Abstract
This study provides new data on chromosomal characteristics and DNA barcoding of three endemic loaches of Iran: spiny southern loach Cobitis linea (Heckel, 1847), Persian stream loach Oxynoemacheilus persa (Heckel, 1848) and Tongiorgi stream loach Oxynoemacheilus tongiorgii (Nalbant & Bianco, 1998). The chromosomes of these fishes were investigated by examining metaphase chromosome spreads obtained from epithelial gill and kidney cells. The diploid chromosome numbers of all three species were 2n=50. The karyotypes of C. linea consisted of 4M + 40SM + 6ST, NF=94; of O. persa by 20M + 22SM + 8ST, NF=90 and of O. tongiorgii by 18M + 24SM + 8ST, NF= 92. Sex chromosomes were cytologically indistinguishable in these loaches. Maximum likelihood-based estimation of the phylogenetic relationships based on the COI barcode region clearly separates the three Iranian loach species of the Kor River basin. All species distinguished by morphological characters were recovered as monophyletic clades by the COI barcodes. The obtained results could be used for population studies, management and conservation programs.
Key Words: Loaches, Phylogenetic relationships, COI barcode region, Idiogram, Iran
INTRODUCTION
The confirmed freshwater ichthyofauna of Iran are represented by 202 species in 104 genera, 28 families, 17 orders and 3 classes found in 19 different basins [1]. The most diverse order is the Cypriniformes with 120 confirmed species (59.4%) including Cyprinidae with 93 confirmed species (46.0%), Nemacheilidae with 22 species (10.9%) and Cobitidae with 5 species (2.5%) [1]. However, a few new and exotic fishes have been recently reported from inland waters of Iran, increasing the number of confirmed species to more than 220 [2-8]. From a cytogenetic point of view, few of these freshwater fish species have been chromosomally characterized [9-12] and cobitid (Cobitidae) and nemacheilid (Nemacheilidae) loaches have not been completely accounted for so far [13].
The Cobitidae family, sometimes called sting-loaches (spiny loaches), is found in Eurasia and Morocco and has about 26 genera with about 177 species [14]. Four Cobitidae species have been recorded from Iran [1, 13]. Loaches of the Nemacheilidae family are a characteristic element of the Eurasian ichthyofauna and occur in nearly every running water. About 30 genera and 720 nominal species are presently known, most of them from South and Southeast Asia. However, a great number of taxa remain to be described [15].
The same situation is observed in Iran and new species are being described whose taxonomic statuses are being reviewed [5, 16-19] mostly based on their morphology and anatomy (gas bladder capsule, gut). The application of non-morphological methods such as cytogenetic and molecular studies may provide a complementary data source for more accurate and precise identification of these fishes. These types of studies have received considerable attention in recent years [19, 20-23]. Fish chromosome data have great importance in studies concerning evolutionary systematics, aquaculture, mutagenesis, genetic control and the rapid production of inbred lines [22, 24]. The study of karyotype is also important in aquaculture in connection with the use of chromosome manipulation techniques, including the induction of polyploidy, gynogenesis, androgenesis and inter or intra-species hybridization [25, 26]. About 3425 freshwater and marine fish species have been reviewed in this respect [22] which is about 10.5% of the 32,700 described fish species. Moreover, recent molecular systematics has enabled the re-assessment of many fish taxa and provided phylogenetic hypotheses for them. In this context, DNA barcoding using short, standardized DNA sequences to identify species by using primers that are applicable to the broadest possible taxonomic group have generated novel insights from faunal assessments. Our main goals are to contribute to the understanding and exploring of cytogenetical data (i.e., diploid chromosome numbers, description of karyotypes, idiograms) and phylogenetic relationships of three endemic loaches of the Kor River basin based on the COI barcode region in order to help future taxonomical and genetic studies.
MATERIALS AND METHODS
Cobitis linea, Oxynoemacheilus persa and Oxynoemacheilus tongiorgii specimens (Fig. 1) ?′?′27] was followed. Colchicine solution was prepared with 0.005 g in a 20 ml physiological serum. The fish were injected intraperitoneally with 0.02 ml of colchicine per gram of body weight using an insulin syringe and taken back to the aquarium for 4-5 hours. They were then anaesthetized using MS222, and their gill filaments and kidneys were removed and placed in hypotonic 0.36% KCl solution for 45 min in room temperature (25˚C). After adding 2-3 drops of fresh and cold Carnoy fixative (1: 3, Acetic acid: Methanol), the solutions were centrifuged for 10 min at 1000 rpm. The supernatants were then discarded and 5ml fresh and cold fixative was added to the sediments, mixed thoroughly and left for 1 hour. The fixation and centrifugation stages were repeated twice. The suspensions now were trickled to cold slides from a height of almost 2 meters. These slides were stained with 10% Giemsa for 20 min. Chromosomes were observed, selected and photographed by an Olympus light microscope mounted with a camera. Karyotypes were prepared by arranging chromosomes in pairs by size. For each chromosome, the average lengths of short and long arms, the arm ratio (the ratio of the length of the long arm to the short arm or r value) and the centromeric index (CI, expressed as the ratio of the length of the short arm to total chromosome length) were calculated and chromosomes were classified according to Levan et al.’s (1964) criteria [28]. Fundamental number (NF) was expressed as twice the atelocentric number plus the number of telocentric chromosomes.
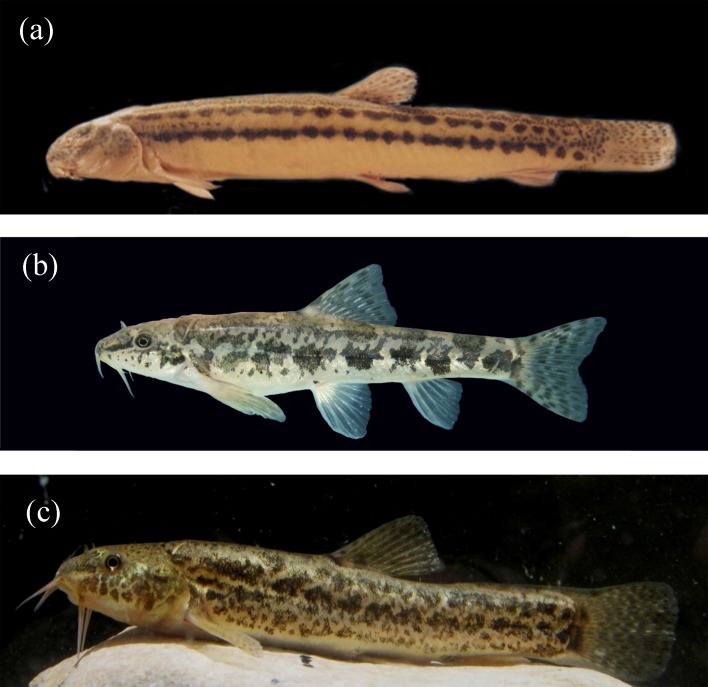
Figure 1.
Endemic loaches of the Kor River basin in Iran. a, Cobitis linea; b, Oxynoemacheilus persa; c, Oxynoemacheilus tongiorgii.
DNA extraction and PCR: Genomic DNA was extracted using Macherey & Nagel NucleoSpin® Tissue kits following the manufacturer’s protocol on an Eppendorf EpMotion® pipetting-roboter with vacuum manifold. The standard vertebrate DNA barcode region of the COI (cytochrome c oxidase subunit 1) was amplified using an M13 tailed primer cocktail including FishF2_t1 (5’TGT AAA ACG ACG GCC AGT CGA CTA ATC ATA AAG ATA TCG GCA C3’), FishR2_t1 (5’CAG GAA ACA GCT ATG ACA CTT CAG GGT GAC CGA AGA ATC AGA A3’), VF2_t1 (5’TGT AAA ACG ACG GCC AGT CAA CCA ACC ACA AAG ACA TTG GCA C3’) and FR1d_t1 (5’CAG GAA ACA GCT ATG ACA CCT CAG GGT GTC CGA ARA AYC ARA A3’) [29]. Sequencing of the ExoSAP-IT (USB) purified PCR product in both directions was conducted at Macrogen Europe Laboratories with forward sequencing primer M13F (5’GTA AAA CGA CGG CCA GT3’) and reverse sequencing primer M13R-pUC (5’CAG GAA ACA GCT ATG AC3’).
Molecular data analysis: Data processing and sequence assembly was carried out in Geneious [30] and Muscle algorithm [31] was chosen to create a DNA sequence alignment. Modeltest [32], implemented in the MEGA 6 software [33], was used to determine the most appropriate sequence evolution model for the given data, treating gaps and missing data with the partial deletion option under 95% site coverage cutoff. The model with the lowest BIC (Bayesian Information Criterion) scores was considered to best describe the substitution pattern. According to Modeltest, the Tamura-Nei model [34] with discrete Gamma distribution (5 categories (+G, parameter = 0.4292)) best represented the COI alignment, and was used to estimate the evolutionary history. We generated maximum likelihood phylogenetic trees with 500 bootstrap replicates to explore species phylogenetic affinities. As an appropriate outgroup to root the constructed phylogenetic hypothesis, we included the loach Misgurnus.
Materials used for molecular COI analysis: Twelve loach specimens from the Kor River basin of Iran: Oxynoemacheilus persa: Kor_ Iran_1983_Ex91E1; KP050538; Oxynoemacheilus persa: Kor_Iran_1983_Ex91E2; KP050531; Oxynoemacheilus persa: Kor_Iran_1983_Ex91E3; KP050533; Oxynoemacheilus persa: Kor Iran_1983_Ex91E4; KP050529; Oxynoemacheilus tongiorgii: Kor_Iran_6_Ex82E10; KP050537; Oxynoemacheilus tongiorgii: Kor_Iran_6_Ex82E11; KP050532; Oxynoemacheilus tongiorgii: Kor_Iran_6_Ex87A2; KP050536; Oxynoemacheilus tongiorgii: Kor_ Iran_6_Ex87A3; KP050535; Oxynoemacheilus tongiorgii: Kor_Iran_6_Ex87A4; KP050534; Oxynoemacheilus tongiorgii: Kor_Iran_6_Ex87A5;KP050540 Cobitis linea: Kor_Iran_1982_Ex82A7; KP050530; Cobitis linea: Kor_Iran_1982_Ex82A6; KP050539.
Comparative material from GenBank: Oxynoemacheilus panthera_KJ554017; Oxynoemacheilus namiri_KJ553891; Oxynoemacheilus angorae_KJ553966; Oxynoemacheilus angorae_KJ553824; Oxynoemacheilus anatolicus_KJ443916; Seminemacheilus ispartensis_KJ554948; Seminemacheilus sp._KJ554960; Cobitis elongatoides_HQ961002; Cobitis vardarensis_HQ600718; Cobitis taenia_KJ128459; Cobitis taenia_KJ128460; Cobitis lutheri_HQ536324; Misgurnus fossilis_JQ011436; Misgurnus fossilis_JQ011436.
RESULTS AND DISCUSSION
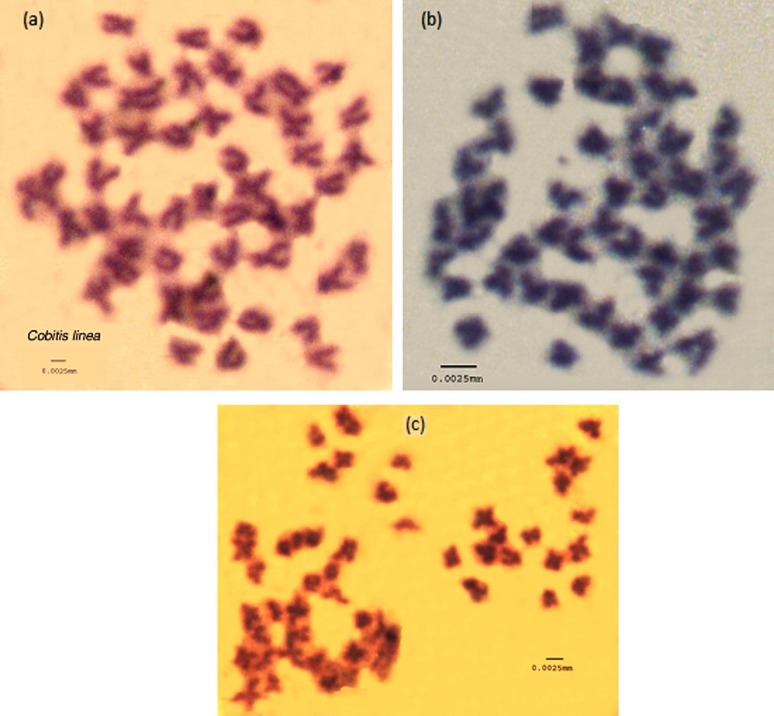
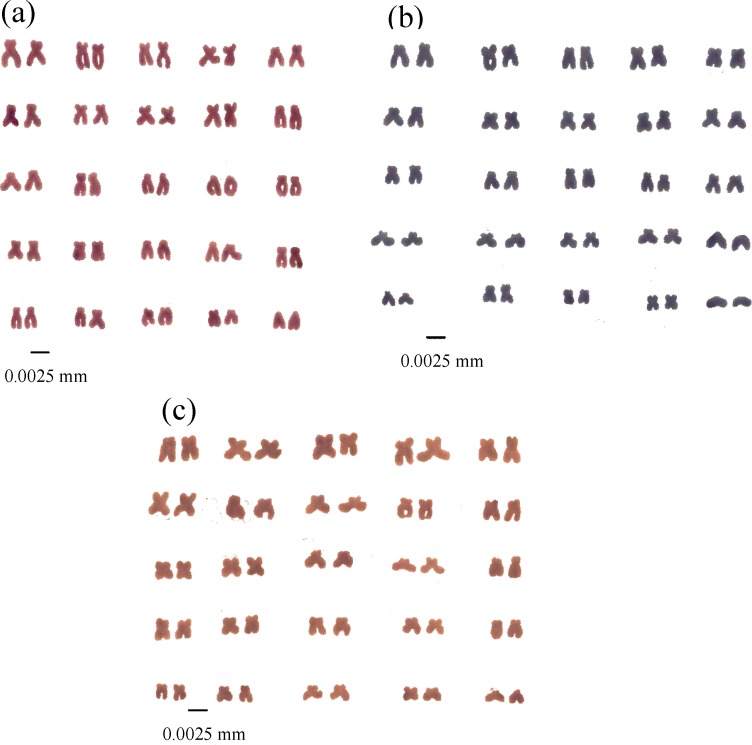
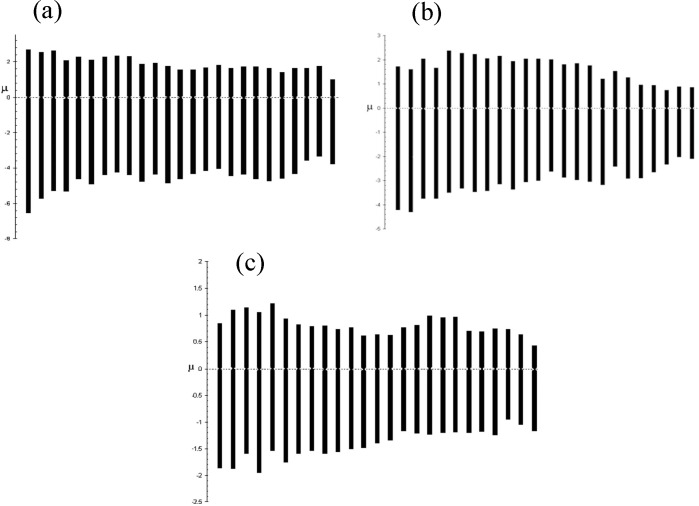
Metaphase spreads of the three species are given in Fig. 2. Diploid chromosome numbers of all three species were 2n=50 (Fig. 3). Quantitative data of the different measurements used to classify chromosomes and idiograms are given in table I and figure 4. The karyotypes consisted of 2 pairs of metacentric, 20 pairs of submetacentric and 3 pairs of subtelocentric chromosomes (4m + 40sm + 6st) in C. linea; 10 metacentric, 11 submetacentric and 4 subtelocentric (20m +22sm + 8st) in O. persa and 9 metacentric, 12 submetacentric and 4 subtelocentric (18m +24sm + 8st) in O. tongiorgii. The arm numbers were 94, 90 and 92 in C. linea, O. persa and O. tongiorgii respectively. Sex chromosomes were cytologically indistinguishable in these loaches.
Figure 2.
Giemsa stained chromosome spreads of three loaches from Iran. . a, Cobitis linea; b, O. persa ; c, O. tongiorgii
Figure 3.
Karyotypes of three loaches from Iran. a, C. linea; b, O. persa ; c, O. tongiorgii
Table 1.
Long arm length, LA (µm); short arm length, SA(µm); total arm length, TA (µm); arm ratio, AR; centromeric index, CI and chromosome type, CT of three endemic loaches of Iran
|
C.linea
|
O.persa
|
O.tangiorgii
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | LA | SA | TA | AR | CI | CT | LA | SA | TA | AR | CI | CT | CT | LA | SA | TA | AR | CI |
| 1 | 6.53 | 2.69 | 9.21 | 2.43 | 29 | Sm | 1.85 | .59 | 2.34 | 3.13 | .25 | St | St | 1.97 | .63 | 2.60 | 3.12 | .24 |
| 2 | 5.72 | 2.51 | 8.22 | 2.28 | .30 | Sm | 1.78 | .98 | 2.76 | 1.82 | .36 | Sm | St | 1.92 | 1.09 | 3.01 | 1.76 | .36 |
| 3 | 5.32 | 2.64 | 7.95 | 2.02 | .33 | Sm | 1.55 | .70 | 2.25 | 2.21 | .31 | Sm | St | 1.59 | 1.13 | 2.72 | 1.41 | .42 |
| 4 | 5.48 | 2.11 | 7.58 | 2.6 | .28 | Sm | 1.52 | .49 | 2.01 | 3.10 | .24 | St | M | 1.97 | 1.05 | 3.02 | 1.88 | .35 |
| 5 | 4.92 | 2.1 | 7.01 | 2.35 | .30 | Sm | 1.35 | .91 | 2.26 | 1.48 | .40 | M | Sm | 1.44 | 1.34 | 2.78 | 1.07 | .48 |
| 6 | 4.64 | 2.29 | 6.94 | 2.03 | .33 | Sm | 1.12 | .90 | 2.02 | 1.24 | .45 | M | M | 1.78 | .93 | 2.71 | 1.91 | .34 |
| 7 | 4.97 | 1.76 | 6.73 | 2.83 | .26 | Sm | 1.27 | .79 | 2.06 | 1.61 | .38 | M | Sm | 1.59 | .82 | 2.41 | 1.94 | .34 |
| 8 | 4.43 | 2.28 | 6.71 | 1.94 | .34 | Sm | 1.39 | .69 | 2.08 | 2.01 | .33 | Sm | Sm | 1.54 | .78 | 2.32 | 1.97 | .34 |
| 9 | 4.86 | 1.58 | 6.7 | 1.63 | .28 | M | 1.34 | .66 | 2.00 | 2.03 | .33 | Sm | M | 1.59 | .80 | 2.39 | 1.99 | .33 |
| 10 | 4.29 | 2.4 | 6.69 | 1.78 | .36 | Sm | 1.29 | .62 | 1.91 | 2.08 | .32 | Sm | Sm | 1.51 | .82 | 2.33 | 1.84 | .35 |
| 11 | 4.36 | 2.3 | 6.69 | 1.91 | .34 | Sm | 1.39 | .62 | 2.01 | 2.24 | .31 | Sm | Sm | 1.51 | .82 | 2.33 | 1.84 | .35 |
| 12 | 4.74 | 1.62 | 6.36 | 2.93 | .25 | Sm | 1.33 | .59 | 1.92 | 2.25 | .31 | Sm | Sm | 1.99 | .60 | 2.59 | 3.31 | .23 |
| 13 | 4.57 | 1.74 | 6.31 | 2.63 | .28 | Sm | 1.59 | .50 | 2.09 | 3.11 | .24 | St | St | 1.42 | .63 | 2.05 | 2.25 | .31 |
| 14 | 4.36 | 1.94 | 6.3 | 2.25 | .31 | Sm | 1.23 | .69 | 1.92 | 1.78 | .36 | Sm | Sm | 1.16 | .62 | 2.04 | 2.29 | .30 |
| 15 | 4.57 | 1.61 | 6.18 | 2.85 | .26 | Sm | 1.19 | .69 | 1.88 | 1.72 | .37 | Sm | Sm | 1.22 | .76 | 1.92 | 1.53 | .40 |
| 16 | 4.44 | 1.7 | 6.14 | 2.61 | .28 | Sm | 1.35 | .62 | 1.97 | 2.18 | .31 | Sm | St | 1.24 | .78 | 2.00 | 1.56 | .39 |
| 17 | 4.62 | 1.46 | 6.08 | 3.16 | .24 | St | 1.21 | .77 | 1.98 | 1.57 | .39 | M | St | 1.18 | 1.09 | 2.33 | 1.14 | .47 |
| 18 | 4.32 | 1.65 | 5.97 | 2.62 | .28 | Sm | 1.13 | .67 | 1.80 | 1.69 | .37 | Sm | Sm | 1.21 | .95 | 2.13 | 1.24 | .45 |
| 19 | 4.59 | 1.35 | 5.94 | 3.41 | .23 | St | 1.18 | .74 | 1.92 | 1.59 | .39 | M | Sm | 1.20 | 1.00 | 2.21 | 1.21 | .45 |
| 20 | 4.05 | 1.85 | 5.9 | 2.2 | .31 | Sm | 1.07 | .79 | 1.86 | 1.35 | .42 | M | M | 1.18 | .70 | 1.90 | 1.71 | .37 |
| 21 | 4.33 | 1.54 | 5.86 | 2.82 | .26 | Sm | 1.04 | .63 | 1.67 | 1.65 | .38 | M | Sm | 1.35 | .64 | 1.82 | 1.84 | .35 |
| 22 | 4.16 | 1.68 | 5.83 | 2.48 | .29 | Sm | 1.04 | .68 | 1.72 | 1.53 | .40 | M | M | .94 | .74 | 2.09 | 1.82 | .35 |
| 23 | 3.69 | 1.7 | 5.36 | 2.15 | .32 | Sm | .95 | .62 | 1.57 | 1.53 | .39 | M | St | 1.05 | .79 | 1.73 | 1.19 | .49 |
| 24 | 3.34 | 1.98 | 5.32 | 1.69 | .37 | M | 1.06 | .56 | 1.62 | 1.89 | .35 | Sm | Sm | 1.05 | .66 | 1.71 | 1.59 | .39 |
| 25 | 4.07 | 99 | 5.06 | 4.11 | .20 | St | 2.13 | .50 | 2.63 | 4.26 | .19 | St | Sm | 1.58 | .42 | 2.00 | 3.76 | .21 |
Figure 4.
Haploid idiograms of three species of loaches from Iran. a, C. linea; b, O. persa ; c, O. tongiorgii.
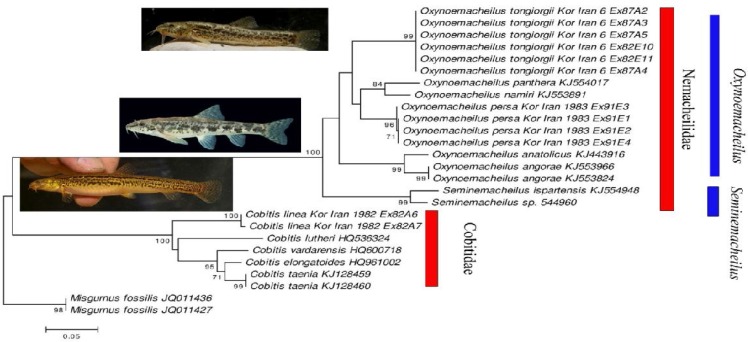
Maximum likelihood based estimation of the phylogenetic relationships based on the COI barcode region clearly separated the three Iranian loach species from the Kor drainage (Fig. 5). All species distinguished by morphological characters are recovered as monophyletic clades by the COI barcodes. K2P distances between (sympatric) species in Kor drainage loaches were found for Cobitis linea to Oxynoemacheilus tongiorgii (41.3 % K2P), Cobitis linea to Oxynoemacheilus persa (38.4% K2P) and Oxynoemacheilus persa to Oxynoemacheilus tongiorgii (10.3 % K2P). Smallest K2P distances between genera were found for Oxynoemacheilus to Seminemacheilus (8.1 % K2P) and Oxynoemacheilus to Cobitis (38.4 % K2P).
Figure 5.
Maximum Likelihood (ML) estimation of the phylogenetic relationships based on the mitochondrial COI barcode region. Nucleotide positions with less than 95 % site coverage were eliminated before analysis. Numbers of major nodes indicate bootstrap values above 65% from 500 pseudo-replicates. All branch lengths are drawn to scale and give number of substitutions per site. The photos show O. tongiorgii, O. persa , and C. linea
The resolution of the mitochondrial COI gene fragment does not, however, allow for unequivocal inference of sister species relationships and the restricted outgroup sampling available for this study sets clear constraints on the phylogenetic interpretation of the results.
In many vertebrate groups, the study of karyotypes and genome size has contributed, along with analyses of mitochondrial and nuclear gene sequences, to the solution of various challenges in biology, systematics and evolution [22]. However, in fishes, which are the most diverse of all vertebrate groups, higher taxa have been traditionally classified largely by morphology and paleontology, with a much smaller input of cytogenetic information., This is partly due to the fact that karyotypes can be obtained only from living specimens, tissues, or cells, which makes it challenging to study the karyotypes of fishes that are difficult to collect alive (e.g. deep-sea fishes). Nevertheless, even fresh material provides no guarantee that reliable chromosome figures can be obtained easily [22]. Karyotypes are descriptions of the number and morphology of chromosomes. The number of chromosomes per cell seems to be a rather conservative characteristic and may thus be used as an indicator of the closeness of species’ interrelationships within families [35]. The diploid chromosome number of fishes varies from 2n= 22-26 in some species of an Antarctic fish group [36], to 2n=240-260 in some anadromous Acipenseridae which show several microchromosomes [37].
According to our observations, the diploid chromosome numbers of all the endemic loach species were 2n= 50, being in conformation with the chromosome number of other species and genera of loaches. Klinkhardt et al., (1995) [38] and Arkhipchuk (1999) [39] reported the chromosome number of Cobitis calderoni (Bacescu, 1962), C. granoei Rendahl, 1935, C. lutheri Rendahl, 1935, C. macroccana Pellegrin, 1929, C. taenia Linnaeus, 1758 and also Niwaella delicata (Niwa, 1937) to be 2n= 50. Other balitorid species such as Barbatula barbatula (Linnaeus, 1758), Acanthocobitis botia (Hamilton, 1822), Triplophysa dorsalis (Kessler, 1872), and T. stoliczkai (Steindachner, 1866) which have been cytologically investigated so far, have the diploid chromosome number 2n=50 [38, 39]. It can be concluded that the chromosome number in this group is conservative. Yet, in few species of Cobitoidea, the diploid chromosome number is reported to vary from 2n=48 to 2n=94 [38, 39]. The chromosome number of C. biwae (Jordan & Snyder, 1901) and Cobitis takatsuensis (Mizuno, 1970) was reported to be 2n= 48 [39], for C. matsubarai (Okada & Ikeda, 1939) it was 2n= 86, 94 [40], and for C. taenia (Linnaeus, 1758) it was 2n=50, 75, 86, 94 [39, 38]. It could be suggested that the most common diploid chromosome number is 2n=50, which is the modal number in loach fishes. However, a cytological indication of polyploidy has been noted in some loach species. Polyploidy has been also noted among members of the Salmonidae, Cyprinidae, Cobitidae and Catostomidae families [22, 41].
When interpreting karyotypic evolution, it is often assumed that the primitive fish karyotype consists of 48 rods from which the karyotypes of all existing fish forms have been derived [41], but this issue is yet to be resolved. The discovery of 48 rather large acrocentric chromosomes in the Pacific hag fish, Eptatretus stoutii, belonging to the order Myxiniformes [42, 43] and the occurrence of 48 rods in the majority of fishes studied prior to 1967 led to the idea that the primitive karyotype of ancestral vertebrates evolved from chordate might consist of 48 rods [41]. Therefore, most subsequent researchers assumed karyotypic evolution in different groups of fishes to be founded on the basic assumption of 48 rods as the primitive number [41]. However, the discovery of 2n=24 rods in two species of freshwater eels [44, 45], 2n=36 rods in two species of Myxine and low diploid numbers ranging between 14- 42 in a large number of fish families showing an NF less than 36 in some cases [41] would possibly call for a more cautious prediction of the primitive karyotype of fish. The karyotype formulas of these loaches were found to be different, being 4m + 40sm + 6st in C. linea; 10m +26sm + 14st in O. persa and 18m +24sm + 8st in O. tongiorgii. The chromosome arm number (NF) of C. linea (94) was larger than that of the three steam loaches. Chromosome arm numbers of 66-152 have been reported for different species of the genus Cobitis [38]. In the present study, no cytological evidence was found for sex chromosome dimorphism in any of these four loaches, which agrees with reports on many other fish species [9-11]. In marine fishes too, despite the large number of living species, the occurrence of cytologically differentiated sex chromosomes appears to be rare [20].
The main obstacles in the study of nemacheilid loaches of the Middle East, including Iran, are the confused definitions of the genera and the large number of poorly diagnosed species described from this area [17, 46]. For a long time, the loaches of the Kor river basin have been considered to belong to two genera with four species:
Cobitis linea, Orthrias persus (Heckel, 1846), Orthrias farsicus (Nalbant & Bianco, 1998) and Seminemacheilus tongiorgii (Nalbant & Bianco, 1998). Stoumboudi et al. (2006) [47] and Prokofiev (2009) [48] placed most nemacheilid loaches from Eastern Europe and the Middle East in the genus Oxynoemacheilus. Recently, Freyhof et al. (2011) [17] reviewed the western Palaearctic Oxynoemacheilus, and Kottelat (2012) [46] listed species of this genus based mostly on the proposals of Prokofiev (2009) [48]. Freyhof et al. (2011) [17] transferred Seminemacheilus and Orthrias to the genus Oxynoemacheilus and considered Orthrias persus (Heckel, 1846), Orthrias farsicus (Nalbant & Bianco, 1998) and Seminemacheilus tongiorgii as synonymous to Oxynoemacheilus persa (Heckel, 1848) and Oxynoemacheilus tongiorgii (Nalbant & Bianco, 1998), respectively [1, 13, 18]. Maximum likelihood estimations of the phylogenetic relationships based on the COI barcode region clearly separate the three Iranian loach species from the Kor drainage and support their independent evolution from other studied loaches. This supports Freyhof et al. (2011) [17] and Kottelat’s (2012) [46] notion of the validity of Oxynoemacheilus tongiorgii and Oxynoemacheilus persa.
Acknowledgments: The authors thank Shiraz University for financial support and J. Freyhof for his comments and the C. linea photo.
Conflict of Interest: The authors declare that they have no competing interest.
References
- 1.Esmaeili HR, Coad BW, Gholamifard A, Nazari N, Teimory A. Annotated checklist of the freshwater fishes of Iran. Zoosyst Rossica. 2010;19:361–386. [Google Scholar]
- 2.Esmaeili HR, Gholamifard A, Sayyadzadeh G, Parsi B, Mirghiyasi S, Ghasemian S. New record of the convict cichlid, Amatitlania nigrofasciata (Günther, 1867), from the Middle East (Actinopterygii: Cichlidae) Aqua Int J Ichthyol. 2013;19:225–229. [Google Scholar]
- 3.EsmaeilI HR, Masoudi M, Mehraban HR. Assignment of Acanthopagrus populations in the Persian Gulf drainage system of Iran to Acanthopagrus arabicus Iwatsuki, 2013 (Perciformes: Sparidae) Iran J Ichthyol. 2014;1:23–28. [Google Scholar]
- 4.Esmaeili HR, Teimory A, Owfi F, Abbasi K, Coad BW. Alien and invasive freshwater fish species in Iran: Diversity, environmental impacts and management. Iran J Ichthyol. 2014;1:62–72. [Google Scholar]
- 5.Esmaeili HR, Sayyadzadeh G, Özuluğ M, Geiger M, Freyhof J. Three new species of Turcinoemacheilus from Iran and Turkey (Teleostei: Nemacheilidae) Ichthyol Explor Fres. 2014;24:257–273. [Google Scholar]
- 6.Esmaeili HR, Teimori A, Gholami Z, Reichenbacher B. Two new species of the tooth-carp Aphanius (Teleostei: Cyprinodontidae) and the evolutionary history of the Iranian inland and inland-related Aphanius species. Zootaxa. 2014;3786:246–268. doi: 10.11646/zootaxa.3786.3.2. [DOI] [PubMed] [Google Scholar]
- 7.Gholami Z, Esmaeili HR, Erpenbeck D, Reichenbacher B. Phylogenetic analysis of Aphanius from the endorheic Kor River Basin in the Zagros Mountains, South-western Iran (Teleostei: Cyprinodontiformes: Cyprinodontidae) J Zool Syst Evol Res. 2014;52:130–141. [Google Scholar]
- 8.Teimori A, Esmaeili HR, Erpenbeck D, Reichenbacher B. A new and unique species of the genus Aphanius Nardo, 1827 (Teleostei: Cyprinodontidae) from Southern Iran: A case of regressive evolution. Zool Anz. 2014;253:327–337. [Google Scholar]
- 9.Esmaeili HR, Piravar Z. First report on karyotype of Cyprinion tenuiradius Heckel, 1849 (Pisces: Cyprinidae), from Southwest of Iran. Zool Middle East. 2006;39:75–80. [Google Scholar]
- 10.Esmaeili HR, Piravar Z, Ebrahimi M. First karyological analysis of Iranian cichlid fish, Iranocichla hormuzensis Coad, 1982 (Perciformes, Cichlidae) from southern Iran. J Appl Anim Res. 2006;30:77–80. [Google Scholar]
- 11.Esmaeili HR, Piravar Z, Shiva AH. Karyological analysis of two endemic tooth-carps, Aphanius persicus and A. sophiae (Pisces: Cyprinodontidae) from Southwest of Iran. Turk J Zool. 2007;31:64–74. [Google Scholar]
- 12.Nasri M, Keivany Y, Dorafshan S. First karyological analysis of smallmouth lotak, Cyprinion kais Heckel, 1843, an endemic Cyprinid fish from the Tigris–Euphrates Basin. Ital J Zool. 2010;77:272–276. [Google Scholar]
- 13.Coad BW. Freshwater fishes of Iran. 2014. http://www.briancoad.com.
- 14.Nelson JS. Fishes of the world. New Jersey: John Wiley & Sons, Inc; 2006. [Google Scholar]
- 15.Bohlen J, Šlechtov V. A new genus and two new species of loaches (Teleostei: Nemacheilidae) from Myanmar. Ichthyol Explor Fres. 2011;22:1–10. [Google Scholar]
- 16.Nalbant TT, Bianco PG. The loaches of Iran and adjacent regions with description of six new species (Cobitoidea) Ital J Zool. 1998;65(Supplement):109–123. [Google Scholar]
- 17.Freyhof J, Erk’akan F, Özeren C, Perdices A. An overview of the western Palaearctic loach genus Oxynoemacheilus (Teleostei: Nemacheilidae) Ichthyol Explor Fres. 2011;22:301–312. [Google Scholar]
- 18.Golzarianpour K, Abdoli A, Freyhof J. Oxynoemacheilus kiabii, a new loach from Karkheh River drainage, Iran (Teleostei:Nemacheilidae) Ichthyol Explor Fres. 2011;22:201–208. [Google Scholar]
- 19.Freyhof J, Esmaeili HR, Sayyadzadeh G, Geiger M. Review of the crested loaches of the genus Paracobitis Bleeker, 1868 from Iran and Iraq with the description of four new species (Teleostei: Nemacheilidae) Ichthyol Explor Fres. 2014;25:11–38. [Google Scholar]
- 20.Galetti PM, Aguliar CT, Molina WF. An overview of marine fish cytogenetics. Hydrobiologia. 2000;420:55–62. [Google Scholar]
- 21.Ozouf-Costaz C, Foresti F. Fish cytogenetic research: advances, application and perspectives. Neth J Zool. 1992;42:277–290. [Google Scholar]
- 22.Arai R. Fish Karyotypes: A Check List. Japan: Springer; 2011. [Google Scholar]
- 23.Geiger MF, Herder F, Monaghan MT. Spatial heterogeneity in the Mediterranean biodiversity hotspot affects barcoding accuracy of its freshwater fishes. Mol Ecol Resour. 2014;14:1210–1221. doi: 10.1111/1755-0998.12257. [DOI] [PubMed] [Google Scholar]
- 24.Al-Sabti K. Handbook of Genetoxic Effects and Fish Chromosomes. Ljubljana: Jozef Stefan Institute; 1991. [Google Scholar]
- 25.Wu C, Ye Y, Chen R. Genome manipulation in carp (Cyprinus carpio L.) Aquaculture. 1986;54:57–61. [Google Scholar]
- 26.Diter A, Quillet E, Chourrout D. Suppression of first egg mitosis induced by heat shocks in the rainbow trout. J Fish Biol. 1993;42:777–786. [Google Scholar]
- 27.Uwa H. Karyotype evolution and geographical distribution in the ricefish, genus Oryzias (Oryziidae) In: Uyeno TR, Arai T, Taniuchi K Matsuura, editors. Indo-Pacific Fish Biology: Proceeding of the Second International Conference on Indo-Pacific Fishes Ichthyological Society of Japan, Tokyo. 1986. pp. 867–876. [Google Scholar]
- 28.Levan A, Fredga K, Sandberg AA. Nomenclature for centromeric positions on chromosomes. Hereditas. 1964;52:201–202. [Google Scholar]
- 29.Ivanova NV, Zemlak TS, Hanner RH, Hebert PDN. Universal primer cocktails for fish DNA barcoding. Molecular Ecology Notes. 2007;7:544–548. [Google Scholar]
- 30.Biomatters. Geneious Pro. 2013. Available: http://www.geneious.com.
- 31.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- 33.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 35.Moyle PB, Cech JJ. Fishes, an introduction to ichthyology. Fifth Edition. New Jersey: Prentice Hall, Englewood cliffs; 2004. [Google Scholar]
- 36.Ozouf-Costaz C, Pisano E, Thaeron C, Hureau GC. Antarctic fish chromosome banding: significance for evolutionary studies. Cybium. 1997;21:399–409. [Google Scholar]
- 37.Fontana F, Rossi R, Lanfredi M, Arlati G, Bronzi P. Cytogenetic chracterictization of cell lines from three sturgeon species. Caryologia. 1997;50:91–95. [Google Scholar]
- 38.Klinkhardt M, Tesche M, Greven H. Database of fish chromosomes. Westarp Wissenschaften. 1995. http://www.fishbase.org.
- 39.Arkhipchuk VV. Chromosome database. Database of Dr. Victor Arkhipchuk. 1999. http://www.fishbase.org.
- 40.Nakabo T. Fishes of Japan with Pictorial Keys to the Species. English edition I. . Japan: Tokai University Press; 2002. [Google Scholar]
- 41.Khuda-Bukhsh AR, Chanda T, Barat A. Karyomorphology and evolution in some Indian hillstream fishes with particular reference to polyploidy in some species. In: Uyeno TR, Arai T, Taniuchi KM, editors. Indo-Pacific Fish Biology: Proceeding of the Second International Conference on Indo-Pacific Fishes Ichthyological Society of Japan, Tokyo. 1986. pp. 886–898. [Google Scholar]
- 42.Taylor KM. The chromosomes of some lower chordates. Chromosoma. 1967;21:181–188. doi: 10.1007/BF00343643. [DOI] [PubMed] [Google Scholar]
- 43.Vasil'ev VP. Chromosome numbers in fish-like vertebrates and fish. J Ichthyol. 1980;20:1–38. [Google Scholar]
- 44.Rishi KK, Haobam MS. Karyotypic. Study on two fresh water mud eels. In: Manna GK, Sinha U, editors. Prospective in Cytology and Genetics. Proc. 4th All Indian Congress of Genetics. India. 1984. pp. 429–432. [Google Scholar]
- 45.Kitada J, Tagawa M. On the chromosomes of two species of Cyclostomata. La Kromosomo. 1973;91:2913–2916. [In Japanese with English summary] [Google Scholar]
- 46.Kottelat M. Conspectus cobitidum: an inventory of the loaches of the world (Teleostei: Cypriniformes: Cobitoidei) Raffles B Zool. 2012;(Suppl. 26):1–199. [Google Scholar]
- 47.Stoumboudi MT, Kottelat M, Barbieri M. The fishes of the inland waters of Lesbos Island, Greece. Ichthyol Explor Freshwater. 2006;17:129–146. [Google Scholar]
- 48.Prokofiev AM. Problems of the classification and phylogeny of nemacheiline loaches of the group lacking the pre-ethmoid I (Cypriniformes: Balitoridae: Nemacheilinae) J Ichthyol. 2009;49:874–898. [Google Scholar]