it as a cutoff point to justify valve placement in patients with prolonged PAL. They also used a flow <100 mL/min during balloon occlusion as an additional criterion, as Cerfolio and Bryant20 suggested that point to be consistent with a small air leak. Their suggestions corroborate with our limited experience in what would constitute a “significant” decrease in air leak, justifying valve placement.
With the overall small sample size, it is hard to draw firm conclusions and perform any statistically relevant comparisons between the subgroups of patients. It is also impossible to recommend whether any of the patients should have undergone surgical exploration as opposed to valve placement. However, it is worthwhile to note that we placed a relatively similar number of valves in the medical and surgical patients (means, 2.3 and 1.75, respectively). Patients with prolonged PAL from a secondary spontaneous pneumothorax usually have more diffuse emphysema and therefore more sources of air leaks, making placement of endobronchial valves more challenging and potentially less successful. Nevertheless, the change in duration of mean air leak was substantially more significant after valve deployment in the medical patients compared with that in the surgical ones in our series. It is interesting, too, that in the anatomic resection subgroup, 3 of 4 patients had a right upper lobectomy, including patient 2 with AIDS and coccidiomycosis, who developed an apical air space and persistent air leak. Because we were unable during surgery to perform a safe peribronchial dissection in a hostile chest, the patient still had a long enough proximal remnant of the right upper lobe bronchus to allow placement of 1 valve and resolution of the air leak. Upper resections are also known to be associated with a residual apical pleural space, predisposing to longer pulmonary air leaks from a poor visceral–parietal pleura apposition.17,22
The limitations of this study are multiple. First, the sample case series was relatively small. Second, the population was heterogeneous. Third, digital monitoring was used in the last 4 patients only and was not used during clinical follow-up on the medical floor or in the outpatient setting. There may have been a value in quantitatively assessing the air leak in the days following valve deployment, to eliminate any variability in its interpretation by the different attending physicians.
These limitations highlight the need for a prospective randomized trial, in which one group of patients with prolonged PAL is followed conservatively, allowing the air leak to run its natural course, and the other group undergoes valve placement. With the difficulty of achieving an appropriate patient accrual, such a study is likely to be lengthy. In addition, and in view of the valve's high price tag (∼$2750), a cost-efficiency analysis would be useful to better demonstrate the efficacy and the advantages of this treatment modality. Such an analysis should take into account the procedural costs, the daily hospital costs, and the need for any reintervention.
In conclusion, placement of endobronchial valves in the management of prolonged PAL is safe, feasible, and seems to shorten the hospital length of stay. Digital monitoring may help guide the valve placement and potentially enable better patient selection, therefore improving the outcomes.
Acknowledgments
Nancy Giebelhaus, RN (for data population).
Contributor Information
Charles Bakhos, Department of Thoracic Medicine and Surgery, Lewis Katz School of Medicine, Temple University, Philadelphia, Pennsylvania..
Peter Doelken, Division of Pulmonary Medicine.
Stevan Pupovac, Department of Cardiothoracic Surgery, Hofstra Northwell School of Medicine, New Hyde Park, New York, USA..
Ashar Ata, Department of Surgery, Albany Medical Center, Albany, New York, USA..
Tom Fabian, Section of Thoracic Surgery.
References:
- 1. Shrager JB, DeCamp MM, Murthy SC. Intraoperative and postoperative management of air leaks in patients with emphysema. Thorac Surg Clin. 2009;19:223–231. [DOI] [PubMed] [Google Scholar]
- 2. Videm V, Pillgram-Larsen J, Oyvind E, et al. Spontaneous pneumothorax in COPD; complications, treatment and recurrences. Eur J Respir Dis. 1987;71:365–371. [PubMed] [Google Scholar]
- 3. Jones DR, Stiles BM, Denlinger CE, et al. Pulmonary segmentectomy: results and complications. Ann Thorac Surg. 2003;76:343–348. [DOI] [PubMed] [Google Scholar]
- 4. Ciccone A, Meyers B, Guthrie T, et al. Long term outcome of bilateral lung volume reduction in 250 consecutive patients with Emphysema. J Thorac Cardiovasc Surg. 2003;125:513–525. [DOI] [PubMed] [Google Scholar]
- 5. Cerfolio RJ, Tummala RP, Holman WL, et al. A prospective algorithm for the management of air leaks after pulmonary resection. Ann Thorac Surg. 1998;66:1726–1731. [DOI] [PubMed] [Google Scholar]
- 6. Wood DE, Cerfolio RJ, Gonzalez X, Springmeyer SC. Bronchoscopic management of prolonged air leak. Clin Chest Med. 2010;31:127–133. [DOI] [PubMed] [Google Scholar]
- 7. Wood DE, McKenna RJ, Jr, Yusen RD, et al. A multicenter trial of an intrabronchial valve for treatment of severe emphysema. J Thorac Cardiovasc Surg. 2007;133:65–73. [DOI] [PubMed] [Google Scholar]
- 8. Toma TP, Hopkinson NS, Hillier J, et al. Bronchoscopic volume reduction with valve implants in patients with severe emphysema. Lancet. 2003;361:931–933. [DOI] [PubMed] [Google Scholar]
- 9. Glover W, Chavis TV, Daniel TM, et al. Fibrin glue application through the flexible fiberoptic bronchoscope: closure of bronchopleural fistulas. J Thorac Cardiovasc Surg. 1987;93:470–472. [PubMed] [Google Scholar]
- 10. Takaoka K, Inoue S, Ohira S. Central bronchopleural fistulas closed by injection of absolute ethanol. Chest 2002;122:374–378. [DOI] [PubMed] [Google Scholar]
- 11. Weinreb N, Riker D, Beamis J, Lamb C. Ease of use of Watanabe spigot for alveolopleural fistulas. J Bronchol Interv Pulmonol. 2009;16:130–132. [DOI] [PubMed] [Google Scholar]
- 12. Travaline JM, McKenna RJ, De Giacomo T, et al. Treatment of persistent pulmonary air leaks using endobronchial valves. Chest. 2009;136:355–360. [DOI] [PubMed] [Google Scholar]
- 13. Reed MF, Gilbert CR, Taylor MD, Toth JW. Endobronchial valves for challenging air leaks. Ann Thorac Surg. 2015;100:1181–1186. [DOI] [PubMed] [Google Scholar]
- 14. Hance JM, Martin JT, Mullett TW. Endobronchial valves in the treatment of persistent air leaks. Ann Thorac Surg. 2015;100:1780–1786. [DOI] [PubMed] [Google Scholar]
- 15. Gillespie CT, Sterman DH, Cerfolio RJ, et al. Endobronchial valve treatment for prolonged air leaks of the lung: a case series. Ann Thorac Surg. 2011;91:270–273. [DOI] [PubMed] [Google Scholar]
- 16. Firlinger I, Stubenberger E, Muller MR, Burghuber OC, Valipour A. Endoscopic one-way valve implantation in patients with prolonged air leak and the use of digital air leak monitoring. Ann Thorac Surg. 2013;95:1243–1249. [DOI] [PubMed] [Google Scholar]
- 17. Brunelli A, Monteverdi M, Borri A, Salati M, Marasco RD, Fianchini A. Predictors of prolonged air leak after pulmonary lobectomy. Ann Thorac Surg. 2004;77:1205–1210. [DOI] [PubMed] [Google Scholar]
- 18. Gilbert C, Casal R, Lee H, et al. Use of one-way intrabronchial valves in air leak management after tube thoracostomy drainage. Ann Thorac Surg. 2016;101:1891–1896. [DOI] [PubMed] [Google Scholar]
- 19. Dooms CA, De Leyn PR, Yserbyt J, Decaluwe H, Ninane V. Endobronchial valves for persistent postoperative pulmonary air leak: accurate monitoring and functional implications. Respiration. 2012;84:329–333. [DOI] [PubMed] [Google Scholar]
- 20. Cerfolio RJ, Bryant AS. The benefits of continuous and digital air leak assessment after elective pulmonary resection: a prospective study. Ann Thorax Surg. 2008;86:396–401. [DOI] [PubMed] [Google Scholar]
- 21. Pompili C, Detterbeck F, Papagiannopoulos K, et al. Multicenter international randomized comparison of objective and subjective outcomes between electronic and traditional chest drainage systems. Ann Thorac Surg. 2014;98:490–497. [DOI] [PubMed] [Google Scholar]
- 22. Abolhoda A, Liu D, Brooks A, Burt M. Prolonged air leak following radical upper lobectomy: an analysis of incidence and possible risk factors. Chest. 1998;113:1507–1510. [DOI] [PubMed] [Google Scholar]