Abstract
The receptor for calcitonin gene-related peptide (CGRP) and adrenomedullin (AM) requires an intracellular peripheral membrane protein named CGRP-receptor component protein (RCP) for signaling. RCP is required for CGRP and AM receptor signaling, and it has recently been discovered that RCP enables signaling by binding directly to the receptor. RCP is present in most immortalized cell lines, but in vivo RCP expression is limited to specific subsets of cells, usually co-localizing with CGRP-containing neurons. RCP protein expression correlates with CGRP efficacy in vivo, suggesting that RCP regulates CGRP signaling in vivo as it does in cell culture. RCP is usually identified in cytoplasm or membranes of cells, but recently has been observed in nucleus of neurons, suggesting an additional transcriptional role for RCP in cell function. Together, these data support an essential role for RCP in CGRP and AM receptor function, in which RCP expression enhances signaling of the CGRP or AM receptor, and therefore increases the efficacy of CGRP and AM in vivo.
Keywords: Calcitonin gene-related peptide, adrenomedullin, calcitonin-like receptor, receptor activity modifying protein, CGRP-receptor component protein, signal transduction, trafficking
INTRODUCTION
The receptor for CGRP is unique for G protein-coupled receptors (GPCR) in that it is a complex of three proteins: the ligand-binding GPCR named calcitonin-like receptor (CLR) which is in a complex with two accessory proteins, receptor activity modulating protein (RAMP1) and CGRP-receptor component protein (RCP). RAMP1 is a single transmembrane protein that aids trafficking of CLR to the cell surface, and confers pharmacologic specificity to CLR [1]. CLR can also interact with the RAMP homologs RAMP2 or RAMP3 to form a high affinity receptor for adrenomedullin (AM). The role for RCP in CGRP function has been less clear. RCP is a soluble protein found in the cytoplasm and in the membrane associated with CLR. In cell culture RCP is required for CLR signaling, and it has recently been discovered that RCP enables signaling by binding directly to CLR. RCP is present in most immortalized cell lines, but in vivo RCP expression is limited to specific subsets of cells, usually co-localizing with CGRP-containing neurons. RCP protein expression correlates with CGRP efficacy in vivo, suggesting that RCP regulates CLR signaling in vivo as it does in cell culture. RCP is usually identified in cytoplasm or membranes of cells, but recently has been observed in nucleus of neurons, suggesting an additional transcriptional role for RCP in cell function. Together, these data support an essential role for RCP in CGRP and AM receptor function, in which RCP expression enhances signaling at CLR, and therefore increases the efficacy of CGRP and AM in vivo. The focus of this review is on the recent data that support an interaction between RCP and CLR, and the physiologic implications of this regulatory role for RCP in CGRP and AM biology.
1. RCP CELLULAR BIOCHEMISTRY
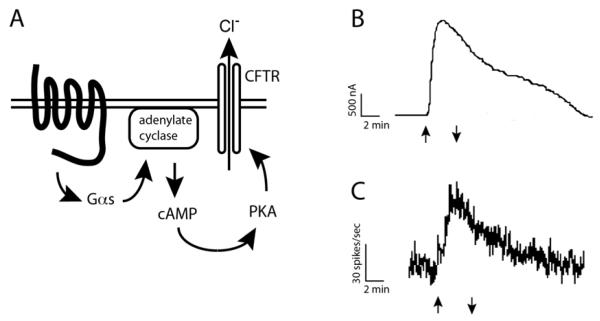
RCP is a 16 kDa intracellular protein that was expression-cloned in a Xenopus oocyte-based assay targeting the CGRP receptor. In this assay, activation of a GPCR was detected by chloride currents from a reporter gene, the cystic fibrosis transmembrane conductance regulator (CFTR). The CFTR is a Protein Kinase A (PKA)-activated chloride channel, and activation of a Gαs-coupled GPCR results in increased PKA phosphorylation of CFTR, resulting in ligand-dependent chloride currents (Fig. 1A) [2-4]. The cDNA for RCP was identified by dividing a cochlear hair cell cDNA library [5] into pools, which were then in vitro transcribed into cRNA, and pools of library cRNA were co-injected with in vitro-transcribed CFTR cRNA into oocytes. Oocytes were screened for receptor activity by voltage-clamp upon incubation with ligand, and activation was detected by increased chloride current [6]. By repeated subdivision of positive pools of cDNA, a final cDNA was identified that conferred CGRP-responsiveness in the oocyte/CFTR assay (Fig. 1B). Contrary to what was predicted, this cDNA did not encode a GPCR; instead it encoded a small hydrophilic 148 aa protein, which contained no obvious protein motifs that would explain how it mediated CGRP signaling. This protein was hypothesized to enhance an endogenous Xenopus CGRP receptor, and was named the CGRP-receptor component protein (RCP). In support of this hypothesis, endogenous CGRP receptor function has been described in oocytes, and CLR, RAMP and RCP have subsequently been identified in Xenopus, and endogenous CGRP activity has also been reported in frog [7-10]. RCP did not act as a transcription factor in this oocyte/CFTR assay, as incubation with CGRP elicited a similar response in enucleated oocytes, or in oocytes pre-treated with the RNA polymerase II inhibitor α-amanitin, when injected with RCP cRNA.
Fig. (1). RCP confers CGRP responsiveness in oocyte/CFTR assay.
A) Oocyte/CFTR assay, showing GPCR, and PKA-dependent signaling pathways. B) CFTR chloride conductance upon incubation with 100 nM CGRP (adapted with permission from Luebke et al, 1996). Arrows indicate time of addition and washout of ligand C) Xenopus lateral line organ hair cell neuron firing rate upon incubation with 100 nM CGRP (adapted with permission from Adams et al., 1987). Arrows indicate time of addition and washout of ligand.
CGRP produced a biphasic chloride current in the oocyte/CFTR assay (Fig. 1B). This is in contrast to what might be expected for activation of a GPCR, which would be predicted to result in a single peak of chloride conductance in the oocyte/CFTR assay, followed by a decrease in chloride conductance as the GPCR was inactivated, as observed for activation of the μ-opioid and adrenergic receptors [11, 12]. Interestingly, the μ-opioid receptor activated CFTR even in the presence of PKA inhibitors, suggesting the potential for an alternate pathway for CFTR activation by GPCR signaling in the oocyte. The biphasic response to CGRP in oocytes may thus be due to a combination of PKA-dependent and PKA-independent signaling pathways. In contrast, a monophasic response to forskolin was observed in oocytes injected with CFTR cRNA alone. Forskolin activates adenylate cyclase directly, bypassing the requirement for a GPCR, suggesting that the biphasic response was not a characteristic of CFTR activation in general. Interestingly, activation of the CGRP receptor in hair cells of the Xenopus lateral line organ showed a similar biphasic response (Fig. 1C), suggesting that the biphasic response observed in the oocyte/CFTR assay was a characteristic of the CGRP receptor, and not of the oocyte/CFTR assay. This biphasic response to CGRP might also be due unique mechanisms of receptor desensitization or CGRP washout, such that the kinetics of CGRP receptor activation would be significantly different from opioid receptor kinetics. However, it should be noted that this biphasic response was not observed in oocytes injected with RAMP2 and CLR when incubated with adrenomedullin [1], even though this study also detected a biphasic response to CGRP with RAMP1 and CLR. Therefore, activation of CLR/RAMP1 by CGRP appears to have different kinetics or incorporate additional signaling pathways than when CLR/RAMP2 is activated by AM, at least in Xenopus.
CLR and RAMP1 were initially thought to be sufficient to generate a high-affinity receptor for CGRP [1]. However, it was subsequently shown that RCP was endogenously expressed in the cell lines used for the initial characterization of CLR and RAMP [13, 14]. Indeed, most stable cell lines now appear to express RCP, making gain-of-function experiments difficult to interpret. Instead, the role of RCP in CLR/RAMP function was first defined in antisense cell lines where RCP expression was inhibited [13, 14]. In these studies, loss of RCP resulted in a loss of CGRP-mediated cAMP signaling, without a loss of apparent cell surface receptors, determined by 125I-CGRP binding. Similarly, loss of RCP decreased AM-mediated signaling in these antisense cells, suggesting that RCP was mediating the effects of ligand binding to CLR, the core GPCR for both CGRP and AM. These affects were specific to CLR, as signaling at two other endogenous GPCRs (β2-adrenergic and A2b adenosine) were not inhibited in the RCP-antisense cells.
RCP is present in a complex with CLR and RAMP1 and RAMP2, as determined by co-immunoprecipitation [14]. RCP co-immunoprecipitates with both RAMP1 and RAMP2, indicating the involvement of RCP in both CGRP and AM receptor function. However, it was not known if RCP interacted directly with CLR, or via an intermediate docking protein. Recently, it has been determined that RCP binds directly to the second intracellular cytoplasmic loop of CLR (ICL2CLR) [15]. Interaction between RCP and ICL2CLR was first identified by yeast two-hybrid assay, where RCP was tested against each of the three intracellular cytoplasmic loops and the carboxyl-tail (C-tail) of CLR. Only ICL2CLR produced a positive result in the yeast two-hybrid assay, and this interaction was recapitulated by co-immunoprecipitation in cell culture when ICL2CLR was transfected as a fusion protein with enhanced green fluorescent protein (EGFP). RCP co-immunoprecipitated with ICL2CLR but not with the other cytoplasmic domains of CLR, or with the ICL2 from the β2-adrenergic receptor (β2AR), a GPCR that does not require RCP for signaling [13]. Furthermore, expression of soluble ICL2CLR acted as a dominant-negative on CLR signaling, sequestering RCP in the cytoplasm, away from membrane-bound CLR. In contrast, expression of soluble ICL2CLR had no effect on β2AR signaling, further indicating the specificity of RCP for CLR. The inhibitory effect of ICL2CLR on CLR signaling was not a general characteristic of expression of soluble GPCR ICL2, as expression of ICL2β2AR had no effect on signaling at either CLR or β2AR. Interestingly, Family B GPCRs can form dimers, and loss of dimerization can lead to lower-affinity monomers. This has been demonstrated for the secretin receptor, where the site of receptor dimerization has been localized to TM4 [16, 17]. The dominant-negative ICL2CLR construct used in the studies identifying interaction between CLR and RCP [15] contained 10 aa of TM4, which does have regions of homology between the secretin receptor and CLR, particularly the “GWG” motif, of which the carboxyl glycine was shown to participate in dimerization of the secretin receptor (Fig. 2). Thus, some of the effect of ICL2CLR expression could be due to inhibition of a conserved dimerization domain, resulting in CLR monomers. However, the inhibitory effect of ICL2CLR expression on CLR signaling was more severe than loss of dimerization was on secretin receptor signaling, suggesting that dimerization alone may not account for the inhibitory effect of ICL2CLR expression.
Fig. (2). Conservation of RCP-interacting domain in GPCRs.
RCP binds to ICL2CLR, which is conserved between CLR from mammals, amphibian and the Drosophila receptor CG17415. Results from alanine-scanning mutagenesis on human CLR by Conner et al. [21] are shown on top, with effects on signaling or cell-surface expression indicated as inhibiting (closed circles) or having no effect (open circle). Other Family B GPCRs are aligned below, including receptors for calcitonin (CTR), secretin, corticotropin releasing hormone (CRH-R), vasopressin (VPAC1-R), glucagon, and parathyroid hormone (PTH-R). Numbering for each sequence begins with initiator methionine.
Interestingly, loss of RCP interaction with CLR did not significantly change the efficacy of CGRP for activating the CLR/RAMP1 dimer. Instead, the maximal signaling response was severely depressed in cells expressing ICL2CLR, but the half-maximal concentration of CGRP remained largely unchanged. These data suggested that in the presence of soluble ICL2CLR there was either a decrease in cell-surface CLR, or a decrease in signaling from a constant number of cell-surface CLR. Trafficking of CLR and RAMP1 to the cell surface appears to be unaffected by loss of interaction with RCP, determined by 125I-CGRP binding to cells with depleted RCP or by cell surface ELISA for CLR and RAMP1 in cells with CLR-RCP interaction disrupted [13, 15]. This is in contrast to RAMP1, which is required for trafficking of CLR to the cell surface [1]. These data also suggest that RCP is not required for CLR/RAMP interaction, as 125I-CGRP binding to CLR requires RAMP1, and this binding was not disrupted in the absence of RCP [13, 14]. Thus, it appears that RCP exerts its effect on CLR by either coupling the CLR/RAMP complex to the cellular signaling proteins, or by sorting the CLR/RAMP complex into membrane sub-domains that can enhance signaling such as lipid rafts [18, 19].
RAMP1 was cloned using an oocyte/CFTR expression-cloning strategy similar to what was first used to identify RCP [1, 6]. Knowing the mechanisms deduced for RCP and RAMP1 in CLR function (signaling and trafficking/pharmacology, respectively), it is now possible to postulate how their isolation was possible using the oocyte/CFTR expression-cloning assay. For RAMP1, increased trafficking of endogenous CLR to peripheral membrane of the oocyte could provide more receptor at the surface and provide pharmacologic specificity for CGRP to endogenous CLR, resulting in increased response in the oocyte/CFTR assay to CGRP. RCP would also result in increased responsiveness to CGRP in the oocyte/CFTR assay, but would instead enhance signaling at a relatively constant number of endogenous CLR/RAMP1 heterodimers already present in the oocyte membrane.
RCP is present in all mammalian species, and is present even in yeast, a single-cell eukaryote. GPCRs that interact with RCP have not been yet identified in all species, but recently a Drosophila GPCR that is dependent upon RCP for signaling has been reported. CG17415 is a Drosophila GPCR with 30% homology to CLR that binds diuretic hormone 31 (DH31), and exhibits enhanced signaling when expressed in the presence of RCP [20]. An approximately 2-fold increase in cAMP production was observed when Drosophila CG17415 was transfected into NIH3T3 cells, which endogenously express RCP, RAMP1, RAMP2, and CLR [13, 14]. In contrast, when transfected into HEK293 cells, which express RCP but not detectable levels of CLR or RAMP1 and RAMP2, no increase in cAMP over basal level was observed, suggesting that CG17415 may require either RAMP1 and RAMP2 or an additional protein expressed in NIH3T3 cells but not in HEK293 cells. A Drosophila homolog of RCP (dRCP) was identified, and when CG17415 was cotransfected with dRCP into HEK293 cells, an approximately 2-fold induction of cAMP signaling over basal levels was observed, with an EC50 of approximately 5 μM. Interestingly, a more potent response was observed when CG17415 was co-transfected with human RCP, resulting in an EC50 of 1.2 nM and a maximal stimulation 7-fold higher than that observed with dRCP. One explanation for the increased signaling by Drosophila CG17415 when co-transfected with human rather than Drosophila RCP in the human cell line HEK293, would be if the interaction between RCP and the cellular signaling proteins had more stringent binding requirements than the interaction between RCP and CLR. The primary role for RCP appears to be coupling the CLR/RAMP heterodimer to the cellular signaling proteins, and dRCP may not interface with human signaling proteins as efficiently as human RCP.
RCP interacts with CLR through ICL2, and ICL2 is highly conserved in CLR from mammals to amphibians (Fig. 2). ICL2 of Drosophila CG17415 has much less homology with the CLR sequence, but does contain several motifs that may be candidates for RCP-interacting domains. This homology includes the “YLH” motif located near the junction of transmembrane domain 3 (TM3), the “GWG” motif towards the junction with TM4, and the “WMLCEG” motif within the flanking TM3 sequence (Fig. 2). Thus, there are at least three conserved regions within CLR that could interact with RCP. Additionally, there is a lysine residue (lysine-248 of mouse CLR) that is conserved between all of the CLR-like sequences. This lysine residue is particularly interesting, because Conner et al. [21] have carried out alanine-scanning mutagenesis studies in ICL2CLR, and have identified 5 amino acids in that inhibit CLR signaling (Fig. 2). Of these residues, 4 also inhibit cell-surface expression of CLR, and presumably inhibit CLR signaling by sequestering CLR from the cell surface, thereby inhibiting the ability of CLR to bind ligand. However, when lysine-248 was changed to alanine, CLR signaling was significantly inhibited while trafficking to the cell surface was not affected. Since loss of RCP interaction also results in decreased signaling but not cell-surface expression of CLR [13, 14], lysine-248 may promote CLR-RCP interaction, either directly as part of an RCP-binding site, or indirectly by contributing to a conformation of ICL2CLR required for RCP binding.
RCP may thus enable signaling at CLR orthologs in species as diverse as Drosophila, Xenopus, and mammals. The mechanism of RCP action is not yet known. It may enable interaction with downstream effector molecules such as G-proteins that mediate or enhance GPCR signaling. In this case, loss of RCP could result in loss of interaction with G-proteins, effectively inhibiting signal transduction.
2. RCP PHYSIOLOGY
Data from cell culture studies supports a role for RCP in CLR signaling, and RCP may thus be an important regulator of CLR function in vivo, and therefore a regulator of CGRP and AM efficacy. To examine this potential role for RCP, several groups have investigated RCP expression in vivo. In general, RCP is expressed in tissue known to express receptors for either AM or CGRP, and RCP is detected co-localized with CGRP in both central and peripheral nervous system, including brain, eye, spinal cord, neuromuscular junction [22-26]. RCP is not detected in all cell types, suggesting that the ubiquitous expression observed in cell culture is probably a result of the immortalization process associated with creating cell lines, and not a general characteristic of mammalian cells. In the brain, RCP is not always co-localized with CGRP binding [22], and this may represent tissues or cells that express AM receptors (CLR + RAMP2 or RAMP3) that also require RCP for signaling.
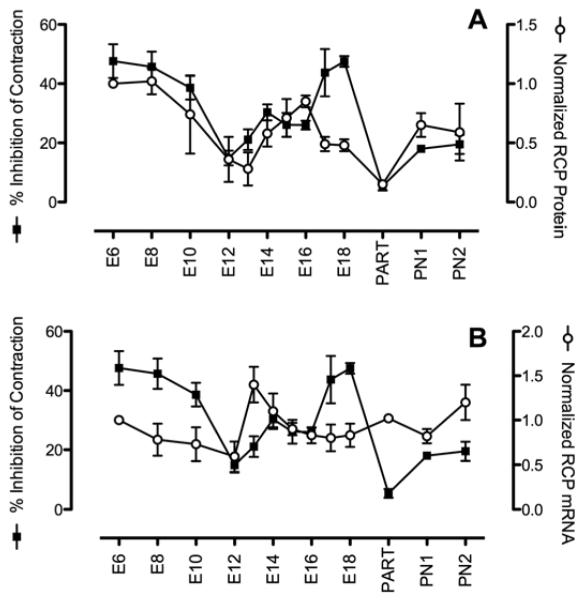
A regulatory role for RCP in CGRP efficacy was first observed in mouse uterus. CGRP inhibits myometrial contraction [27], and it was found that CGRP inhibited acetylcholine-induced contraction of myometrial tissue isolated during pregnancy in mouse [28]. In this study expression of RCP correlated with CGRP efficacy during gestation, particularly at parturition, where RCP expression was lowest, and contraction was minimally inhibited by CGRP (Fig. 3A). This corresponds well with physiology, as myometrial contraction is required for delivery. Importantly, expression of RCP protein was an accurate predictor of CGRP receptor function, and therefore CGRP efficacy. In contrast, RCP mRNA did not vary significantly during gestation, and more importantly did not correlate with receptor function, especially not at parturition (Fig. 3B). These data suggest that RCP is primarily regulated at the level of translation or protein degradation, and that mRNA levels, whether detected by northern blot or RT-PCR, may not be an accurate predictor of RCP function.
Fig. (3). RCP protein expression correlates with CGRP efficacy.
Myometrial strips were isolated from pregnant mice during the stages of gestation indicated, and tested for contraction when incubated with 10 μM acetylcholine. Acetylcholine was then washed out and strips were incubated with 100 nM CGRP, and then retested for contraction with 10 μM acetylcholine in the presence of CGRP. Percent inhibition was calculated as contraction observed with ACh + CGRP divided by contraction with ACh alone. A) Inhibition of contraction plotted with RCP protein expression, detected by western blot and normalized to RCP expressed at E6 B) Inhibition of contraction plotted with RCP mRNA expression, detected by northern blot and normalized to mRNA expressed at E6 (adapted with permission from Naghashpour et al, 1997).
RCP protein expression in myometrium also correlates with CGRP efficacy during the estrus cycle. CGRP was most potent at inhibiting myometrial contraction during metestrus, then diestrus, then proestrus, and weakest during estrus [29]. In these studies the EC50 of CGRP did not significantly change between estrus (weakest effect) and metestrus (most potent effect), suggesting that the affinity of CLR/RAMP1 for CGRP did not change during the estrus cycle. In contrast, the maximal effect of CGRP was 3-fold higher during metestrus compared to estrus. Since receptor affinity for CGRP did not change between metestrus and estrus, these data suggest that there were either more CLR/RAMP1 heterodimers on the cell surface during metestrus, or that a constant number of cell-surface CLR/RAMP1 heterodimers were more efficiently coupled to the cellular signaling pathway. These studies in myometrium are similar to what was observed in cell culture when CLR/RAMP1 were uncoupled from RCP: decreased maximal signal, with unchanged EC50 [15].
CGRP had little inhibitory effect on myometrial contraction in overectomized mice, but this inhibitory effect could be rescued by treatment of overectomized mice with progesterone, which resulted in increased expression of RCP protein. Estrogen had little effect in this experimental paradigm on either contraction or RCP protein expression, suggesting that progesterone is one positive modulator of RCP expression, and that increased RCP expression will enhance CGRP receptor function independent of expression of CLR and RAMP1.
RCP also regulates CGRP receptor function in the vasculature. CGRP was first recognized for its potent vasodilatory effect in the central and peripheral vasculature [30-34]. CGRP-immunoreactive neurons innervate blood vessels, and CLR, RAMP1 and RAMP2 and RCP are detected in the vascular endothelial cells [24, 35]. Furthermore, elevated blood pressure has been reported in CGRP knockout mice [36]. Several animal models for hypertension have established a role for CGRP in anti-hypertensive responses; spontaneously hypertensive rat (SHR) show decreased CGRP expression in neurons that innervate the vasculature [37, 38], and in mineralocorticoid-salt induced hypertension there is an increase in CGRP biosynthesis, suggesting a counter-regulatory role for CGRP in hypertension [39, 40].
Hyper-responsiveness of the vasculature to CGRP has been reported in conjunction with decreased CGRP production in spontaneously hypertensive rats [41]. In these experiments, pial arteries from hypertensive rats dilated significantly greater than control in response to CGRP, suggesting that the efficacy of CGRP was increased, possibly as a mechanism to counter decreased circulating CGRP. An increase in CGRP efficacy has also been reported for a third animal model for hypertension, the subtotal nephrectomy-salt hypertensive rat (SN-salt) [42]. In this animal model, mean arterial pressure was elevated in the SN-salt animals, and isolated mesenteric arteries dilated significantly more in response to CGRP in SN-salt rats than in control rats, suggesting that the vasculature was hyper-responsive to CGRP. This was not a general increase in vascular responsiveness, as incubation with a broad spectrum vasodilator such as sodium nitroprusside did not show a change in efficacy between SN-salt and control animals. This increased responsiveness to CGRP was accompanied by increased RCP expression. Western blots of mesenteric arteries showed that protein levels of RCP increased by 40% in the SN-salt animals, while no significant change was observed for CLR or for RAMP1, RAMP2, or RAMP3.
The increased efficacy of CGRP in the vasculature of the SN-salt rats, combined with the elevated levels of RCP protein and the unchanged protein levels of CLR and RAMP1, suggest that the same number of CGRP receptors were present in the vasculature of control and SN-salt animals, but the receptors were more efficiently coupled to the cellular signaling pathway that in the SN-salt condition, resulting in increased responsiveness to CGRP. Importantly, the EC50 for CGRP was not significantly different between control and SN-salt rats, but instead the maximal stimulation by CGRP was increased in the SN-salt rats. This increased maximal stimulation is in agreement with the previous cell culture data, where loss of RCP interaction had little effect on EC50, but decreased maximal stimulation [15]. Interestingly, a similar increase in AM efficacy was not observed in the SN-salt rats. Since AM binds to the same core GPCR (CLR), but in combination with RAMP2 or RAMP3 instead of RAMP1, and since RAMP2 and RAMP3 were detected in the mesenteric arteries tested, these results suggest that the enhanced vasodilatory effect of CGRP was due either to CGRP receptor (CLR/RAMP1) signaling through different or additional pathways compared to the AM receptor (CLR/RAMP2 or RAMP3), or that the CGRP receptors were in a membrane environment more conducive to signal transduction, such as has been proposed for lipid rafts [18, 19]. A caveat to these observations is that immunohistochemistry for RCP and the RAMPs was not carried out in the SN-salt experiments. If RAMP1 was expressed in cells distinct from those expressing RAMP2 and RAMP3, and if elevated expression of RCP occurred only in cells expressing RAMP1, then enhanced efficacy of CGRP over AM could be explained by increased RCP expression alone.
3. ALTERNATE SIGNALING PATHWAYS MEDIATED BY RCP
One role for RCP in CLR function could be to enable or regulate the ability of CLR to utilize multiple signaling pathways. Binding of CGRP to its receptor is most commonly associated with enhanced cAMP production via the GαS-mediated pathway [43-45]. However, CGRP binding has also been associated with production of nitrous oxide (NO) [46], activation of K-ATP channels [8, 47], increased intracellular calcium [48-50], and activation of the MAP Kinase pathway [51, 52]. One role for RCP could be to couple the CLR/RAMP1 heterodimer to distinct signaling effector molecules. For example, RCP is known to be required for cAMP-mediated signaling at CLR in cell culture [13-15]. One role for RCP in this signaling pathway could be to increase CLR interaction with GαS resulting in increased activation of adenylate cyclase upon activation of CLR/RAMP1 by CGRP. In this case, removal of RCP may unmask alternate signaling pathways, such as MAPK. CGRP can activate MAPK signaling [52, 53] and this was tested in NIH3T3 cells. However, no change in MAPK signaling was observed upon disruption of CLR-RCP interaction, monitored using ERK phosphorylation as a readout [15]. This has only been tested in one cell line to date, and will likely depend upon the cellular environment for expression of downstream effectors.
One alternate cellular function that may be affected by RCP is transcription in the nucleus. RCP has been identified in nuclei of motor neurons in the anterior horn of the spinal cord and hypoglossal nucleus of the medulla [22]. Interestingly, RCP was present in all of the motor neurons in these regions, but in non-neuronal cells RCP was present only in the cytoplasm. This data suggests that there may be a cell- or tissue-specific subcellular distribution of RCP, and one potential effect could be transcriptional regulation in cells where RCP is observed in the nucleus. This would be similar to what has recently been described for β-arrestin, a protein that was initially characterized for its ability to be recruited to activated GPCR and its role in GPCR inactivation [54, 55]. However, it has recently been shown that upon GPCR activation β-arrestin can also translocate to the nucleus [56, 57]. For the δ-opioid receptor, activation of the GPCR results in translocation of β-arrestin-1 to the nucleus where it results in histone acetylation and recruitment of transcription factors to the promoter for c-fos, inducing expression of this immediate early gene transcription factor [56]. Translocation of a GPCR-associated protein to the nucleus thus represents an additional layer of transcriptional regulation that can be coupled to GPCR activation.
In support of this nuclear role for RCP, RCP has been identified as a component of a transcription complex in yeast [58, 59]. The yeast homolog of RCP was first identified as clone named YJL011C, which encodes a protein named C17. RNA polymerases are holoenzymes, composed of multiple proteins that together form a functional complex. C17 is a component of yeast RNA Polymerase III complex, and is required for transcription initiation. Yeast RCP (C17) is found primarily in the nucleus, in contrast to mammalian cells, where RCP is found primarily in the cytoplasm or peripheral membrane. Yeast with C17 deleted were not viable, but could be rescued by introduction of human RCP [59], suggesting that human RCP can assume the nuclear role C17 in the yeast. When RNA Polymerase III was immunoprecipitated from yeast lacking endogenous C17 but expressing exogenous human RCP, human RCP was detected in the pellet, indicating that human RCP could efficiently integrate into the yeast RNA Polymerase holoenzyme. Furthermore, RCP was identified by mass-spectroscopy from purified human RNA Polymerase III. Interestingly, RCP has been described in the human RNA Polymerase III holoenzyme, as a protein named RPC9 [60]. While a role for RCP in GPCR function has not been identified yet in yeast, RCP may have dual roles in mammalian cells involving both GPCR signaling at the peripheral membrane and transcriptional regulation in the nucleus.
CONCLUSION
RCP is an important regulator of CLR signaling. Direct interaction between RCP and CLR is required for signaling at CLR, the core receptor for CGRP and AM. The mechanism of RCP function is still being elucidated, but RCP is required for either coupling CLR to the cellular signaling proteins or for sorting CLR/RAMP into membrane compartments that facilitate signaling. RCP is thus in a unique position to coordinate cellular processes with CLR, potentially increasing the readout from CGRP binding CLR. At the organismal level, RCP regulation of CLR signaling directly influences CGRP and AM efficacy, and RCP protein is an indicator of CGRP-responsiveness of cells and tissue. Additionally, work from in vitro studies in Xenopus oocytes and from in vivo studies in hypertensive rats suggests that the CLR/RAMP1 complex may signal through additional pathways than CLR/RAMP2, and that RCP may preferentially enable CLR/RAMP1. There is emerging evidence for additional roles for RCP in both transcription and for activation of additional receptors. Thus, RCP may have pleiotropic influences on cellular physiology, and may coordinate multiple cellular responses to activation of neuropeptide hormone receptors. The in vivo role for RCP in whole animal physiology is predicted to be complex, involving at least the CGRP and AM receptors, which has regulatory implications for a diverse array of physiologic systems, including regulation of vascular tone [42, 61-63], migraine [64-67], inflammation [68, 69] and obesity [70, 71]. The interaction between these physiologic systems is predicted to be complex, and a more complete analysis for the in vivo role of RCP will require production of knockout or conditional knockout mice for RCP. Future animal studies inhibiting expression of RCP should provide valuable information on the correlation between CGRP and AM receptor signaling and the physiologic response to CGRP and AM.
ACKNOWLEDGEMENTS
This work was funded in part by NIH DK52328 and a grant from the Schmitt Program for Integrative Brain Research.
LIST OF ABBREVIATIONS
- CGRP
Calcitonin gene-related peptide
- AM
Adrenomedullin
- GPCR
G protein-coupled receptor
- CLR
Calcitonin-like receptor
- ICL
Intracellular cytoplasmic loop
- RAMP
Receptor activity modifying protein
- RCP
CGRP-receptor component protein
- PKA
Protein kinase A
- CFTR
Cystic fibrosis transmembrane conductance regulator
- cAMP
Cyclic adenosine monophosphate
- MAPK
Mitogen-activated protein kinase
- ERK
Extracellular signal-regulated kinases
- kDa
Kilo Dalton
- ELISA
Enzyme-linked immunosorbent assay
- CRH-R
Corticotropin releasing hormone receptor
- VPAC1-R
Vasopressin receptor
- PTH-R
Parathyroid receptor
- CTR
Calcitonin receptor
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
REFERENCES
- [1].McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPs regulate the transport and ligand specificity of the calcitonin- receptor-like receptor. Nature. 1998;393(6683):333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- [2].Baukrowitz T, Hwang TC, Nairn AC, Gadsby DC. Coupling of CFTR Cl- channel gating to an ATP hydrolysis cycle. Neuron. 1994;12(3):473–482. doi: 10.1016/0896-6273(94)90206-2. [DOI] [PubMed] [Google Scholar]
- [3].Bear CE, Duguay F, Naismith AL, Kartner N, Hanrahan JW, Riordan JR. Cl- channel activity in Xenopus oocytes expressing the cystic fibrosis gene. J. Biol. Chem. 1991;266(29):19142–19145. [PubMed] [Google Scholar]
- [4].Uezono Y, Bradley J, Min C, McCarty NA, Quick M, Riordan JR, Chavkin C, Zinn K, Lester HA, Davidson N. Receptors that couple to 2 classes of G proteins increase cAMP and activate CFTR expressed in Xenopus oocytes. Receptors Channels. 1993;1(3):233–241. [PubMed] [Google Scholar]
- [5].Wilcox ER, Fex J. Construction of a cDNA library from microdissected guinea pig organ of Corti. Hear. Res. 1992;62(1):124–126. doi: 10.1016/0378-5955(92)90208-5. [DOI] [PubMed] [Google Scholar]
- [6].Luebke AE, Dahl GP, Roos BA, Dickerson IM. Identification of a protein that confers calcitonin gene-related peptide responsiveness to oocytes by using a cystic fibrosis transmembrane conductance regulator assay. Proc. Natl. Acad. Sci. USA. 1996;93(8):3455–3460. doi: 10.1073/pnas.93.8.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Adams JC, Mroz EA, Sewell WF. A possible neurotransmitter role for CGRP in a hair-cell sensory organ. Brain Res. 1987;419(1-2):347–351. doi: 10.1016/0006-8993(87)90606-8. [DOI] [PubMed] [Google Scholar]
- [8].Guillemare E, Lazdunski M, Honore E. CGRP-induced activation of KATP channels in follicular Xenopus oocytes. Pflugers Arch. 1994;428(5-6):604–609. doi: 10.1007/BF00374584. [DOI] [PubMed] [Google Scholar]
- [9].Klein SL, Strausberg RL, Wagner L, Pontius J, Clifton SW, Richardson P. Genetic and genomic tools for Xenopus research, The NIH Xenopus initiative. Dev. Dyn. 2002;225(4):384–391. doi: 10.1002/dvdy.10174. [DOI] [PubMed] [Google Scholar]
- [10].Kline LW, Kaneko T, Chiu KW, Harvey S, Pang PK. Calcitonin gene-related peptide in the bullfrog, Rana catesbeiana, localization and vascular actions. Gen. Comp. Endocrinol. 1988;72(1):123–129. doi: 10.1016/0016-6480(88)90187-6. [DOI] [PubMed] [Google Scholar]
- [11].Birnbaum AK, Wotta DR, Law PY, Wilcox GL. Functional expression of adrenergic and opioid receptors in Xenopus oocytes, interaction between alpha 2- and beta 2-adrenergic receptors. Brain Res. Mol. Brain Res. 1995;28(1):72–80. doi: 10.1016/0169-328x(94)00185-h. [DOI] [PubMed] [Google Scholar]
- [12].Wotta DR, Birnbaum AK, Wilcox GL, Elde R, Law PY. Mu-opioid receptor regulates CFTR coexpressed in Xenopus oocytes in a cAMP independent manner. Brain Res. Mol. Brain Res. 1997;44(1):55–65. doi: 10.1016/s0169-328x(96)00189-1. [DOI] [PubMed] [Google Scholar]
- [13].Evans BN, Rosenblatt MI, Mnayer LO, Oliver KR, Dickerson IM. CGRP-RCP, a novel protein required for signal transduction at calcitonin gene-related peptide and adrenomedullin receptors. J. Biol. Chem. 2000;275(40):31438–31443. doi: 10.1074/jbc.M005604200. [DOI] [PubMed] [Google Scholar]
- [14].Prado MA, Evans-Bain B, Oliver KR, Dickerson IM. The role of the CGRP-receptor component protein (RCP) in adrenomedullin receptor signal transduction. Peptides. 2001;22(11):1773–1781. doi: 10.1016/s0196-9781(01)00517-4. [DOI] [PubMed] [Google Scholar]
- [15].Egea SC, Dickerson IM. Direct interactions between calcitonin-like receptor (CLR) and CGRP-receptor component protein (RCP) regulate CGRP receptor signaling. Endocrinology. 2012;153(4):1850–1860. doi: 10.1210/en.2011-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gao F, Harikumar KG, Dong M, Lam PC, Sexton PM, Christopoulos A, Bordner A, Abagyan R, Miller LJ. Functional importance of a structurally distinct homodimeric complex of the family B G protein-coupled secretin receptor. Mol. Pharmacol. 2009;76(2):264–274. doi: 10.1124/mol.109.055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Harikumar KG, Pinon DI, Miller LJ. Transmembrane segment IV contributes a functionally important interface for oligomerization of the Class II G protein-coupled secretin receptor. J. Biol. Chem. 2007;282(42):30363–30372. doi: 10.1074/jbc.M702325200. [DOI] [PubMed] [Google Scholar]
- [18].Ilegems E, Iwatsuki K, Kokrashvili Z, Benard O, Ninomiya Y, Margolskee RF. REEP2 enhances sweet receptor function by recruitment to lipid rafts. J. Neurosci. 2010;30(41):13774–13783. doi: 10.1523/JNEUROSCI.0091-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Simons K, Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1(1):31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- [20].Johnson EC, Shafer OT, Trigg JS, Park J, Schooley DA, Dow JA, Taghert PH. A novel diuretic hormone receptor in Drosophila, evidence for conservation of CGRP signaling. J. Exp. Biol. 2005;208(Pt 7):1239–1246. doi: 10.1242/jeb.01529. [DOI] [PubMed] [Google Scholar]
- [21].Conner AC, Simms J, Howitt SG, Wheatley M, Poyner DR. The second intracellular loop of the calcitonin gene-related peptide receptor provides molecular determinants for signal transduction and cell surface expression. J. Biol. Chem. 2006;281(3):1644–1651. doi: 10.1074/jbc.M510064200. [DOI] [PubMed] [Google Scholar]
- [22].Ma W, Chabot JG, Powell KJ, Jhamandas K, Dickerson IM, Quirion R. Localization and modulation of calcitonin gene-related peptide-receptor component protein-immunoreactive cells in the rat central and peripheral nervous systems. Neuroscience. 2003;120(3):677–694. doi: 10.1016/s0306-4522(03)00159-3. [DOI] [PubMed] [Google Scholar]
- [23].Oliver KR, Wainwright A, Kinsey AM, Heavens RP, Sirinathsinghji DJ, Hill RG. Regional and cellular localization of calcitonin gene-related peptide- receptor component protein mRNA in the guinea-pig central nervous system. Brain Res. Mol. Brain Res. 1999;66(1-2):205–210. doi: 10.1016/s0169-328x(99)00036-4. [DOI] [PubMed] [Google Scholar]
- [24].Pokabla MJ, Dickerson IM, Papka RE. Calcitonin gene-related peptide-receptor component protein expression in the uterine cervix, lumbosacral spinal cord, and dorsal root ganglia. Peptides. 2002;23(3):507–514. doi: 10.1016/s0196-9781(01)00638-6. [DOI] [PubMed] [Google Scholar]
- [25].Rosenblatt MI, Dahl GP, Dickerson IM. Characterization and localization of the rabbit ocular calcitonin gene- related peptide (CGRP)-receptor component protein (RCP) Invest. Ophthalmol. Vis. Sci. 2000;41(5):1159–1167. [PubMed] [Google Scholar]
- [26].Rossi SG, Dickerson IM, Rotundo RL. Localization of the calcitonin gene-related peptide receptor complex at the vertebrate neuromuscular junction and its role in regulating acetylcholinesterase expression. J. Biol. Chem. 2003;278(27):24994–5000. doi: 10.1074/jbc.M211379200. [DOI] [PubMed] [Google Scholar]
- [27].Samuelson UE, Dalsgaard CJ, Lundberg JM, Hokfelt T. Calcitonin gene-related peptide inhibits spontaneous contractions in human uterus and fallopian tube. Neurosci. Lett. 1985;62(2):225–230. doi: 10.1016/0304-3940(85)90359-3. [DOI] [PubMed] [Google Scholar]
- [28].Naghashpour M, Rosenblatt MI, Dickerson IM, Dahl GP. Inhibitory effect of calcitonin gene-related peptide on myometrial contractility is diminished at parturition. Endocrinology. 1997;138(10):4207–4214. doi: 10.1210/endo.138.10.5447. [DOI] [PubMed] [Google Scholar]
- [29].Naghashpour M, Dahl G. Sensitivity of myometrium to CGRP varies during mouse estrous cycle and in response to progesterone. Am. J. Physiol. Cell Physiol. 2000;278(3):C561–C569. doi: 10.1152/ajpcell.2000.278.3.C561. [DOI] [PubMed] [Google Scholar]
- [30].Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313(5997):54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- [31].Krootila K, Uusitalo H, Palkama A. Effect of neurogenic irritation and calcitonin gene-related peptide (CGRP) on ocular blood flow in the rabbit. Curr. Eye Res. 1988;7(7):695–703. doi: 10.3109/02713688809033199. [DOI] [PubMed] [Google Scholar]
- [32].Nuki C, Kawasaki H, Kitamura K, Takenaga M, Kangawa K, Eto T, Wada A. Vasodilator effect of adrenomedullin and calcitonin gene-related peptide receptors in rat mesenteric vascular beds. Biochem. Biophys. Res. Commun. 1993;196(1):245–251. doi: 10.1006/bbrc.1993.2241. [DOI] [PubMed] [Google Scholar]
- [33].Gao YJ, Nishimura Y, Suzuki A, Nakai Y. Calcitonin gene-related peptide-induced relaxation in isolated small superior mesenteric arteries from adult stroke-prone spontaneously hyper-tensive rats. Clin. Exp. Pharmacol. Physiol. 1995;22(Suppl 1):S109–S111. doi: 10.1111/j.1440-1681.1995.tb02842.x. [DOI] [PubMed] [Google Scholar]
- [34].Champion HC, Akers DL, Santiago JA, Lambert DG, McNamara DB, Kadowitz PJ. Analysis of responses to human synthetic adrenomedullin and calcitonin gene-related peptides in the hindlimb vascular bed of the cat. Mol. Cell Biochem. 1997;176(1-2):5–11. [PubMed] [Google Scholar]
- [35].Oliver KR, Wainwright A, Edvinsson L, Pickard JD, Hill RG. Immunohistochemical localization of calcitonin receptor-like receptor and receptor activity-modifying proteins in the human cerebral vasculature. J. Cereb. Blood Flow Metab. 2002;22(5):620–629. doi: 10.1097/00004647-200205000-00014. [DOI] [PubMed] [Google Scholar]
- [36].Gangula PR, Zhao H, Supowit SC, Wimalawansa SJ, Dipette DJ, Westlund KN, Gagel RF, Yallampalli C. Increased blood pressure in alpha-calcitonin gene-related peptide/calcitonin gene knockout mice. Hypertension. 2000;35(1 Pt 2):470–475. doi: 10.1161/01.hyp.35.1.470. [DOI] [PubMed] [Google Scholar]
- [37].Supowit SC, Ramana CV, Westlund KN, DiPette DJ. Calcitonin gene-related peptide gene expression in the spontaneously hypertensive rat. Hypertension. 1993;21(6 Pt 2):1010–1014. doi: 10.1161/01.hyp.21.6.1010. [DOI] [PubMed] [Google Scholar]
- [38].Westlund KN, DiPette DJ, Carson J, Holland OB. Decreased spinal cord content of calcitonin gene-related peptide in the spontaneously hypertensive rat. Neurosci. Lett. 1991;131(2):183–186. doi: 10.1016/0304-3940(91)90609-w. [DOI] [PubMed] [Google Scholar]
- [39].Supowit SC, Gururaj A, Ramana CV, Westlund KN, DiPette DJ. Enhanced neuronal expression of calcitonin gene-related peptide in mineralocorticoid-salt hypertension. Hypertension. 1995;25(6):1333–1338. doi: 10.1161/01.hyp.25.6.1333. [DOI] [PubMed] [Google Scholar]
- [40].Supowit SC, Zhao H, Hallman DM, DiPette DJ. Calcitonin gene-related peptide is a depressor of deoxycorticosterone-salt hypertension in the rat. Hypertension. 1997;29(4):945–950. doi: 10.1161/01.hyp.29.4.945. [DOI] [PubMed] [Google Scholar]
- [41].Hong KW, Yu SS, Shin YW, Kim CD, Rhim BY, Lee WS. Decreased CGRP level with increased sensitivity to CGRP in the pial arteries of spontaneously hypertensive rats. Life Sci. 1997;60(10):697–705. doi: 10.1016/s0024-3205(97)00001-5. [DOI] [PubMed] [Google Scholar]
- [42].Supowit SC, Katki KA, Hein TW, Gupta P, Kuo L, Dickerson IM, Dipette DJ. Vascular reactivity to calcitonin gene-related peptide is enhanced in subtotal nephrectomy-salt induced hypertension. Am. J. Physiol. Heart Circ. Physiol. 2011;301(3):H683–H688. doi: 10.1152/ajpheart.00598.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chiba T, Yamaguchi A, Yamatani T, Nakamura A, Morishita T, Inui T, Fukase M, Noda T, Fujita T. Calcitonin gene-related peptide receptor antagonist human CGRP-(8-37) Am. J. Physiol. 1989;256(2 Pt 1):E331–E335. doi: 10.1152/ajpendo.1989.256.2.E331. [DOI] [PubMed] [Google Scholar]
- [44].Han ZQ, Coppock HA, Smith DM, Van Noorden S, Makgoba MW, Nicholl CG, Legon S. The interaction of CGRP and adrenomedullin with a receptor expressed in the rat pulmonary vascular endothelium. J. Mol. Endocrinol. 1997;18(3):267–272. doi: 10.1677/jme.0.0180267. [DOI] [PubMed] [Google Scholar]
- [45].Hasbak P, Eskesen K, Schifter S, Edvinsson L. Increased alphaCGRP potency and CGRP-receptor antagonist affinity in isolated hypoxic porcine intramyocardial arteries. Br. J. Pharmacol. 2005;145(5):646–655. doi: 10.1038/sj.bjp.0706232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Vause CV, Durham PL. CGRP stimulation of iNOS and NO release from trigeminal ganglion glial cells involves mitogen-activated protein kinase pathways. J. Neurochem. 2009;110(3):811–821. doi: 10.1111/j.1471-4159.2009.06154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zhang L, Bonev AD, Mawe GM, Nelson MT. Protein kinase A mediates activation of ATP-sensitive K+ currents by CGRP in gallbladder smooth muscle. Am. J. Physiol. 1994;267(3 Pt 1):G494–G499. doi: 10.1152/ajpgi.1994.267.3.G494. [DOI] [PubMed] [Google Scholar]
- [48].Drissi H, Lasmoles F, Mellay V.; Le, Marie PJ, Lieber-herr M. Activation of phospholipase C-beta1 via Galphaq/11 during calcium mobilization by calcitonin gene-related peptide. J. Biol. Chem. 1998;273(32):20168–20174. doi: 10.1074/jbc.273.32.20168. [DOI] [PubMed] [Google Scholar]
- [49].Drissi H, Lieberherr M, Hott M, Marie PJ, Lasmoles F. Calcitonin gene-related peptide (CGRP) increases intracellular free Ca2+ concentrations but not cyclic AMP formation in CGRP receptor-positive osteosarcoma cells (OHS-4) Cytokine. 1999;11(3):200–207. doi: 10.1006/cyto.1998.0415. [DOI] [PubMed] [Google Scholar]
- [50].Schiess MC, Poindexter BJ, Brown BS, Bick RJ. The effects of CGRP on calcium transients of dedifferentiating cultured adult rat cardiomyocytes compared to non-cultured adult cardiomyocytes, possible protective and deleterious results in cardiac function. Peptides. 2005;26(3):525–530. doi: 10.1016/j.peptides.2004.10.020. [DOI] [PubMed] [Google Scholar]
- [51].Wang Z, Ma W, Chabot JG, Quirion R. Cell-type specific activation of p38 and ERK mediates calcitonin gene-related peptide involvement in tolerance to morphine-induced analgesia. FASEB J. 2009;23(8):2576–86. doi: 10.1096/fj.08-128348. [DOI] [PubMed] [Google Scholar]
- [52].Yu XJ, Li CY, Wang KY, Dai HY. Calcitonin gene-related peptide regulates the expression of vascular endothelial growth factor in human HaCaT keratinocytes by activation of ERK1/2 MAPK. Regul. Pep. 2006;137(3):134–139. doi: 10.1016/j.regpep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- [53].Schaeffer C, Vandroux D, Thomassin L, Athias P, Rochette L, Connat JL. Calcitonin gene-related peptide partly protects cultured smooth muscle cells from apoptosis induced by an oxidative stress via activation of ERK1/2 MAPK. Biochim. Biophys. Acta. 2003;1643(1-3):65–73. doi: 10.1016/j.bbamcr.2003.09.005. [DOI] [PubMed] [Google Scholar]
- [54].Gurevich VV, Dion SB, Onorato JJ, Ptasienski J, Kim CM, Sterne-Marr R, Hosey MM, Benovic JL. Arrestin interactions with G protein-coupled receptors. Direct binding studies of wild type and mutant arrestins with rhodopsin, beta 2- adrenergic, and m2 muscarinic cholinergic receptors. J. Biol. Chem. 1995;270(2):720–731. doi: 10.1074/jbc.270.2.720. [DOI] [PubMed] [Google Scholar]
- [55].Gurevich VV, Gurevich EV. The molecular acrobatics of arrestin activation. Trends Pharmacol. Sci. 2004;25(2):105–11. doi: 10.1016/j.tips.2003.12.008. [DOI] [PubMed] [Google Scholar]
- [56].Kang J, Shi Y, Xiang B, Qu B, Su W, Zhu M, Zhang M, Bao G, Wang F, Zhang X, Yang R, Fan F, Chen X, Pei G, Ma L. A nuclear function of beta-arrestin1 in GPCR signaling, regulation of histone acetylation and gene transcription. Cell. 2005;123(5):833–847. doi: 10.1016/j.cell.2005.09.011. [DOI] [PubMed] [Google Scholar]
- [57].Reiter E, Lefkowitz RJ. GRKs and beta-arrestins, roles in receptor silencing, trafficking and signaling. Trends Endocrinol. Metab. 2006;17(4):159–165. doi: 10.1016/j.tem.2006.03.008. [DOI] [PubMed] [Google Scholar]
- [58].Ferri ML, Peyroche G, Siaut M, Lefebvre O, Carles C, Conesa C, Sentenac A. A novel subunit of yeast RNA polymerase III interacts with the TFIIB-related domain of TFIIIB70. Mol. Cell Bio. 2000;20(2):488–495. doi: 10.1128/mcb.20.2.488-495.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Siaut M, Zaros C, Levivier E, Ferri ML, Court M, Werner M, Callebaut I, Thuriaux P, Sentenac A, Conesa C. An Rpb4/Rpb7-like complex in yeast RNA polymerase III contains the orthologue of mammalian CGRP-RCP. Mol. Cell Biol. 2003;23(1):195–205. doi: 10.1128/MCB.23.1.195-205.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hu P, Wu S, Sun Y, Yuan CC, Kobayashi R, Myers MP, Hernandez N. Characterization of human RNA polymerase III identifies orthologues for Saccharomyces cerevisiae RNA polym-erase III subunits. Mol. Cell Biol. 2002;22(22):8044–8055. doi: 10.1128/MCB.22.22.8044-8055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Nandha KA, Taylor GM, Smith DM, Owji AA, By-field PG, Ghatei MA, Bloom SR. Specific adrenomedullin binding sites and hypotension in the rat systemic vascular bed. Regul. Pept. 1996;62(2-3):145–151. doi: 10.1016/0167-0115(96)00017-1. [DOI] [PubMed] [Google Scholar]
- [62].Shindo T, Kurihara Y, Nishimatsu H, Moriyama N, Kakoki M, Wang Y, Imai Y, Ebihara A, Kuwaki T, Ju KH, Minamino N, Kangawa K, Ishikawa T, Fukuda M, Akimoto Y, Kawakami H, Imai T, Morita H, Yazaki Y, Nagai R, Hirata Y, Kurihara H. Vascular abnormalities and elevated blood pressure in mice lacking adrenomedullin gene. Circulation. 2001;104(16):1964–1971. doi: 10.1161/hc4101.097111. [DOI] [PubMed] [Google Scholar]
- [63].Uddman R, Edvinsson L, Ekblad E, Hakanson R, Sundler F. Calcitonin gene-related peptide (CGRP), perivascular distribution and vasodilatory effects. Regul. Pept . 1986;15(1):1–23. doi: 10.1016/0167-0115(86)90071-6. [DOI] [PubMed] [Google Scholar]
- [64].Edvinsson L. Clinical data on the CGRP antagonist BIBN4096BS for treatment of migraine attacks. CNS Drug Rev. 2005;11(1):69–76. doi: 10.1111/j.1527-3458.2005.tb00036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Goadsby PJ, Lipton RB, Ferrari MD. Migraine--current understanding and treatment. N. Engl. J. Med. 2002;346(4):257–70. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- [66].Ho TW, Mannix LK, Fan X, Assaid C, Furtek C, Jones CJ, Lines CR, Rapoport AM. Randomized controlled trial of an oral CGRP antagonist, MK-0974, in acute treatment of migraine. Neurology. 2007 doi: 10.1212/01.WNL.0000286940.29755.61. [DOI] [PubMed] [Google Scholar]
- [67].Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22(1):54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- [68].Clementi G, Caruso A, Cutuli VM, Prato A, de Bernardis E, Fiore CE, Amico-Roxas M. Anti-inflammatory activity of amylin and CGRP in different experimental models of inflammation. Life Sci. 1995;57(14):PL193–197. doi: 10.1016/0024-3205(95)02100-w. [DOI] [PubMed] [Google Scholar]
- [69].Gomes RN, Castro-Faria-Neto HC, Bozza PT, Soares MB, Shoemaker CB, David JR, Bozza MT. Calcitonin gene-related peptide inhibits local acute inflammation and protects mice against lethal endotoxemia. Shock. 2005;24(6):590–594. doi: 10.1097/01.shk.0000183395.29014.7c. [DOI] [PubMed] [Google Scholar]
- [70].Gram DX, Hansen AJ, Wilken M, Elm T, Svendsen O, Carr RD, Ahren B, Brand CL. Plasma calcitonin gene-related peptide is increased prior to obesity, and sensory nerve desensitization by capsaicin improves oral glucose tolerance in obese Zucker rats. Eur. J.; Endocrinol. 2005;153(6):963–969. doi: 10.1530/eje.1.02046. [DOI] [PubMed] [Google Scholar]
- [71].Zelissen PM, Koppeschaar HP, Lips CJ, Hackeng WH. Calcitonin gene-related peptide in human obesity. Peptides. 1991;12(4):861–863. doi: 10.1016/0196-9781(91)90147-h. [DOI] [PubMed] [Google Scholar]