Abstract
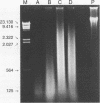
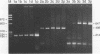
AIMS--To evaluate the ability of four rapid DNA extraction methods to provide DNA for the polymerase chain reaction (PCR) from routinely fixed, paraffin wax embedded archival tissues. METHODS--Eighteen blocks of various tissues, 18 blocks of cervical cancer specimens, and nine blocks of B cell lymphomas were investigated. Both normal and biopsy specimen sized tissues were studied. DNA was extracted using four methods: boiling for 20 minutes in distilled water; boiling for 20 minutes in 5% Chelex-100 resin solution; 3-hour proteinase K digestion; and 3-hour proteinase K digestion, followed by boiling in 5% Chelex-100. Different exons of the p53 gene, human papillomavirus type 16 (HPV 16) sequence, and immunoglobulin heavy chain (IgH) gene rearrangement were amplified from the extracts. RESULTS--The Chelex boiling, proteinase K digestion, and proteinase K digestion-Chelex boiling methods produced DNA suitable for amplification in all of the 45 samples. Boiling in water yielded insufficient template for the PCR in three of the 45 cases (7%), and in six of 42 positive cases (14%) much fainter bands were observed, mostly when the processed material was either biopsy specimen sized or a B cell lymphoma sample. Fragments of the p53 gene were successfully amplified up to 408 base pairs in water boiled extracts, up to 647 in Chelex boiled preparates, and up to 984 in proteinase K digested and proteinase K digested-Chelex boiled samples, although with decreased sensitivity in the last case. All of the templates were reusable after 3 months of storage at -20 degrees C. CONCLUSIONS--Chelex boiling, proteinase K digestion, and proteinase K digestion followed by Chelex boiling produce suitable templates for the PCR from a large variety of paraffin wax embedded tissues. As the simple 20 minute boiling method in 5% Chelex-100 solution requires minimal manipulation and time, it could be useful, especially in the routine processing of large amounts of material.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen M. L., Shieh Y. S., Shim K. S., Gerber M. A. Comparative studies on the detection of hepatitis B virus DNA in frozen and paraffin sections by the polymerase chain reaction. Mod Pathol. 1991 Sep;4(5):555–558. [PubMed] [Google Scholar]
- Coates P. J., d'Ardenne A. J., Khan G., Kangro H. O., Slavin G. Simplified procedures for applying the polymerase chain reaction to routinely fixed paraffin wax sections. J Clin Pathol. 1991 Feb;44(2):115–118. doi: 10.1136/jcp.44.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubeau L., Chandler L. A., Gralow J. R., Nichols P. W., Jones P. A. Southern blot analysis of DNA extracted from formalin-fixed pathology specimens. Cancer Res. 1986 Jun;46(6):2964–2969. [PubMed] [Google Scholar]
- Figueroa M. E., Rasheed S. Molecular pathology and diagnosis of infectious diseases. Am J Clin Pathol. 1991 Apr;95(4 Suppl 1):S8–21. [PubMed] [Google Scholar]
- Gill P., Kimpton C. P., Sullivan K. A rapid polymerase chain reaction method for identifying fixed specimens. Electrophoresis. 1992 Mar;13(3):173–175. doi: 10.1002/elps.1150130135. [DOI] [PubMed] [Google Scholar]
- Goelz S. E., Hamilton S. R., Vogelstein B. Purification of DNA from formaldehyde fixed and paraffin embedded human tissue. Biochem Biophys Res Commun. 1985 Jul 16;130(1):118–126. doi: 10.1016/0006-291x(85)90390-0. [DOI] [PubMed] [Google Scholar]
- Heller M. J., Robinson R. A., Burgart L. J., TenEyck C. J., Wilke W. W. DNA extraction by sonication: a comparison of fresh, frozen, and paraffin-embedded tissues extracted for use in polymerase chain reaction assays. Mod Pathol. 1992 Mar;5(2):203–206. [PubMed] [Google Scholar]
- Impraim C. C., Saiki R. K., Erlich H. A., Teplitz R. L. Analysis of DNA extracted from formalin-fixed, paraffin-embedded tissues by enzymatic amplification and hybridization with sequence-specific oligonucleotides. Biochem Biophys Res Commun. 1987 Feb 13;142(3):710–716. doi: 10.1016/0006-291x(87)91472-0. [DOI] [PubMed] [Google Scholar]
- Jackson D. P., Lewis F. A., Taylor G. R., Boylston A. W., Quirke P. Tissue extraction of DNA and RNA and analysis by the polymerase chain reaction. J Clin Pathol. 1990 Jun;43(6):499–504. doi: 10.1136/jcp.43.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiene P., Milde-Langosch K., Runkel M., Schulz K., Löning T. A simple and rapid technique to process formalin-fixed, paraffin-embedded tissues for the detection of viruses by the polymerase chain reaction. Virchows Arch A Pathol Anat Histopathol. 1992;420(3):269–273. doi: 10.1007/BF01600280. [DOI] [PubMed] [Google Scholar]
- Kogan S. C., Doherty M., Gitschier J. An improved method for prenatal diagnosis of genetic diseases by analysis of amplified DNA sequences. Application to hemophilia A. N Engl J Med. 1987 Oct 15;317(16):985–990. doi: 10.1056/NEJM198710153171603. [DOI] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Lench N., Stanier P., Williamson R. Simple non-invasive method to obtain DNA for gene analysis. Lancet. 1988 Jun 18;1(8599):1356–1358. doi: 10.1016/s0140-6736(88)92178-2. [DOI] [PubMed] [Google Scholar]
- Miller C. W., Simon K., Aslo A., Kok K., Yokota J., Buys C. H., Terada M., Koeffler H. P. p53 mutations in human lung tumors. Cancer Res. 1992 Apr 1;52(7):1695–1698. [PubMed] [Google Scholar]
- Ramasamy I., Brisco M., Morley A. Improved PCR method for detecting monoclonal immunoglobulin heavy chain rearrangement in B cell neoplasms. J Clin Pathol. 1992 Sep;45(9):770–775. doi: 10.1136/jcp.45.9.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Shibata D. K., Arnheim N., Martin W. J. Detection of human papilloma virus in paraffin-embedded tissue using the polymerase chain reaction. J Exp Med. 1988 Jan 1;167(1):225–230. doi: 10.1084/jem.167.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A., Raoult D. A simple method for amplification of DNA from paraffin-embedded tissues. Nucleic Acids Res. 1992 Oct 11;20(19):5237–5238. doi: 10.1093/nar/20.19.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh P. S., Metzger D. A., Higuchi R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques. 1991 Apr;10(4):506–513. [PubMed] [Google Scholar]
- Wan J. H., Trainor K. J., Brisco M. J., Morley A. A. Monoclonality in B cell lymphoma detected in paraffin wax embedded sections using the polymerase chain reaction. J Clin Pathol. 1990 Nov;43(11):888–890. doi: 10.1136/jcp.43.11.888. [DOI] [PMC free article] [PubMed] [Google Scholar]