Abstract
IMPORTANCE
Large patient cohorts are necessary to validate the efficacy of transoral robotic surgery (TORS) in the management of head and neck cancer.
OBJECTIVES
To review oncologic outcomes of TORS from a large multi-institutional collaboration and to identify predictors of disease recurrence and disease-specific mortality.
DESIGN, SETTING, AND PARTICIPANTS
A retrospective review of records from 410 patients undergoing TORS for laryngeal and pharyngeal cancers from January 1, 2007, through December 31, 2012, was performed. Pertinent data were obtained from 11 participating medical institutions.
INTERVENTIONS
Select patients received radiation therapy and/or chemotherapy before or after TORS.
MAIN OUTCOMES AND MEASURES
Locoregional control, disease-specific survival, and overall survival were calculated. We used Kaplan-Meier survival analysis with log-rank testing to evaluate individual variable association with these outcomes, followed by multivariate analysis with Cox proportional hazards regression modeling to identify independent predictors.
RESULTS
Of the 410 patients treated with TORS in this study, 364 (88.8%) had oropharyngeal cancer. Of these 364 patients, information about post-operative adjuvant therapy was known about 338: 106 (31.3) received radiation therapy alone, and 72 (21.3%) received radiation therapy with concurrent chemotherapy. Neck dissection was performed in 323 patients (78.8%). Mean follow-up time was 20 months. Local, regional, and distant recurrence occurred in 18 (4.4%), 15 (3.7%), and 10 (2.4%) of 410 patients, respectively. Seventeen (4.1%) died of disease, and 13 (3.2%) died of other causes. The 2-year locoregional control rate was 91.8% (95% CI, 87.6%-94.7%), disease-specific survival 94.5% (95% CI, 90.6%-96.8%), and overall survival 91% (95% CI, 86.5%-94.0%). Multivariate analysis identified improved survival among women (P = .05) and for patients with tumors arising in tonsil (P = .01). Smoking was associated with worse overall all-cause mortality (P = .01). Although advanced age and tobacco use were associated with locoregional recurrence and disease-specific survival, they, as well as tumor stage and other adverse histopathologic features, did not remain significant on multivariate analysis.
CONCLUSIONS AND RELEVANCE
This large, multi-institutional study supports the role of TORS within the multidisciplinary treatment paradigm for the treatment of head and neck cancer, especially for patients with oropharyngeal cancer. Favorable oncologic outcomes have been found across institutions. Ongoing comparative clinical trials funded by the National Cancer Institute will further evaluate the role of robotic surgery for patients with head and neck cancers.
In 2015 nearly 60 000 patients in the United States will be diagnosed as having head and neck cancer (HNC).1 Despite decreasing smoking rates,2 oropharyngeal cancer is increasing, especially in men, and appears related to high-risk human papillomavirus (HPV).3 In a population-based study4 that used the Surveillance, Epidemiology, and End Results database from 1988 to 2004, the incidence of HPV-positive cancers increased by 225%, whereas the incidence of HPV-negative cancers decreased by 50%. The HPV-associated oropharyngeal cancers have a better overall prognosis when compared with HPV-negative cancers and tend to present with smaller primary tumor burden.5 In addition, HPV may be associated with a surprisingly few nonoropharyngeal cancers in the larynx and pharynx.6
Excluding the oral cavity, patients with HNC have typically been treated with radiation with or without chemotherapy because of the potential morbidities associated with traditional, transmandibular, or transcervical open approaches. Because of the changing epidemiology of this disease2,3 and concerns about the late toxic effects of radiation,7 the role of primary surgery has been revisited.
In 2005, two groups8,9 presented the first experiences in robotic head and neck surgery. The term transoral robotic surgery (TORS) was coined by Weinstein et al10 at The University of Pennsylvania, and since then many single institutions have found that TORS is safe and feasible and has good functional outcomes.10-13 In 2009, the US Food and Drug Administration approved the use of the da Vinci Robotic Surgical System for transoral otolaryngologic procedures, including selected benign and malignant T1 to T2 tumors.14,15 Although several institutions have reported oncologic results after TORS,10-13,16-24 many of these reports are limited by small numbers, limited follow-up, or heterogeneity within the study population.
To better understand the oncologic outcomes after TORS for HNC, larger, more homogenous studies are needed. We report the results of a multi-institutional working group study to better evaluate the oncologic outcomes, to better understand patterns of recurrence and mortality, and to identify the risk factors associated with each. A better understanding of these outcomes might serve as the basis for future prospective studies.
Methods
Patients
For selected patients with squamous cell carcinoma of the head and neck arising from the posterior oral cavity, oropharynx, larynx, and hypopharynx, TORS was performed at 11 institutions from January 1, 2007, through December 31, 2012. Participating institutions included Albert Einstein College of Medicine, Université Catholique de Louvain at Dinant Godinne Namur, Memorial Sloan-Kettering Cancer Center, New York Head and Neck Institute, Mount Sinai Medical Center, Stanford University, University of Alabama at Birmingham, University of California, San Francisco, Mayo Clinic, Rochester, Minnesota, The University of Texas MD Anderson Cancer Center, University of Pittsburgh Medical Center, Veterans Affairs Pittsburgh Healthcare, and Yonsei University School of Medicine. This study was endorsed by the Research Committee of the American Head and Neck Society in 2011.
Treatment
Treatment specifics for patients were institution dependent and usually determined in consultation with a multidisciplinary tumor board. In general, surgery was performed using a da Vinci robotic system (Intuitive Surgical Inc) with techniques previously described.10,11,13,22,25 Surgical candidates generally had smaller volume tumors amenable to surgery, although large tumors were not necessarily a contraindication for surgery and were not excluded from the analysis. Access to the oropharynx was achieved with the Feyh-Kastenbauer, Weinstein-O’Malley-Feyh-Kastenbauer, Crowe-Davis, Laryngeal Advanced Retractor System, or Dingman retractor. Tumors were resected using oncologic principles with attempts to achieve negative surgical margins on frozen-section pathologic analysis. The institutions within this study used differing designations to define negative and/or close margins. Some centers used a 2 mm cutoff while others used 5 mm. A close margin was defined as less than a negative margin but without tumor at the cut edge of the specimen on final pathology, which was defined as a positive margin. Neck dissections were performed for clinically positive neck disease and patients at high risk for occult nodal disease.
In this multicenter review, postoperative adjuvant therapy was not standardized and was given in accordance with local institutional multidisciplinary tumor board recommendations. Patients with adverse pathologic features after surgery received adjuvant treatment with radiation alone or combined chemoradiation. Adverse pathologic features included positive or close margins, extracapsular nodal spread, lymphovascular invasion, perineural invasion, multiple positive lymph nodes, or advanced T and/or N classification. Radiotherapy and chemotherapy protocols varied by institution. Radiation dosage varied based on the presence of persistent disease, high risk of disease, and low risk of disease.
Data Collection
Institutional review board approval for a waiver of consent for this retrospective review was obtained for each institution. De-identified data were pooled. Outcomes were retrospectively collected using a standardized data collection form across institutions. Outcomes pertaining to patient demographics, disease, treatment, and oncologic outcomes were collected. Individual institutions collected data, and analysis was pooled across institutions.
Statistical Analysis
Data were analyzed using SPSS statistical software, version 20 (SPSS Inc), and Stata SE, version 13 (StataCorp). Descriptive statistics were used to summarize data where applicable. Kaplan-Meier methods were used for oncologic outcomes with the log-rank test to compare recurrence and survival outcomes between groups. Multivariate analysis was performed to identify independent risk factors for recurrence and survival using Cox proportional hazards regression models. We selected potential independent risk factors for multivariate analysis based on their significance in univariate testing. For locoregional recurrence, these risk factors included age older than 60 years, a positive smoking history, positive margins, and oropharyngeal wall or faucial arch subsite. For overall survival, multivariate analysis included the risk factors of age older than 60 years, male sex, smoking history, oropharyngeal wall or faucial arch subsite, and tongue base subsites.
Results
Patients
In this multicenter study, TORS was performed on 410 patients, most of whom were men (338 [82.4%]) with tumors classified as T1 (170 [41.5%]) or T2 (172 [42.0%]). TORS was most often selected for patients with oropharyngeal cancer (364 [88.8%] of 410): 45.4% arising in the tonsil, 31.7% in the tongue base, 8.0% in or across the faucial arch or oropharyngeal walls, and 3.4% in the soft palate. A more complete clinical and demographic profile of the study population is presented in Table 1. The mean follow-up was 20 months (range, 1-74 months).
Table 1.
Patient and Tumor Characteristics
| Characteristic | Value (N = 410)a |
|---|---|
| Age, mean (SD), y | 59.6 (10.8) |
|
| |
| Sex | |
|
| |
| Male | 338 (82.4) |
|
| |
| Female | 72 (17.6) |
|
| |
| Smoking history | |
|
| |
| Yes | 224 (54.6) |
|
| |
| No | 114 (27.8) |
|
| |
| Unknown | 72 (17.6) |
|
| |
| Alcohol use history | |
|
| |
| Yes | 166 (40.5) |
|
| |
| No | 86 (21.0) |
|
| |
| Quit | 50 (12.2) |
|
| |
| Unknown | 108 (26.3) |
|
| |
| Subsite | |
|
| |
| Oropharynx | 364 (88.8) |
|
| |
| Supraglottic larynx | 24 (5.9) |
|
| |
| Hypopharynx | 9 (2.2) |
|
| |
| Posterolateral oral cavity | 9 (2.2) |
|
| |
| Other | 4 (1.0) |
|
| |
| Pathologic tumor stage | |
|
| |
| Tx | 18 (4.4) |
|
| |
| T0 | 12 (2.9) |
|
| |
| T1 | 170 (41.5) |
|
| |
| T2 | 172 (42.0) |
|
| |
| T3 | 28 (6.8) |
|
| |
| T4 | 10 (2.4) |
|
| |
| Pathologic nodal stage | |
|
| |
| N0 | 120 (29.2) |
|
| |
| N1 | 57 (13.9) |
|
| |
| N2a | 64 (15.6) |
|
| |
| N2b | 110 (26.8) |
|
| |
| N2c | 16 (3.9) |
|
| |
| N3 | 7 (1.7) |
|
| |
| Nx | 36 (8.8) |
|
| |
| Positive margins | |
|
| |
| Yes | 39 (9.9) |
|
| |
| No | 354 (86.3) |
|
| |
| Unknown | 17 (4.1) |
|
| |
| Close margins (1-5 mm) | |
|
| |
| Yes | 86 (21.0) |
|
| |
| No | 170 (41.5) |
|
| |
| Not specified | 154 (37.6) |
|
| |
| Perineural invasion | |
|
| |
| Yes | 67 (16.3) |
|
| |
| No | 234 (57.0) |
|
| |
| Not specified | 109 (26.6) |
|
| |
| Lymphovascular invasion | |
|
| |
| Yes | 78 (19.0) |
|
| |
| No | 217 (52.9) |
|
| |
| Not specified | 115 (28.0) |
|
| |
| Multiple nodal positivity | |
|
| |
| Yes | 110 (26.8) |
|
| |
| No | 241 (58.8) |
|
| |
| Unknown | 59 (14.4) |
|
| |
| Extracapsular spread | |
|
| |
| Yes | 58 (14.2) |
|
| |
| No | 100 (24.4) |
|
| |
| Unknown | 252 (61.5) |
|
| |
| HPV status (229 patients tested) | |
|
| |
| Negative | 70 (17.1) |
|
| |
| Positive | 159 (38.8) |
|
| |
| Unknown | 181 (44.1) |
|
| |
| p16 positivity (219 patients tested) | |
|
| |
| Negative | 61 (14.9) |
|
| |
| Positive | 158 (38.5) |
|
| |
| Unknown | 191 (46.6) |
|
| |
| Neck dissection | |
|
| |
| Yes | 323 (78.8) |
|
| |
| No | 77 (18.8) |
|
| |
| Unknown | 10 (2.4) |
|
| |
| Adjuvant treatment (338 patients) | |
|
| |
| Radiotherapy | 106 (25.9) |
|
| |
| Chemoradiotherapy | 72 (17.6) |
|
| |
| No adjuvant treatment received | 160 (39.0) |
|
| |
| Unknown | 72 (17.6) |
Abbreviation: HPV, human papillomavirus.
Data are presented as number (percentage) of patients unless otherwise indicated.
Smoking status was known for 338 (82.4%) of 410 patients. Of these, 224 (66.3%) had smoked at some point in their lives. Of the smokers, 10.3% were pipe or cigar smokers, 14.7% smoked 10 pack-years or less, 27.2% smoked 11 through 19 pack-years, 28.1% smoked 20 through 40 pack-years, and 19.6% smoked more than 40 pack-years. Alcohol use was common in the study population: 166 (40.5%) actively consumed alcohol, 50 (12.2%) had quit, and 86 (26.3%) did not consume alcohol. Of these, 55.0% currently consumed alcohol, 16.6% had quit, and 28.5% had no history of alcohol use.
The HPV status was known in 229 (55.9%) of 410 patients. Of these, 159 patients (69.4%) were HPV positive by HPV polymerase chain reaction assay or in situ hybridization techniques. The p16 status was known for 219 (53.4%) of 410 patients. Of the 219 patients with known p16 status, 158 (72.1%) were p16-positive by immunohistochemical staining.
Treatment
All patients underwent TORS for resection of the primary tumor. Eleven (2.7%) of 410 patients previously received radiation alone or chemoradiation and underwent TORS as a salvage procedure. Of the 393 patients (95.9%) who had known margin status, 39 (9.9%) had positive margins.
Four hundred patients had information regarding whether a neck dissection was performed. Neck dissection was performed in 323 patients (78.8%). Of those who underwent neck dissections, 226 (70.0%) underwent the dissection concurrently with TORS surgery, and 97 (30.0%) underwent neck dissection in a staged fashion. Adjuvant treatment data were unknown for 72 (17.6%) of 410 patients. Of those with known adjuvant treatment status, TORS alone was performed in 160 (47.3%) of 338 patients, 106 (31.4%) of 338 underwent adjuvant radiotherapy, and 72 (21.3%) of 338 patients underwent adjuvant chemoradiation. Therefore, we had available adjuvant treatment data on 338 patients (82.4%), of whom 52.7% received adjuvant therapy.
Recurrences
Forty-three patients (10.5%) experienced recurrences. Thirty-three patients (8.0%) experienced local or regional recurrences. Ten patients (2.4%) experienced distant metastases. The median time to locoregional recurrence was 16.4 months. Twenty-one patients (5.1%) experienced recurrences within the first 2 years after surgery. In patients experiencing recurrences, 8 of 130 tongue base primary tumors (6.2%), 5 of 186 tonsil primary tumors (2.7%), 6 of 34 oropharyngeal wall or faucial arch primary tumors (17.6%), and 2 of 14 soft-palate primary tumors (14.3%) recurred. Tumor recurred in 13 (8.1%) of 160 patients treated with TORS alone without adjuvant treatment.
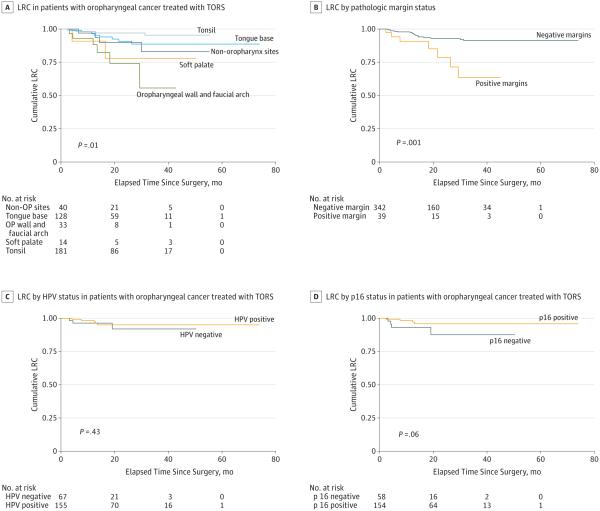
The 2-year and 3-year locoregional control rates for the entire cohort were 91.8% (95% CI, 87.6%-94.7%) and 88.8% (95% CI, 83.5%-92.4%) (Figure 1). Because 88.8% of the patients in our cohort presented with oropharyngeal primary tumors, we evaluated locoregional control by oropharyngeal subsite, HPV status, and p16 status (Figure 1). Tonsil primary tumors had the highest rate of locoregional control by log-rank testing (P = .01). We did not observe differences in locoregional control associated with known HPV or p16 status. The greatest difference in locoregional control was seen when comparing patients with positive margins and those with clear margins (P = .001) (Figure 1). At 2-year follow-up, the locoregional control rate for patients with positive margins during TORS was 78.6% compared with 92.9% when surgical margins were negative. At 3-year follow-up, the locoregional control rate for patients with positive margins during TORS was 63.5% compared with 91.2% when surgical margins were negative. Risk factors for locoregional recurrence are outlined in Table 2. In univariate analysis, older age, smoking status, oropharyngeal wall or faucial arch primary tumor site, and positive margins were risk factors for disease recurrence. Tonsil primary site predicted a lower risk of recurrence compared with all sites. In multivariate analysis, none of these predictors remained a significant predictor of recurrence (Table 3). Although comparison of oropharyngeal subsites revealed differences in locoregional recurrence rates, we did not observe differences in recurrence rates between overall head and neck sites (ie, oropharynx, oral cavity, supraglottic larynx, hypopharynx).
Figure 1. Locoregional Control (LRC) for Patients Treated With Transoral Robotic Surgery (TORS).
Locoregional control by oropharyngeal subsite in all patients and pathologic margin status, human papillomavirus (HPV) status, and p16 status in patients with oropharyngeal primary tumors.
Table2.
Factors Associated With Locoregional Control and Overall Survival (N=410)
| Factor | 2-Year Locoregional Control, % |
P Value for Log-Rank Testa |
2-Year Overall Survival, % |
P Value for Log-Rank Testa |
|---|---|---|---|---|
| Age, y | ||||
|
| ||||
| <60 | 94.6 | 87.6 | ||
|
|
.01 |
|
.01 | |
| ≥60 | 88.7 | 93.9 | ||
|
| ||||
| Sex | ||||
|
| ||||
| Male | 91.8 | 89.1 | ||
|
|
.94 |
|
.03 | |
| Female | 91.9 | 98.6 | ||
|
| ||||
| Smoking history | ||||
|
| ||||
| Yes | 88.8 | 86.0 | ||
|
|
.01 |
|
.001 | |
| No | 96.9 | 97.2 | ||
|
| ||||
| HPV | ||||
|
| ||||
| Positive | 95.0 | 96.7 | ||
|
|
.52 |
|
.23 | |
| Negative | 92.0 | 93.7 | ||
|
| ||||
| p16 | ||||
|
| ||||
| Positive | 96.9 | 100 | ||
|
|
.08 |
|
.13 | |
| Negative | 87.6 | 94.9 | ||
|
| ||||
| Subsite | ||||
|
| ||||
| Tonsil vs all other | 97.1 | .01 | 95.4 | .01 |
|
| ||||
| BOT vs all other | 90.7 | .99 | 92.1 | .83 |
|
| ||||
| Oropharyngeal wall and faucial arch vs all others |
74.1 | .001 | 81.8 | .04 |
|
| ||||
| Soft palate vs all other sites | 77.9 | .19 | 100 | .26 |
|
| ||||
| Positive margins | ||||
|
| ||||
| Yes | 78.6 | 93.9 | ||
|
|
.001 |
|
.36 | |
| No | 92.9 | 90.9 | ||
|
| ||||
| Perineural invasion | ||||
|
| ||||
| Yes | 94.4 | 86.3 | ||
|
|
.82 |
|
.55 | |
| No | 91.0 | 90.7 | ||
|
| ||||
| Lymphovascular invasion | ||||
|
| ||||
| Yes | 100 | 88.6 | ||
|
|
.01 |
|
.94 | |
| No | 89.2 | 90.0 | ||
|
| ||||
| Tumor stage | ||||
|
| ||||
| T1-T2 | 91.7 | 90.1 | ||
|
|
.50 |
|
.86 | |
| T3-T4 | 96.1 | 92.6 | ||
|
| ||||
| Nodal stage | ||||
|
| ||||
| N0 | 90.7 | .16 | 91.0 | .53 |
|
| ||||
| N1 | 93.6 | .47 | 84.9 | .12 |
|
| ||||
| N2a | 94.7 | .40 | 92.4 | .72 |
|
| ||||
| N2b | 100 | .01 | 90.6 | .88 |
|
| ||||
| N2c | 100 | .27 | 90.9 | .68 |
|
| ||||
| N3 | 100 | .47 | 100 | .41 |
|
| ||||
| Nx | 42.6 | <.001 | 100 | .52 |
|
| ||||
| Adjuvant treatment | ||||
|
| ||||
| None | 86.6 | .12 | 93.5 | .58 |
|
| ||||
| Radiotherapy | 98.9 | .40 | 95.1 | .14 |
|
| ||||
| Chemoradiotherapy | 96.5 | .16 | 81.0 | .03 |
Abbreviations: BOT, base of tongue; HPV, human papillomavirus.
Log-rank test.
Table 3.
Multivariate Analysis of Risk Factors for Locoregional Recurrence and All-Cause Mortality
| Factor | HR (95% CI) | P Valuea |
|---|---|---|
| Risk Factors for Locoregional Recurrence | ||
|
| ||
| Age >60 y | 2.49 (0.90-6.92) | .08 |
|
| ||
| Smoking history | 3.60 (0.81-15.9) | .09 |
|
| ||
| Positive margins | 2.43 (0.92-6.47) | .07 |
|
| ||
| Tonsil primary site | 0.28 (0.08-1.00) | .05 |
|
| ||
| Oropharyngeal wall, faucial wall primary site | 2.51 (0.87-7.28) | .09 |
|
| ||
| Risk Factors for All-Cause Mortality | ||
|
| ||
| Age >60 y | 1.76 (0.79-3.96) | .17 |
|
| ||
| Female sex | 0.18 (0.02-0.99) | .05 |
|
| ||
| Smoking history | 6.90 (1.57-28.9) | .01 |
|
| ||
| Tonsil primary site | 0.18 (0.07-0.65) | .01 |
|
| ||
| Oropharyngeal wall, faucial wall primary site | 0.91 (0.36-3.23) | .90 |
|
| ||
| Tongue base primary site | 0.53 (0.26-1.68) | .39 |
Abbreviation: HR, hazard ratio.
Cox proportional hazards model.
Survival
Overall, there were 30 deaths (7.3%) in the cohort. There was a single mortality related to surgery due to postoperative hemorrhage on the night of surgery. Seventeen patients (4.2%) died of their disease, and 13 patients (3.2%) died without any evidence of disease. Of those who died without any evidence of disease, 8 died of unknown causes, and 4 died of causes unrelated to cancer, including cardiac arrest (n = 1), cerebrovascular accident (n = 1), complication of gastrostomy tube placement (n = 1), and ruptured aortic aneurysm (n = 1).
The 2- and 3-year overall survival rates were 91% (95% CI, 86.5%-94.0%) and 87.1% (95% CI, 81.4%-91.2%), respectively (Figure 2). The 2- and 3-year disease-specific survival rates were 94.5% (95% CI, 90.6%-96.8%) and 92.5% (95% CI, 87.8%-95.5%), respectively. Again, because most patients presented with oropharyngeal primary tumors, we compared overall and disease-specific survival rates based on HPV and p16 status. No significant differences were observed. On univariate analysis, risk factors for death of any cause included age, male sex, tobacco history, and oropharyngeal wall or faucial arch primary site (Table 2). On multivariate analysis, only tobacco smoking history predicted worse overall survival, whereas tonsil primary site was associated with improved overall survival (Table 3). Unfortunately, tobacco smoking as a negative risk predictor may have been confounded by limited HPV data in this study; thus, we were cautious to draw any conclusion about the influence of tobacco itself. We did not perform a survival analysis comparing overall head and neck sites because no differences in locoregional recurrence rates were observed (eFigure in the Supplement). Furthermore, because most patients (88.8%) in this study had oropharyngeal primary tumors, a robust survival comparison that included nonoropharyngeal sites was not possible.
Figure 2. Overall Survival (OS) and Disease-Specific Survival (DSS) for Patients Treated With Transoral Robotic Surgery (TORS).
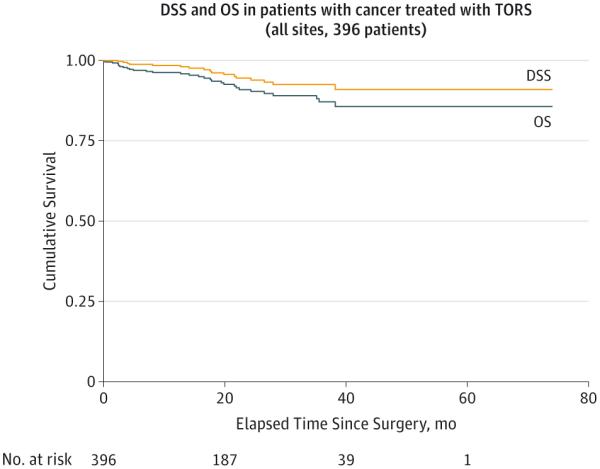
Overall survival and DSS in all 396 study patients with head and neck cancer.
Discussion
To our knowledge, this study reports the largest series of patients undergoing TORS for the treatment of HNC. Our collaboration across 11 medical centers draws on a diverse experience to further elucidate the role of TORS across various multidisciplinary treatment paradigms. Informative data on adjuvant treatment regimens for most of these patients were available to comprehensively review patients’ treatment plans. The findings from the present study support the use of TORS as a treatment alternative for select HNCs. Ongoing clinical trials will better help delineate its role.
This study presents a broad description of TORS applied to the treatment of HNC. However, as reported, 88.8% of patients had oropharyngeal primary tumors. Our rationale for the inclusion of other sites (ie, supraglottic larynx, hypopharynx, posterolateral oral cavity) was to determine where TORS fits into the multidisciplinary treatment paradigm across institutions. On the basis of this experience, TORS appears to play the greatest role in the multidisciplinary management of patients with oropharyngeal cancer. Furthermore, we were able to evaluate morbidity and mortality from postoperative bleeding experienced by the various participating centers. Fatal post-operative hemorrhage was encountered in 1 (0.2%) of 410 patients. This death occurred the night of surgery in a patient who had previously received preoperative chemotherapy (cetuximab) but no radiation therapy. This is an important adverse event relevant to all sites amenable to transoral endoscopic head and neck surgery. Patients should be counseled about this rare but catastrophic surgical complication but reminded that primary chemoradiation protocols carry an approximately 2% treatment-related mortality risk.5
The pathophysiologic basis of oropharyngeal cancers has changed markedly during the past several decades. In a review of the National Cancer Institute’s Surveillance, Epidemiology, and End Results database, Chaturvedi et al4 found that from the years 1973 to 2004 the incidence of high-risk HPV-associated oropharyngeal cancers increased by 225%, whereas HPV-negative cancers decreased by 50%. It is now estimated that more than 70% of oropharyngeal cancers have evidence of an HPV infection. These virally mediated cancers tend to present with smaller primary tumor volume and more advanced nodal disease.21,26,27 This changing pattern of the disease with fewer locally advanced tumors may then be more amenable to surgical resection without rendering patients functionally disabled from high-volume resections. Indeed, of the 364 patients (88.8%) in this study treated for oropharyngeal primary tumors, 314 had pathologic stage T2 or lower disease.
In this present study, 338 patients had available data regarding adjuvant therapy. Of these, 106 (31.3%) received radiation therapy alone and 72 (21.3%) received combined chemoradiotherapy. In aggregate, 50.2% had N2a or greater nodal disease with negative primary margins or positive margins with any nodal status, both disease categories that would commonly warrant at least adjuvant radiation therapy.
The early functional outcomes studies for TORS suggest good postoperative results with early oral alimentation and limited long-term sequelae.7,11-13 In a prospective comparative study,11 patients treated with TORS had significantly better eating ability and dietary intake 2 weeks after treatment compared with patients treated with chemoradiation. In addition, surgical patients experienced a return of their oral dietary intake 1 year after surgery, whereas patients treated with chemoradiation continued to have decreased oral diet as assessed by the Performance Status Scale questionnaire. In the absence of persistent disease after surgery, those who receive adjuvant radiotherapy do so at a reduced dose compared with definitive therapy, which may reduce the early and late toxic effects of radiotherapy.28
With the emergence of TORS in the treatment of oropharyngeal cancer in the last decade, reporting of oncologic results was relatively limited to informative but smaller cohort sizes. Weinstein et al10 reported cancer oncologic results for 47 patients undergoing TORS for oropharyngeal cancer. In their study, the 2-year disease-specific survival was 90%, and local recurrences occurred in 2% of patients, regional recurrences in 4%, and distant metastases in 9%. White et al24 reported 1- and 2-year oncologic outcomes in a cohort that included all head and neck sites (including larynx and oral cavity). Among the 89 patients included in the analysis, there were 11 recurrences (3 local, 7 regional, and 1 distant) and an overall recurrence-free survival rate of 86.5%. Genden et al17 reported oncologic results on 30 patients with head and neck squamous cell carcinomas treated with TORS. They described an 18-month locoregional control rate of 91%, a disease-free survival rate of 78%, and an overall survival rate of 90%. Moore et al16 followed up 66 patients for a minimum of 2 years and reported a 2-year disease-specific survival rate of 95.1% and a recurrence-free survival rate of 92.4%, with 3-year local and regional control rates of 97.0% and 94.0%, respectively. More recently, Weinstein et al15 reported on the largest multicenter series of patients with cancer undergoing TORS (N = 177), with the primary oncologic outcomes of interest being margin status. Seven (4.3%) of 161 patients with available margin data had positive final margins. In this largest reported series of patients with cancer undergoing TORS, similar findings are observed: obtaining negative margins is feasible in most properly selected patients. Low rates of positive margins in our current study also demonstrate favorable rates of locoregional control and disease-specific mortality in a larger cohort of patients treated with TORS.
Our results also compare favorably with oncologic outcome data for definitive radiotherapy. Eisbruch et al29 and the Radiation Therapy Oncology Group described a locoregional control rate of 91% for patients treated with accelerated hypo-fractionated intensity-modulated radiation therapy without chemotherapy for T1 and T2 oropharyngeal cancers with N0 or N1 nodal disease. Garden et al30 found a locoregional control rate of 94%, a recurrence-free survival rate of 88%, and an overall survival rate of 94% in a similar cohort of patients treated with intensity-modulated radiation therapy for small primary disease. Mendenhall et al31 found a 5-year local control rate of 93% for T1 and 91% for T2 oropharyngeal cancers treated with intensity-modulated radiation therapy. Sher et al32 found similar results with a 3-year locoregional control rate of 97% and 79% for T1 and T2 oropharyngeal cancers, respectively, treated with concurrent chemoradiotherapy or with induction chemotherapy followed by concurrent chemoradiotherapy.
To our knowledge, this is the largest study reporting oncologic outcomes for oropharyngeal cancers treated with TORS. The size of the cohort enabled us to better delineate patterns of recurrence in this population and risk factors for recurrence. Overall, 43 patients (10.5%) experienced a recurrence, with 17 patients (4.2%) dying of disease after treatment. No independent predictors of recurrence remained significant in multivariate analysis although positive margin status neared significance (P = .06). Positive margin status is a well-known predictor of local recurrence in oropharyngeal cancer.33,34 In our study, there was a higher incidence of positive margins in patients with oropharyngeal wall or faucial arch tumors; however, only 34 of 410 patients had this primary subsite, making interpretation of these results challenging.
Unfortunately, this registry study did not have adequate HPV status information for 181 of 410 patients (44.1%). This limits the ability to accurately compare oncologic outcomes based on HPV status. Our finding that HPV-negative tumors did not have a higher likelihood of recurrence or mortality compared with those with HPV-positive tumors requires further validation. Cohen et al16 also did not observe a difference in locoregional control or survival in patients with HPV-associated tumors treated with TORS. In our study, because most patients had low primary tumor volume irrespective of HPV status, this factor may explain why local control rates and overall survival did not differ based on HPV status. Ultimately, our results indicate favorable oncologic outcomes for HPV-positive and HPV-negative groups using a TORS approach.
There are limitations to this study. As a retrospective study, variability in reporting across the centers and thus missing data elements pertaining to pathologic variables limit definitive comparative analyses. As a result, many subgroup analyses may be underpowered to detect significant differences. Only prospective studies with standardized end points and subsequent pathologic analysis can circumvent this issue. In addition, with a mean follow-up time of 20 months, the follow-up time may limit definitive conclusions about oncologic outcomes after TORS. However, our results suggest that most recurrences occur within the first 2 years. Last, the data collected here represent the results of 11 institutions with expertise in TORS. The results may not necessarily be applicable to new adopters of this technology because there is a learning curve to become technically facile. Future prospective studies are needed to better delineate the role of TORS in oropharyngeal cancer35 and to compare the functional outcomes of this approach and definitive radiotherapy. Results of study 3311 from the Eastern Cooperative Oncology Group, a National Cancer Institute–funded clinical trial in transoral endoscopic head and neck surgery, may help to further elucidate the role of TORS within the HPV-positive oropharyngeal cancer. The present study serves to support favorable oncologic results achieved with patients treated with TORS.
Conclusions
This large, multicenter collaborative study supports the role of transoral robotic head and neck surgery within the multidisciplinary treatment paradigm for the treatment of HNC, especially for patients with oropharynx cancer. Pathologic staging obtained from TORS may allow multidisciplinary teams to administer adjuvant therapy using a precision-medicine approach. Favorable oncologic outcomes have been found across institutions. Ongoing prospective clinical trials will provide further information regarding integrating robotic surgery into a treatment algorithm for optimal patient care.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported in part by funds from the Bureau of Labor Statistics, Research and Development, Department of Veterans Affairs (Dr Duvvuri).
Footnotes
Author Contributions: Drs de Almeida and Holsinger had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: de Almeida, Miles, White, Duvvuri, Ferris, Wang, Genden, Holsinger.
Acquisition, analysis, or interpretation of data: de Almeida, Li, Magnuson, Smith, Moore, Lawson, Remacle, Ganly, Kraus, Teng, Miles, Duvvuri, Ferris, Mehta, Kiyosaki, Damrose, Wang, Kupferman, Koh, Holsinger.
Drafting of the manuscript: de Almeida, Li, Teng, Miles, Genden, Holsinger.
Critical revision of the manuscript for important intellectual content: de Almeida, Li, Magnuson, Smith, Moore, Lawson, Remacle, Ganly, Kraus, Teng, White, Duvvuri, Ferris, Mehta, Kiyosaki, Damrose, Wang, Kupferman, Koh.
Statistical analysis: de Almeida, Li, Ganly, Kiyosaki, Holsinger.
Obtained funding: Duvvuri, Holsinger.
Administrative, technical, or material support: de Almeida, Smith, Lawson, Remacle, Duvvuri, Ferris, Mehta, Kiyosaki, Damrose, Kupferman, Holsinger.
Study supervision: de Almeida, Magnuson, Moore, Teng, Miles, Ferris, Koh, Genden, Holsinger.
Conflict of Interest Disclosures: None reported.
Role of the Funder/Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Previous Presentation: Early results of this study were presented at the Eighth International Conference on Head and Neck Cancer; July 22, 2012; Toronto, Ontario, Canada. Final results were presented at the Annual Meeting of the American Head and Neck Society; April 22, 2015; Boston, Massachusetts.
Disclaimer: This work does not represent the views of the US government or the US Department of Veterans Affairs.
Supplemental content at jamaotolaryngology.com
Contributor Information
John R. de Almeida, Department of Otolaryngology–Head and Neck Surgery, Princess Margaret Cancer Center, Toronto, Ontario, Canada.
Ryan Li, Division of Head and Neck Surgery, Department of Otolaryngology–Head and Neck Surgery, Stanford University, Palo Alto, California.
J. Scott Magnuson, Department of Otolaryngology–Head and Neck Surgery, University of Alabama at Birmingham.
Richard V. Smith, Department of Otorhinolaryngology–Head and Neck Surgery, Montefiore Medical Center, Albert Einstein College of Medicine, New York, New York.
Eric Moore, Department of Otolaryngology–Head and Neck Surgery, Mayo Clinic, Rochester, Minnesota.
Georges Lawson, Catholic University of Louvain at Mont-Godinne, Yvoir, Belgium.
Marc Remacle, Catholic University of Louvain at Mont-Godinne, Yvoir, Belgium.
Ian Ganly, Department of Surgery, Head and Neck Oncology, Memorial Sloan Kettering Cancer Center, New York, New York.
Dennis H. Kraus, New York Head and Neck Institute, North Shore Health System, New York, New York.
Marita S. Teng, Department of Otolaryngology Head and Neck Surgery, Icahn School of Medicine at Mount Sinai, New York, New York.
Brett A. Miles, Department of Otolaryngology Head and Neck Surgery, Icahn School of Medicine at Mount Sinai, New York, New York.
Hilliary White, Department of Otolaryngology–Head and Neck Surgery, University of Alabama at Birmingham.
Umamaheswar Duvvuri, Department of Otolaryngology–Head and Neck Surgery, University of Pittsburgh Medical Center, Veterans Affairs Pittsburgh HealthCare System, Pittsburgh, Pennsylvania.
Robert L. Ferris, Department of Otolaryngology–Head and Neck Surgery, University of Pittsburgh Medical Center, Veterans Affairs Pittsburgh HealthCare System, Pittsburgh, Pennsylvania.
Vikas Mehta, Department of Otolaryngology–Head and Neck Surgery, University of Pittsburgh Medical Center, Veterans Affairs Pittsburgh HealthCare System, Pittsburgh, Pennsylvania.
Krista Kiyosaki, Division of Head and Neck Surgery, Department of Otolaryngology–Head and Neck Surgery, Stanford University, Palo Alto, California.
Edward J. Damrose, Division of Head and Neck Surgery, Department of Otolaryngology–Head and Neck Surgery, Stanford University, Palo Alto, California.
Steven J. Wang, Department of Otolaryngology–Head and Neck Surgery, University of California, San Francisco.
Michael E. Kupferman, Department of Head and Neck Surgery, MD Anderson Cancer Center, Houston, Texas.
Yoon Woo Koh, Severance Hospital, Yonsei University School of Medicine, Seoul, Korea.
Eric M. Genden, Department of Otolaryngology Head and Neck Surgery, Icahn School of Medicine at Mount Sinai, New York, New York.
F. Christopher Holsinger, Division of Head and Neck Surgery, Department of Otolaryngology–Head and Neck Surgery, Stanford University, Palo Alto, California; Department of Head and Neck Surgery, MD Anderson Cancer Center, Houston, Texas.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Sturgis EM, Cinciripini PM. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer. 2007;110(7):1429–1435. doi: 10.1002/cncr.22963. [DOI] [PubMed] [Google Scholar]
- 3.Ryerson AB, Peters ES, Coughlin SS, et al. Burden of potentially human papillomavirus-associated cancers of the oropharynx and oral cavity in the US, 1998-2003. Cancer. 2008;113(10):2901–2909. doi: 10.1002/cncr.23745. suppl. [DOI] [PubMed] [Google Scholar]
- 4.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung CH, Zhang Q, Kong CS, et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol. 2014;32(35):3930–3938. doi: 10.1200/JCO.2013.54.5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutcheson KA, Holsinger FC, Kupferman ME, Lewin JS. Functional outcomes after TORS for oropharyngeal cancer: a systematic review. Eur Arch Otorhinolaryngol. 2015;272(2):463–471. doi: 10.1007/s00405-014-2985-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hockstein NG, Nolan JP, O’Malley BW, Jr, Woo YJ. Robot-assisted pharyngeal and laryngeal microsurgery: results of robotic cadaver dissections. Laryngoscope. 2005;115(6):1003–1008. doi: 10.1212/01.WNL.0000164714.90354.7D. [DOI] [PubMed] [Google Scholar]
- 9.McLeod IK, Melder PC. Da Vinci robot-assisted excision of a vallecular cyst: a case report. Ear Nose Throat J. 2005;84(3):170–172. [PubMed] [Google Scholar]
- 10.Weinstein GS, O’Malley BW, Jr, Cohen MA, Quon H. Transoral robotic surgery for advanced oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2010;136(11):1079–1085. doi: 10.1001/archoto.2010.191. [DOI] [PubMed] [Google Scholar]
- 11.Genden EM, Desai S, Sung CK. Transoral robotic surgery for the management of head and neck cancer: a preliminary experience. Head Neck. 2009;31(3):283–289. doi: 10.1002/hed.20972. [DOI] [PubMed] [Google Scholar]
- 12.Iseli TA, Kulbersh BD, Iseli CE, Carroll WR, Rosenthal EL, Magnuson JS. Functional outcomes after transoral robotic surgery for head and neck cancer. Otolaryngol Head Neck Surg. 2009;141(2):166–171. doi: 10.1016/j.otohns.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Moore EJ, Olsen KD, Kasperbauer JL. Transoral robotic surgery for oropharyngeal squamous cell carcinoma: a prospective study of feasibility and functional outcomes. Laryngoscope. 2009;119(11):2156–2164. doi: 10.1002/lary.20647. [DOI] [PubMed] [Google Scholar]
- 14.US Food and Drug Administration . 501(k) Summary: Indications for Use for Intuitive Surgical Endoscopic Instrument Control System for Transoral Otolaryngology Procedures. US Food and Drug Administration; Silver Spring, MD: 2009. [Google Scholar]
- 15.Weinstein GS, O’Malley BW, Jr, Magnuson JS, et al. Transoral robotic surgery: a multicenter study to assess feasibility, safety, and surgical margins. Laryngoscope. 2012;122(8):1701–1707. doi: 10.1002/lary.23294. [DOI] [PubMed] [Google Scholar]
- 16.Cohen MA, Weinstein GS, O’Malley BW, Jr, Feldman M, Quon H. Transoral robotic surgery and human papillomavirus status: Oncologic results. Head Neck. 2011;33(4):573–580. doi: 10.1002/hed.21500. [DOI] [PubMed] [Google Scholar]
- 17.Genden EM, Kotz T, Tong CC, et al. Transoral robotic resection and reconstruction for head and neck cancer. Laryngoscope. 2011;121(8):1668–1674. doi: 10.1002/lary.21845. [DOI] [PubMed] [Google Scholar]
- 18.Hurtuk A, Agrawal A, Old M, Teknos TN, Ozer E. Outcomes of transoral robotic surgery: a preliminary clinical experience. Otolaryngol Head Neck Surg. 2011;145(2):248–253. doi: 10.1177/0194599811402172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore EJ, Olsen SM, Laborde RR, et al. Long-term functional and oncologic results of transoral robotic surgery for oropharyngeal squamous cell carcinoma. Mayo Clin Proc. 2012;87(3):219–225. doi: 10.1016/j.mayocp.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Malley BW, Jr, Weinstein GS, Hockstein NG. Transoral robotic surgery (TORS): glottic microsurgery in a canine model. J Voice. 2006;20(2):263–268. doi: 10.1016/j.jvoice.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Ukpo OC, Pritchett CV, Lewis JE, Weaver AL, Smith DI, Moore EJ. Human papillomavirus-associated oropharyngeal squamous cell carcinomas: primary tumor burden and survival in surgical patients. Ann Otol Rhinol Laryngol. 2009;118(5):368–373. doi: 10.1177/000348940911800509. [DOI] [PubMed] [Google Scholar]
- 22.Vergez S, Lallemant B, Ceruse P, et al. Initial multi-institutional experience with transoral robotic surgery. Otolaryngol Head Neck Surg. 2012;147(3):475–481. doi: 10.1177/0194599812443221. [DOI] [PubMed] [Google Scholar]
- 23.Weinstein GS, O’Malley BW, Jr, Snyder W, Sherman E, Quon H. Transoral robotic surgery: radical tonsillectomy. Arch Otolaryngol Head Neck Surg. 2007;133(12):1220–1226. doi: 10.1001/archotol.133.12.1220. [DOI] [PubMed] [Google Scholar]
- 24.White HN, Moore EJ, Rosenthal EL, et al. Transoral robotic-assisted surgery for head and neck squamous cell carcinoma: one- and 2-year survival analysis. Arch Otolaryngol Head Neck Surg. 2010;136(12):1248–1252. doi: 10.1001/archoto.2010.216. [DOI] [PubMed] [Google Scholar]
- 25.Holsinger FC, McWhorter AJ, Ménard M, Garcia D, Laccourreye O. Transoral lateral oropharyngectomy for squamous cell carcinoma of the tonsillar region, I: technique, complications, and functional results. Arch Otolaryngol Head Neck Surg. 2005;131(7):583–591. doi: 10.1001/archotol.131.7.583. [DOI] [PubMed] [Google Scholar]
- 26.Dahlstrom KR, Calzada G, Hanby JD, et al. An evolution in demographics, treatment, and outcomes of oropharyngeal cancer at a major cancer center: a staging system in need of repair. Cancer. 2013;119(1):81–89. doi: 10.1002/cncr.27727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pytynia KB, Dahlstrom KR, Sturgis EM. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014;50(5):380–386. doi: 10.1016/j.oraloncology.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maciejewski B, Withers HR, Taylor JM, Hliniak A. Dose fractionation and regeneration in radiotherapy for cancer of the oral cavity and oropharynx, part 2: normal tissue responses: acute and late effects. Int J Radiat Oncol Biol Phys. 1990;18(1):101–111. doi: 10.1016/0360-3016(90)90273-m. [DOI] [PubMed] [Google Scholar]
- 29.Eisbruch A, Harris J, Garden AS, et al. Multi-institutional trial of accelerated hypofractionated intensity-modulated radiation therapy for early-stage oropharyngeal cancer (RTOG 00-22) Int J Radiat Oncol Biol Phys. 2010;76(5):1333–1338. doi: 10.1016/j.ijrobp.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garden AS, Morrison WH, Wong PF, et al. Disease-control rates following intensity-modulated radiation therapy for small primary oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2007;67(2):438–444. doi: 10.1016/j.ijrobp.2006.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendenhall WM, Amdur RJ, Morris CG, Kirwan JM, Li JG. Intensity-modulated radiotherapy for oropharyngeal squamous cell carcinoma. Laryngoscope. 2010;120(11):2218–2222. doi: 10.1002/lary.21144. [DOI] [PubMed] [Google Scholar]
- 32.Sher DJ, Thotakura V, Balboni TA, et al. Treatment of oropharyngeal squamous cell carcinoma with IMRT: patterns of failure after concurrent chemoradiotherapy and sequential therapy. Ann Oncol. 2012;23(9):2391–2398. doi: 10.1093/annonc/mdr609. [DOI] [PubMed] [Google Scholar]
- 33.Laccourreye O, Hans S, Ménard M, Garcia D, Brasnu D, Holsinger FC. Transoral lateral oropharyngectomy for squamous cell carcinoma of the tonsillar region, II: an analysis of the incidence, related variables, and consequences of local recurrence. Arch Otolaryngol Head Neck Surg. 2005;131(7):592–599. doi: 10.1001/archotol.131.7.592. [DOI] [PubMed] [Google Scholar]
- 34.Zieske LA, Johnson JT, Myers EN, Thearle PB. Squamous cell carcinoma with positive margins: surgery and postoperative irradiation. Arch Otolaryngol Head Neck Surg. 1986;112(8):863–866. doi: 10.1001/archotol.1986.03780080063014. [DOI] [PubMed] [Google Scholar]
- 35.Adelstein DJ, Ridge JA, Brizel DM, et al. Transoral resection of pharyngeal cancer: summary of a National Cancer Institute Head and Neck Cancer Steering Committee Clinical Trials Planning Meeting, November 6-7, 2011, Arlington, Virginia. Head Neck. 2012;34(12):1681–1703. doi: 10.1002/hed.23136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.