In children and adolescents undergoing evaluation for Lyme disease, the C6 enzyme immunoassay had similar sensitivity but lower specificity than standard 2-tiered testing. This assay could be used to guide initial management decisions, but supplemental immunoblot should still be performed.
Keywords: Lyme disease, Lyme disease diagnostics, C6, pediatrics
Abstract
Background. The commercially-available C6 Lyme enzyme immunoassay (EIA) has been approved to replace the standard whole-cell sonicate EIA as a first-tier test for the diagnosis of Lyme disease and has been suggested as a stand-alone diagnostic. However, the C6 EIA has not been extensively studied in pediatric patients undergoing evaluation for Lyme disease.
Methods. We collected discarded serum samples from children and adolescents (aged ≤21 years) undergoing conventional 2-tiered testing for Lyme disease at a single hospital-based clinical laboratory located in an area endemic for Lyme disease. We performed a C6 EIA on all collected specimens, followed by a supplemental immunoblot if the C6 EIA result was positive but the whole-cell sonicate EIA result was negative. We defined a case of Lyme disease as either a clinician-diagnosed erythema migrans lesion or a positive standard 2-tiered serologic result in a patient with symptoms compatible with Lyme disease. We then compared the performance of the C6 EIA alone and as a first-tier test followed by immunoblot, with that of standard 2-tiered serology for the diagnosis of Lyme disease.
Results. Of the 944 specimens collected, 114 (12%) were from patients with Lyme disease. The C6 EIA alone had sensitivity similar to that of standard 2-tiered testing (79.8% vs 81.6% for standard 2-tiered testing; P = .71) with slightly lower specificity (94.2% vs 98.8% 2; P < .002). Addition of a supplemental immunoblot improved the specificity of the C6 EIA to 98.6%.
Conclusions. For children and adolescents undergoing evaluation for Lyme disease, the C6 EIA could guide initial clinical decision making, although a supplemental immunoblot should still be performed.
Lyme disease is a tick-borne spirochetal infection that affects hundreds of thousands of persons each year in the United States alone [1], with a peak in incidence in the school-age and adolescent age groups [2]. Current serologic diagnosis of Lyme disease consists of a standardized 2-tiered testing protocol: a whole-cell sonicate (WCS) enzyme immunoassay (EIA) followed by supplemental immunoglobulin G (IgG) and immunoglobulin M (IgM) immunoblots after a positive or equivocal EIA [3]. Many clinical laboratories send patient specimens to commercial reference laboratories for immunoblots, often resulting in a several-day delay for results to be available [4]. Thus, standard 2-tiered Lyme disease serology has limited utility in the acute-care setting where real-time clinical management decisions must be made for pediatric patients with suspected Lyme disease.
The C6 Lyme EIA measures antibody reactivity to a synthetic peptide corresponding to the sixth invariable region of VlsE, a highly conserved surface protein of the causative Borrelia burgdorferi bacterium [5]. The currently available commercial C6 EIA can be run in house in as little as 1 hour [6] and is cleared by the Food and Drug Administration as a first-tier test to be followed by a supplemental immunoblot [4, 7–9]. In adults, the C6 EIA alone has demonstrated superior sensitivity and comparable specificity compared with standard 2-tiered serology [4, 7–9]. However, its performance has not been rigorously studied in pediatric patients undergoing evaluation for suspected Lyme disease.
To this end, we conducted a cross-sectional study of children and adolescents undergoing evaluation for Lyme disease. Our objectives were to evaluate the performance of the C6 EIA both as a stand-alone test and as part of several 2-tiered testing strategies for the diagnosis of Lyme disease in pediatric patients.
METHODS
Study Design
We performed a cross-sectional study of children and adolescents (aged ≤21 years) undergoing serologic evaluation for Lyme disease between June 2014 and December 2015. Our study was limited to tests ordered at a single hospital-based laboratory located in an area endemic for Lyme disease. This laboratory receives specimens from a variety of clinical settings, including inpatient units, the emergency department, and primary care and subspecialty clinics. The institutional review board approved the study protocol with a waiver of informed consent.
Study Patients
We collected a convenience sample of available discarded serum samples from patients in whom standard 2-tiered Lyme disease testing was ordered by their treating clinician. We required a minimum of 25 µL of available serum. We included multiple tests from the same patient if obtained ≥30 days apart. To serve as an asymptomatic control group, we also included a convenience sample of discarded serum specimens from patients undergoing allergy testing with clinical radioallergosorbent testing (RAST) who did not have symptoms compatible with Lyme disease.
Data Collection
We abstracted the following data points from the hospital electronic medical record: patient demographics, duration of symptoms (≤30 vs >30 days) [3], results of standard 2-tiered Lyme disease serology, and clinical indication for testing. When clinical history was not available in the medical record, we contacted the ordering provider to determine the duration of symptoms as well as the clinical indication for testing. If we were unable to determine the clinical indication for testing, we excluded the specimen from our analysis.
Routine Clinical Testing
Our institution sends clinical specimens to a single commercial laboratory (ARUP National Laboratories). According to clinical protocol, a standard WCS Lyme EIA (MarDx; Trinity Biotech) had been previously performed for each clinical specimen. All specimens with Lyme EIA index values ≥1.2 (positive) or 1.0–1.19 (equivocal) were reflexively evaluated using both IgG and IgM Western immunoblots (MarDx; Trinity Biotech). Immunoblots were scored as positive or negative by the diagnostic laboratory, according to Centers for Disease Control and Prevention recommendations for interpretation [3].
Research Testing
After collection, we labeled study specimens with study numbers and stored them at −80°C. We performed the commercially available C6 Lyme EIA test (Immunetics) in the Branda Laboratory (Massachusetts General Hospital). The C6 assay provides a quantitative result, which we interpreted according to a priori cut points provided by the manufacturer: C6 EIA index value ≥1.10 (positive), 0.91–1.09 (equivocal), or ≤0.90 (negative). We classified both positive and equivocal tests as positive in our analysis. For specimens with positive or equivocal C6 EIA but negative WCS EIA, we performed Lyme disease IgG and IgM immunoblots (MarDx, Trinity Biotech) at the same commercial laboratory (ARUP National Laboratories), using standardized interpretation criteria [3]. For a single serum specimen with insufficient remaining volume to perform the ARUP immunoblot, we performed a ViraStripe Lyme immunoblot (Viramed Biotech) in the Steere Laboratory (Massachusetts General Hospital).
Outcome Measure
We defined a case of Lyme disease as a clinician-diagnosed erythema migrans (EM) lesion or a positive 2-tiered serologic result in the presence of a Lyme disease-associated clinical syndrome [3, 10]. A positive 2-tiered serologic result was defined as a positive or equivocal WCS EIA result followed by a positive IgG or IgM immunoblot. Patients with a positive IgM immunoblot alone were considered to be serologically positive only if the duration of symptoms was ≤30 days [3, 11–13]. The following were considered clinical syndromes compatible with Lyme disease by stage: early (single EM lesion), early disseminated (multiple EM lesions, cranial neuritis, meningitis, carditis), and late (arthritis).
Control Groups
We defined 3 control groups: symptomatic, nonspecific symptoms, and asymptomatic. “Symptomatic” control subjects had clinical symptoms compatible with Lyme disease but did not meet our Lyme disease case definition (ie, no EM lesion and negative serologic results). “Nonspecific symptoms” control subjects had Lyme disease testing ordered by their treating clinician in the absence of a Lyme disease–associated clinical syndrome, as defined above, and thus did not meet our Lyme disease case definition, regardless of serologic test results. Typically, these subjects had nonspecific constitutional symptoms, such as fever or fatigue. “Asymptomatic” control subjects had RAST ordered by their treating clinician to assess for environmental or food allergies.
Sample Size
We powered our study to compare the sensitivity of the C6 EIA alone with that of conventional 2-tiered testing. Based on previous local experience, we assumed that approximately 20% of collected specimens would be from patients with confirmed Lyme disease, of whom approximately 25% would have EM. To demonstrate a 30% difference in sensitivity between C6 EIA and standard 2-tiered testing for the diagnosis of early Lyme disease [9] with an α of .05 and power of 80%, we estimated that 840 serum samples would be needed from patients undergoing evaluation for Lyme disease.
Statistical Analysis
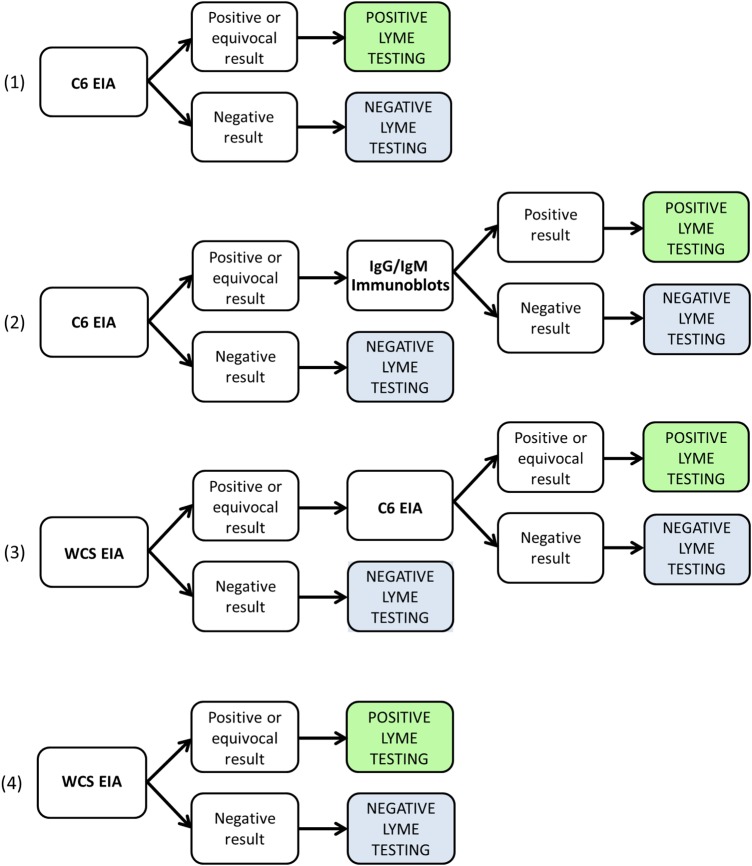
First, we compared the clinical characteristics and indications for testing between patients with captured and those with missed eligible specimens. We compared medians with the Mann–Whitney test and proportions with the χ2 test. Second, we evaluated the performance of the following testing strategies in the specimens collected from patients undergoing serologic evaluation for Lyme disease, as well as the specimens from asymptomatic control patients: (1) C6 EIA alone, (2) C6 EIA followed by supplemental IgG and IgM immunoblots, (3) WCS EIA followed by supplemental C6 EIA, and (4) WCS EIA alone controls (Figure 1). Finally, we compared the performance of these 4 testing strategies with that of standard 2-tiered Lyme serologic testing using the χ2 test. We used the Statistical Program for the Social Sciences, version 23.0 (SPSS).
Figure 1.
Alternate testing strategies: (1) C6 enzyme immunoassay (EIA) alone, (2) 2-tiered algorithm with C6 EIA as first-tier test followed by supplemental immunoblots, (3) 2-tiered algorithm with whole-cell sonicate (WCS) EIA followed by supplemental C6 EIA, and (4) WCS EIA alone. Abbreviations: IgG, immunoglobulin G; IgM, immunoglobulin M.
RESULTS
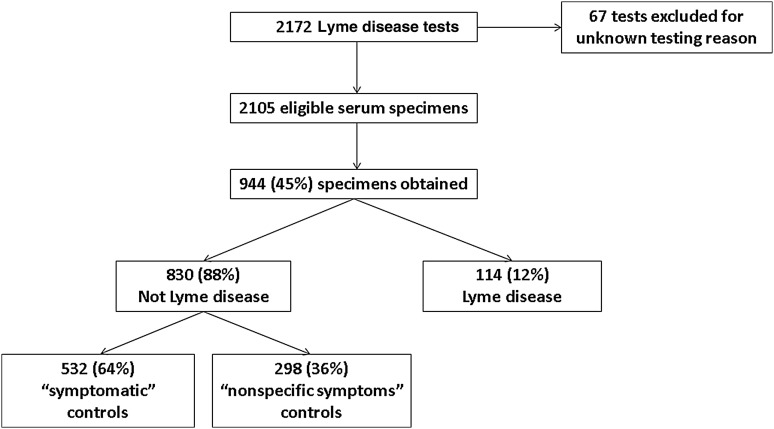
During the study period, 2172 Lyme disease tests were ordered from patients ≤21 years of age (Figure 2). We excluded 67 tests without available clinical information. Of the remaining 2105 tests, we were able to collect 944 discarded serum specimens (45% of eligible tests ordered).
Figure 2.
Study specimens.
Of the 944 study specimens, 114 (12%) were from patients with Lyme disease, 532 (56%) were from patients with symptoms compatible with Lyme disease who tested negative for Lyme disease (“symptomatic” control group), and 298 (32%) were from patients with nonspecific symptoms (“nonspecific symptoms” control group). We also included 101 specimens from unique patients undergoing RAST (“asymptomatic” control group).
We compared children and adolescents with available study specimens with those in whom no specimens were collected (Table 1). Patients with captured specimens were slightly younger and more likely to be female than those whose serum was not collected. The distribution of clinical presentations as well as the proportion of patients with Lyme disease did not differ between groups.
Table 1.
Characteristics of Pediatric Patients With Versus Without Discarded Serum Samples Obtained
| Characteristic | Patients, No. (%)a |
P Value | |
|---|---|---|---|
| Samples Obtained (n = 944) | Samples Not Obtained (n = 1161) | ||
| Age, median (IQR), y | 10.9 (6.4–15.2) | 11.6 (7.0–15.5) | .04 |
| Male sex | 421 (45) | 571 (49) | .04 |
| Stage | .21 | ||
| Early | 16 (2) | 18 (2) | |
| Early disseminated | 223 (23) | 283 (24) | |
| Late | 407 (43) | 541 (46) | |
| Nonspecific symptoms | 298 (32) | 319 (27) | |
| Lyme disease | 114 (12) | 152 (13) | .51 |
Abbreviation: IQR, interquartile range.
a Data represent No. (%) of patients, unless otherwise specified.
Of the 114 specimens obtained from patients with Lyme disease, 16 (14%) were from patients with early Lyme disease, 41 (36%) from those with early disseminated disease, and 57 (50%) from those with late disease. The Lyme disease diagnosis was made as follows: 21 (18% of Lyme disease cases) based on EM alone, 84 (74%) based on positive results of standard 2-tiered serology with a Lyme disease–associated clinical syndrome, and 9 (8%) based on both EM and positive results of 2-tiered serology.
We report the performance of the 3 C6 EIA testing strategies as well as standard 2-tiered serologic testing and WCS EIA alone (Table 2). All 5 testing strategies had similar overall sensitivity. C6 EIA followed by immunoblot (strategy 2) had overall specificity similar to that of standard 2-tiered serologic testing. However, C6 EIA alone (strategy 1) and WCS EIA followed by C6 EIA (strategy 3) had slightly lower specificity. WCS EIA alone (strategy 4) had substantially lower specificity than any of the other testing strategies. Although negative predictive values did not differ between testing strategies, positive predictive values were substantially lower in the strategies without supplemental immunoblots.
Table 2.
Test Characteristics of Standard 2-Tiered Serology Versus Alternate Testing Strategies in Patients With Lyme Disease Testing Ordered as Part of Clinical Care
| Testing Strategy | Proportion of Samplesa (%; 95% CI) |
||||
|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
| Standard 2-tiered testing | 93/114 (81.6; 73.0–88.0) | 820/830 (98.8; 97.7–99.4) | 93/103 (90.3; 82.5–95.0) | 820/841 (97.5; 96.1–98.4) | 913/944 (96.7; 95.4–97.7) |
| C6 EIA alone | 91/114 (79.8; 71.1–86.5) | 782/830 (94.2; 92.3–95.7)b | 91/139 (65.5; 56.9–73.2)b | 782/805 (97.1; 95.7–98.1) | 873/944 (92.5; 90.6–94.0)b |
| C6 EIA with immunoblot | 89/114 (78.1; 69.2–85.1) | 818/830 (98.6; 97.4–99.2) | 89/101 (88.1; 79.8–93.4) | 819/843 (97.0; 95.6–98.0) | 907/944 (96.1; 94.8–97.2) |
| WCS EIA with C6 EIA | 91/114 (79.8; 71.1–86.5) | 801/830 (96.5; 95.0–97.6)b | 91/120 (75.8; 67.0–83.0)b | 801/824 (97.2; 95.8–98.2) | 892/944 (94.5; 92.9–95.8)b |
| WCS EIA alone | 100/114 (87.7; 79.9–92.9) | 670/830 (80.7; 77.8–83.3)b | 100/260 (38.5; 32.6–44.7)b | 670/684 (98.0; 96.5–98.8) | 770/944 (81.2; 79.0–83.9)b |
Abbreviations: CI, confidence interval; EIA, enzyme immunoassay; NPV, negative predictive value; PPV, positive predictive value; WCS, whole-cell sonicate.
a Proportions represent the following: sensitivity, true-positives/total with disease; specificity, true-negatives/total without disease; PPV, true-positives/total positive results; and NPV, true-negatives/total negative results.
b P < .05 for comparison with standard 2-tiered testing.
Finally, we report the specificity of the testing strategies in the control subjects. In all 3 control groups, C6 EIA alone and WCS EIA alone were significantly less specific than standard 2-tiered testing (Table 3). Addition of a supplemental immunoblot increased the specificity of the C6-based strategy to a level similar to that of standard 2-tiered testing.
Table 3.
Specificity of Each Testing Strategy Among 3 Control Groups: Symptomatic, Nonspecific Symptoms, and Asymptomatic
| Testing Strategy | Specificity by Control Group, Proportion of Samplesa (%; 95% CI) |
||
|---|---|---|---|
| Symptomatic Controls | Controls With Nonspecific Symptoms | Asymptomatic Controls | |
| Standard 2-tiered testing | 532/532 (100; 99.1–100) | 288/298 (96.6; 93.7–98.3) | 99/101 (98.0; 92.3–99.7) |
| C6 EIA alone | 505/532 (94.9; 92.6–96.6)b | 277/298 (93.0; 89.3–95.5)b | 88/101 (87.1; 78.6–92.7)b |
| C6 EIA with immunoblot | 529/532 (99.4; 98.2–99.9) | 289/298 (97.0; 94.1–98.5) | 98/101 (97.0; 90.9–99.2) |
| WCS EIA with C6 EIA | 519/532 (97.6; 95.7–98.6)b | 282/298 (94.6; 91.3–96.8) | 98/101 (97.0; 90.9–99.2) |
| WCS EIA alone | 442/532 (83.1; 79.6–86.1)b | 228/298 (76.5; 71.2–81.1)b | 88/101 (87.1; 78.6–92.7)b |
Abbreviations: CI, confidence interval; EIA, enzyme immunoassay; WCS, whole-cell sonicate.
a Proportions for specificity represent true-negatives/total without disease.
b P < .05 for comparison with standard 2-tiered testing.
DISCUSSION
We performed a cross-sectional study using discarded serum samples from children and adolescents tested for Lyme disease at a single hospital laboratory in an endemic area. To our knowledge, our study is the first to evaluate the C6 EIA in pediatric patients. We found that the C6 EIA followed by supplemental immunoblot, as the assay is currently approved, performed similarly to standard 2-tiered Lyme disease serologic testing. The C6 EIA alone had similar sensitivity compared with standard 2-tiered testing. However, given the lower specificity of the C6 EIA alone in all control groups, a supplemental immunoblot should still be performed for children or adolescents with a positive or equivocal C6 EIA result.
Several previous studies have evaluated the performance of the C6 EIA in adults [4, 8, 9, 14, 15]. In the largest study to date, the C6 EIA was evaluated as a stand-alone test in serum samples from 528 well-characterized patients with Lyme disease as well as from healthy blood donors [9]. The C6 EIA had superior sensitivity in patients with early disease (a single EM lesion) when compared with standard 2-tiered testing (58% vs 27%, respectively; P < .001), whereas sensitivity was similar for the 2 approaches in patients with later stages of disease [9]. Overall sensitivity in our study did not differ significantly between any of the strategies evaluated. However, our study was inadequately powered to show a sensitivity benefit of the C6 EIA in early Lyme disease, because only 14% of the serum samples from patients with Lyme disease came from those with solitary EM lesions. In keeping with findings from previous studies [4, 7, 9, 16], we found that the C6 EIA alone was less specific than standard 2-tiered testing. The specificity of a 2-tiered strategy with C6 EIA followed by supplemental immunoblot did not differ from that of standard WCS EIA based 2-tiered testing.
Several studies have evaluated a 2-EIA strategy for Lyme disease diagnosis, consisting of WCS EIA as the first-tier test, with positive and equivocal results followed by supplemental C6 EIA [4, 8, 15]. This 2-EIA testing strategy offered the sensitivity benefits of EIA-based testing but preserved the specificity of standard 2-tiered testing. Importantly, the 2-EIA model was found to be more cost-effective than immunoblot-based testing, owing to both its lower cost and its increased ability to be performed in house [8]. In our pediatric study, the sensitivity of the 2-EIA model was similar to that of other testing strategies. However, its specificity was slightly lower in the “symptomatic” control group. Because EIA-based approaches are more sensitive than immunoblots in early disease [4, 8, 15], this finding may reflect false-negative conventional 2-tiered test results in children with true early or early-disseminated Lyme disease (and therefore miscategorization of these patients as negative control subjects) rather than false-positive 2-EIA test results [16].
Children and adolescents presenting with potential Lyme disease often pose diagnostic challenges. Clinical prediction rules can assist decision making in children with facial palsy [17], meningitis [18–20], or arthritis [21], but there remains considerable overlap in clinical presentation between Lyme disease and its mimics. Thus, a rapid and accurate diagnostic test for Lyme disease would be an asset in acute-care settings. Given the high negative predictive value in our study population, the C6 EIA could be used to rule out Lyme disease in the appropriate clinical scenario. For example, C6 EIA should be performed in a patient with aseptic meningitis. If the patient appears well, is at low risk for Lyme meningitis by the “Rule of Sevens” [19], and has a negative C6 EIA, the treating clinician could consider discharge with supportive care. As always, if there is persistent clinical concern for Lyme disease, repeated serologic testing could be considered. In addition, although the positive predictive value of the C6 EIA alone was substantially lower than that of testing strategies that included supplemental immunoblot, a positive C6 EIA result could also be used to guide initial therapy. For example, a well-appearing child with monoarticular arthritis and a positive C6 EIA result could be observed without arthroscopic irrigation or parenteral antibiotic therapy while awaiting supplemental immunoblots.
Although the C6 EIA may be useful in making initial management decisions, we do not recommend its use as the sole definitive diagnostic test for Lyme disease. A recent study of 7 large commercial laboratories in the United States found that 3.4 million serologic tests for Lyme disease were sent annually from 2.4 million patients [1]. Thus, even a small decrement in specificity would lead to an unacceptable increase in the number of false-positive results. For the patients tested by the laboratories included in the above-mentioned study, the observed 4.6% decrease in specificity between the C6 EIA and standard 2-tiered testing would result in >100 000 additional false-positive Lyme disease test results annually. Overdiagnosis of Lyme disease may result in unnecessary antibiotic administration as well as other adverse events [22–24]. For this reason, we still recommend that a supplemental immunoblot be performed for all children and adolescents with a positive C6 EIA result.
Our study has several limitations. First, not all patients tested for Lyme disease had discarded serum samples available. However, the differences between the captured and missed eligible patients were clinically insignificant. Secondly, as mentioned previously, we obtained a lower than expected number of specimens from patients with solitary EM lesions, in whom the C6 EIA has shown the greatest increase in sensitivity over 2-tiered testing. Third, although exposure history is required for the diagnosis of Lyme disease, we were unable to ascertain patients' potential exposure from available medical records. However, because the study laboratory was located in an area endemic for Lyme disease, we assumed that most patients had potential exposure. Fourth, although interpretation of Lyme disease immunoblots can vary between diagnostic laboratories [25], all but 1 were performed in the same laboratory. Next, our asymptomatic control patients were being evaluated clinically for food or environmental allergies, and we cannot exclude the possibility that the frequency of false-positive Lyme disease serologic results might differ in these patients.
Most importantly, our study was limited by our comparison of testing strategies to a flawed diagnostic reference standard. First, because the appearance of an EM lesion is not always classic and overlaps with many other non–Lyme disease diagnoses [26], some patients’ lesions may have been misdiagnosed as early Lyme disease. Second, 2-tiered serologic testing can be falsely negative in early and early-disseminated disease [16]. Approximately 20% of study specimens categorized as having a falsely positive C6 EIA were from patients with suspected early-disseminated Lyme disease in whom results of conventional 2-tiered testing were negative. Although we were unable to obtain convalescent serum samples in these patients, some of them may have had early disseminated Lyme disease and thus a true-positive C6 EIA result. Third, Lyme disease serologic findings may remain positive after previous exposure to Borrelia spp., even after adequate treatment, and thus some patients categorized as having Lyme disease on this basis may have had past infection [12]. However, even considering the above limitations, our Lyme disease case definition represents the best available diagnostic standard and replicates current clinical practice. Further investigations should focus on novel approaches to improve the diagnosis of Lyme disease.
In summary, the C6 Lyme EIA alone has equivalent sensitivity with a modest decrease in specificity when compared with standard 2-tiered testing for the diagnosis of Lyme disease in children and adolescents. A testing strategy based solely on the C6 EIA would lead to overtreatment if used on a national level but could be useful to guide initial management of a child or adolescent presenting with signs or symptoms consistent with potential Lyme disease. Although supplemental immunoblots are still required to confirm a Lyme disease diagnosis, our study supports using the C6 EIA as a first-line diagnostic test in pediatric patients undergoing evaluation for Lyme disease. In the appropriate clinical scenario, the C6 EIA could limit unnecessary procedures and allow for prompt initiation of appropriate therapy.
Notes
Acknowledgments. Immunetics provided reagents and software for the C6 enzyme immunoassay.
Disclaimer. Immunetics had no input into the study design, data analysis, or manuscript preparation.
Financial support. This work was supported by Boston Children's Hospital (House Officers research grant and Michael Shannon research grant to S. C. L. and Research Faculty Council pilot grant to L. E. N.) and Harvard Medical School (Harvard Catalyst Early Clinical Data grant to L. E. N.).
Potential conflicts of interest. J. A. B. reports receiving grant support for other research investigations from Immunetics, bioMérieux, Alere, DiaSorin, the Bay Area Lyme Foundation, and the National Institutes of Health; he is also a paid consultant for T2 Biosystems and has been a paid consultant for AdvanDx. A. J. M. serves as a scientific advisory board member to BacterioScan. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hinckley AF, Connally NP, Meek JI et al. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis 2014; 59:676–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson CA, Saha S, Kugeler KJ et al. Incidence of clinician-diagnosed Lyme disease, United States, 2005–2010. Emerg Infect Dis 2015; 21:1625–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 1995; 44:590–1. [PubMed] [Google Scholar]
- 4.Branda JA, Linskey K, Kim YA, Steere AC, Ferraro MJ. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis 2011; 53:541–7. [DOI] [PubMed] [Google Scholar]
- 5.Liang FT, Steere AC, Marques AR, Johnson BJ, Miller JN, Philipp MT. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi vlsE. J Clin Microbiol 1999; 37:3990–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Immunetics Web site. C6 (B. burgdorferi) Lyme ELISA Available at: http://www.immunetics.com/lyme.html Accessed 7 April 2016.
- 7.Branda JA, Aguero-Rosenfeld ME, Ferraro MJ, Johnson BJ, Wormser GP, Steere AC. 2-Tiered antibody testing for early and late Lyme disease using only an immunoglobulin G blot with the addition of a VlsE band as the second-tier test. Clin Infect Dis 2010; 50:20–6. [DOI] [PubMed] [Google Scholar]
- 8.Wormser GP, Levin A, Soman S, Adenikinju O, Longo MV, Branda JA. Comparative cost-effectiveness of two-tiered testing strategies for serodiagnosis of Lyme disease with noncutaneous manifestations. J Clin Microbiol 2013; 51:4045–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wormser GP, Schriefer M, Aguero-Rosenfeld ME et al. Single-tier testing with the C6 peptide ELISA kit compared with two-tier testing for Lyme disease. Diagn Microbiol Infect Dis 2013; 75:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Lyme disease (Borrelia burgdorferi): 2011 case definition. Available at: http://www.cdc.gov/nndss/conditions/lyme-disease/case-definition/2011/. Accessed 5 July 2016.
- 11.Lantos PM, Lipsett SC, Nigrovic LE. False positive Lyme disease IgM immunoblots in children. J Pediatr 2016; 174:267–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalish RA, McHugh G, Granquist J, Shea B, Ruthazer R, Steere AC. Persistence of immunoglobulin M or immunoglobulin G antibody responses to Borrelia burgdorferi 10–20 years after active Lyme disease. Clin Infect Dis 2001; 33:780–5. [DOI] [PubMed] [Google Scholar]
- 13.Seriburi V, Ndukwe N, Chang Z, Cox ME, Wormser GP. High frequency of false positive IgM immunoblots for Borrelia burgdorferi in clinical practice. Clin Microbiol Infect 2012; 18:1236–40. [DOI] [PubMed] [Google Scholar]
- 14.Wormser GP, Tang AT, Schimmoeller NR et al. Utility of serodiagnostics designed for use in the United States for detection of Lyme borreliosis acquired in Europe and vice versa. Med Microbiol Immunol 2014; 203:65–71. [DOI] [PubMed] [Google Scholar]
- 15.Branda JA, Strle F, Strle K, Sikand N, Ferraro MJ, Steere AC. Performance of United States serologic assays in the diagnosis of Lyme borreliosis acquired in Europe. Clin Infect Dis 2013; 57:333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steere AC, McHugh G, Damle N, Sikand VK. Prospective study of serologic tests for Lyme disease. Clin Infect Dis 2008; 47:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nigrovic LE, Thompson AD, Fine AM, Kimia A. Clinical predictors of Lyme disease among children with a peripheral facial palsy at an emergency department in a Lyme disease-endemic area. Pediatrics 2008; 122:e1080–5. [DOI] [PubMed] [Google Scholar]
- 18.Avery RA, Frank G, Glutting JJ, Eppes SC. Prediction of Lyme meningitis in children from a Lyme disease-endemic region: a logistic-regression model using history, physical, and laboratory findings. Pediatrics 2006; 117:e1–7. [DOI] [PubMed] [Google Scholar]
- 19.Cohn KA, Thompson AD, Shah SS et al. Validation of a clinical prediction rule to distinguish Lyme meningitis from aseptic meningitis. Pediatrics 2012; 129:e46–53. [DOI] [PubMed] [Google Scholar]
- 20.Garro AC, Rutman M, Simonsen K, Jaeger JL, Chapin K, Lockhart G. Prospective validation of a clinical prediction model for Lyme meningitis in children. Pediatrics 2009; 123:e829–34. [DOI] [PubMed] [Google Scholar]
- 21.Deanehan JK, Kimia AA, Tan Tanny SP et al. Distinguishing Lyme from septic knee monoarthritis in Lyme disease-endemic areas. Pediatrics 2013; 131:e695–701. [DOI] [PubMed] [Google Scholar]
- 22.Ettestad PJ, Campbell GL, Welbel SF et al. Biliary complications in the treatment of unsubstantiated Lyme disease. J Infect Dis 1995; 171:356–61. [DOI] [PubMed] [Google Scholar]
- 23.Ramsey AH, Belongia EA, Chyou PH, Davis JP. Appropriateness of Lyme disease serologic testing. Ann Fam Med 2004; 2:341–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson AD, Cohn KA, Shah SS et al. Treatment complications in children with Lyme meningitis. Pediatr Infect Dis J 2012; 31:1032–5. [DOI] [PubMed] [Google Scholar]
- 25.Bakken LL, Case KL, Callister SM, Bourdeau NJ, Schell RF. Performance of 45 laboratories participating in a proficiency testing program for Lyme disease serology. JAMA 1992; 268:891–5. [PubMed] [Google Scholar]
- 26.Tibbles CD, Edlow JA. Does this patient have erythema migrans? JAMA 2007; 297:2617–27. [DOI] [PubMed] [Google Scholar]