Abstract
Background
Cancer pain, especially the one caused by metastasis in bones, is a severe type of pain. Pain becomes chronic unless its causes and consequences are resolved. With improvements in cancer detection and survival among patients, pain has been considered as a great challenge because traditional therapies are partially effective in terms of providing relief. Cancer pain mechanisms are more poorly understood than neuropathic and inflammatory pain states. Chronic inflammatory pain and neuropathic pain are influenced by NB001, an adenylyl cyclase 1 (AC1)-specific inhibitor with analgesic effects. In this study, the analgesic effects of NB001 on cancer pain were evaluated.
Results
Pain was induced by injecting osteolytic murine sarcoma cell NCTC 2472 into the intramedullary cavity of the femur of mice. The mice injected with sarcoma cells for four weeks exhibited significant spontaneous pain behavior and mechanical allodynia. The continuous systemic application of NB001 (30 mg/kg, intraperitoneally, twice daily for three days) markedly decreased the number of spontaneous lifting but increased the mechanical paw withdrawal threshold. NB001 decreased the concentrations of cAMP and the levels of GluN2A, GluN2B, p-GluA1 (831), and p-GluA1 (845) in the anterior cingulate cortex, and inhibited the frequency of presynaptic neurotransmitter release in the anterior cingulate cortex of the mouse models.
Conclusions
NB001 may serve as a novel analgesic to treat bone cancer pain. Its analgesic effect is at least partially due to the inhibition of AC1 in anterior cingulate cortex.
Keywords: NB001, bone cancer pain, adenylyl cyclase 1, anterior cingulate cortex
Introduction
Similar to the liver and lungs, bones are easily attacked by malignant tumors; as a consequence, this condition exacerbates and progresses to bone cancer because bones contain a richer blood supply than other organs do. Either primary or secondary (metastatic) bone cancer usually causes severe pain in early stages. Pain gradually worsens as cancer progresses and thus seriously reduces the quality of life of patients. As a type of chronic pain, bone cancer pain manifests in three forms, namely, basic continuous pain, spontaneous or incident-breakthrough pain, and allodynia. Although the exact mechanism of bone cancer pain remains unclear, symptoms may generally be related to local nerve damage (neuropathic pain) and inflammatory environmental stimuli (inflammatory pain).1,2
Classic treatment approaches for bone cancer pain are usually combined with analgesic drugs and radiotherapy, but the overall efficiency is less than satisfactory. Three types of analgesic drugs are available. (a) Non-steroidal anti-inflammatory drugs (NSAIDs) are represented by COX-2 inhibitors, such as celecoxib. These drugs provide advantages, including few side effects. However, these drugs also exhibit disadvantages, including limited efficacy when they are used alone. (b) Opioids include morphine, which is a classic medication in this field. On the one hand, opioids provide several benefits, including rapid action and targeted effect; on the other hand, opioids cause disadvantages, such as dose dependence and highly addictive effects. As a consequence, the long-term use of opioids induces drowsiness, constipation, tolerance, and other adverse reactions. (c) Bisphosphonate drugs, such as pamidronic acid and zoledronic acid, confer many advantages, such as increase in bone mass, bone metastasis inhibition, and pain relief. However, positive outcomes occur gradually; for instance, analgesic properties require at least six months to take effect. Thus far, none of the available drugs elicit efficacious analgesic effects with minimal significant side effects on bone cancer pain.3–5
The development of chronic pain is closely related to neuronal synaptic plasticity in the central nervous system (CNS), including the anterior cingulate cortex (ACC).6–8 Furthermore, ionotropic glutamate receptor channels play an important role in the transmission of pain signals.9 Peripheral pain stimulation prompts the release of presynaptic glutamate in the CNS; as a result, postsynaptic ionotropic glutamate receptor channels open and promote calcium influx. High calcium concentrations in the postsynaptic intracellular compartment activates nine membrane-bound adenylate cyclase (AC) isoforms, which catalyze the production of the second messenger cyclic adenosine monophosphate (cAMP).10 The latter activates protein kinase A (PKA),11 which then triggers ionotropic glutamate receptor channels and increases their expression in the postsynaptic membrane through synaptic plasticity.12 With these cascade effects caused by positive feedback, signals from chronic pain, including bone cancer pain, are delivered and amplified.
The inhibition of the AC activity, especially AC1, provides well-documented benefits that alleviate chronic pain and neuronal excitotoxicity.13–16 These roles have been considered to develop isoform-selective AC inhibitors. Insufficient amounts of these inhibitors currently limit the exploration of functions and treatment of dysfunctions involving the AC/cAMP signaling in the CNS, especially in the ACC. Among these inhibitors, the AC1 inhibitor NB001 blocks chronic muscle, neuropathic, and inflammatory pain in rodents without inducing evident side effects.16,17 However, the analgesic effect of NB001 on cancer pain remains unknown.
We examined the potential analgesic effects of the AC1 inhibitor NB001 on bone cancer pain by using a mouse model.18 We found that the continuous systemic application of an increased dose of NB001 (30 mg/kg, intraperitoneal (ip), twice per day for three days) can effectively decrease the concentrations of cAMP, GluN2A, GluN2B, p-GluA1 (831), and p-GluA1 (845) and the frequency of presynaptic neurotransmitter release (mEPSC) in the ACC of the mouse models. As a consequence, NB001 alleviates bone cancer pain, as shown by various behavioral tests.
Materials and methods
Animals
We used male adult C3H/HeJ mice weighted 25–30 g which were purchased from Nanjing Biomedical Research Institute of Nanjing University. Mice were housed, in accordance with the National Institutes of Health guidelines, in boxes of temperature- and humidity-controlled environment, and maintained on a 12 h light/dark cycle with free access to chow and water. All procedures were approved by the Animal Care and Use Committee of the Fourth Military Medical University.
Cell culture
Osteolytic murine sarcoma cells (NCTC 2472, American Type Culture Collection (ATCC), Rockville, MD, USA) were cultured in NCTC 135 medium (Invitrogen) containing 10% horse serum (Gibco) and passaged two times weekly according to ATCC guidelines. For their administration, cells were detached by scraping and then centrifuged at 1000 × g. The pellet was suspended in fresh NCTC 135 medium (105 cells/10 µL) and then used for intramedullary femur inoculation.
Group and surgery
Mice were randomly divided into five groups, with eight mice per group: the “sham” group (group A), the “model with saline” group (group B), the “model with 10 mg/kg NB001 × once” group (group C), the “model with 30 mg/kg NB001 × once” group (group D), and the “model with 30 mg/kg NB001 × 6 times” group (group E). For tumor cell inoculation, mice were anesthetized with pentobarbital sodium (Bioszune, USA, 40 mg/kg) i.p. An arthrotomy was performed as previous described.5,19 A minimal skin incision was made and the patellar ligaments were cut, exposing the condyles of the distal femur. A 23-gauge needle was inserted at the level of the intercondylar notch and the intramedullary canal of the femur to create a cavity for injection of the cells. Approximately 1 × 105 cells in 10 µL of medium were injected unilaterally into the intramedulary cavity of the femur using a syringe. To prevent leakage of cells outside the bone, the injection site was sealed with dental acrylic (Paladur, Heraeus Kulzer, GmbH, Wehrheim, Germany).
Drug treatment
On the day 29 after the surgery (the day after the operated femurs of the model groups appear to obvious destruction shown by X-ray), mice were injected intraperitoneally with the AC1 inhibitor, NB001 dissolved in saline. For the C, D, and E groups, NB001 were used as 10 mg/kg (once), 30 mg/kg (once), and 30 mg/kg (twice daily for three days). Groups A and B were administered with saline only.
Determination of bone destruction
Radiographs were taken on day 7, 14, 21, 28, 35, and 42 after the surgery using an X-ray machine with images captured by a digital camera. All the mice were anesthetized with pentobarbital before the determination.
Pain behavior assessments
Animals were tested for spontaneous pain (spontaneous lifting), movement-evoked pain (limb-use on rotarod), and mechanical hypersensitivity (calibrated von Frey filaments) before surgery (baseline) and at day 29 following surgery in a blinded fashion (tester was blind to treatment groups). Because the best analgesic effect time of NB001 is between 45 min to 2 h after used systemically,16 all testing was performed 1 h after drug treatment.
Spontaneous lifting
Spontaneous lifting of the hind paws was measured as described by Mouedden et al.20 Spontaneous lifting was performed at room temperature and before each test animals were habituated to the laboratory room for at least 30 min. For testing itself, animals were placed and habituated in a transparent acrylic cylinder of 20 cm diameter put on the surface of a glass plate. The animals were observed for 4 min for spontaneous lifting behavior of the left hind paw. Data were expressed as percentage withdrawal time over total session time. Zero percent was the normal value observed in most nonoperated and sham-operated animals.
Limb-use on rotarod
Limb-use on rotarod was measured as previously described.20 After spontaneous lifting assessment, animals were immediately placed on a mouse rotarod (ENV-575 M®, Med Associates Inc., GA, USA) at a speed of 16 r/min for 2 min and limb-use during the forced ambulation was scored according to the following criterion: 4 = normal; 3 = limping; 2 = partial non-use of left hind paw; 1 = substantial non-use of left hind paw; and 0 = non-use of left hind paw. The sedative effects of drugs were also examined by determining animals’ ability to support their own body weights when forced to run on rotarod at a speed of 16 r/min. Animals that fell from the rotarod within 1 min were considered as positive for sedation/motor impairment.
Mechanical hypersensitivity
Paw withdrawal thresholds in response to probing with calibrated von Frey filaments were determined in the manner described by Chaplan et al.21 Mice were kept in suspended cages with wire mesh floors and the von Frey filament applied perpendicularly to the plantar surface of the ipsilateral paw until it buckled slightly and was held for 3–6 s. A positive response was indicated by a sharp withdrawal of the paw. An initial probe equivalent to 0.6 g was applied and if the response was negative, the stimulus was incrementally increased until a positive response was obtained, then decreased until a negative result was obtained. This up–down method was repeated until three changes in behavior were determined, and the pattern of positive and negative responses was tabulated. The 50% paw withdrawal threshold was determined by the non-parametric method of Dixon.22
Whole-cell patch-clamp recordings
On the day 29 after the surgery, the brains were removed and coronal brain slices (300 µm) containing the ACC were prepared as described previously.23 Experiments were performed in a recording chamber on the stage of an Axioskop 2FS microscope (Zeiss, Oberkochen, Germany) with infrared differential interference contrast optics for visualization of whole-cell patch-clamp recording. EPSCs were recorded from layer II–III neurons with an Axopatch 200B amplifier (Molecular Devices, Palo Alto, CA), and the stimulations were delivered by a bipolar tungsten stimulating electrode placed in layer V of the ACC. The recording pipettes (3–5 MΩ) were filled with solution containing the following (in mM): 145 K-gluconate, 5 NaCl, 1 MgCl2, 0.2 EGTA, 10 HEPES, 2 Mg-ATP, and 0.1 Na3-GTP, adjusted to pH 7.2 with KOH. For miniature EPSC (mEPSC) recording, 0.5 μM TTX was added in the perfusion solution. Picrotoxin (100 μM) was always present to block GABAA receptor-mediated inhibitory synaptic currents. Access resistance was 15–30 MΩ and monitored throughout the experiment. Data were discarded if access resistance changed > 15% during an experiment.
cAMP detection by ELISA
Cyclic AMP assay was carried out as reported.24,25 Briefly, ACC were homogenated in 0.1 M HCl. The levels were detected by using enzyme-linked immunosorbent assay (ELISA) kits (Westang, Shanghai, China). Absorbance values were read at 450 nm using the ELISA plate reader (Denley Dragon Wellscan MK 3, Finland).
Western blot analysis
ACC tissues were homogenized in ice-cold RIPA lysis buffer containing protease inhibitor cocktail. Equivalent amounts of protein were performed on SDS-PAGE gels and transferred to a nitrocellulose membrane. The following primary antibodies were used: anti-GluN2A (1:300; Millipore, MAB MAB5216), anti-GluN2B (1:400; Millipore, MAB5780), anti-GluA1 (1:300; Abcam, ab31232), anti-Ser845-phospho-GluA1 (1:400; Abcam, ab76321), anti-Ser831-phospho-GluA1 (1:1000; Abcam, ab109464), and anti-β-actin (1:10000; Sigma, A5316). After incubation with the appropriate HRP-coupled secondary antibody, the proteins were observed using enhanced chemiluminescence (GE Healthcare) according to the manufacturer’s instructions. The density of immunoblots was conducted using ChemiDoc XRS (Bio-Rad, Hercules) and quantified using Quantity One version 4.1.0 (Bio-Rad).
Hematoxylin–eosin staining
The femurs were rinsed in water and infused by 40% formaldehyde solution to fix for seven days, placed in Decal solution (RDO-Apex, Aurora, IL) for 1 h for decalcification and embedded in paraffin for sectioning. Femora were cut in the frontal plane 3 µm thick and stained with hematoxylin and eosin (H&E) to visualize normal marrow elements and cancer cells under bright field microscopy on a Nikon E800 at 4 × magnification.
Statistical analysis
Results were expressed as the mean ± SE. Data were evaluated using one-way analysis of variance (ANOVA) for post hoc comparisons (SPSS 19.0). The data that passed the homogeneity test were analyzed by the one-way ANOVA least significant difference (LSD) test. In all cases, p < 0.05 was considered statistically significant.
Results
Transplantation of sarcoma cells in the femur causes bone loss and fracture
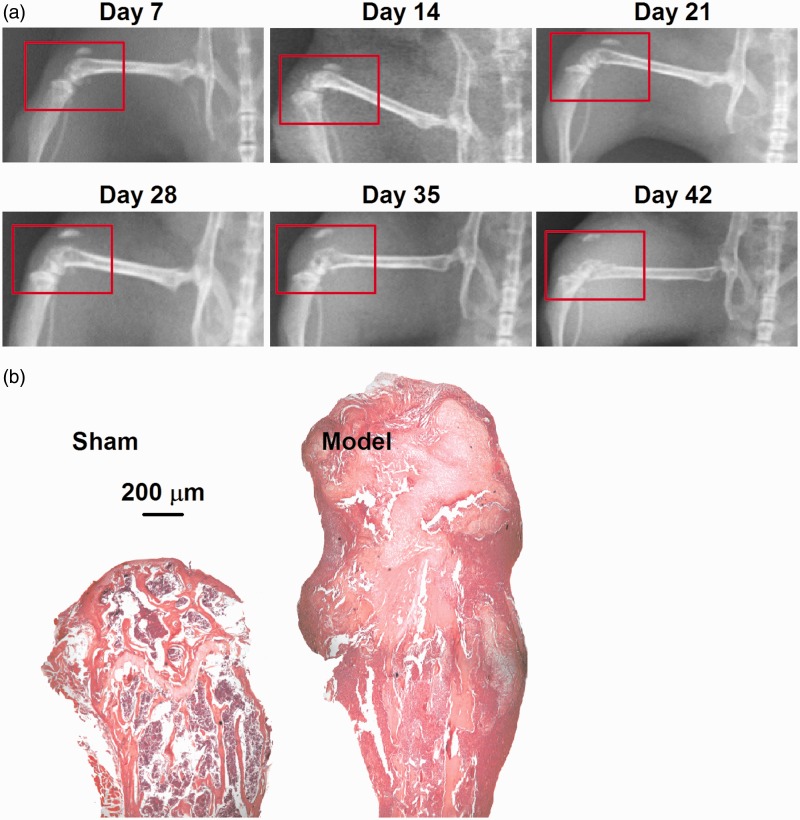
After osteolytic murine sarcoma was injected into the distal femur, the model mice were evaluated through X-ray every seven days. As shown in Figure 1(a), the distal femur of the model mouse was destroyed, and the damage gradually aggravated from the 28th day after surgery. Cancellous bone increasingly hollowed, and cortical bone thinned with time. On day 42, a broken distal femur was observed. The tumor invaded the surrounding knee, and elevated the patella. Hematoxylin and eosin (H&E) staining demonstrated that the sarcoma cells invaded the femoral cavity and thus induced bone destruction and fracture (Figure 1(b)).
Figure 1.
Establishment of bone cancer model. (a) X-ray images of the model femur showed the progressive loss of mineralized bone after the injection of sarcoma cells. Numbers represent days after surgery. Red circles indicate the operative side. (b) H&E staining showed the pathological structure of femora. In the sham bone (left), there is a clear separation of mineralized bone (normal, pink) and marrow cells (with large number of inflammatory cells infiltration, purple). In the model bone (right), the smaller and more densely packed cancer cells (purple) have largely replaced the marrow cells and destructed the mineralized bone to fracture (pink) in the intramedullary space.
NB001, an AC1 inhibitor, attenuates bone-cancer–induced pain
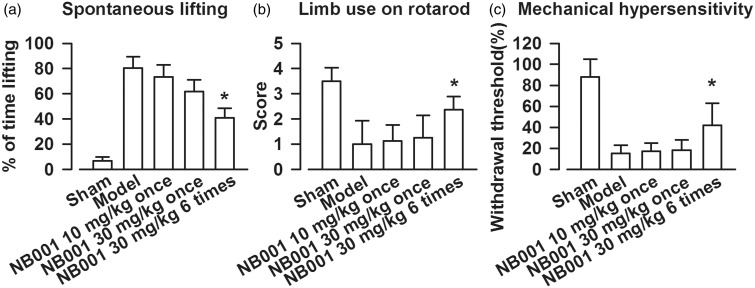
Spontaneous lifting was evaluated to determine the effects of NB001 on spontaneous bone cancer pain. Bone cancer induced a significant increase in the time of lifting. Single systemic dose of NB001 (10 or 30 mg/kg) did not attenuate spontaneous lifting. However, repeated injections of 30 mg/kg NB001 (twice per day for three days) significantly decreased spontaneous lifting (Figure 2(a)). To determine whether NB001 relieves incident-breakthrough pain and allodynia, we evaluated limb usage on a rotarod and mechanical hypersensitivity. The systemic administration of NB001 (30 mg/kg, twice per day for three days) improved the limb use on the forced ambulatory rotarod and reversed mechanical hypersensitivity of the treated mice compared with the saline-treated tumor-bearing mice (Figure 2(b) and (c)). The single dose of NB001 (10 or 30 mg/kg) did not elicit analgesic effects on cancer pain.
Figure 2.
Assessments of bone cancer-induced pain behavior. (a) Systemic administration of NB001 (30 mg/kg, ip, twice per day for three days) attenuated sarcoma-induced spontaneous lifting compared to saline treated model mice. (b) NB001 treatment improved limb-use on rotarod compared to saline treated model mice. (c) NB001 treatment reversed the mechanical hypersensitivity compared to saline treated model mice. Data are presented as mean ± SE, *p < 0.05 versus model.
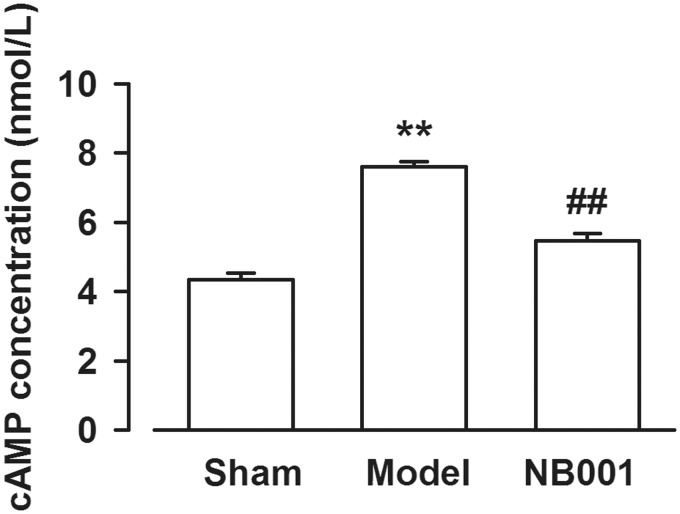
Considering that NB001 is an inhibitor of AC1, we determined whether NB001 inhibits cAMP production. The cAMP concentration in the ACC of the mice was determined, and the results revealed that the cAMP levels decreased after a continuous systemic treatment of a higher dose of NB001 (30 mg/kg, ip, twice per day for three days) was administered than that of the saline-treated models (Figure 3). This result suggested that cAMP involved in bone-cancer–induced pain can be considered as a possible analgesic target.
Figure 3.
The cAMP levels in the ACC. Levels of cAMP in the ACC were decreased by NB001 (30 mg/kg, ip, twice per day for three days) compared with that in models. Data are presented as mean ± SE, **p < 0.01 vs. Sham; ##p < 0.01 vs. model.
NB001 treatment reduces the activity of the ionotropic glutamate receptor
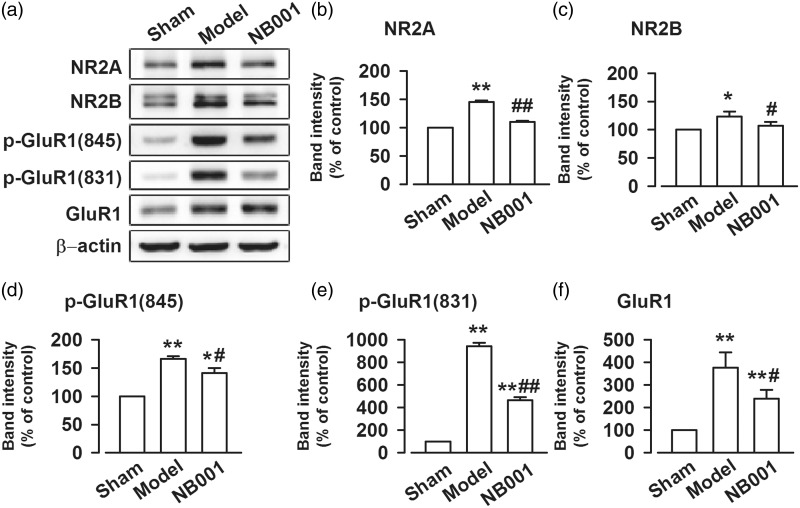
In the AC1/cAMP/PKA signaling pathway, an increase in cAMP concentrations activates PKA. The latter then activates ionotropic glutamate receptor channels and increases their expression in the postsynaptic membrane. To determine whether NB001 affects the activation of NMDAR by inhibiting cAMP production, we detected GluN2A and GluN2B subunits in ACC. GluN2A and GluN2B immunoreactivities were significantly increased in the mice suffering from cancer pain. The continuous systemic administration of a higher dose of NB001 (30 mg/kg, ip, twice per day for three days) decreased GluN2A and GluN2B concentrations compared with that of the saline-treated models (Figure 4(a) to (c)). We then measured the amount of AMPAR in the ACC after bone cancer pain was induced. Likewise, the phosphorylation levels of GluA1 (at serine 831 and 845) were increased in bone-cancer–induced pain but were decreased by the continuous systemic administration of the higher dose of NB001 (Figure 4(a), (e), and (f)). However, NB001 could not change the increased level of GluR1 in the model group (Figure 4(a) and (d)).
Figure 4.
The expression of NMDAR and AMPAR in bone cancer pain. (a) Representative bands of GluN2A, GluN2B, GluA1, p-GluA1(S831), and p-GluA1(S845) after saline or NB001 treatment in bone cancer mice. (b) to (f) Western blot analysis GluN2A, GluN2B, GluA1, p-GluA1(S831), and p-GluA1(S845) in total homogenates of ACC in bone cancer mice. Data are presented as mean ± SE, *p < 0.05 **p < 0.01 vs. Sham; #p < 0.05, ##p < 0.01 vs. model.
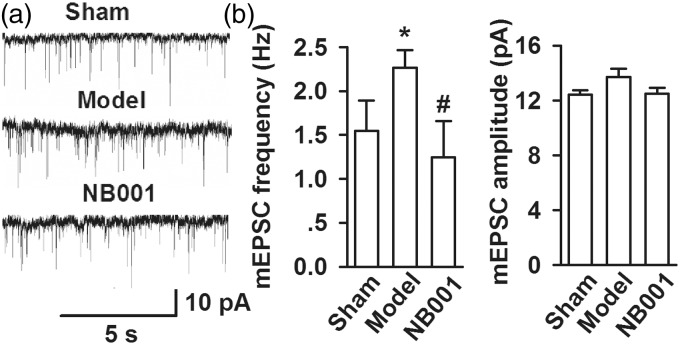
NB001 inhibits the presynaptic glutamate release in ACC
To determine whether the inhibition of the AC1/cAMP/PKA signal pathway by NB001 (30 mg/kg, ip, twice per day for three days) weakens presynaptic or postsynaptic transmitter release, we determined ionotropic glutamate receptor-mediated mEPSCs (Figure 5(a)). This finding suggested the release of single quantum of neurotransmitters. The mEPSC frequency in the mouse model significantly increased compared with that in the shams/models. By contrast, the mEPSC frequency in the mouse model was decreased by NB001 (sham, 1.55 ± 0.35 Hz; model, 2.27 ± 0.20 Hz; NB001, 1.25 ± 0.41 Hz; p < 0.05) (Figure 5(b) and (c)). However, the amplitude of mEPSCs did not differ between groups (sham, 12.41 ± 0.31 pA; model, 13.72 ± 0.58 pA; NB001, 12.48 ± 0.45 pA; p > 0.05; Figure 5(b) and (c)).
Figure 5.
Miniature EPSCs in ACC neurons. (a) Representative mEPSCs recorded in pyramidal neurons at a holding potential of −70 mV from sham (saline), model (saline), and NB001 (30 mg/kg, ip, twice per day for three days) mice. (b) Cumulative frequency (left) and amplitude (right) histogram of the mEPSCs in neurons from sham, model, and NB001 mice. Values were obtained by normalizing the mean peak currents at each holding potential to a holding potential of −70 mV. Inset shows the mEPSCs recording at +50 and −70 mV. Data are presented as mean ± SE, *p < 0.05 versus sham, #p < 0.05 versus model.
Discussion
In this study, bone cancer induced by osteolytic murine sarcoma cells was considered to evaluate the analgesic effects of NB001. The systemic use of NB001 (30 mg/kg, ip, twice per day for three days) significantly increased the mechanical paw withdrawal threshold and decreased the cAMP, GluN2A, GluN2B, and p-GluA1 concentrations (831/845) in the ACC. Electrophysiological recordings revealed the inhibitory effects of NB001 on the presynaptic glutamate release in the ACC of bone cancer pain models. Thus, it seems that both presynaptic and postsynaptic mechanisms were involved in its mechanism. mEPSC recordings in the ACC showed that the frequency was significantly enhanced by cancer pain. However, the amplitude of mEPSC was only slightly increased with no significance. This is not consistent with the increases of GluN2A, GluN2B, and p-GluA1 (831 and 845) in the ACC of mice with cancer pain. More experiments are needed to differentiate the location (i.e., presynaptic or postsynaptic, intrasynaptic, or extrasynaptic) of GluN2A, GluN2B, and p-GluA1 (831 and 845) in the ACC synapse of cancer pain animals.
In patients suffering from chronic pain, injuries trigger a series of plastic changes by activating the ACs/cAMP/PKA signaling pathways in pain-related cortical areas, especially the ACC.26–29 In neurons, activity-dependent cAMP synthesis is primarily mediated by ACs stimulated by membrane-bound Ca2+/calmodulin.30 Thus far, ten members of the AC family have been identified. Among these ACs, AC isoforms 1–9 are present in the brain.31,32 Two of these isoforms, namely, AC1 and AC8, are activated by calcium via a calcium-binding protein calmodulin.32 These enzymes link activity-dependent increase in intracellular calcium to intracellular cAMP production. In addition, these neuronal ACs are viewed as coincidence detectors because of their specific interaction with ionotropic glutamate receptor channels, such as NMDARs, AMPARs, and voltage-dependent calcium channels at the neuronal membrane.
Although AC1 and AC8 are the two major calcium-stimulated ACs in the CNS, they play important roles in synaptic plasticity and persistent pain.27 AC1 is more sensitive to calcium than AC8; AC1, not AC8, contributes to intracellular cAMP production after NMDAR is activated by glutamate.14,33 Therefore, AC1 is a good target to treat chronic pain, and this result is confirmed in AC1 knockout (AC1-KO) mice. Zhuo et al.14 found that the behavioral nociceptive responses of late-phase acute muscle pain and chronic muscle inflammatory pain are significantly reduced in AC1-KO mice. The analgesic effects of AC1 genetic reduction are also caused by the inhibition of synaptic plasticity in the ACC neurons associated with pain.17 Zhuo et al. further demonstrated that the genetic deletion of AC1 significantly attenuates neuronal death induced by glutamate in primary cultures of cortical neurons; by contrast, AC8 deletion does not elicit any significant effect. MMDA-induced cortical lesions are significantly reduced in AC1 but not in AC8 knockout mice.14
As an AC1 inhibitor, NB001 causes good analgesic effects on mouse models of chronic muscle,17 neuropathic, and inflammatory pain16 when this substance is administered systemically once at a low dose (1–3 mg/kg, peritoneal cavity, intraperitoneally, or orally). This substance does not induce any significant side effect on anxiety, motor function, or fear memory, although the inhibitory effects of NB001 on inflammatory pain are weaker than those on neuropathic pain. Furthermore, NB001 possible elicits inhibitory effects similar to those found in the AC1-KO mice. The strong analgesic effect produced by the systemic administration of NB001 is due to the inhibition of chronic-pain–related plasticity within the cortical and subcortical neural networks, including ACC. AC1 is expressed widely in the CNS including the spinal cord. In present study, we focus on the alteration of synaptic transmission in the ACC area. Other brain regions such as spinal cord and insular cortex may also involve in the chronic pain process. Thus, we could not rule out the possibility that the inhibition of AC1 in other brain regions also contribute to the analgesic effect of NB001.
In general, the two main components of bone cancer pain are neuropathic and inflammatory pain, and NB001 provides good analgesic effects on these types of pain. Hence, NB001 could effectively alleviate bone cancer pain. Among the drugs administered to treat bone cancer pain, morphine elicits the strongest analgesic effect. However, Luger et al.5 found that the doses of morphine required to alleviate bone cancer pain are generally 10-fold greater than those required to alleviate pain behaviors of comparable magnitude generated by the inflammatory pain model. In this regard, the required dose of morphine reaches up to 10–30 mg/kg.5 Considering that the analgesic inhibitory mechanism of the AC1/cAMP/PKA signaling pathway may be caused by synaptic plasticity inhibition, we aimed to establish the continuous application of NB001 in the experimental design. The results confirmed that the continuous systemic use of high-dose NB001 caused satisfactory analgesic effects on the mouse models of bone cancer pain.
Studies on bone cancer pain via animal models have revealed that evident bone destruction initially appears from days 12–14 and fracture occurs on days 22–24 after surgery; the most sensitive behavioral time window is observed from days 14–22 after surgery.19,20,34,35 In our study, bone destruction and fracture occurred on days 28 and 35 after surgery, respectively. By contrast, the most sensitive behavioral time window was detected between days 29 and 34 after surgery. These differences may be related to fewer tumor cells used in modeling (105 cells/10 µL); thus, bone cancer growth in animal models is delayed.
Studies have yet to determine whether NB001 directly targets AC1 to reduce cAMP levels.36 Nevertheless, other studies have demonstrated that this drug inhibits the AC1 activity and causes a series of downstream effects. NB001 also alleviates chronic pain in different animal models, including the mouse model of bone cancer pain in our study, without causing significant side effects. Considering that none of the currently available drugs achieve the desired treatment of bone cancer pain, we propose NB001 as a promising agent that can be administered continuously and systemically at a high dose.
Authors’ contributions
WBK, QC, XML, and JT carried out behavioral experiments. QY, LW and DSW carried biological experiments. YYG carried out electrophysiological experiments. JNZ and GL analyzed the data and drafted the manuscript, MGZ and MZ designed and finished the final draft of the manuscript. All authors read and approved the final manuscript. WBK, QY, and YYG contributed equally to this work.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (No. 81325022), Clinical Science and Technology Project Funding in Jiangsu Province (No. BL2012002), China Postdoctoral Science Foundation (2015M572814) and Six Talent Project in Jiangsu Province (No. WSW-091).
References
- 1.Falk S, Dickenson AH. Pain and nociception: mechanisms of cancer-induced bone pain. J Clin Oncol 2014; 32: 1647–1654. [DOI] [PubMed] [Google Scholar]
- 2.Middlemiss T, Laird BJ, Fallon MT. Mechanisms of cancer-induced bone pain. Clin Oncol (R Coll Radiol) 2011; 23: 387–392. [DOI] [PubMed] [Google Scholar]
- 3.Mantyh PW. Bone cancer pain: from mechanism to therapy. Curr Opin Support Palliat Care 2014; 8: 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Honore P, Mantyh PW. Bone cancer pain: from mechanism to model to therapy. Pain Med 2000; 1: 303–309. [DOI] [PubMed] [Google Scholar]
- 5.Luger NM, Mach DB, Sevcik MA, et al. Bone cancer pain: from model to mechanism to therapy. J Pain Symptom Manage 2005; 29: S32–S46. [DOI] [PubMed] [Google Scholar]
- 6.Zhuo M. Cortical plasticity as a new endpoint measurement for chronic pain. Mol Pain 2011; 7: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhuo M. Neural mechanisms underlying anxiety-chronic pain interactions. Trends Neurosci 2016; 39: 136–145. [DOI] [PubMed] [Google Scholar]
- 8.Liu SB, Zhang MM, Cheng LF, et al. Long-term upregulation of cortical glutamatergic AMPA receptors in a mouse model of chronic visceral pain. Mol Brain 2015; 8: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bleakman D, Alt A, Nisenbaum ES. Glutamate receptors and pain. Semin Cell Dev Biol 2006; 17: 592–604. [DOI] [PubMed] [Google Scholar]
- 10.Sutherland EW, Rall TW. Fractionation and characterization of a cyclic adenine ribonucleotide formed by tissue particles. J Biol Chem 1958; 232: 1077–1091. [PubMed] [Google Scholar]
- 11.Aley KO, Levine JD. Role of protein kinase A in the maintenance of inflammatory pain. J Neurosci 1999; 19: 2181–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009; 10: 895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao MG, Ko SW, Wu LJ, et al. Enhanced presynaptic neurotransmitter release in the anterior cingulate cortex of mice with chronic pain. J Neurosci 2006; 26: 8923–8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Gong B, Vadakkan KI, et al. Genetic evidence for adenylyl cyclase 1 as a target for preventing neuronal excitotoxicity mediated by N-methyl-D-aspartate receptors. J Biol Chem 2007; 282: 1507–1517. [DOI] [PubMed] [Google Scholar]
- 15.Qiu S, Zhang M, Liu Y, et al. GluA1 phosphorylation contributes to postsynaptic amplification of neuropathic pain in the insular cortex. J Neurosci 2014; 34: 13505–13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Xu H, Wu LJ, et al. Identification of an adenylyl cyclase inhibitor for treating neuropathic and inflammatory pain. Sci Transl Med 2011; 3: 65ra63. [DOI] [PubMed] [Google Scholar]
- 17.Vadakkan KI, Wang H, Ko SW, et al. Genetic reduction of chronic muscle pain in mice lacking calcium/calmodulin-stimulated adenylyl cyclases. Mol Pain 2006; 2: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwei MJ, Honore P, Rogers SD, et al. Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J Neurosci 1999; 19: 10886–10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King T, Vardanyan A, Majuta L, et al. Morphine treatment accelerates sarcoma-induced bone pain, bone loss, and spontaneous fracture in a murine model of bone cancer. Pain 2007; 132: 154–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Mouedden M, Meert TF. Evaluation of pain-related behavior, bone destruction and effectiveness of fentanyl, sufentanil, and morphine in a murine model of cancer pain. Pharmacol Biochem Behav 2005; 82: 109–119. [DOI] [PubMed] [Google Scholar]
- 21.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 22.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 1980; 20: 441–462. [DOI] [PubMed] [Google Scholar]
- 23.Zhao MG, Toyoda H, Lee YS, et al. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron 2005; 47: 859–872. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Wu LJ, Kim SS, et al. FMRP acts as a key messenger for dopamine modulation in the forebrain. Neuron 2008; 59: 634–647. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Wu LJ, Zhang F, et al. Roles of calcium-stimulated adenylyl cyclase and calmodulin-dependent protein kinase IV in the regulation of FMRP by group I metabotropic glutamate receptors. J Neurosci 2008; 28: 4385–4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoeger-Bement MK, Sluka KA. Phosphorylation of CREB and mechanical hyperalgesia is reversed by blockade of the cAMP pathway in a time-dependent manner after repeated intramuscular acid injections. J Neurosci 2003; 23: 5437–5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei F, Qiu CS, Kim SJ, et al. Genetic elimination of behavioral sensitization in mice lacking calmodulin-stimulated adenylyl cyclases. Neuron 2002; 36: 713–726. [DOI] [PubMed] [Google Scholar]
- 28.Zhuo M. Central plasticity in pathological pain. Novartis Found Symp 2004; 261: 132–145. discussion 145–154. [PubMed] [Google Scholar]
- 29.Toyoda H, Zhao MG, Zhuo M. Enhanced quantal release of excitatory transmitter in anterior cingulate cortex of adult mice with chronic pain. Mol Pain 2009; 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liauw J, Wu LJ, Zhuo M. Calcium-stimulated adenylyl cyclases required for long-term potentiation in the anterior cingulate cortex. J Neurophysiol 2005; 94: 878–882. [DOI] [PubMed] [Google Scholar]
- 31.Sunahara RK, Taussig R. Isoforms of mammalian adenylyl cyclase: multiplicities of signaling. Mol Interv 2002; 2: 168–184. [DOI] [PubMed] [Google Scholar]
- 32.Xia Z, Storm DR. Calmodulin-regulated adenylyl cyclases and neuromodulation. Curr Opin Neurobiol 1997; 7: 391–396. [DOI] [PubMed] [Google Scholar]
- 33.Gong B, Wang H, Gu S, et al. Genetic evidence for the requirement of adenylyl cyclase 1 in synaptic scaling of forebrain cortical neurons. Eur J Neurosci 2007; 26: 275–288. [DOI] [PubMed] [Google Scholar]
- 34.Saito O, Aoe T, Yamamoto T. Analgesic effects of nonsteroidal antiinflammatory drugs, acetaminophen, and morphine in a mouse model of bone cancer pain. J Anesth 2005; 19: 218–224. [DOI] [PubMed] [Google Scholar]
- 35.Mouedden ME, Meert TF. Pharmacological evaluation of opioid and non-opioid analgesics in a murine bone cancer model of pain. Pharmacol Biochem Behav 2007; 86: 458–467. [DOI] [PubMed] [Google Scholar]
- 36.Brand CS, Hocker HJ, Gorfe AA, et al. Isoform selectivity of adenylyl cyclase inhibitors: characterization of known and novel compounds. J Pharmacol Exp Ther 2013; 347: 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]