Abstract
Quorum sensing controls the luminescence of Vibrio fischeri through the transcriptional activator LuxR and the specific autoinducer signal produced by luxI. Amino acid sequences of these two genes were analyzed using bioinformatics tools. LuxI consists of 193 amino acids and appears to contain five α-helices and six ß-sheets when analyzed by SSpro8. LuxI belongs to the autoinducer synthetase family and contains an acetyltransferase domain extending from residues 24 to 110 as MOTIF predicted. LuxR, on the other hand, contains 250 amino acids and has ten α-helices and four ß-sheets. MOTIF predicted LuxR to possess functional motifs; the inducer binding site extending from amino acid residues 23 to 147 and the LuxR activator site extending between amino acids 182 and 236. The InterProScan5 server identified a winged helix- turn-helix DNA binding motif.
Key Words: Quorum sensing, Vibrio fischeri, Motifs
INTRODUCTION
Environmental changes lead to morphological and physiological responses in bacteria through signal transduction pathways that send signals for transcription, causing gene expression to adapt the organism to the new environment. The signals originate from diffusible molecules synthesized by the cells themselves and serve to provide means for cell-to-cell communication and function as a mechanism for coordinating gene expression in response to cell population density in a phenomenon known as quorum sensing [1].
Signal molecules regulating quorum sensing are synthesized from cellular precursors by a synthase protein I and interact with a transcriptional activating R protein to provoke the expression of different target genes [2]. These signal molecules only act after bacterial population reaches a certain level so that the concentration of the signaling molecules reaches a threshold value [3].
Quorum sensing was first found to control the luminescence of Vibrio fischeri, a bacterium that forms symbiosis with certain marine animals to produce light [4, 5]. V. fischeri has been shown to regulate the expression of the lux operon through the transcriptional activator LuxR and the specific autoinducer produced by luxI [6]. This system exists in both Gram-negative and Gram-positive bacteria to control functions such as bioluminescence, virulence, biofilm formation and antibiotic production [7-9].
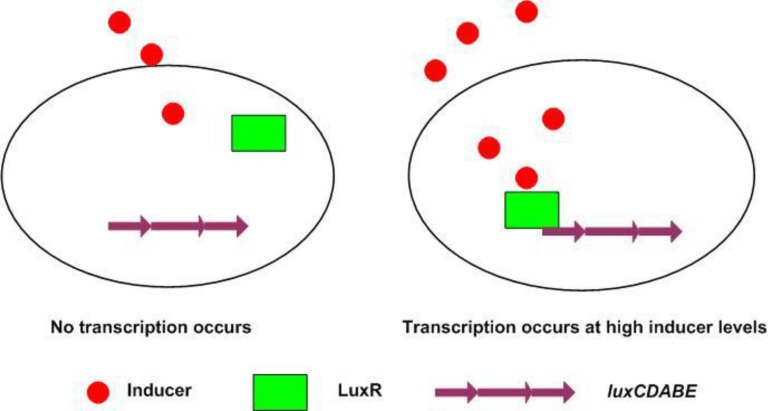
In V. fischeri, quorum sensing is regulated by two proteins; LuxI and LuxR (Fig. 1) [10]. The LuxI protein is an autoinducer synthase responsible for the production of the autoinducer signal molecule, an acylated-homoserine lactone (AHL) which diffuses through the cell membrane [11]. The second protein, LuxR, is a regulatory protein which binds to the autoinducer and DNA [12]. Engebrecht and Silverman discovered both regulatory components (luxI and luxR) and the luciferase structural genes (luxCDABE) [10, 13]. In low concentrations, V. fischeri produces almost no light; however, when the cell density increases, the autoinducer accumulates so that a critical concentration of the inducer is reached, activating the expression of the lux-ICDABE operon. An exponential increase in autoinducer production occurs from the increase of luxI transcriptions, and due to the fact that the luciferase structural genes luxCDABE are located downstream to the luxI, an exponential increase in light production takes place [14]. The aim of the present study is to characterize the components of this system structurally and functionally.
Figure 1.
Quorum sensing involves two regulatory components: the transcriptional activator protein (R protein) and the inducer produced by the autoinducer synthase. Accumulation of the inducer takes place in a cell-density-dependent manner until a threshold level is achieved. At this time the inducer binds to and activates the R protein, which in turn causes gene expression (15).
MATERIALS AND METHODS
Amino acid sequences, LuxI (Accesssion No. sp|P35328) and LuxR (Accession No. sp|P35327), were obtained from the uniprot database (available at http://www.uniprot.org) and formatted as FASTA files to be analyzed using the following online programs:
1- Compute pI/Mw, a tool which computes theoretical isoelectric points (pI) and molecular weights (Mw), available at http://web.expasy.org/compute_pi,
2- SSpro8 of SCRATCH, a program for predicting secondary and disordered regions, available at http://scratch.proteomics.ics.uci.edu/ [16]. The output of this program, according to Kabsch and Sander [17] is H: alpha-helix, G: 3-10-helix, E: extended strand, T: turn, S: bend, C: the rest,
3- PHYRE2, or Protein Homology/analog Y Recognition Engine, which determines protein tertiary structures, [18] available at http://www.sbg. bio.ic.ac.uk/phyre 2/html/ page.cgi?id= index,
4- MOTIF, a program to identify motifs from GenomeNet, Japan, using Pfam and Prosite databases, available at http:// www.genome. jp/tools/motif. This program depends on Pfam, a database of protein domain alignments derived from the protein sequence secondary database of the Swiss Institute of Bioinformatics (SWISS-Prot), and translates nucleic acid secondary databases stored in the European Molecular Biology Laboratory Database (TrEMBL) [19].
The e-value provides information about the likelihood of a given sequence match obtained by chance. This value is calculated by the program to indicate the probability of the motif in the sequenc significantly. If the e-value is small, the match is significant because it is less likely to be a result of random chance. If e < 1 × 10−50, the database match is most likely to be a result of a homologous relationships. If e is between 0.01 and 1×10−50, the match can be considered as homology. If e is between 0.01 and 10, the match is considered insignificant, but may point to a tentative remote homology relationship [20].
5-InterProScan5, a program from the European Bioinformatics Institute, United Kingdom, available at http://www.ebi.ac.uk/interpro. InterProScan 5 uses several databases such as PROSITE, Pfam, PRINTS, ProDom and SMART to identify signature protein motifs [21].
RESULTS AND DISCUSSION
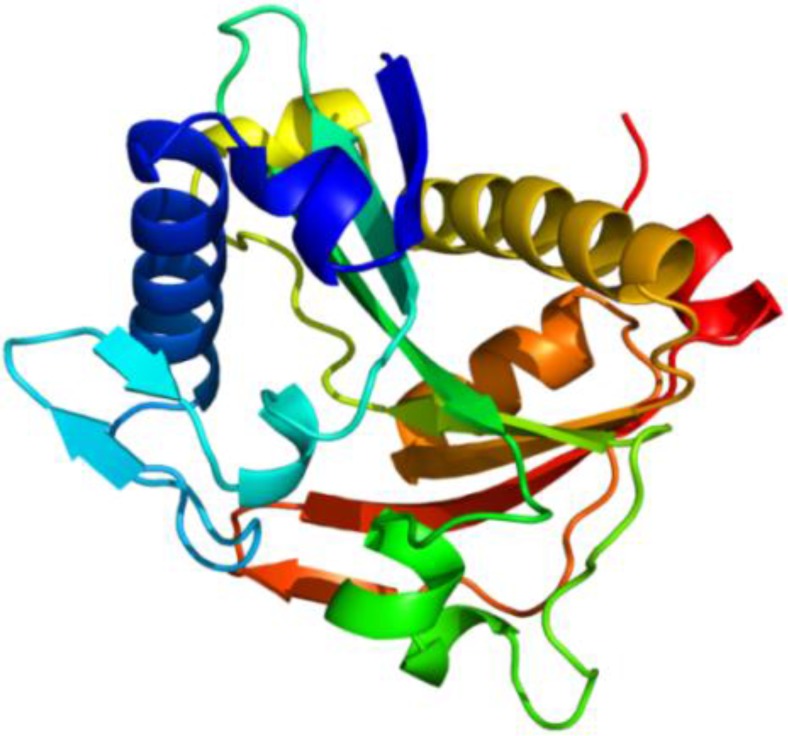
LuxI consists of 193 amino acids and has Mw of 22014.15 Dalton and pI of 5.70 as estimated by the Compute pI/Mw software online. Using the SSpro8 program that predicts secondary structure (Fig. 2B), it appears that the molecule consists of five distinct α helices: α1 15→32, α2 79→82, α3 120→137, α4 149→157 and α5 187→195 and six ß sheets: ß1 2→7, ß2 54→61, ß3 64→73, ß4 102→109, ß5 142→147, ß6 176→184. Phyre2 was used to predict the tertiary structure (Fig. 3).
Figure 2.
(A) The amino acid sequence of LuxI in V. fischeri ES114 showing acetyltransferase domain in red (B) predicted secondary structure by SSpro8 where H: alpha-helix, G: 3-10-helix, E: extended strand, T: turn, S: bend, C: the rest
Figure 3.
LuxI tertiary structure is composed of five α-helices and six β-strands connected by loops as predicted by Phyr2
To predict functional motifs and domains in LuxI, MOTIF software was used. Results show that LuxI had an autoinducer synthetase family signature domain (e- value=3.1×10-34) and contained an acetyltransferase domain extending from residues 24 to 110 (e-value = 9.9×10-9) (Fig. 2A).
Members of the LuxI family of proteins are synthases that catalyze the production of acylated homoserine lactones (acyl-HSLs). The acyl portion of the acyl-HSL is derived from fatty-acid precursors conjugated to the Acyl carrier protein (acyl-ACP), and the HSL moiety is derived from S-adenosylmethionine (SAM) [22, 23]. The LuxI enzyme promotes the formation of an amide bond joining the acyl side chain from the acyl-ACP to SAM. Lactonization of the ligated intermediate together with the subsequent release of methylthioadenosine (MTA) results in the formation of acyl-HSLs [22].
Many different LuxI type proteins which were 190–230 amino acids long and shared 30–35% similarity were identified in Proteobacteria. Ten residues were conserved within most LuxI proteins in the amino terminal 110 amino acids, but no correlation was found between the synthesized acyl-HSL and the level of sequence similarity among the proteins. Nevertheless, it has been previously noted that many of the LuxI proteins that direct the synthesis of 3-oxoacyl-HSLs have a conserved threonine residue at the 143 position [24].
Recent structural and mutational analyses of the acyl-HSL synthase from Pantoea stewartii indicates that this threonine residue might be involved in stabilizing interactions with fatty-acyl biosynthetic precursors carrying a carbonyl group at the third position in the acyl chain [25]. Crystal structures have shown that acyl-HSL synthases have structural similarities with N-acetyltransferases of eukaryotes [24].
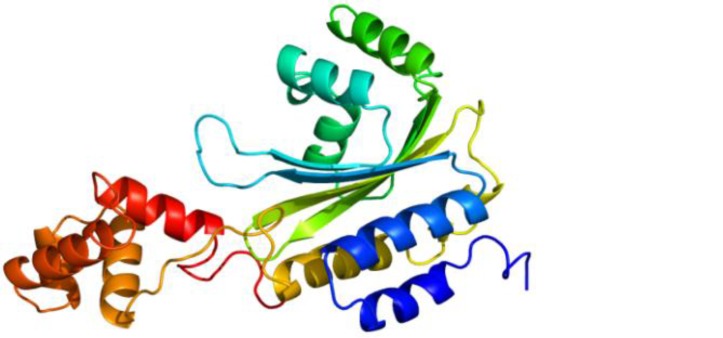
LuxR, on the other hand, contains 250 amino acids (Fig. 4A), and has an estimated Mw of 28519.63 Dalton and pI of 8.54. The molecule has ten α helices: α1 3→19, α2 22→35, α3 64→73, α4 80→87, α5 104→115, α6 147→173, α7 185→195, α8 200→207, α9 211→224 and α10 230→239 and four ß sheets: ß1 40→46, ß2 56→60, ß3 92→95 and ß4 133→139 [Fig. 4B]. Phyr2 demonstrated the tertiary structure prediction of the molecule (Fig. 5).
Figure 4.
Amino acid sequence of LuxR and secondary structure of LuxR predicted by SSpro8. H:alpha-helix, E: extended strand, T: turn, S: bend, C: the rest.
Figure 5.
LuxR tertiary structure is composed of ten α-helices and four β-strands connected by loops as predicted by Phyr2.
MOTIF was also used to analyze LuxR (Table 1). The results indicate that the inducer binding site extended from amino acid residues 23 to 147, while the LuxR activator site extended between amino acids 182 and 236. The homeodomain fold is a protein structural domain that binds DNA and is found in transcription factors. The fold consists of a 60-amino acid helix-turn-helix structure on which three alpha helices are connected by short loop regions. The two N-terminal helices are antiparallel, and a longer C-terminal helix is perpendicular to them, interacting directly with the DNA [26, 27].
Table 1.
Functional motifs in LuxR predicted by MOTIF program
| Motif | Position/(e-value) * | Recognition sequence |
|---|---|---|
| Autoinducer binding domain | 23...147/ (1.3×10-26) | KDINQCLSEIAKIIHCEYYLFAIIYPHSIIK PDVSIIDNYPEKWRKYYDDAGLLEYDP VVDYSKSHHSPINWNVFEKKTIKKESPN VIKEAQESGLITGFSFPIHTASNGFGMLSFAHSDK DIYT |
| Bacterial regulatory proteins, luxR family | 182...236/ (1.2×10-20) | ILTKREKECLAWASEGKSTWDISKIL GCSERTVTFHLTNTQMKLNTTNRCQSISK |
| Homeodomain-like domain | 191...215/ (0.002) | LAWASEGKSTWDISKILGCSERTVT |
| Sigma-70, region 4 | 183...219/ (0.0051) | LTKREKECLAWASEGKSTWDISKILGCSERT VTFHLT |
| Helix-turn-helix domain | 190...214/ (0.48) | CLAWASEGKSTWDISKILGCSERTV |
| HTH DNA binding domain | 201...225/ (0.41) | WDISKILGCSERTVTFHLTNTQMKL |
e-value represent the probability that a sequence could arise randomly by chance, values below 0.01 could be of random appearance.
DNA-binding proteins play a crucial role in the biology of the cell, being responsible for the transfer of biological information from genes to proteins [28, 29]. A large number of DNA-binding proteins are deposited in the Protein Data Bank (PDB) [30] and the Nucleic Acid Database [31]. HTH is a short motif consisting of a first alpha- helix, a connecting turn and a second recognition helix which interacts with the DNA. The two alpha-helices extend from the domain surface and make a convex unit to fit into the major groove of DNA [23, 33, 34].
Protein families with DNA-binding HTHs are greatly diverged, showing immense variation in amino acid sequences, sequence portions of DNA-binding domains and structural elements outside the DNA-binding motif [35]. It can be concluded from the analysis performed by MOTIF that LuxR possesses a regulatory domain extending along the C-terminal region, and an HTH structure which binds DNA and contains a sequence similarity to region 4 of the sigma factor belonging to RNA polymerase.
LuxR is a member of a family of transcriptional activators defined by sequence similarities in a C-terminal helix-turn-helix containing region [36]. Previous studies indicated two regions of the LuxR protein necessary for activity; one extending between residues 79-127 involved in autoinducer binding, and the other, between residues 184-230, supposedly involved in DNA binding [37, 38].
Residues 184-230 form the helix-turn-helix, which is a highly conserved region characterizing the LuxR family [36]. The C-terminal region of the LuxR family members also shows significant sequence similarity to region 4 bacterial RNA polymerase σ factors [39, 40]. Region 4 is a helix-turn-helix-containing region considered to recognize the -35 sequences of promoters [41].
InterProScan 5 program was used to explore further the different motifs in LuxR shown by MOTIF. The results showed four motifs; (1) transcription regulator LuxR, C- terminal (2) transcription factor LuxR-like autoinducer-binding domain (3) winged helix-turn-helix DNA binding motif (4) signal transduction response regulator, C- terminal effector, (which is an unrelated motif since it is a fragment of the two- component signal transduction system). Accordingly, LuxR is a transcription regulator containing an autoinducer binding site and of winged helix-turn-helix configuration.
The winged helix proteins constitute a subfamily of helix-turn-helix proteins. A large number of such related proteins with diverse biological functions have been characterized by X-ray crystallography and solution NMR spectroscopy. Studies of winged helix proteins and their complexes with DNA have shown that the motif isextremely variable, exhibits two different modes of DNA binding, and can participate in protein–protein interactions [42].
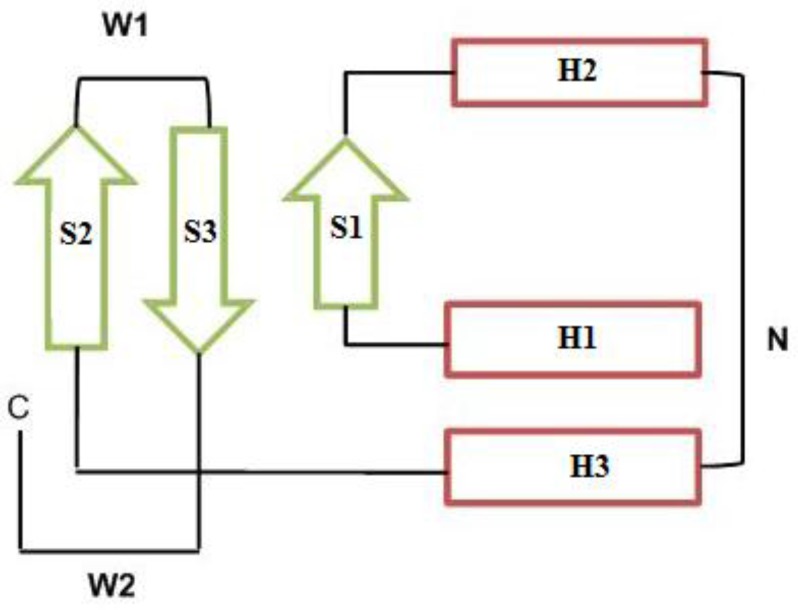
The winged helix motif is a compact α/ß structure composed of two wings (W1 and W2), three α-helices (H1, H2 and H3) and three ß-strands (S1, S2 and S3), arranged in the order H1-S1-H2-H3-S2-W1-S3-W2 (Fig. 6). The N-terminal half of the motif is helical, whereas the C-terminal half is composed of two of the three strands forming the twisted antiparallel ß-sheet and the two large loops or wings, W1 and W2. Wing W1 connects strands S2 and S3, and wing W2 extends from strand S3 to the C terminal of the DNA binding domain. These loops flank helix H3 like the wings of a butterfly, inspiring the name “winged helix motif” [43].
Figure 6.
Winged helix-turn-helix topology consists of two wings: W1 and W2, three α-helices (H1, H2 and H3) and three ß-strands (S1, S2 and S3). The N-terminal half (N) is helical, whereas the C- terminal half (C) is composed of the twisted antiparallel ß-sheet and the two large wings (see text below) (41
The results of this study show that the quorum sensing system of V. fischeri is composed of two proteins; the LuxI, a synthease which produces the autoinducer and contains the Acyltranseferase motif, and the LuxR, which is a transcription regulator containing LuxR-like autoinducer-binding sites and possess a winged helix-turn-helix DNA binding domain.
Conflict of Interest: The authors declare that they have no competing interest.
References
- 1.Fuqua WC, Winans SC, Greenberg EP. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuqua WC, Winans SC, Greenberg EP. Census and consensus in bacterial ecosystems: The luxR-luxI family of quorum-sensing transcriptional regulators. Annu Rev Micrbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 3.Miller MB, Bassler , BL Quorum sensing in bacteria. Annu Rev Micrbiol. 2001;55:165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- 4.Nealson KH, Hastings JW. Bacterial bioluminescence: its control and ecological significance. Microbiol Rev. 1979;43:469–518. doi: 10.1128/mr.43.4.496-518.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruby EG. Lessons from a cooperative bacterial-animal association: the Vibrio fischeri-Euprymna scolopes light organ symbioses. Annu Rev Microbiol. 1996;50:591–624. doi: 10.1146/annurev.micro.50.1.591. [DOI] [PubMed] [Google Scholar]
- 6.Dunlap PV, Greenberg EP. Control of Vibrio fischeri lux gene transcription by a cyclic AMP receptor protein-LuxR protein regulatory circuit. J Bacteriol. 1988;170:4040–4046. doi: 10.1128/jb.170.9.4040-4046.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henke JM, Bassler BL. Bacterial social engagements. Trends cell Biol. 2004;14:648–656. doi: 10.1016/j.tcb.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Waters CM, Bassler , BL Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 9.Bassler BL, Losick R. Bacterial speaking. Cell. 2006;125:237–246. doi: 10.1016/j.cell.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Engebrecht J, Silverman M. Nucleotide sequence of the regulatory locus controlling expression of bacterial genes for bioluminescence. Nucleic acid Res. 1987;15:10455–10467. doi: 10.1093/nar/15.24.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan HB, Greenberg EP. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J Bacteriol. 1985;163:1210–1214. doi: 10.1128/jb.163.3.1210-1214.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salmond GPC, Bycraft BW, Stewart GS, Williams P. The bacterial „enigma‟:Cracking the code of cell-cell communication. Mol Micrbiol. 995;16:615–624. doi: 10.1111/j.1365-2958.1995.tb02424.x. [DOI] [PubMed] [Google Scholar]
- 13.Engebrecht J, Silverman M. Identification of genes and gene products necessary for bacterial bioluminescence. Proc Natl Acad Sci USA. 1984;81:4154–4158. doi: 10.1073/pnas.81.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engebrecht J, Nealson K, Silverman M. Bacterial bioluminescence: Isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983;32:773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- 15.Kievit TR, Iglewski BH. Bacterial quorum sensing in pathogenic relationships. Infect Immun. 2000;68:4839–4849. doi: 10.1128/iai.68.9.4839-4849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng J, Randall AZ, Sweredoski MJ, Baldi P. SCRATCH: a protein structure and structural feature prediction server. Nucleic Acids Res. 2005;33:W72–W76. doi: 10.1093/nar/gki396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kabsch W, Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983;22:2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- 18.Kelley LA, Sternberg MJ. Protein structure prediction on the Web: a case study using the Phyre server. Nature Protocols. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 19.Bateman A, Birney E, Cerrruti L, Durbin R, Etwiller L, Eddy SR, Griffiths-Jones S, Howe KL, Marshal M, Sonnhammer ELL. The Pfam protein families‟ database. Nucleic Acid Res. 2000;30:276–280. doi: 10.1093/nar/30.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiong J. ”Essential Bioinformatics”. Cambridge: Cambridge University Press; 2006. pp. 85–94. [Google Scholar]
- 21.Zdobnov EM, Apweiler R. InterProScan--an integration platform for the signature- recognition methods in InterPro. Bioinformatics. 2001;17:847–848. doi: 10.1093/bioinformatics/17.9.847. [DOI] [PubMed] [Google Scholar]
- 22.Morè MI, Finger LD, Stryker JL, Fuqua C, Eberhard A, Winans SC. Enzymatic synthesis of a quorum-sensing autoinducer through use of defined substrates. Science. 1996;272:1655–1658. doi: 10.1126/science.272.5268.1655. [DOI] [PubMed] [Google Scholar]
- 23.Schaefer AL, Val DL, Hanzelka BL, Cronan JE Jr, Greenberg EP. Generation of cell-to-cell signals in quorum sensing: acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein. Proc Natl Acad Sci USA. 1996;93:9505–9509. doi: 10.1073/pnas.93.18.9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuqua C, Greenberg EP. Listening in on bacteria: Acylhomoserine lactone signaling. Mol Cell Biol. 2002;3:685–695. doi: 10.1038/nrm907. [DOI] [PubMed] [Google Scholar]
- 25.Watson WT, Minogue TD, Val DL, von Bodman SB, Churchill MEA. Structural basis and specificity of Acyl-homoserine lactone signal production in bacterial quorum sensing. Mol Cell. 2002;9:685–694. doi: 10.1016/s1097-2765(02)00480-x. [DOI] [PubMed] [Google Scholar]
- 26.Luscombe NM, Austin SE, Berman HM, Thornton JM. An overview of the sruc- tures of protein-DNA complexes. Genome Biol. 2001;1:1–37. doi: 10.1186/gb-2000-1-1-reviews001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones S, Thornton JM. Protein–DNA Interactions: The story so far and a new method for prediction. Comp Funct Genomics. 2003;4:428–431. doi: 10.1002/cfg.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acid Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berman HM, Olson WK, Beveridge DL, Westbrook J, Gelbin A, Demeny T, Hsieh S -H, Srinivasan AR, Schneider B. A comprehensive relational database of three- dimensional structures of nucleic acids. Biophs J. 1992;63:751–759. doi: 10.1016/S0006-3495(92)81649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pabo CO, Sauer RT. Transcription factors: structural families and principles of DNA recognition. Annu Rev Biochem. 1992;61:1053–1095. doi: 10.1146/annurev.bi.61.070192.005201. [DOI] [PubMed] [Google Scholar]
- 31.Steitz TA, Ohlendorf DH, Mckay DB, Anderson WF, Mathews BW. Structural similarity in the DNA-binding domains of catabolite gene activator and cro repressor protein. Proc Natl Acad Sci USA. 1982;79:3097–3100. doi: 10.1073/pnas.79.10.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berman RG. DNA recognition by the helix–turn–helix motif. Curr Opin Struct Biol. 1992;2:100–108. [Google Scholar]
- 33.Rosinski JA, Atchley WR. Molecular evolution of helix–turn–helix proteins. J Mol Evol. 1999;49:301–309. doi: 10.1007/pl00006552. [DOI] [PubMed] [Google Scholar]
- 34.Gehring WJ. The homeobox in perspective. Trends Biochem Sci. 1992;17:277–280. doi: 10.1016/0968-0004(92)90434-b. [DOI] [PubMed] [Google Scholar]
- 35.Gehring WJ. Exploring the homeobox. Gene. 1993;135:215–221. doi: 10.1016/0378-1119(93)90068-e. [DOI] [PubMed] [Google Scholar]
- 36.Henikoff S, Wallace JC, Brown JP. Finding protein similarities with nucleotide sequence databases. Methods Enzymol. 1990;183:111–132. doi: 10.1016/0076-6879(90)83009-x. [DOI] [PubMed] [Google Scholar]
- 37.Shadel GS, Young R, Baldwin TO. Use of regulated cell lysis in a lethal genetic selection in Escherichia coli: identification of the autoinducer-binding region of the LuxR protein from Vibrio fischeri ATCC 7744. J Bacteriol. 1990;172:3980–3987. doi: 10.1128/jb.172.7.3980-3987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slock J, Van Riet D, Kolibackuk D, Greenberg EP. Critical regions of the Vibrio fischeri luxR protein defined by mutational analysis. J Bacteriol. 1990;172:3974–3979. doi: 10.1128/jb.172.7.3974-3979.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stout V, Torres-Cabassa A, Maurizi MR, Gutnick D, Gottesman S. RcsA, an unstable positive regulator of capsular polysaccharide synthesis. J Bacteriol. 1991;173:1738–1747. doi: 10.1128/jb.173.5.1738-1747.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kahn D, Ditta G. Modular structure of FixJ: homology of the transcriptional activator domain with the - 35 binding domain of sigma factors. Mol Micrbiol. 1991;5:987–997. doi: 10.1111/j.1365-2958.1991.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 41.Helman JO, Chamberlin MJ. Structure and function of bacterial sigma factors. Annu Rev Biochem. 1988;57:839–872. doi: 10.1146/annurev.bi.57.070188.004203. [DOI] [PubMed] [Google Scholar]
- 42.Gajiwala K, Burley SK. Winged helix proteins. Curr Opin Str Biol. 2000;10:110–116. doi: 10.1016/s0959-440x(99)00057-3. [DOI] [PubMed] [Google Scholar]
- 43.Clark KL, Halay ED, Lai E, Burley SK. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature. 1993;364:412–420. doi: 10.1038/364412a0. [DOI] [PubMed] [Google Scholar]