Abstract
Background
Genetic variation in the cluster on chromosome 15, encoding the nicotinic acetylcholine receptor subunits (CHRNA5‐CHRNA3‐CHRNB4), has shown strong associations with tobacco consumption and an additional risk increase in smoking‐related diseases such as chronic obstructive pulmonary disease (COPD), peripheral artery disease and lung cancer.
Objectives
To test whether rs1051730 (C/T), a tag for multiple variants in the CHRNA5‐CHRNA3‐CHRNB3 cluster, is associated with a change in risk of smoking‐related mortality and morbidity in the Malmö Diet and Cancer study, a population‐based prospective cohort study.
Methods
At baseline participants were classified as current (n = 6951), previous (n = 8426) or never (n = 9417) smokers. Cox‐proportional hazards models were used to determine the correlation between rs1051730 and incidence of first COPD, tobacco‐related cancer, other cancer and cardiovascular disease (CVD), and total mortality due to these causes, during approximately 14 years of follow‐up.
Results
Amongst current smokers there were 480 first incident COPD events, 852 tobacco‐related cancers, 810 other cancers and 1022 CVD events. A total of 1508 deaths occurred, including 500 due to CVD, 102 due to respiratory diseases and 677 due to cancer. In adjusted additive models, an increasing number of T alleles were associated with a gradual increase in total mortality, incident COPD and tobacco‐related cancer, even after adjustment for smoking quantity. No significant associations were observed amongst never smokers.
Conclusion
Our data suggest that gene variance in the CHRNA5‐CHRNA3‐CHRNB4 cluster is associated with an increased risk of death, incidence of COPD and tobacco‐related cancer in smokers. These findings indicate an individual susceptibility to tobacco use and its complications; this may be important when targeting and designing smoking cessation therapies.
Keywords: CHRNA, COPD, epidemiology, smoking genetics, tobacco‐related cancer
Introduction
More than 1 billion people around the world are smokers 1. Negative health consequences such as cancer, heart disease, stroke and respiratory diseases are well‐known complications, and cigarette smoking is responsible for about 5 million deaths annually (Data from the World Health Organization). Recent genomewide association studies have shown convincing associations between a number of genetic variations and both nicotine dependence (ND) and smoking behaviour 2, 3, 4, 5, 6. The synonymous single nucleotide polymorphism (SNP) rs1051730 on chromosome 15q25, in the gene for the nicotinic acetylcholine receptor (nAChr) subunit CHRNA3, showed the strongest association. For this SNP, which shows a risk allele frequency of approximately 38% in European populations, each copy of the risk allele corresponded to an increase in smoking quantity of 1 cigarette per day (CPD) 3. It is interesting that the cluster of genes on chromosome 15q25, encoding the nACHr subunits CHRNA5‐CHRNA3‐CHRNB4, has been shown to be associated not only with smoking quantity and ND but also with smoking‐related diseases such as chronic obstructive pulmonary disease (COPD), lung cancer, peripheral artery disease and bladder cancer 7. Not all smokers develop these smoking‐related diseases, a fact that might indicate that genetic differences also contribute to individual susceptibility.
This gene cluster of nACHrs is also known to be an area of high correlation and, according to the international HapMap project (http://hapmap.ncbi.nlm.nih.gov), the rs1051730 is in almost perfect correlation with rs16969968 (CHRNA5) in European populations, and therefore, these variants are considered to be essentially interchangeable. The rs16969968 is a coding variant, and rs1051730 should be considered as a surrogate marker 8.
The purpose of this study was to determine whether genetic variations in the 15q25 locus affect the risk of smoking‐related complications amongst smokers in the Malmö Diet and Cancer study.
Methods
Study population
The prospective population‐based Malmö Diet and Cancer study included a total of 18 326 women born between 1923 and 1950 and 12 121 men born between 1923 and 1945 in Malmö, Sweden 9. Participants were recruited from 1991 to 1996. At the baseline examination, anthropometric variables and blood pressure were measured and blood samples were collected and stored for later analysis. Additionally, subjects were asked to complete a self‐administered questionnaire of health‐ and lifestyle‐related factors including current and previous disease, medication, smoking and socioeconomic factors. All participants provided written, informed consent, and study protocols were approved by the ethics committee at Lund University, Lund, Sweden.
DNA extraction and genotyping
DNA was available for 28 564 subjects, and genotyping of rs1051730 was successfully performed for 26 471 subjects (success rate 92.7%). Genotyping was performed using TaqMan (Applied Biosystems, Foster City, CA, USA) with primers and conditions according to the manufacturer′s recommendations.
Baseline variables
Blood pressure was measured once in the supine position, after a 5‐min rest, using a mercury sphygmomanometer. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg, or ongoing therapy with antihypertensive medication. Use of antihypertensive or lipid‐lowering treatment (LLT), history of diabetes and smoking status were assessed from the Malmö Diet and Cancer study baseline questionnaire. Information about age, sex, rs1051730, body mass index (BMI), hypertension, previous diabetes diagnosis and LLT was available for all subjects included in the analyses (see Fig. 1).
Figure 1.
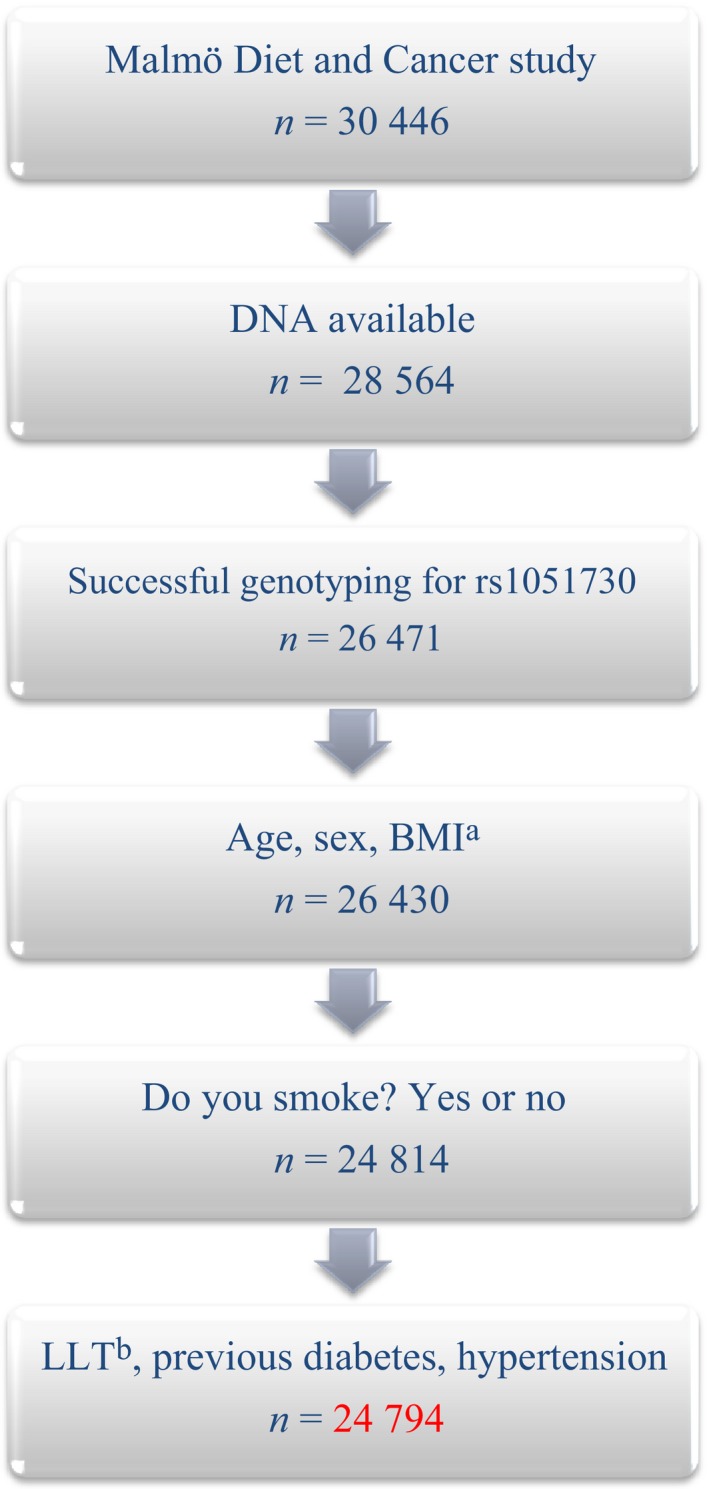
Selection of study subjects. aBMI, Body Mass Index; bLLT, Lipid‐lowering therapy.
Study participants who reported smoking daily or sometimes within the past year were classified as current smokers. Previous smokers reported smoking cessation at least 1 year before baseline and never smokers reported never having smoked. Current and previous smokers were included in the subgroup of ever smokers.
Data regarding CPD were available for current smokers, and additionally adjusted for as a continuous variable.
Assessment of end‐points
The following end‐points were examined: (i) incident COPD, (ii) incident tobacco‐related cancer, (iii) incident non‐tobacco‐related cancer, (iv) incident cardiovascular disease (CVD), (v) total mortality, (vi) CVD mortality, (vii) cancer mortality and (viii) respiratory disease mortality.
Information about mortality end‐points during follow‐up was retrieved through linkage of the 10‐digit civil registration number with the Swedish National Cause of Death Register (SNCDR). The SNCDR has previously been validated 10, 11. Mortality was classified as attributable to cardiovascular causes for main International Classification of Diseases (ICD) ninth and 10th revision (ICD9 and ICD10, respectively) codes 390–459 (ICD9) or I00–I99 (ICD10) and was attributable to cancer when the code was given as 140–239 (ICD9) or C00–C97 (ICD10) on the cause of death certificate.
CVD was defined as fatal or nonfatal myocardial infarction (MI), stroke or death due to ischaemic heart disease from the Swedish Hospital Discharge Register or SNCDR. MI was defined as codes 410 (ICD9) or I21 (ICD10), death due to ischaemic heart disease was defined as codes 412 and 414 (ICD9) or I22–I23 and I25 (ICD10) and stroke as codes 430, 431, 434 and 436 (ICD9) or I60–I61, I63 and I64 (ICD10). Respiratory disease mortality was defined as codes 460–519 (ICD9) or J00‐99 (ICD10), and incident COPD as codes 490–496 (ICD9) or J40–44 (ICD10).
Tobacco‐related cancers were defined by the International Agency for Research on Cancer (IARC) 12: cancer of the oral cavity [ICD seventh revision (ICD7) code 140–144], oropharynx (ICD7 145, 147 and 148), nasopharynx (ICD7 146), oesophagus (ICD7 150), stomach (ICD7 151), colon (ICD7 153), rectum (ICD7 154), liver (ICD7 155 and 156), pancreas (ICD7 157), nose and sinuses (ICD7 160), larynx (ICD7 161), lung (ICD7 162 and 163), uterine cervix (ICD7 171), ovary (ICD7 175), kidney (ICD7 180) and lower urinary tract (ICD7 181) and myeloid leukaemia (ICD7 205).
Non‐tobacco‐related (‘other’) cancers included cancers of the breast (ICD7 170), prostate (ICD7 177), skin including malignant melanoma (ICD7 190–191) and nervous system (ICD7 193) and malignant lymphoma (ICD7 200–201).
Follow‐up for the end‐points incident COPD and CVD, and total, CVD, respiratory disease and cancer mortality, extended until 31 December 2009. Follow‐up for the end‐points incident tobacco‐related and non‐tobacco‐related cancers extended until 31 December 2010.
Statistical analysis
Continuous variables are reported as means (SD) and dichotomous variables as numbers (%). The SNP rs1051730 was coded additively (CC=0, CT=1, TT=2) in all analyses. Cross‐sectional relationships between genotype and smoking status were evaluated using logistic regression. Cox‐proportional hazard models were used to calculate hazard ratios (HR) and 95% confidence intervals (CIs) for rs1051730 in relation to first event of each end‐point during follow‐up.
All P‐values reported are two‐sided. P‐values were not adjusted for multiple tests. SPSS version 22.0 (IBM Corporation, New York, NY, USA) was used for all calculations.
Results
Baseline characteristics
Complete data were retrieved for 24 794 study participants (Fig. 1). Baseline data of the study participants are presented in Table 1. Smoking data and genotype distribution are presented in Table 2. There was no significant deviation from Hardy–Weinberg equilibrium in any of the groups studied (P > 0.05).
Table 1.
Baseline characteristics of the study population
| Female | Male | All | |
|---|---|---|---|
| Study participants, n (%) | 15 094 (60.9) | 9700 (39.1) | 24 794 (100) |
| Age, years | 57 (±8) | 59 (±7) | 58 (±8) |
| BMI, kg/m2 | 25 (±4) | 26 (±4) | 26 (±4) |
| Hypertensiona, n (%) | 8569 (56.8) | 6655 (68.6) | 15 224 (61.4) |
| Lipid‐lowering therapy, n (%) | 320 (2) | 475 (5) | 795 (3) |
| Previous diabetes, n (%) | 480 (3.2) | 558 (5.8) | 1038 (4.2) |
| CPDb | 13 (±7) | 16 (±9) | 14 (±8) |
Data are presented as mean (±SE) unless otherwise stated.
aAntihypertensive treatment and/or systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg. bData available for 6260 study participants. CPD, cigarettes per day.
Table 2.
Distribution of rs1051730 in the overall study population and stratified by smoking status
| Genotype | CC | CT | TT |
|---|---|---|---|
| All genotyped subjects n = 26 471 (%) | 12 092 (45.7) | 11 442 (43.2) | 2937 (11.1) |
| Analysed subjectsa n = 24 794 (%) | 11 311 (45.6) | 10 732 (43.3) | 2751 (11.1) |
| Current smokers n = 6951 (%) | 3109 (44.7) | 3041 (43.8) | 801 (11.5) |
| Previous smokers n = 8426 (%) | 3916 (46.5) | 3611 (42.9) | 899 (10.7) |
| Never smokers n = 9417 (%) | 4286 (45.5) | 4080 (43.3) | 1051 (11.2) |
aWith complete data for age, sex, rs1051730, body mass index, smoking status, hypertension and lipid‐lowering treatment.
rs1051730 polymorphism and relation to smoking behaviour and risk factors
In additive models adjusted for age and sex, the risk allele (T) showed an association with current smoking (compared to nonsmokers, i.e. never and former smokers combined) [odds ratio (OR) 1.042, 95% CI 1.000–1.087; P = 0.050]. Conversely, the polymorphism was associated with a lower probability of being a former smoker (OR 0.961, 95% CI 0.923–1.000; P = 0.048). No associations were observed between the polymorphism and ever or never smoking status (OR, 95% CI: 0.994, 0.965–1.033; P = 0.752 and 1.002, 0.964–1.042; P = 0.911, respectively). Furthermore, within the group of ever smokers (i.e. never smokers excluded), the OR for current smoking compared with previous smoking per T allele was 1.057 (95% CI 1.007–1.109; P = 0.024).
The risk allele showed a strong linear association with smoking quantity (β = 1.137 CPD per allele; P = 9 × 10−15). There were no significant correlations between rs1051730 and BMI, diabetes, hypertension or LLT (data not shown).
CHRNA polymorphism and smoking‐related complications in current, previous and never smokers
Multivariable‐adjusted HRs and 95% CIs per genotype from categorical models are shown in Tables 3, 4, 5, where carriers of CT and TT are compared with CC carriers (defined as the reference group: HR 1.0). P‐values for trend (from additive models), number of events, total cases and event rates per 1000 person‐years for all end‐points are also shown in Tables 3, 4, 5.
Table 3.
Multivariable‐adjusted hazard ratios per genotype in current smokers
| Genotype | CC | CT | TT | Ptrenda | Ptrendb | Ptrendc | ||
|---|---|---|---|---|---|---|---|---|
| End‐points | Events (total casesa , b) | Events/1000 p‐ys | HR 95% CIa | |||||
| Incident COPD | 480 (6931/6241c) | 4.97 | 1.0 (ref) |
1.281 1.053–1.559* |
1.657 1.266–2.169* |
<0.001* | <0.001* | 0.001* |
| Incident tobacco‐related cancer | 852 (6304/5645c) | 9.33 | 1.0 (ref) |
1.139 0.985–1.317 |
1.385 1.126–1.705* |
0.002* | 0.002* | 0.043* |
| Incident other cancer | 810 (6676/6015c) | 8.48 | 1.0 (ref) |
1.098 0.981–1.271 |
1.058 0.842–1.331 |
0.350 | 0.358 | 0.769 |
| Incident CVD | 1022 (6760/6084c) | 11.21 | 1.0 (ref) |
0.956 0.840–1.089 |
0.958 0.780–1.177 |
0.534 | 0.683 | 0.618 |
| Total mortality | 1508 (6951/6260c) | 15.71 | 1.0 (ref) |
1.036 0.930–1.155 |
1.258 1.076–1.472* |
0.014* | 0.009* | 0.065 |
| CVD mortality | 500 (6951/6260c) | 5.37 | 1.0 (ref) |
0.893 0.738–1.079 |
1.181 0.904–1.544 |
0.674 | 0.574 | 0.666 |
| Respiratory disease mortality | 102 (6946/6256c) | 1.06 | 1.0 (ref) |
1.534 0.995–2.364 |
1.827 1.009–3.311* |
0.022* | 0.017* | 0.061 |
| Cancer mortality | 677 (6946/6255c) | 7.05 | 1.0 (ref) |
1.113 0.947–1.307 |
1.145 0.897–1.463 |
0.161 | 0.160 | 0.496 |
COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; p‐ys, patient‐years; HR, hazard ratio; CI, confidence interval.
aAdjusted for age and sex. bAdjusted for age, sex, body mass index (BMI), hypertension, previous diabetes and lipid‐lowering treatment. cAdjusted for age, sex, BMI, hypertension, previous DM, LLT and cigarettes per day (available cases 6260).
*P < 0.05.
Table 4.
Multivariable‐adjusted hazard ratios per genotype in previous smokers
| Genotype | CC | CT | TT | Ptrenda | Ptrendb | ||
|---|---|---|---|---|---|---|---|
| End‐point | Events (total casesa , b) | Event/1000 py‐s | HR 95% CIa | ||||
| Incident COPD | 211 (8394) | 1.73 | 1.0 (ref) |
1.086 0.814–1.449 |
1.265 0.819–1.955 |
0.293 | 0.292 |
| Incident tobacco‐related cancer | 693 (7919) | 5.80 | 1.0 (ref) |
1.101 0.939–1.290 |
1.241 0.975–1.581 |
0.063 | 0.062 |
| Incident other cancer | 1218 (8071) | 10.36 | 1.0 (ref) |
0.917 0.814–1.033 |
0.934 0.766–1.140 |
0.206 | 0.212 |
| Incident CVD | 1047 (8025) | 9.33 | 1.0 (ref) |
1.007 0.885–1.144 |
0.991 0.805–1.219 |
0.987 | 0.865 |
| Total mortality | 1425 (8426) | 11.90 | 1.0 (ref) |
1.165 1.044–1.301* |
1.214 1.020–1.444* |
0.004* | 0.004* |
| CVD mortality | 488 (8425) | 4.18 | 1.0 (ref) |
1.373 1.137–1.659* |
1.281 0.945–1.736 |
0.006* | 0.005* |
| Respiratory disease mortality | 70 (8390) | 0.59 | 1.0 (ref) |
1.379 0.832–2.285 |
1.543 0.725–3.283 |
0.160 | 0.166 |
| Cancer mortality | 614 (8426) | 5.13 | 1.0 (ref) |
1.044 0.882–1.236 |
1.195 0.923–1.547 |
0.216 | 0.218 |
COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; p‐ys, patient‐years; HR, hazard ratio; CI, confidence interval.
aAdjusted for age and sex. bAdjusted for age, sex, body mass index (BMI), hypertension, previous diabetes and lipid‐lowering treatment.
*P < 0.05.
Table 5.
Multivariable‐adjusted hazard ratios per genotype in never smokers
| Genotype | CC | CT | TT | Ptrenda | Ptrendb | ||
|---|---|---|---|---|---|---|---|
| End‐point | Events (total casesa , b) | Events/1000 p‐ys | HR 95% CIa | ||||
| Incident COPD | 79 (9314) | 0.56 | 1.0 (ref) |
1.269 0.801–2.013 |
0.731 0.306–1.744 |
0.990 | 0.987 |
| Incident tobacco‐related cancer | 559 (8975) | 4.27 | 1.0 (ref) |
1.079 0.906–1.286 |
0.974 0.733–1.293 |
0.789 | 0.790 |
| Incident other cancer | 1233 (8906) | 9.20 | 1.0 (ref) |
0.983 0.874–1.106 |
0.860 0.708–1.044 |
0.200 | 0.213 |
| Incident CVD | 955 (9268) | 7.18 | 1.0 (ref) |
0.935 0.818–1.070 |
0.878 0.708–1.089 |
0.176 | 0.180 |
| Total mortality | 1143 (9416) | 8.34 | 1.0 (ref) |
0.965 0.854–1.091 |
0.909 0.746–1.107 |
0.327 | 0.309 |
| CVD mortality | 350 (9415) | 2.60 | 1.0 (ref) |
0.814 0.650–1.018 |
0.894 0.634–1.261 |
0.184 | 0.152 |
| Respiratory disease mortality | 33 (9310) | 0.24 | 1.0 (ref) |
0.792 0.385–1.632 |
0.572 0.206–2.390 |
0.460 | 0.452 |
| Cancer mortality | 520 (9406) | 3.80 | 1.0 (ref) |
1.033 0.863–1.237 |
0.811 0.595–1.106 |
0.416 | 0.414 |
COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; p‐ys, patient‐years; HR, hazard ratio; CI, confidence interval.
aAdjusted for age and sex. bAdjusted for age, sex, body mass index (BMI), hypertension, previous diabetes and lipid‐lowering treatment.
The HRs and 95% CIs presented in the following section are average values per T allele calculated in additive models. The mean follow‐up times for all end‐points are shown in Table S1.
Current smokers
Incident COPD
In model 1, adjusted for age and sex, each copy of the risk allele was associated with a significant increase in COPD incidence (HR 1.290, 95% CI 1.130–1.463). The increased risk remained significant in model 2, with further adjustments for BMI, diabetes, LLT and hypertension (HR 1.286, 95% CI 1.131–1.463), as well as in model 3 after adjustments for CPD (HR 1.249, 95% CI 1.091–1.429).
Incident tobacco‐related cancer
Significant associations between the risk allele and tobacco‐related cancer were seen in models 1, 2 and 3 (HR, 95% CI: 1.167, 1.058–1.287; 1.116, 1.057–1.286; and 1.114, 1.004–1.237, respectively).
Incident other cancers
No significant associations between the risk allele and other cancers were seen in model 1 (HR 1.050, 95% CI 0. 948–1.162), model 2 (HR 1.050, 95% CI 0. 948–1.162) or model 3 (HR 1.017, 95% CI 0.911–1.314).
Incident CVD
No significant relation between the risk allele and incident CVD was observed in model 1 (HR 0.971, 95% CI 0.886–1.065), model 2 (HR 0.981, 95% CI 0.895–1.076) or model 3 (HR 0.975, 95% CI 0.884–1.076).
Total mortality
In models 1 and 2, the risk allele was significantly associated with total mortality (HR, 95% CI: 1.098, 1.019–1.182 and 1.103, 1.025–1.188, respectively). In model 3, the association was nearly significant (HR 1.077, 95% CI 0.995–1.166).
CVD mortality
No significant association between the risk allele and CVD mortality was seen in any model (HR, 95% CI: 1.028, 0.903–1.171; 1.038, 0.912–1.181 and 1.031, 0.898–1.182, in models 1–3, respectively).
Respiratory disease mortality
In models 1 and 2, the risk allele was significant correlated with respiratory disease mortality (HR, 95% CI: 1.384, 1.048–1.827 and 1.376, 1.043–1.815, respectively). After adjusting for CPD in model 3, the association was no longer significant (HR 1.318, 95% CI 0.987–1.359).
Cancer mortality
The associations between the risk allele and cancer mortality were not significant, with identical results in models 1 and 2 (HR 1.083, 95% CI 0.969–1.210) or in model 3 (HR 1.033, 95% CI 0.917–1.163).
Previous smokers
Incident disease
In contrast to current smokers, no significant relation was observed between the risk allele and incident COPD in model 1 (HR 1.113, 95% CI 0.911–1.359) or model 2 (HR 1.114, 95% CI 0.912–1.360). A nearly significant association was seen when analysing the association with incident tobacco‐related cancer in models 1 and 2 (HR, 95% CI: 1.110, 0.994–1.239 and 1.111, 0.995–1.240) but not with incident other cancers (0.946, 0.869–1.031 and 0.947, 0.869–1.032) or CVD (0.999, 0.912–1.095 and 0.992, 0.905–1.087, respectively).
Mortality end‐points
All‐cause mortality events were analysed in previous smokers and, in line with the results from current smokers, significant associations between the risk allele and mortality end‐points were found in both models 1 and 2 (HR, 95% CI: 1.122, 1.038–1.212 and 1.119, 1.036–1.208). Moreover, a significant association was seen for mortality caused by CVD in these two models, respectively (HR, 95% CI: 1.202, 1.055–1.369 and 1.205, 1.057–1.374.). Respiratory disease mortality was not significantly associated with the allele (HR, 95% CI: 1.277, 0.908–1.797 and 1.273, 0.904–1.792); similarly, there was no significant association with death from cancer (HR, 95% CI: 1.077, 0.957–1.212 and 1.077, 0.957–1.211, respectively).
Never smokers
Incident disease
No associations were observed in models 1 and 2 between the risk allele and incident COPD (HR, 95% CI: 0.998, 0.718–1.386 and 1.003, 0.722–1.393), tobacco‐related cancer (HRs 1.017, 95% CIs 0.899–1.151 in both models), incident other cancer (HR, 95% CI: 0.947 0.871–1.029 and 0.948, 0.872–1.031) or incident CVD (HR, 95% CI: 0.936, 0.851–1.030 and 0.937, 0.852–1.031, respectively).
Mortality end‐points
No significant correlations were observed in models 1 and 2 between the risk allele and all‐cause mortality (HR, 95% CI: 0.957, 0.878–1.044 and 0.956, 95% CI 0.876–1.043), CVD mortality (0.956, 0.876–1.043 and 0.891, 0.760–1.045) or respiratory disease mortality (0.819, 0.483–1.390 and 0.816, 0.481–1.385). There was also no correlation between the SNP and cancer mortality (HR, 95% CI: 0.948, 0.833–1.079 and 0.947, 0.832–1.078).
Additional analyses
We could not confirm the association between the risk allele and bladder cancer 7. Incident bladder cancer was analysed separately with 117 (1.28 event/1000 person‐years) cases in the current smoking group (n = 6304) and 98 (1.20/1000 person‐years) cases with CPD data. No risk increase was seen in models 1, 2 or 3 (HR per allele, 95% CI: 0.808, 0.625–1.044; 0.763, 0.584–0.997; and 0.768, 0.566–1.043, respectively). Lung cancer incidence was also analysed separately, and a significant risk increase was seen in current smokers with 277 events (3.03/1000 person‐years) reported in 6304 participants in models 1 and 2 (HR, 95% CI: 1.289, 1.088–1.527 and 1.283, 1.083–1.519, respectively). After adjusting for CPD in model 3 (n = 5641) with 251 events (3.08/1000 person‐years), the association was no longer significant (HR 1.176, 95% CI 0.982–1.408). Furthermore, as we excluded lung cancer diagnosis from the end‐point tobacco‐related cancers, the associations were no longer significant (data not shown).
The median age of death stratified by genotype was also analysed, focusing on potential differences between subjects with low‐ (CC) and high‐risk genotypes (TT). In current smokers, the median age of death was 1.4 years lower in TT carriers compared to CC carriers, and in previous smokers the median age of death was 0.2 years lower amongst TT carriers compared to CC carriers. By contrast, in the never smoking group, the median age of death was 2.1 years higher in subjects with the TT genotype compared to those with the CC genotype.
Discussion
The novel finding of the present study is that genetic variance in the 15q25 locus predicts an increased risk of death amongst smokers. We also confirmed the associations between this variance and incident COPD 4, 13, 14, tobacco‐related cancers 7, 15, 16, lung cancer 4, 7, 15, 17, 18, 19, 20, 21 and smoking quantity 2, 3, 7, indicating an exciting overlap of genetic influence on ND and smoking‐related diseases. As mentioned above, this region of the nAChRs is characterized by high correlation and the results should be interpreted as an association with the cluster instead of the rs1051730.
The additional risk increase in all‐cause mortality was observed in both current and previous smokers. To illustrate this from another perspective, the median age at death amongst current smokers was 1.4 years lower in subjects with the risk genotype (TT) compared to subjects with the CC genotype.
There was no association between the SNP and mortality amongst never smokers, despite only a slightly lower number of events in this subgroup. Furthermore, with regard to the specific causes of mortality, the SNP was significantly associated with increased respiratory disease mortality amongst current smokers. There was no such significant association amongst former smokers; however as the number of events was more than 30% lower in this subgroup, the lack of association between the SNP and respiratory disease mortality amongst former smokers may be due to a lack of power. Furthermore, the SNP was associated significantly with CVD mortality amongst former smokers, whereas there was no such significant association amongst current smokers, despite a similar number of events. It may be speculated that because current smoking is itself a strong risk factor for CVD mortality, genetic influences on the nicotine receptor may affect the risk of CVD to a lesser extent in this subgroup (i.e. the risk increase in CVD mortality caused by smoking is large regardless of genotype). However, whether the differences in cause‐specific mortality in relation to the SNP amongst the subgroups of current and former smokers could be attributed to different pathophysiological implications of the SNP in different diseases could not be determined with certainty from the current analyses. In general, we acknowledge that the power for interpretation of cause‐specific mortality in the different subgroups is likely to be limited.
It is likely that the increased risk of total mortality as a result of the SNP amongst current and former smokers could be attributed to multiple potential mechanisms. There may be an interaction between the inhaled substances and the receptor that in a later process results in pathophysiological changes causing disease. It may be hypothesized that genetic changes in inflammatory responses could increase the risk of many smoking‐related diseases, thus also suggesting a possible common cause of the modification of the consequences of smoking by genetic influences. Yet, whether this is a direct association, or only a proxy for the increased exposure to tobacco carcinogens, remains controversial as the risk allele is also related to smoking quantity.
The association with lung cancer has been investigated in many studies and, in line with our results, it has been argued that the increased CPD is not the sole explanation. Investigation of nicotinic receptor function and distribution may theoretically increase understanding of this clinically interesting relationship. The nAchRs are present in the central nervous system and in peripheral organs such as the lung, and nicotine addiction is mediated through nAChRs in the mesolimbic dopaminergic system. In bronchial cells, the receptors are involved in remodelling airway epithelium and in the regulation of inflammation and immunity. In theory, nicotine consequently acts as a suppressor of the immune response, and could affect the clearance of transformed cells and participate in the emergence of neoplastic lesions 22.
In the present study, although the mortality risk is higher in both current and former smokers carrying the risk allele, the data highlight the benefits of smoking cessation. In ex‐smokers, the risk allele no longer confers an additional risk of COPD incidence or tobacco‐related cancers.
As briefly mentioned above, whether or not the increased risk due to the polymorphism in 15q25 in smokers could be the sole consequence of increase in CPD, is worth considering. The strong correlation between genetic variants in CHRNA5‐CHRNA3‐CHRNB4, here represented by rs1051730, and CPD suggests that the minor allele (T) could be associated with reduced sensitivity to plasma nicotine levels, leading to increased tobacco consumption 23. Smokers homozygous for the minor allele inhale more often than noncarriers and heterozygous smokers 7. Keskitalo et al. 24 measured CPD and the serum levels of cotinine, a metabolite of nicotine, in 560 daily smokers from a Finnish population. Both cotinine levels and CPD were strongly association with rs1051730, with effect sizes of 0.30 and 0.13, respectively. Hence, the authors concluded that the nicotinic receptor polymorphism influences cotinine/nicotine levels, and appears to be involved in nicotine metabolism and/or regulation. The effect size for cotinine levels is greater than that for CPD, and the authors suggested that the nAChR polymorphism influences nicotine levels more than smoking quantity, or at least that cotinine is a better measure of nicotine intake. Moreover, Timofeeva et al. 20 investigated the effect of the polymorphism on smoking behaviour and lung cancer by measuring circulating cotinine levels in lung cancer patients within the The European Prospective Investigation into Cancer and Nutrition cohort. No association was seen with smoking behaviour but the association between increased cotinine levels and the minor allele of rs16969968 (CHRNA5) was confirmed. The authors concluded that the use of crude measures such as CPD could underestimate the established effects of the SNP on chromosome 15q25 on lung cancer risk mediated by smoking.
In addition to smoking, obesity is a well‐known risk factor for many diseases 25. Nicotine acts on the reward system of the brain and, because eating and smoking are behavioural attributes that at least in part are controlled by the same mechanisms 26, Thorgeirsson et al. 27 investigated whether SNPs associated with BMI also have an impact on smoking behaviour. In several cases, the studied variants that were correlated with elevated BMI, increased the propensity to smoke and/or increased smoking quantity. Although no association between BMI and rs1051730 was seen in our cohort, Thorgeirsson et al. demonstrated a significant correlation with lower BMI in smokers but not in never smokers. The authors suggested that the influence on BMI is probably through the effect of the polymorphism on smoking behaviour and consequently the increase in metabolic rate and appetite suppression attributable to nicotine.
A limitation of our study is that cotinine levels were not measured. In addition, the burden of nicotine after baseline examinations is not known. It could have been of interest to analyse individuals exposed to passive smoking. Moreover, because this genetic variance in the α5‐α3‐β4 nAChr gene cluster seems to increase the need for nicotine, there might be a residual confounding effect of under‐reporting smoking quantity in carriers of the risk allele, compared to noncarriers. A further limitation is that our definition of cause‐specific mortality is based on the main underlying cause of death as listed on the Swedish death certificate. Autopsy rates are relatively low (27%) leading to some uncertainty about the specific cause of death. We did note a slightly higher proportion of cancer deaths than we intuitively expected. Finally, we acknowledge that the power for interpreting cause‐specific mortality in the subgroups is likely to be limited.
Conclusion
In this large, prospective study we have shown that smoking and gene variance in the CHRNA5‐CHRNA3‐CHRNB4 cluster correlate with increased cigarette intake, mortality and incident tobacco‐related diseases. Tobacco use is a leading cause of morbidity and mortality worldwide and our results confirm the notion that genetic factors partly contribute to the development of nicotine addiction and its complications. Understanding the genetics of dependence could lead to optimization of targeted treatments and prevention of disability and death. In the future, further genetic variants and molecular pathways need to be identified, in order to develop a more individualized intervention therapy, and possibly also to help motivate smoking cessation in high‐risk individuals.
Conflicts of interest statement
None declared.
Supporting information
Table S1. Mean follow‐up time (years).
Acknowledgements
Funding was received from the European Research Council (StG‐282255), the Swedish Heart and Lung Foundation, the Swedish Research Council, the Novo Nordisk Foundation, Skåne University Hospital donation funds, the Medical Faculty of Lund University, governmental funding for clinical research within the National Health Services, the Albert Påhlsson Research Foundation, Region Skåne, the King Gustaf V and Queen Victoria Foundation, and the Marianne and Marcus Wallenberg Foundation.
Halldén S, Sjögren M, Hedblad B, Engström G, Hamrefors V, Manjer J, Melander O (Lund University; Skåne University Hospital Malmö, Malmö, Sweden; Skåne University Hospital; and Skåne University Hospital Malmö, Malmö, Sweden). Gene variance in the nicotinic receptor cluster (CHRNA5‐CHRNA3‐CHRNB4) predicts death from cardiopulmonary disease and cancer in smokers. J Intern Med 2016; 279: 388–398.
References
- 1. Secretan B, Straif K, Baan R, et al A review of human carcinogens–Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol 2009; 10: 1033–4. [DOI] [PubMed] [Google Scholar]
- 2. Thorgeirsson TE, Gudbjartsson DF, Surakka I, et al Sequence variants at CHRNB3‐CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet 2010; 42: 448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Consortium TaG . Genome‐wide meta‐analyses identify multiple loci associated with smoking behavior. Nat Genet 2010; 42: 441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saccone NL, Culverhouse RC, Schwantes‐An TH, et al Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta‐analysis and comparison with lung cancer and COPD. PLoS Genet 2010; 6: e1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bierut LJ, Madden PA, Breslau N, et al Novel genes identified in a high‐density genome wide association study for nicotine dependence. Hum Mol Genet 2007; 16: 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saccone NL, Wang JC, Breslau N, et al The CHRNA5‐CHRNA3‐CHRNB4 nicotinic receptor subunit gene cluster affects risk for nicotine dependence in African‐Americans and in European‐Americans. Cancer Res 2009; 69: 6848–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaur‐Knudsen D, Bojesen SE, Tybjaerg‐Hansen A, Nordestgaard BG. Nicotinic acetylcholine receptor polymorphism, smoking behavior, and tobacco‐related cancer and lung and cardiovascular diseases: a cohort study. J Clin Oncol 2011; 29: 2875–82. [DOI] [PubMed] [Google Scholar]
- 8. Bierut LJ, Stitzel JA, Wang JC, et al Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry 2008; 165: 1163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berglund G, Elmstahl S, Janzon L, Larsson SA. The Malmo diet and cancer study. Design and feasibility. J Intern Med 1993; 233: 45–51. [DOI] [PubMed] [Google Scholar]
- 10. Inghammar M, Engstrom G, Lofdahl CG, Egesten A. Validation of a COPD diagnosis from the Swedish Inpatient Registry. Scand J Public Health 2012; 40: 773–6. [DOI] [PubMed] [Google Scholar]
- 11. Hammar N, Alfredsson L, Rosen M, et al A national record linkage to study acute myocardial infarction incidence and case fatality in Sweden. Int J Epidemiol 2001; 30(Suppl 1): S30–4. [DOI] [PubMed] [Google Scholar]
- 12. Agudo A, Bonet C, Travier N, et al Impact of cigarette smoking on cancer risk in the European prospective investigation into cancer and nutrition study. J Clin Oncol 2012; 30: 4550–7. [DOI] [PubMed] [Google Scholar]
- 13. Kaur‐Knudsen D, Bojesen SE, Nordestgaard BG. CHRNA3 and CYP3A5*3 genotype, lung function and chronic obstructive pulmonary disease in the general population. Pharmacogenet Genomics 2014; 24: 220–9. [DOI] [PubMed] [Google Scholar]
- 14. Pillai SG, Ge D, Zhu G, et al A genome‐wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet 2009; 5: e1000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lips EH, Gaborieau V, McKay JD, et al Association between a 15q25 gene variant, smoking quantity and tobacco‐related cancers among 17 000 individuals. Int J Epidemiol 2010; 39: 563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Russo P, Cardinale A, Margaritora S, Cesario A. Nicotinic receptor and tobacco‐related cancer. Life Sci 2012; 91: 1087–92. [DOI] [PubMed] [Google Scholar]
- 17. Amos CI, Wu X, Broderick P, et al Genome‐wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet 2008; 40: 616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hung RJ, McKay JD, Gaborieau V, et al A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature 2008; 452: 633–7. [DOI] [PubMed] [Google Scholar]
- 19. Rafnar T, Sulem P, Besenbacher S, et al Genome‐wide significant association between a sequence variant at 15q15.2 and lung cancer risk. Cancer Res 2011; 71: 1356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Timofeeva MN, McKay JD, Smith GD, et al Genetic polymorphisms in 15q25 and 19q13 loci, cotinine levels, and risk of lung cancer in EPIC. Cancer Epidemiol Biomarkers Prev 2011; 20: 2250–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spitz MR, Amos CI, Dong Q, Lin J, Wu X. The CHRNA5‐A3 region on chromosome 15q24‐25.1 is a risk factor both for nicotine dependence and for lung cancer. J Natl Cancer Inst 2008; 100: 1552–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tournier JM, Birembaut P. Nicotinic acetylcholine receptors and predisposition to lung cancer. Curr Opin Oncol 2011; 23: 83–7. [DOI] [PubMed] [Google Scholar]
- 23. Wang JC, Cruchaga C, Saccone NL, et al Risk for nicotine dependence and lung cancer is conferred by mRNA expression levels and amino acid change in CHRNA5. Hum Mol Genet 2009; 18: 3125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Keskitalo K, Broms U, Heliovaara M, et al Association of serum cotinine level with a cluster of three nicotinic acetylcholine receptor genes (CHRNA3/CHRNA5/CHRNB4) on chromosome 15. Hum Mol Genet 2009; 18: 4007–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haslam DW, James WP. Obesity. Lancet 2005; 366: 1197–209. [DOI] [PubMed] [Google Scholar]
- 26. Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci 2008; 363: 3191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thorgeirsson TE, Gudbjartsson DF, Sulem P, et al A common biological basis of obesity and nicotine addiction. Transl Psychiatry 2013; 3: e308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Mean follow‐up time (years).


