Abstract
In 2011, one in every four (26%) children under 5 years of age worldwide was stunted. The realization that most stunting cannot be explained by poor diet or by diarrhoea, nor completely reversed by optimized diet and reduced diarrhoea has led to the hypothesis that a primary underlying cause of stunting is subclinical gut disease. Essentially, ingested microbes set in motion two overlapping and interacting pathways that result in linear growth impairment. Firstly, partial villous atrophy results in a reduced absorptive surface area and loss of digestive enzymes. This in turn results in maldigestion and malabsorption of much needed nutrients. Secondly, microbes and their products make the gut leaky, allowing luminal contents to translocate into systemic circulation. This creates a condition of chronic immune activation, which (i) diverts nutrient resources towards the metabolically expensive business of infection fighting rather than growth; (ii) suppresses the growth hormone‐IGF axis and inhibits bone growth, leading to growth impairment; and (iii) causes further damage to the intestinal mucosa thereby exacerbating the problem. As such, the unhygienic environments in which infants and young children live and grow must contribute to, if not be the overriding cause of, this environmental enteric dysfunction. We suggest that a package of baby‐WASH interventions (sanitation and water improvement, handwashing with soap, ensuring a clean play and infant feeding environment and food hygiene) that interrupt specific pathways through which feco‐oral transmission occurs in the first two years of a child's life may be central to global stunting reduction efforts.
Keywords: stunting, nutrition, disease, infant and child nutrition, early growth, sanitation
The stunting dilemma
In 2011, 165 million (26%) children under 5 years of age worldwide were stunted as indicated by a height‐for‐age Z‐score (HAZ) of −2 or lower (Black et al. 2013). This stunting is associated with greater risk of death from infectious diseases in childhood (Caulfield et al. 2004; Pelletier et al. 1995), poorer cognition, (Grantham‐McGregor et al. 2007; Walker et al. 2011) poorer educational outcomes (Alderman et al. 2006; Maluccio et al. 2009) and lower adult earnings (Hoddinott et al. 2013). For these compelling reasons, normalizing child growth during the window of opportunity – between conception and the first two years of postnatal life (Victora et al. 2010) – represents an important long‐term investment.
Analyses of the effects of improved dietary intake on child growth suggest that a nutritionally adequate diet is necessary but not sufficient for ensuring optimal linear growth. A comprehensive review of complementary feeding interventions (Dewey and Adu‐Afarwuah, 2008) revealed a growth effect (mean effect size) of 0.0–0.64 length‐for‐age Z‐scores. A comparable linear growth effect (standard mean difference) of 0.08–0.62 length‐for‐age Z‐scores was reported in a recent series of reviews (Bhutta et al. 2013; Imdad et al. 2011; Lassi et al. 2013) of 16 randomized and quasi‐randomized complementary feeding intervention studies. While statistically significant, these analyses demonstrate that the growth effect of the most efficacious of these interventions was +0.7 Z‐scores, which translates to a modest 30% reduction in stunting because the average linear growth deficit is −2.0 HAZ by 24 months among African and Asian children (Victora et al. 2010).
Similarly, disease explains only a part of the variation in stunting. Infection has long been understood to be central to the interactive relationship between disease and nutrition (Scrimshaw et al. 1968), with infectious disease episodes increasing the risk of a child being undernourished and vice versa. However, the association between diarrhoea, the most studied and most frequent infection in developing countries, and linear growth is modest, particularly because of catch‐up growth after illness episodes (Briend, 1990; Briend et al. 1990). In a pooled analysis of nine studies, a higher cumulative burden of diarrhoea before 24 months was associated with greater odds of being stunted at 24 months (Checkley et al. 2008). Additional analyses of seven of these studies (Richard et al. 2013; Richard et al. 2014) revealed that the association between diarrhoea burden and linear growth is small (0.38 cm, which translates to about 1/15th of the average height deficit at 2 years of age among African and Asian children), and provided further evidence of catch‐up growth: when diarrhoeal episodes were followed by diarrhoea‐free periods in the first two years of life, catch‐up growth allowed children to regain their initial trajectories. The findings of these analyses are consistent with other observations that reductions in clinic presentations of diarrhoea were not associated by improvements in nutritional status (Poskitt et al. 1999); and the introduction of a highly effective programme to treat infectious diseases dramatically reduced infant mortality but had no effect on growth (Rousham and Gracey, 1997).
Key messages.
The recalcitrance of stunting to diet and disease control interventions has led to the hypothesis that a primary underlying cause of stunting is subclinical gut disease (EED).
In the context of marginal diets and recurrent infections, EED likely explains a significant portion of the unresolved stunting affecting one in every three children in developing countries.
Avoiding ingestion of enteric pathogens and other causative microbes by infants and young children could preventmost of the EED burden.
Interventions aimed at preventing and reducing EED, particularly through babyߚtargetedWASH interventions, may be critical to global stunting reduction efforts.
The realization that most stunting cannot be explained by poor diet or by diarrhoea, nor completely reversed by optimized diet and reduced diarrhoea has led researchers to reexamine papers published over the past several decades that have posited a linkage between unsanitary living environments leading to an acquired asymptomatic but chronic gut injury with systemic immunostimulation and poor growth (Rosenberg and Solomons, 1978; Rosenberg et al. 1974; Solomons, 2003; Solomons et al. 1993). Frequently, researchers have assumed that any growth benefits of WASH interventions are mediated through reduced diarrhoea. Accordingly, because the linkage between diarrhoea and growth is so weak, the 2008 Lancet Nutrition series estimated that WASH interventions implemented at scale would reduce stunting by only 2.5%, when they modelled the effect through diarrhoea. Other observations suggest the effect of WASH on linear growth may be independent of diarrhoea: in a cross‐sectional analysis of DHS data from eight countries, Esrey (1996) noted that optimum water and sanitation were more strongly associated with linear growth than it was with diarrhoea. In a longitudinal cohort of Gambian children from age 8 to 64 weeks, measures of chronic immunostimulation were highly correlated with measures of enteropathy, and together were strongly predictive of poor linear growth (Campbell et al. 2003) further strengthening the hypothesized role of enteropathy and immunostimulation in stunting. Solomons draws parallels between the conditions of these infants and animal husbandry (Solomons, 2003; Solomons et al. 1993); noting that either cleaning up the environmental conditions for chickens or adding antibiotics to pig fodder can improve growth and enhance meat production, he presents a major clue that the cause of enteropathy is environmental.
Humphrey (2009) integrated this longstanding literature into a hypothesis that exposure to poor sanitation and hygiene causes this enteropathy, now termed environmental enteric dysfunction (EED; Keusch et al. 2013) and that this EED (rather than diarrhoea) is the primary causal pathway from poor sanitation and hygiene to stunting. A more recent observational study supports this hypothesis: Bangladeshi children living in environmentally clean households had less severe EED and higher HAZ than children from contaminated households (Lin et al. 2013).
Environmental enteric dysfunction as a modulator of growth
The healthy gut
The healthy small intestine is a complex organ consisting of multiple functional elements: a mucus layer containing defensins and immunoglobulins, a single layer of epithelial cells sealed by tight junctions, and the lamina propia and submucosa containing immune cells (Hodin and Matthews, 2008; McKay et al. 2010). The intestinal epithelium, illustrated in Fig. 1a [adapted from (Sandler and Douek, 2012)] forms a one‐cell‐thick interface between the internal organism and external luminal environment. It also comprises two major compartments, the villus and the crypt, which play major roles in the digestion and absorption of nutrients, in absorption and secretion of water and electrolytes and in intestinal immune function (Hodin and Matthews, 2008; Jaladanki and Wang, 2011; Peterson and Artis, 2014). The villous compartment comprises mature, absorptive cells that form finger‐like projections extending into the intestinal lumen thereby amplifying the absorptive surface area 20‐fold. The crypt is a contiguous pocket of epithelial cells at the base of the villus that is populated by younger epithelial cells involved primarily in secretion of antimicrobial proteins and stem cells that continually divide and migrate towards the villous surface. Collectively, these features are responsible for the barrier, immune and absorptive functions of the small intestine.
Figure 1.
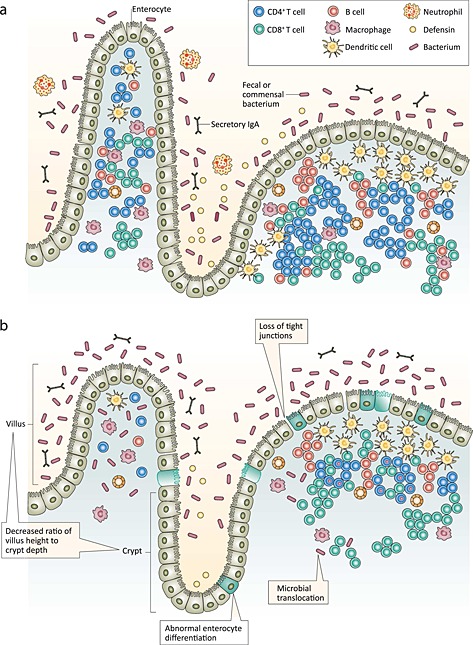
The intestinal epithelium in health (a) and with environmental enteric dysfunction (b). Adapted and reprinted with permission from Macmillan Publishers Ltd: Nature Reviews Microbiology: Sandler and Douek (2012).
The ‘impoverished’ gut: environmental enteric dysfunction
What is it?
Environmental enteric dysfunction is a subclinical disorder of the small intestine that is characterized by villous atrophy, crypt elongation, inflammatory cells infiltrating the crypts and a loss of barrier function or increased permeability (Keusch et al. 2014; Prendergast and Kelly, 2012). The term refers to a phenomenon of impaired intestinal function rather than a clinical syndrome or entity with diagnostic criteria (McKay et al. 2010). The nomenclature for this subclinical condition has evolved with improved understanding from tropical enteropathy to environmental (acquired) enteropathy and is currently referred to as EED (to focus on the functional alterations) (Keusch et al. 2013).
Epidemiology
Initially described as a condition of the tropics (Desai et al. 1969), enteropathy is virtually ubiquitous among persons living in conditions of poverty (Baker, 1976; Keusch, 1972; Menzies et al. 1999). Any explanation of the ubiquity of this condition in developing countries must take into account the observation that the intestinal morphology of stillborn fetuses and newborns in these contexts are normal, thereby demonstrating that the disorder is acquired and not genetic (Cook et al. 1969; Stanfield et al. 1965). South Asian adults with this condition and Peace Corps volunteers who had spent some time in such environments were observed to ‘recover’ when they spent a duration of time away (Gerson et al. 1971; Lindenbaum et al. 1971), suggesting that the causative factor was environmental. However, recovery rates between these groups differ: South Asian adults who migrated to Europe or the United States had enteropathy on arrival, which resolved within ~5 years (Gerson et al. 1971), while asymptomatic American soldiers in Vietnam (Sheehy et al. 1968) and Peace Corps volunteers in Pakistan (Lindenbaum et al. 1971) who acquired enteropathy within a few month's residence in these settings recovered in the course of 4–5 months after returning to the United States. This suggests that enteropathy is reversible, but that recovery is relatively slow, especially among people who have lived in an unsanitary environment and presumably had the condition throughout their lifetime.
Aetiology
Although the pathogenesis of EED is unclear, it has been linked to environmental contamination in general and faecal contamination in particular (Baker, 1976; Lindenbaum et al. 1972) and likely represents an adaptive response to a contaminated environment. In response to a prolonged and persistent exposure to enteric pathogens and enterotoxins expressed by pathogenic bacteria, intestinal morphology is altered in a number of ways; the most frequently observed being crypt hyperplasia and villous atrophy. In EED, illustrated in Fig. 1b, crypts are elongated with rapidly increased cell production rate (Cook et al. 1969; Veitch et al. 2001). This crypt hyperplasia is etiologically related to partial villous atrophy (Desai et al. 1969; Fagundes‐Neto et al. 1984) or shortening, fusing and broadening of the villi – resulting in the architecture of the gut becoming flatter and blunted with an appearance of leaves and ridges rather than the typical finger‐like projections (Haghighi and Wolf, 1997; Prendergast and Kelly, 2012). The main functional implication of these changes in intestinal architecture is reduced absorptive capacity secondary to the diminished surface area. Because absorptive cells are concentrated in the villous section, shorter villi and deeper crypts have fewer absorptive, and more secretory, cells further compromising nutrient absorption (Nabuurs et al. 1993).
At the cellular level, hyperstimulation of enteric T‐cells appears to be important in the pathogenesis of EED (Veitch et al. 2001). In a human fetal intestinal explant model, a cell‐mediated immune response was elicited by stimulating T‐cells resulting in a 10‐fold increase in the rate of crypt epithelial cell proliferation and subsequently shorter villi, demonstrating that activation of T‐cells is important in the pathogenesis of EED and that crypt hyperplasia precedes villous atrophy (Ferreira et al. 1990). In similar studies of induced T‐cell hypersensitivity (Lionetti et al. 1993; MacDonald and Spencer, 1988), some explants showed villous atrophy and crypt hyperplasia, whereas in others, there was mucosal damage, which increased in severity with increasing specimen age. This suggests that crypt hyperplasia, villous atrophy and mucosal damage represent a continuum of adaptive to destructive responses to hyperstimulation by abnormally high levels of ingested bacteria.
What triggers T‐cell hyperstimulation?
This is the same cellular mechanism underlying inflammatory bowel diseases such as celiac disease or Crohn's disease. However, in EED, these are normal reactions to abnormally high concentrations of bacteria, whereas in these diseases, T‐cells are abnormally hyperreactive to normal stimuli. For example, in celiac patients, this stimulus is gluten, a normal antigen in healthy people, but an abnormal antigen for people with this genetic defect. In impoverished populations, most authors suggest the primary cause is high concentrations of faecal microorganisms. Accordingly, in this paper, we focus on the prevention of EED through reducing exposure to faecal microorganisms. It is important to note, however, that other causative factors may be important in the pathogenesis of EED in some contexts; these include mycotoxin exposure (Smith et al. 2012), severe nutritional deficiency (Guerrant et al. 2008), human immunodeficiency virus (Kelly et al. 1997), and diarrhoea (Behrens et al. 1987; Mondal et al. 2012). As such, EED prevention in these contexts could require different interventions.
Environmental enteric dysfunction is self‐perpetuating!
The intestinal epithelium selectively limits permeation of potentially harmful luminal substances (Hodin and Matthews, 2008). However, certain microbes or bacterial toxins (endotoxins) perturb barrier function either directly through loosening of the tight junctions or by activating various cytokine, neutrophil and proinflammatory mediators (Arrieta et al. 2006; Meddings, 2010). This permeable gut allows luminal contents, including microbes (microbial translocation) to cross the epithelial barrier and into systemic circulation (Brenchley and Douek, 2012). Chronic exposure to these insults – among Gambian infants 2–15 months of age (Lunn et al. 1991), increased intestinal permeability was observed in 700 out of 922 dual sugar absorption tests (i.e. for 76% of the time) – create a condition of low‐level chronic immune activation.
Worryingly, EED can be self‐perpetuating once it develops, especially if the host and causative agent are not separated (Gerson et al. 1971; Lindenbaum et al. 1971). Experimental intravenous infusion of bacterial endotoxin administered to healthy humans increased gut permeability (O'Dwyer et al. 1988), suggesting a vicious cycle.
How might environmental enteric dysfunction cause stunting?
Most of the research linking EED and linear growth faltering has been undertaken among rural Gambian infants and young children over the past two decades. In one of their earlier studies (Lunn et al. 1991), investigators in the Dunn Nutrition Laboratory monitored infants over a mean of 7.5 months. In addition to the negative correlation between intestinal permeability and monthly length gain (corrected for age), they calculated that impaired intestinal permeability accounted for 43% of linear growth faltering during this period. In a subsequent study (Campbell et al. 2003), three different markers of intestinal function (lactulose/mannitol ratio, IgG anti‐endotoxin titers and plasma immunoglobulin concentrations) were individually associated with growth faltering and showed substantial degree of overlap in their relationship with growth. In semi‐partial regression analysis, the three markers were calculated to explain up to 55% of linear growth faltering, suggesting that intestinal permeability, microbial translocation and inflammatory and immune response are all part of a single mechanism that overall predicted up to 55% of the growth faltering observed in Gambian children. This supports the mechanism of translocation of luminal bacteria or bacterial products across a compromised gut mucosa, leading to stimulation of systemic immune/inflammatory processes and subsequent growth impairment. This growth impairment arises in three ways. First, chronic immune activation is metabolically expensive, resulting in the diversion of nutrients to fuel the immune response (Ganeshan and Chawla, 2014). Second, chronic overproduction of proinflammatory cytokines (e.g. interleukin‐6) causes growth impairment that is mediated by a decrease in circulating insulin‐like growth factor (IGF‐1) levels (De Benedetti et al. 1997). Third, proinflammatory cytokines (e.g. interleukin‐1; tumour necrosis factor, TNF; interferon gamma, IFNγ) may directly impede linear growth by inhibiting the process of bone remodelling that is required for long bone growth (Bertolini et al. 1986; Skerry, 1994; Stephensen, 1999).
In the context of high nutrient requirements (Butte et al. 2000; Dewey, 2013) and rapid linear growth in infancy and early childhood (Dewey et al. 1992), EED may cause nutrient malabsorption that could further exacerbate its effects on growth. Morphologic data from nutritionally depleted and nondepleted patients suggested that a decrease in villous height is at least partially responsible for the changes in lactulose/mannitol (L/M) ratio, the dual sugar test of absorption and intestinal permeability (van der Hulst et al. 1998). Decreased mannitol absorption is a result of a diminished absorptive area, while increased permeation of lactulose may, in theory, be due to a facilitated diffusion of lactulose into the crypt region as a consequence of decreased villous height in addition to permeation due to loosening of the tight junctions. In the Gambian studies (Campbell et al. 2002; Campbell et al. 2003), the increase in L/M ratio that strongly predicted growth faltering was due to both reduced M and elevated L uptake, indicating that both barrier and absorptive functions of the small intestine were compromised. Furthermore, the loss of enterocyte brush border enzymes required for digestion and absorption (e.g. lactase) that results from villous atrophy can lead to maldigestion and malabsorption (Lunn, 2000).
Recent studies both confirm and provide further insights on these mechanisms. In the Global Enteric Multicenter Study, 83% of children with moderate‐to‐severe diarrhoea seeking care (cases) had at least one pathogen in their stool, as did 72% of matched controls (Kotloff et al. 2013) confirming that sub‐Saharan African and south Asian children 0–59 months old are infected with multiple enteric pathogens even when they do not have diarrhoea. In the MAL‐ED study, measures of intestinal inflammation were associated with linear growth faltering, even after controlling for diarrheal illness (Kosek et al. 2013) providing longitudinal support for the EED‐stunting pathway. In a case–control study (Prendergast et al. 2014) of Zimbabwean infants who were stunted (HAZ < −2; cases) compared with non‐stunted (HAZ > −0.5 controls) at 18 months, an association was observed between stunting and low‐grade inflammation in the first year of life and perturbation of the growth hormone‐IGF axis. Chronic inflammation due to microbial ingestion likely underlies a great deal of the prevalent and intractable stunting in developing countries.
The mechanisms linking environmental contamination, EED and stunting are illustrated in Fig. 2. In summary, ingested microbes set in motion two overlapping and interacting pathways that result in a linear growth impairment. Firstly, partial villous atrophy results in a reduced absorptive surface area and loss of digestive enzymes. This in turn results in maldigestion and malabsorption of nutrients. Secondly, microbes and their products impair the barrier function, causing a ‘leaky gut’, that allows luminal contents to translocate into systemic circulation. Chronic exposure to the microbes creates a condition of chronic immune activation, which (i) diverts nutrient resources (that are both scarce and in high demand) towards the metabolically expensive business of infection fighting rather than growth; (ii) suppresses the growth hormone‐IGF axis, and inhibits bone growth and remodelling, leading to growth impairment; and (iii) causes further damage to the intestinal mucosa thereby exacerbating the problem.
Figure 2.
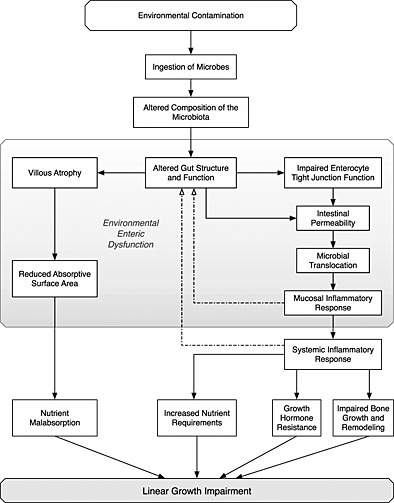
Biological mechanisms linking environmental contamination, environmental enteric dysfunction and linear growth impairment.
There is surprisingly little data in the literature on the relative importance of these pathways (Baker, 1976), especially the malabsorption pathway. We suggest that an effect of malabsorption on growth is plausible only if the amount of nutrients lost in stools is so high that it cannot be compensated by increased food intake. For example, in children, celiac disease can result in stunting of growth without the more classic malabsorptive symptoms of steatorrhoea (Murray, 1999). Also, an effect of infection on growth has been reported during pregnancy (Kayentao et al. 2013; Luntamo et al. 2013), suggesting a direct effect of inflammation as neither nutrient malabsorption nor poor appetite is likely to play a role during intrauterine life. While it is important to ascertain the full nutritional significance of malabsorption in populations where intake is marginal, the effect of chronic immune activation is likely to be the predominant mechanism.
Preventing environmental enteric dysfunction
Based on the observational and mechanistic evidence available, poor sanitation and hygiene contribute to, and are possibly the overriding cause of, EED [Lunn, 2000]. Plausibly, avoiding the ingestion of enteric pathogens and any other causative microbes by infants and young children could prevent most of the EED burden.
Can improvements in WASH prevent or mitigate environmental enteric dysfunction?
The animal literature provides the strongest evidence that cleaning up the environment improves growth. Miller et al. (1986) reported increased crypt depth in pigs that were weaned into a ‘dirty’ environment compared with pigs weaned into a ‘clean’ environment. Also, chicks that are raised in environments with faeces, dust and dander (‘dirty chicks’) have higher circulating IL‐1, a major mediator of the immune response, and slower growth than ‘clean chicks’ that are raised in steam‐cleaned cages (Roura et al. 1992; Solomons, 2003). This effect is observed even in the absence of pathogenic agents, suggesting an immune response to normally nonpathogenic organisms or other environmental immunogens such as dust and dander. Notably, clean chicks both grow faster and use nutrients more efficiently than dirty chicks (Edwards et al. 1960; Roura et al. 1992).
Human evidence is limited to observational studies. In a study of Zambian adults (Kelly et al. 2004), a hygiene score below the median was independently associated with crypt depth above the median and intestinal permeability (measured by a sugar test of absorption and permeability) above the median. In a recent study (Lin et al. 2013), children in environmentally clean Bangladeshi households had lower levels of parasitic infection, less severe EED (lower L : M ratios, −0.32 SD; lower IgG EndoCAb titers, −0.24 SD) and better indicators of attained linear growth (22% points lower stunting prevalence and 0.54 SDs higher HAZ) than children from environmentally contaminated households. In this study, researchers deliberately selected households representing two extremes of the distribution of household environments in order to maximize observed differences, suggesting that it is possible to improve EED and growth within the continuum between these two environmental extremes of rural Bangladesh.
Which WASH interventions could reduce environmental enteric dysfunction and stunting?
Water, sanitation and hygiene (WASH) interventions have the express objective of preventing the ingestion of harmful microbes by interrupting faecal oral transmission. In recent reviews, Curtis et al. (2000) and Brown et al. (2013) discuss the variety of these excreta‐related transmission routes, either as a result of direct transmission through contaminated hands or indirect transmission via contamination of drinking water, soil, utensils, food and flies, and acknowledge that the importance of each transmission route varies between pathogens and settings, and that different pathogens are more prevalent in some populations. As such, effective interventions need to address the predominant transmission routes for the target population and context. Specifically, WASH interventions seeking to prevent EED should address specific pathways through which feco‐oral transmission occurs in the first two years of a child's life.
A recent study illustrates the difficulty of aligning interventions with the causal pathways of target problems. Researchers enrolled households in a slum area in Kathmandu, Nepal, and randomly assigned geographic areas to a handwashing promotion intervention (Langford et al. 2011). The study reported large reductions in diarrhoea but no improvements in HAZ or EED (mucosal integrity) markers. Additionally, IgG levels rose with age and WAZ and WHZ worsened with age at a faster rate in the intervention group relative to controls. However, there were statistically significant differences between overcrowding and biofuel use in intervention and control areas, which suggests that the poorer health trajectory of children in the intervention group may perhaps reflect conditions of over‐crowding and poverty, leading to greater pathogen exposure through pathways unaffected by handwashing behaviours. The authors suggested in conclusion that ‘for children living in highly contaminated, over‐crowded environments, with poor access to clean water and sanitation, handwashing may be necessary, but not sufficient to reduce levels of subclinical mucosal damage and immune stimulation that are strongly associated with growth faltering’.
In an in‐depth observational study in rural Zimbabwe, infants were found to ingest soil and chicken faeces during exploratory play and mouthing behaviours (Ngure et al. 2013; Ngure et al. 2014). Using measurements of Escherischia coli as a marker, we found that active exploratory ingestion of soil (2100 E. coli cfu) and chicken faeces (10 000 000 E. coli cfu) posed greater risk of faecal bacteria exposure in terms of microbial load compared with fingers (no E coli cfu estimated), food (no E. coli cfu estimated) and drinking water (800 E. coli cfu). Similar observations of faecal contamination of the play and feeding areas of infants and young children, and ingestion of chicken faeces have been reported in Peruvian slums and Bangladeshi households (Marquis et al. 1990). In a recent prospective cohort study of Bangladeshi children, a significant association was observed between caregiver‐reported geophagy and elevated EED disease activity scores (defined as a composite score derived from faecal markers of intestinal inflammation), providing additional evidence that soil might be a direct exposure route for faecal pathogens (George et al. 2015). Based on these observations, Table 1 presents a framework for ‘baby‐WASH’ interventions that target the primary feco‐oral microbial transmission pathways among infants and young children. This framework can be used to either guide the formulation of context relevant interventions or as a checklist to ensure that interventions address all relevant pathways through which infants and young children are exposed to and ingest microbes.
Table 1.
Framework for a package of baby‐WASH interventions to interrupt feco‐oral transmission in the first two years of life
| Intervention objective | Timing | Hardware – inputs | Software – behaviour change messages | ||
|---|---|---|---|---|---|
| Access (provision, demand creation) | Practical/technical considerations | Utilization (encouragement, demand creation) | Triggers/motivators | ||
| Reduce faecal load in living environment | Always | Household sanitary facility (toilet). | Preferably one that facilitates or ensures fly control. | Use of sanitary facilities by all household members. Safe disposal of child faeces. | Disgust has been shown to be an effective trigger for behaviour change. |
| Reduce faecal transmission via hands | Always | Handwashing facility, soap/scrubbing agent, water (quantity) | Placement of the handwashing facility – (visual) cue to behaviour. Availability of soap or other scrubbing agent (e.g. ash) near handwashing facility. | Handwashing with soap by all household members (including children) at key potential contamination events (e.g. after faecal contact, before handling food and before feeding) | Disgust is also effective in triggering hand washing. |
| Exclusive breastfeeding | First 6 months | N/A | N/A | Breastfeeding only, to the exclusion of non‐breastmilk items fed for either nutritive or protective (prevention or treatment of perceived childhood illnesses) | Nurture, with a focus on protecting children from potentially harmful non‐breastmilk liquids, foods and traditional remedies |
| Improvement of drinking water quality | 6 months (after 6 months EBF) | Safe water source. Drinking water storage containers. Treatment agent/model (e.g. solar, chlorine) at the point of use. | Water treatment agent should meet organoleptic (taste and smell) expectations of household members. | Water treatment at the point of use. Drinking of treated water by all household members. | Associated taste and smell of treated water with cleanliness. Nurture is an effective motivator for promoting provision of treated water to children |
| Avoidance of child faecal ingestion during mouthing and exploratory play (e.g. geophagy, consumption of chicken faeces) | 2–4 months (crawling and mouthing) | A clean play and infant feeding environment. (Household improvised or technology, such as a protective play space) | The play space should ensure that the child is protected from contamination while ensuring their developmental needs for exploration and interaction are met. Any benefits should outweigh technical and sociocultural burdens of any new technology introduced. | Awareness of risks associated with playing in an environmentally contaminated environment, e.g. geophagy, direct/indirect consumption of animal faeces. | Risk awareness. Nurture. |
| Hygienic handling and preparation of complementary foods | 6 months (after 6 months EBF) | N/A | N/A | Hygienic handling and preparation of complementary foods. Provision of freshly prepared foods as much as possible. | Risk awareness. Nurture. |
Cluster‐randomized trials are currently being conducted to ascertain the effects of WASH and improved nutrition (independently and in combination) on EED and stunting in Zimbabwe (clinicaltrials.gov identifier NCT01824940), Kenya (NCT01704105) and Bangladesh (NCT01590095). These studies will provide much needed causal and mechanistic evidence on the role of WASH in stunting reduction. However, additional complementary questions warrant further research:
What microbes are important in the aetiology of EED, i.e. commensal vs. pathogenic microbes (Korpe and Petri, 2012)? This question is important in defining the range of environmental microbes and microbial products that interventions should address. Among 40 asymptomatic infants with EED living in an urban Sau Paulo slum, 63% had colonic bacterial proliferation of the small bowel (Fagundes Neto et al. 1994). Moreover, because only pathogenic bacteria cause clinical diarrhoea, sanitation/hygiene messages have often particularly focused on avoiding exposure to faeces of young children – the major carriers of these organisms (Jinadu, 2004; Lanata et al. 1998). However, it may be that high concentrations of any bacteria in the small bowel can cause EED: in experiments comparing germ‐free with conventional animals, commensal bacteria in low concentrations exert a trophic effect on the intestinal epithelium shifting morphology from ‘supranormal’ (i.e. very tall villi) to normal, which results in a structurally and functionally competent immune system (Sprinz et al. 1961). Thus, high exposure to all faeces (including those from healthy people and animals) may contribute to EED.
What are the potential roles of antibiotics, probiotics, other anti‐inflammatory agents, and changes in the composition of the microbiome in treating and preventing EED (Petri et al. 2014)? These preventive and therapeutic options for EED represent an area of active research (Galpin et al. 2005; Jones et al. 2014; Ryan et al. 2014; Trehan et al. 2009) and discussion (Petri et al. 2014; Prendergast and Humphrey, 2014), as we simultaneously seek to better understand the pathogenesis, mitigation and measurement of EED.
What is the role of maternal EED on linear growth faltering that occurs in utero? Approximately 25% of the stunting observed at 2 years occurs in utero, i.e. born shorter than they should be: as such, understanding the contribution of maternal EED would aid the formulation of antenatal, and possibly pre‐conception, interventions. This is being investigated through observational and case–control designs within the SHINE trial in Zimbabwe (NCT01824940).
Which biomarker or biomarkers of EED should be used as (i) normative standards and cutoff points for ‘counting the affected’; (ii) indicators of risk for screening populations and targeting interventions; and (iii) indicators for measuring response(s) to interventions (Habicht and Stoltzfus, 1997; Habicht et al. 1982)? No single, validated biomarker of EED is available – currently available biomarkers indicate its structural and functional characteristics relative to the interacting and overlapping processes illustrated in Fig. 2. The process of identifying effective biomarkers and defining measurement protocols continues to evolve with heightened interest in the causes, pathogenesis, effects and responsiveness, of EED (Guerrant et al. 2013; Keusch et al. 2013; Keusch et al. 2014; Korpe and Petri, 2012; Kosek et al. 2014; Petri et al. 2014). This process would benefit from taking these objectives and applications of the ‘best’ indicator or indicators (Habicht and Pelletier, 1990) into cognizance.
Conclusion
Chronic exposure to a contaminated environment creates a constant state of survival responses characterized by loss, malabsorption, maldigestion and inefficient utilization of nutrients. In the context of marginal diets and recurrent infections, this ‘impoverished gut’ condition likely explains a significant portion of the unresolved stunting affecting one in every three children in developing countries. To prevent stunting, we need to prevent the onset of EED because (i) EED is self‐perpetuating once it has developed; (ii) recovery from EED is relatively slow even when there is a dramatic change in environment; and (iii) the window for critical growth and development is short (between conception and the first two years of postnatal life). Interventions such as baby‐WASH, aimed at preventing and reducing environmental enteric dysfunction, may be central to global stunting reduction efforts.
Sources of funding
This publication is based on research funded by the UK Department for International Development/Zimbabwe [AG 201 854–101] and the Bill and Melinda Gates Foundation [OPP1021542].
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
MNNM wrote the first draft of the manuscript, which was critically revised by JHH.
Acknowledgements
We gratefully acknowledge Zandile Mbuya, Laura E. Smith, Andrew J. Prendergast and Nkosinathi V. N. Mbuya for their critical review of specific sections and draft versions of the manuscript. We also thank the SHINE Trial Group for their contributions in the evolution of the ideas presented, and the two anonymous reviewers for their insightful comments and suggestions.
Mbuya, M. N. N. , and Humphrey, J. H. (2016) Preventing environmental enteric dysfunction through improved water, sanitation and hygiene: an opportunity for stunting reduction in developing countries. Maternal & Child Nutrition, 12: 106–120. doi: 10.1111/mcn.12220.
References
- Alderman H., Hoddinott J. & Kinsey B. (2006) Long term consequences of early childhood malnutrition. Oxford Economic Papers 58, 450–474. [Google Scholar]
- Arrieta M.C., Bistritz L. & Meddings J.B. (2006) Alterations in intestinal permeability. Gut 55, 1512–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S.J. (1976) Subclinical intestinal malabsorption in developing countries. Bulletin of the World Health Organization 54, 485–494. [PMC free article] [PubMed] [Google Scholar]
- Behrens R.H., Lunn P.G., Northrop C.A., Hanlon P.W. & Neale G. (1987) Factors affecting the integrity of the intestinal mucosa of Gambian children. American Journal of Clinical Nutrition 45, 1433–1441. [DOI] [PubMed] [Google Scholar]
- Bertolini D.R., Nedwin G.E., Bringman T.S., Smith D.D. & Mundy G.R. (1986) Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature 319, 516–518. [DOI] [PubMed] [Google Scholar]
- Bhutta Z.A., Das J.K., Rizvi A., Gaffey M.F., Walker N., Horton S., et al. (2013) Evidence‐based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet 382, 452–477. [DOI] [PubMed] [Google Scholar]
- Black R.E., Victora C.G., Walker S.P., Bhutta Z.A., Christian P., de Onis M., et al. (2013) Maternal and child undernutrition and overweight in low‐income and middle‐income countries. Lancet 382, 427–451. [DOI] [PubMed] [Google Scholar]
- Brenchley J.M. & Douek D.C. (2012) Microbial translocation across the GI tract. Annual Review of Immunology 30, 149–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briend A. (1990) Is diarrhoea a major cause of malnutrition among the under‐fives in developing countries? A review of available evidence. European Journal of Clinical Nutrition 44, 611–628. [PubMed] [Google Scholar]
- Briend A., Hasan K.Z., Aziz K.M. & Hoque B.A. (1990) Diarrhoea and catch‐up growth. Lancet 335, 1157–1158. [DOI] [PubMed] [Google Scholar]
- Brown J., Cairncross S. & Ensink J.H. (2013) Water, sanitation, hygiene and enteric infections in children. Archives of Disease in Childhood 98, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butte N.F., Wong W.W., Hopkinson J.M., Heinz C.J., Mehta N.R. & Smith E.O. (2000) Energy requirements derived from total energy expenditure and energy deposition during the first 2 y of life. American Journal of Clinical Nutrition 72, 1558–1569. [DOI] [PubMed] [Google Scholar]
- Campbell D.I., Lunn P.G. & Elia M. (2002) Age‐related association of small intestinal mucosal enteropathy with nutritional status in rural Gambian children. British Journal of Nutrition 88, 499–505. [DOI] [PubMed] [Google Scholar]
- Campbell D.I., Elia M. & Lunn P.G. (2003) Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. Journal of Nutrition 133, 1332–1338. [DOI] [PubMed] [Google Scholar]
- Caulfield L.E., De Onis M., Blossner M. & Black R.E. (2004) Undernutrition as an underlying cause of child associated with diarrhea, pneumonia, malaria, and measles. American Journal of Clinical Nutrition 80, 193–198. [DOI] [PubMed] [Google Scholar]
- Checkley W., Buckley G., Gilman R.H., Assis A.M., Guerrant R.L., Morris S.S., et al. (2008) Multi‐country analysis of the effects of diarrhoea on childhood stunting. International Journal of Epidemiology 37, 816–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook G.C., Kajubi S.K. & Lee F.D. (1969) Jejunal morphology of the African in Uganda. Journal of Pathology 98, 157–169. [DOI] [PubMed] [Google Scholar]
- Curtis V., Cairncross S. & Yonli R. (2000) Domestic hygiene and diarrhoea – pinpointing the problem. Tropical Medicine and International Health 5, 22–32. [DOI] [PubMed] [Google Scholar]
- De Benedetti F., Alonzi T., Moretta A., Lazzaro D., Costa P., Poli V., et al. (1997) Interleukin 6 causes growth impairment in transgenic mice through a decrease in insulin‐like growth factor‐I. A model for stunted growth in children with chronic inflammation. Journal of Clinical Investigation 99, 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai H.G., Borkar A.V., Pathare S.M., Dighe P.K. & Jeejeebhoy K.N. (1969) ‘Flat’ jejunal mucosa in the tropics. Indian Journal of Medical Sciences 23, 1–5. [PubMed] [Google Scholar]
- Dewey K.G. (2013) The challenge of meeting nutrient needs of infants and young children during the period of complementary feeding: an evolutionary perspective. Journal of Nutrition. 143, 2050–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey K.G. & Adu‐Afarwuah S. (2008) Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Maternal & Child Nutrition 4 Suppl 1, 24–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey K.G., Heinig M.J., Nommsen L.A., Peerson J.M. & Lonnerdal B. (1992) Growth of breast‐fed and formula‐fed infants from 0 to 18 months: the DARLING Study. Pediatrics 89, 1035–1041. [PubMed] [Google Scholar]
- Edwards H.M., Fuller H.L. & Hess C.W. (1960) The effect of environment on chick growth. Journal of Nutrition 70, 302–306. [DOI] [PubMed] [Google Scholar]
- Esrey S.A. (1996) Water, waste, and well‐being: a multicountry study. American Journal of Epidemiology 143, 608–623. [DOI] [PubMed] [Google Scholar]
- Fagundes Neto U., Martins M.C., Lima F.L., Patricio F.R. & Toledo M.R. (1994) Asymptomatic environmental enteropathy among slum‐dwelling infants. Journal of the American College of Nutrition 13, 51–56. [DOI] [PubMed] [Google Scholar]
- Fagundes‐Neto U., Viaro T., Wehba J., Patricio F.R.d.S. & Machado N.L. (1984) Tropical Enteropathy (environmental enteropathy) in early childhood: a syndrome caused by contaminated environment. Journal of Tropical Pediatrics 30, 204–209. [DOI] [PubMed] [Google Scholar]
- Ferreira R.C., Forsyth L.E., Richman P.I., Wells C., Spencer J. & MacDonald T.T. (1990) Changes in the rate of crypt epithelial cell proliferation and mucosal morphology induced by a T‐cell‐mediated response in human small intestine. Gastroenterology 98, 1255–1263. [DOI] [PubMed] [Google Scholar]
- Galpin L., Manary M.J., Fleming K., Ou C.N., Ashorn P. & Shulman R.J. (2005) Effect of lactobacillus GG on intestinal integrity in Malawian children at risk of tropical enteropathy. American Journal of Clinical Nutrition 82, 1040–1045. [DOI] [PubMed] [Google Scholar]
- Ganeshan K. & Chawla A. (2014) Metabolic regulation of immune responses. Annual Review of Immunology 32, 609–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George C.M., Oldja L., Biswas S., Perin J., Lee G.O., Kosek M., et al. (2015) Geophagy is associated with environmental enteropathy and stunting in children in Rural Bangladesh. American Journal of Tropical Medicine and Hygiene 92, 1117–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerson C.D., Kent T.H., Saha J.R., Siddiqi N. & Lindernbaum J. (1971) Recovery of small‐intestinal structure and function after residence in the tropics: II. Studies in Indians and Pakistanis living in New York City. Annals of Internal Medicine 75, 41–48. [DOI] [PubMed] [Google Scholar]
- Grantham‐McGregor S., Cheung Y.B., Cueto S., Glewwe P., Richter L., Strupp B., et al. (2007) Developmental potential in the first 5 years for children in developing countries. Lancet 369, 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R.L., Oria R.B., Moore S.R., Oria M.O. & Lima A.A. (2008) Malnutrition as an enteric infectious disease with long‐term effects on child development. Nutrition Reviews 66, 487–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R.L., DeBoer M.D., Moore S.R., Scharf R.J. & Lima A.A. (2013) The impoverished gut – a triple burden of diarrhoea, stunting and chronic disease. Nature Reviews Gastroenterology & Hepatology 10, 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habicht J.P. & Pelletier D.L. (1990) The importance of context in choosing nutritional indicators. Journal of Nutrition 120 (Suppl 11), 1519–1524. [DOI] [PubMed] [Google Scholar]
- Habicht J.P. & Stoltzfus R.J. (1997) What do indicators indicate? American Journal of Clinical Nutrition 60, 190–191. [DOI] [PubMed] [Google Scholar]
- Habicht J.P., Meyers L.D. & Brownie C. (1982) Indicators for identifying and counting the improperly nourished. American Journal of Clinical Nutrition 35, 1241–1254. [DOI] [PubMed] [Google Scholar]
- Haghighi P. & Wolf P.L. (1997) Tropical sprue and subclinical enteropathy: a vision for the nineties. Critical Reviews in Clinical Laboratory Sciences 34, 313–341. [DOI] [PubMed] [Google Scholar]
- Hoddinott J., Behrman J.R., Maluccio J.A., Melgar P., Quisumbing A.R., Ramirez‐Zea M., et al. (2013) Adult consequences of growth failure in early childhood. American Journal of Clinical Nutrition 98, 1170–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodin R. & Matthews J. (2008) Small Intestine In: Surgery. (eds Norton J., Barie P., Bollinger R.R., Chang A., Lowry S., Mulvihill S., et al.). Springer: New York. [Google Scholar]
- van der Hulst R.R., von Meyenfeldt M.F., van Kreel B.K., Thunnissen F.B., Brummer R.J., Arends J.W., et al. (1998) Gut permeability, intestinal morphology, and nutritional depletion. Nutrition 14, 1–6. [DOI] [PubMed] [Google Scholar]
- Humphrey J.H. (2009) Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet 374, 1032–1035. [DOI] [PubMed] [Google Scholar]
- Imdad A., Yakoob M.Y. & Bhutta Z.A. (2011) Impact of maternal education about complementary feeding and provision of complementary foods on child growth in developing countries. BMC Public Health 11 (Suppl 3), S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaladanki R.N. & Wang J.‐Y. (2011) Regulation of gastrointestinal mucosal growth. Colloquium Series on Integrated Systems Physiology: From Molecule to Function 3, 1–114. [Google Scholar]
- Jinadu M.K. (2004) Disposal of children's faeces and implications for the control of childhood diarrhoea. The Journal of the Royal Society for the Promotion of Health 124, 276–279. [DOI] [PubMed] [Google Scholar]
- Jones K.D., Hunten‐Kirsch B., Laving A.M., Munyi C.W., Ngari M., Mikusa J., et al. (2014) Mesalazine in the initial management of severely acutely malnourished children with environmental enteric dysfunction: a pilot randomized controlled trial. BMC Medicine 12, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayentao K., Garner P., van Eijk A.M., Naidoo I., Roper C., Mulokozi A., et al. (2013) Intermittent preventive therapy for malaria during pregnancy using 2 vs 3 or more doses of sulfadoxine‐pyrimethamine and risk of low birth weight in Africa: systematic review and meta‐analysis. JAMA 309, 594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P., Davies S.E., Mandanda B., Veitch A., McPhail G., Zulu I., et al. (1997) Enteropathy in Zambians with HIV related diarrhoea: regression modelling of potential determinants of mucosal damage. Gut 41, 811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P., Menzies I., Crane R., Zulu I., Nickols C., Feakins R., et al. (2004) Responses of small intestinal architecture and function over time to environmental factors in a tropical population. American Journal of Tropical Medicine and Hygiene 70, 412–419. [PubMed] [Google Scholar]
- Keusch G.T. (1972) Subclinical malabsorption in Thailand. I. Intestinal absorption in Thai children. American Journal of Clinical Nutrition 25, 1062–1066. [DOI] [PubMed] [Google Scholar]
- Keusch G.T., Rosenberg I.H., Denno D.M., Duggan C., Guerrant R.L., Lavery J.V., et al. (2013) Implications of acquired environmental enteric dysfunction for growth and stunting in infants and children living in low‐ and middle‐income countries. Food and Nutrition Bulletin 34, 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keusch G.T., Denno D.M., Black R.E., Duggan C., Guerrant R.L., Lavery J.V., et al. (2014) Environmental enteric dysfunction: pathogenesis, diagnosis, and clinical consequences. Clinical Infectious Diseases 59 Suppl 4, S207–S212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpe P.S. & Petri W.A., Jr. (2012) Environmental enteropathy: critical implications of a poorly understood condition. Trends in Molecular Medicine 18, 328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosek M., Haque R., Lima A., Babji S., Shrestha S., Qureshi S., et al. (2013) Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. American Journal of Tropical Medicine and Hygiene 88, 390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosek M., Guerrant R.L., Kang G., Bhutta Z., Yori P.P., Gratz J., et al. (2014) Assessment of environmental enteropathy in the MAL‐ED cohort study: theoretical and analytic framework. Clinical Infectious Diseases 59 Suppl 4, S239–S247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotloff K.L., Nataro J.P., Blackwelder W.C., Nasrin D., Farag T.H., Panchalingam S., et al. (2013) Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case–control study. Lancet 382, 209–222. [DOI] [PubMed] [Google Scholar]
- Lanata C.F., Huttly S.R. & Yeager B.A. (1998) Diarrhea: whose feces matter? Reflections from studies in a Peruvian shanty town. Pediatric Infectious Disease Journal 17, 7–9. [DOI] [PubMed] [Google Scholar]
- Langford R., Lunn P. & Panter‐Brick C. (2011) Hand‐washing, subclinical infections, and growth: a longitudinal evaluation of an intervention in Nepali slums. American Journal of Human Biology 23, 621–629. [DOI] [PubMed] [Google Scholar]
- Lassi Z.S., Das J.K., Zahid G., Imdad A. & Bhutta Z.A. (2013) Impact of education and provision of complementary feeding on growth and morbidity in children less than 2 years of age in developing countries: a systematic review. BMC Public Health 13 (Suppl 3), S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A., Arnold B.F., Afreen S., Goto R., Huda T.M., Haque R., et al. (2013) Household environmental conditions are associated with enteropathy and impaired growth in rural Bangladesh. American Journal of Tropical Medicine and Hygiene 89, 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbaum J., Greson C.D. & Kent T.H. (1971) Recovery of small‐intestinal structure and function after residence in the tropics: I. Studies in Peace Corps volunteers. Annals of Internal Medicine 74, 218–222. [DOI] [PubMed] [Google Scholar]
- Lindenbaum J., Harmon J.W. & Gerson C.D. (1972) Subclinical malabsorption in developing countries. American Journal of Clinical Nutrition 25, 1056–1061. [DOI] [PubMed] [Google Scholar]
- Lionetti P., Breese E., Braegger C.P., Murch S.H., Taylor J. & MacDonald T.T. (1993) T‐cell activation can induce either mucosal destruction or adaptation in cultured human fetal small intestine. Gastroenterology 105, 373–381. [DOI] [PubMed] [Google Scholar]
- Lunn P.G. (2000) The impact of infection and nutrition on gut function and growth in childhood. Proceedings of the Nutrition Society 59, 147–154. [DOI] [PubMed] [Google Scholar]
- Lunn P.G., Northrop‐Clewes C.A. & Downes R.M. (1991) Intestinal permeability, mucosal injury, and growth faltering in Gambian infants. Lancet 338, 907–910. [DOI] [PubMed] [Google Scholar]
- Luntamo M., Kulmala T., Cheung Y.B., Maleta K. & Ashorn P. (2013) The effect of antenatal monthly sulphadoxine‐pyrimethamine, alone or with azithromycin, on foetal and neonatal growth faltering in Malawi: a randomised controlled trial. Tropical Medicine and International Health 18, 386–397. [DOI] [PubMed] [Google Scholar]
- MacDonald T.T. & Spencer J. (1988) Evidence that activated mucosal T cells play a role in the pathogenesis of enteropathy in human small intestine. Journal of Experimental Medicine 167, 1341–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maluccio J.A., Hoddinott J., Behrman J.R., Martorell R., Quisumbing A.R. & Stein A.D. (2009) The impact of improving nutrition during early childhood on education among Guatemalan adults. The Economic Journal 119, 734–763. [Google Scholar]
- Marquis G.S., Ventura G., Gilman R.H., Porras E., Miranda E., Carbajal L., et al. (1990) Fecal contamination of shanty town toddlers in households with non‐corralled poultry, Lima, Peru. American Journal of Public Health 80, 146–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay S., Gaudier E., Campbell D.I., Prentice A.M. & Albers R. (2010) Environmental enteropathy: new targets for nutritional interventions. International Health 2, 172–180. [DOI] [PubMed] [Google Scholar]
- Meddings J.B. (2010) Review article: intestinal permeability in Crohn's disease. Alimentary Pharmacology & Therapeutics 11, 47–56. [DOI] [PubMed] [Google Scholar]
- Menzies I.S., Zuckerman M.J., Nukajam W.S., Somasundaram S.G., Murphy B., Jenkins A.P., et al. (1999) Geography of intestinal permeability and absorption. Gut 44, 483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B.G., James P.S., Smith M.W. & Bourne F.J. (1986) Effect of weaning on the capacity of pig intestinal villi to digest and absorb nutrients. The Journal of Agricultural Science 107, 579–590. [Google Scholar]
- Mondal D., Minak J., Alam M., Liu Y., Dai J., Korpe P., et al. (2012) Contribution of enteric infection, altered intestinal barrier function, and maternal malnutrition to infant malnutrition in Bangladesh. Clinical Infectious Diseases 54, 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J.A. (1999) The widening spectrum of celiac disease. The American Journal of Clinical Nutrition 69(3), 354–365. [DOI] [PubMed] [Google Scholar]
- Nabuurs M.J.A., Hoogendoorn A., Van Der Molen E.J. & Van Osta A.L.M. (1993) Villus height and crypt depth in weaned and unweaned pigs, reared under various circumstances in the Netherlands. Research in Veterinary Science 55, 78–84. [DOI] [PubMed] [Google Scholar]
- Ngure F.M., Humphrey J.H., Mbuya M.N., Majo F., Mutasa K., Govha M., et al. (2013) Formative research on hygiene behaviors and geophagy among infants and young children and implications of exposure to fecal bacteria. American Journal of Tropical Medicine and Hygiene 89, 709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngure F.M., Reid B.M., Humphrey J.H., Mbuya M.N., Pelto G. & Stoltzfus R.J. (2014) Water, sanitation, and hygiene (WASH), environmental enteropathy, nutrition, and early child development: making the links. Annals of the New York Academy of Sciences 1308, 118–128. [DOI] [PubMed] [Google Scholar]
- O'Dwyer S.T., Michie H.R., Ziegler T.R., Revhaug A., Smith R.J. & Wilmore D.W. (1988) A single dose of endotoxin increases intestinal permeability in healthy humans. Archives of Surgery 123, 1459–1464. [DOI] [PubMed] [Google Scholar]
- Pelletier D.L., Frongillo E.A., Jr. , Schroeder D.G. & Habicht J.P. (1995) The effects of malnutrition on child mortality in developing countries. Bulletin of the World Health Organization 73, 443–448. [PMC free article] [PubMed] [Google Scholar]
- Peterson L.W. & Artis D. (2014) Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nature Reviews Immunology 14, 141–153. [DOI] [PubMed] [Google Scholar]
- Petri W.A., Naylor C. & Haque R. (2014) Environmental enteropathy and malnutrition: do we know enough to intervene? BMC Medicine 12, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskitt E.M., Cole T.J. & Whitehead R.G. (1999) Less diarrhoea but no change in growth: 15 years' data from three Gambian villages. Archives of Disease in Childhood 80, 115–119; discussion 119–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast A.J. & Humphrey J.H. (2014) The stunting syndrome in developing countries. Paediatrics and International Child Health 34, 250–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast A. & Kelly P. (2012) Enteropathies in the developing world: neglected effects on global health. American Journal of Tropical Medicine and Hygiene 86, 756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast A.J., Rukobo S., Chasekwa B., Mutasa K., Ntozini R., Mbuya M.N., et al. (2014) Stunting is characterized by chronic inflammation in Zimbabwean infants. PLoS One 9, e86928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard S.A., Black R.E., Gilman R.H., Guerrant R.L., Kang G., Lanata C.F., et al. (2013) Diarrhea in early childhood: short‐term association with weight and long‐term association with length. American Journal of Epidemiology 178, 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard S.A., Black R.E., Gilman R.H., Guerrant R.L., Kang G., Lanata C.F., et al. (2014) Catch‐up growth occurs after diarrhea in early childhood. Journal of Nutrition 144, 965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg I.H. & Solomons N.W. (1978) The potential for antidiarrheal and nutrient‐sparing effects of oral antibiotic use in children: a position paper. American Journal of Clinical Nutrition 31, 2202–2207. [DOI] [PubMed] [Google Scholar]
- Rosenberg I.H., Beisel W.R. & Gordon J.E. (1974) Infant and child enteritis‐malabsorption‐malnutrition: the potential of limited studies with low‐dose antibiotic feeding. American Journal of Clinical Nutrition 27, 304–309. [DOI] [PubMed] [Google Scholar]
- Roura E., Homedes J. & Klasing K.C. (1992) Prevention of immunologic stress contributes to the growth‐permitting ability of dietary antibiotics in chicks. Journal of Nutrition 122, 2383–2390. [DOI] [PubMed] [Google Scholar]
- Rousham E.K. & Gracey M. (1997) Persistent growth faltering among aboriginal infants and young children in north‐west Australia: a retrospective study from 1969 to 1993. Acta Paediatrica 86, 46–50. [DOI] [PubMed] [Google Scholar]
- Ryan K.N., Stephenson K.B., Trehan I., Shulman R.J., Thakwalakwa C., Murray E., et al. (2014) Zinc or albendazole attenuates the progression of environmental enteropathy: a randomized controlled trial. Clinical Gastroenterology and Hepatology 12, 1507–1513 e1501. [DOI] [PubMed] [Google Scholar]
- Sandler N.G. & Douek D.C. (2012) Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nature Reviews Microbiology 10, 655–666. [DOI] [PubMed] [Google Scholar]
- Scrimshaw N.S., Taylor C.E. & Gordon J.E. (1968) Interactions of nutrition and infection. Monograph Series. World Health Organization 57, 3–329. [PubMed] [Google Scholar]
- Sheehy T.W., Legters L.J. & Wallace D.K. (1968) Tropical jejunitis in Americans serving in Vietnam. American Journal of Clinical Nutrition 21, 1013–1022. [DOI] [PubMed] [Google Scholar]
- Skerry T.M. (1994) The effects of the inflammatory response on bone growth. European Journal of Clinical Nutrition 48 (Suppl 1), S190–S197; discussion S198. [PubMed] [Google Scholar]
- Prendergast L.E., Stoltzfus R.J. & Prendergast A. (2012) Food chain mycotoxin exposure, gut health, and impaired growth: a conceptual framework. Advances in Nutrition 3, 526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomons N.W. (2003) Environmental contamination and chronic inflammation influence human growth potential. Journal of Nutrition 133, 1237. [DOI] [PubMed] [Google Scholar]
- Solomons N.W., Mazariegos M., Brown K.H. & Klasing K. (1993) The underprivileged, developing country child: environmental contamination and growth failure revisited. Nutrition Reviews 51, 327–332. [DOI] [PubMed] [Google Scholar]
- Sprinz H., Kundel D.W., Dammin G.J., Horowitz R.E., Schneider H. & Formal S.B. (1961) The response of the germ‐free guinea pig to oral bacterial challenge with Escherichia coli and Shigella flexneri: with special reference to lymphatic tissue and the intestinal tract. The American Journal of Pathology 39, 681–695. [PMC free article] [PubMed] [Google Scholar]
- Stanfield J.P., Hutt M.S.R. & Tunnicliffe R. (1965) Intestinal biopsy in kwashiorkor. Lancet 2, 519–523. [DOI] [PubMed] [Google Scholar]
- Stephensen C.B. (1999) Burden of infection on growth failure. Journal of Nutrition 129, 534S–538S. [DOI] [PubMed] [Google Scholar]
- Trehan I., Shulman R.J., Ou C.N., Maleta K. & Manary M.J. (2009) A randomized, double‐blind, placebo‐controlled trial of rifaximin, a nonabsorbable antibiotic, in the treatment of tropical enteropathy. The American Journal of Gastroenterology 104, 2326–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veitch A.M., Kelly P., Zulu I.S., Segal I. & Farthing M.J. (2001) Tropical enteropathy: a T‐cell‐mediated crypt hyperplastic enteropathy. European Journal of Gastroenterology and Hepatology 13, 1175–1181. [DOI] [PubMed] [Google Scholar]
- Victora C.G., de Onis M., Hallal P.C., Blossner M. & Shrimpton R. (2010) Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics 125, e473–e480. [DOI] [PubMed] [Google Scholar]
- Walker S.P., Wachs T.D., Grantham‐McGregor S., Black M.M., Nelson C.A., Huffman S.L., et al. (2011) Inequality in early childhood: risk and protective factors for early child development. Lancet 378, 1325–1338. [DOI] [PubMed] [Google Scholar]


