Abstract
Background
To study potential ischemic effects of intravitreal Bevacizumab (IVB) on unaffected retina in treatment-naive eyes with macular edema secondary to branch retinal vein occlusion (BRVO) and contralateral eyes secondary to systemic absorption.
Methods and Findings
Prospective, interventional series included 27 treatment-naive eyes with BRVO and macular edema. Exclusion criteria: Eyes with diabetic retinopathy, glaucoma, vasculitides, papilledema or systemic neurologic condition. Subjects underwent complete ophthalmological examination including fluoroscein angiography (FA), optical coherence tomography (OCT) and multifocal electroretinogram (mf-ERG). All subjects received single 1.25 mg/0.05ml IVB injection. Two observers measured all parameters; inter-observer agreements were expressed as kappa values. Paired t-test was used to compare values at baseline and follow-up. The statistical analysis was done using SPSS for Windows, Version 14.0. (Chicago, SPSS Inc.) Presenting mean CFT (central foveal thickness) was 499.5(+/-229.7) μm, mean BCVA (best corrected visual acuity) was 0.64(+/-0.41) logMAR. At last follow-up, mean CFT was 267.9(+/-159.3) μm (P<0.001), 95% CI [127.18, 422.32]; mean BCVA was 0.28(+/-0.24) logMAR. Respectively, mean N1 and P1 amplitudes of mfERG in 'unaffected quadrant' at presentation were -6.10(+/-4.00) nV/deg2 and 17.17(+/-11.54)nV/deg2; and -5.33(+/-1.30)nV/deg2 and 15.29(+/-4.69)nV/deg2 at final follow-up (P = 0.631 and 0.197, respectively), (95% CIs [-0.93, 1.42] and [-4.22, 1.08] respectively). In fundus quadrant of fellow eyes corresponding to unaffected quadrant in treated eyes, mean N1 and P1 amplitudes at presentation were -5.39(+/-1.56)nV/deg2 and 15.89(+/-3.89)nV/deg2; and -5.39(+/-1.90)nV/deg2 and 15.9(+/-5.52)nV/deg2 (P = 0.380 and 0.208), (95% CIs [-0.57, 1.28] and [-4.1, 1.1]) at last follow-up, respectively. Limitations: This study analysed the effects with a single injection of bevacizumab. However, whether ischemic adverse effects will emerge with repeated IVB injections as a consequence of cumulative dosing needs further investigation. The setting of our study being a tertiary care centre, the numbers of fresh BRVO cases without prior intervention were limited. Thus, the limitations of our study include a small sample size with a small follow-up period. No major ocular/systemic adverse event was observed in the study period.
Conclusion
No evidence of progressive ischaemia attributable to single bevacizumab treatment was observed in this study. However, a larger prospective study involving subjects with cumulative dosing of bevacizumab and a longer follow-up could provide a better understanding of the potential ischaemic effects of bevacizumab or other anti-VEGF agents.
Introduction
Branch retinal vein obstruction (BRVO) is the second most common retinal vascular disorder after diabetic retinopathy. [1] Visual loss is usually caused by macular edema, macular ischemia, or vitreous hemorrhage. Conventionally, laser photocoagulation has been used to treat macular edema but is now rapidly shifting to intravitreal anti-vascular (VEGF). [2–4] Safety data ranging from histology to functional retinal testing to adverse event reporting in larger trials is continuously accumulating. Various theories and reports suggest variable potential of anti-VEGFs in causing an ischemic effect; most probably due to interference with the action of the natural vasogenic compound i.e. VEGF. [5–8] In mouse retina, a significant increase in apoptosis of cells in the inner and outer nuclear layers, causing reduced thickness of the inner and outer nuclear layers and a decline in retinal function as measured by electroretinograms was noted after 14 days of VEGF neutralisation. [9]
A sudden drop in effective VEGF concentration may be responsible for the closure of the normal capillaries. [10] There are reports of development of anterior ischemic optic neuropathy after intravitreal injections of bevacizumab. [11, 12] Long-term neutralization of retinal VEGF is noted to increase the risk of circulation disturbances in the choriocapillaris. [13] Rouvas et al reported a case of retinal angiomatous proliferation in a patient with AMD treated with a combination of photodynamic therapy (PDT) and intravitreal bevacizumab. [14] Yokomaya et al described a case of extensive occlusion of both retinal arteries and veins 4 weeks after intracameral administration of bevacizumab in a patient with neovascular glaucoma and diabetic retinopathy. [15] Pieramici et al found increased macular ischemia in FFA and increase in the extent of retinal hemorrhages in a patient with perfused CRVO following repeated intravitreal injections of ranibizumab. [16] In order to study the potential ischemic effects of IVB, we undertook a prospective (pilot) study designed to monitor the anatomical and functional status of the normal and affected retinal areas in treatment-naive eyes presenting with BRVO and macular edema. The fellow eye was monitored in all subjects to look for any potential changes due to systemic absorption of IVB.
Methods
This was a prospective interventional case series. This project was approved by Ethics subcommittee of Vision Research Foundation, Chennai in July 2011. All clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. Written informed consent was obtained from all the participants. This study included subjects with BRVO and associated macular edema with best corrected visual acuity (BCVA) worse than Snellen’s acuity 6/6 (i.e. logMAR 0.0). Patients were screened for systemic risk factors of BRVO. Each patient underwent complete ophthalmological examination including BCVA, noncontact slit-lamp biomicroscopy, retinal examination with indirect ophthalmoscopy, fundus photography, fundus fluorescein angiography (FFA using Zeiss Digital Angiography software; FF 450plus IR Fundus Camera, VISUPAC Version 4.43), spectral-domain optical coherence tomography (SD-OCT; Topcon 3D OCT-1000) and multifocal electroretinogram (mf-ERG; Veris ScienceTM 5.2.2X, EDI) before receiving intravitreal bevacizumab (IVB) injection. All patients were treated with the same dose of 1.25 mg/0.05ml IVB in affected eye. Following injection, follow-up were scheduled at day 1, month 1, and month 3. Investigations were repeated on each monthly visit (Fig 1). Pre- and post-injection parameters were analyzed to study the effects on structural (OCT and FFA) and functional (BCVA and mf- ERG) outcomes. Analyzed OCT parameters included central foveal thickness (CFT) in affected eye, maximum retinal thickness in affected eye, CFT of contralateral eye, thickness of unaffected part of retina at 2500 microns from fovea in a vertical scan measuring normal retinal thickness (NRT), and signs of acute retinal ischemia like hyper-reflectivity of inner retina and appearance of middle limiting membrane ‘MLM’ sign. [17–19]
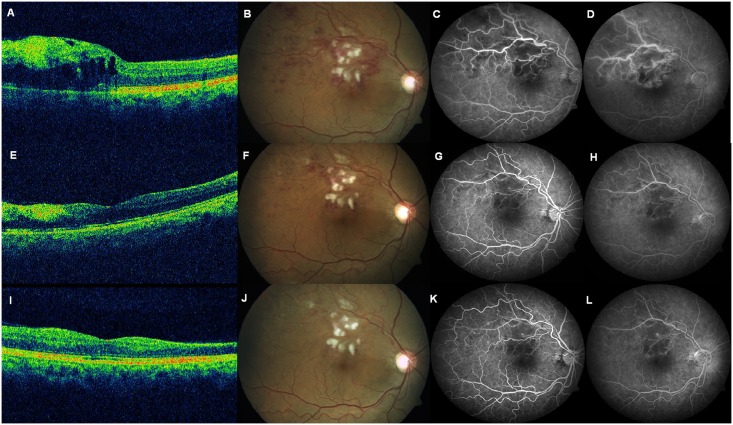
Fig 1. Composite image of OCT, colour fundus photo, and fluorescein angiography in an eye with superotemporal branch vein occlusion.
This figure shows findings at baseline (A-D), month 1 (E-H), and month 3 (I-L). At baseline, Optical coherence tomography (OCT) reveals cystoid macular thickening (A) due to superotemporal branch vein occlusion (B), with fluorescein angiography revealing capillary non-perfusion (C) and late leakage (D). At 1 month, OCT reveals resolution of macular edema with inner retinal thinning (E), resolving retinal haemorrhages (F), with reduction in area of capillary non-perfusion (G) and reduced leakage (H). At 3 months, OCT reveals slight increase in macular thickening (I), resolving retinal haemorrhages (J), with further reduction in area of capillary non-perfusion (K) and persistent late leakage (L).
Analyzed FFA parameters included outline of foveal avascular zone (FAZ), area of FAZ, outline of half of FAZ in unaffected side of retina (using freehand drawing tool of VISUPAC Version 4.43 software on early fluorescein angiography frames with 50° images), capillary non-perfusion (CNP) areas in unaffected side of retina, CNP areas in the contralateral eye. [20, 21] BCVA was recorded at each visit. Analyzed multifocal electroretinogram (mf-ERG) parameters included N1 amplitude, N1 implicit time, P1 amplitude and P1 implicit time in ‘affected quadrant’ and ‘unaffected quadrant’. [22–26] In the contralateral eye, same parameters were analysed from the quadrant corresponding to the defined ‘unaffected quadrant’ in affected eye. An average of 10 hexagonal points in perifoveal zone (to avoid spill-over as far as possible) was considered as shown in Fig 2. Exclusion criteria ruled out eyes with central retinal vein occlusion (CRVO), diabetic retinopathy, sickle cell retinopathy, systemic vasculitic conditions, papilledema or sequelae of disc edema. The statistical analysis was done using SPSS for Windows, Version 14.0. (Chicago, SPSS Inc.).
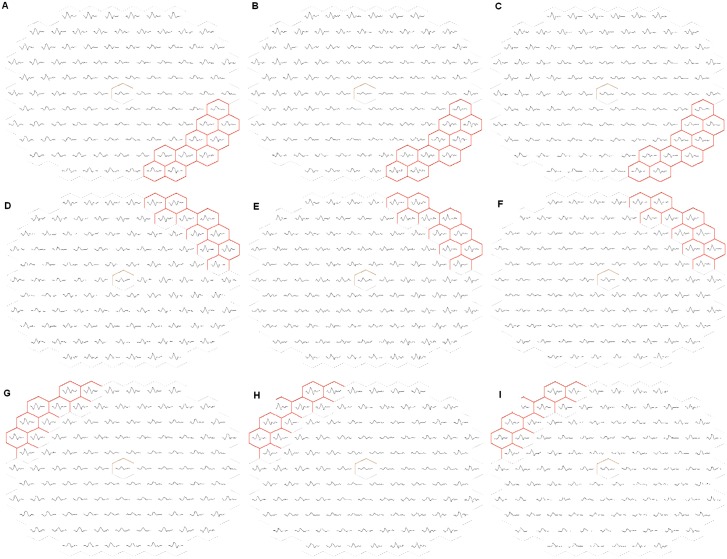
Fig 2. Multifocal ERG plot of a subject with inferotemporal BRVO in left eye.
Plot recorded at baseline (A), month 1 (B), and month 3 (C). Corresponding points in the superotemporal quadrant of the affected eye are recorded at same time intervals (D, E, and F, respectively). Corresponding points in the superotemporal quadrant of the contralateral eye are also recorded at same time intervals (G, H, and I, respectively).
Results
Overall, 27 patients were recruited over a period of 18 months (October 2011 to March 2013). The mean age was 54.4 years (+/-10 years). There were 22 (81.5%) male and 5 (18.5%) female subjects. 18 (66.7%) patients presented with BRVO in right eye, whereas left eye was affected in 9 (33.3%) of the patients. The mean duration of symptoms was 9.7 (+/-9.9) weeks; the most common symptom being diminution of vision—present in 26 (96.30%) patients. Only one patient complained of metamorphopsia and visual field defects. Associated systemic conditions included hypertension (HTN, n = 16), diabetes mellitus (DM, n = 11), hyperlipidemia (n = 5), homocystinuria (n = 1) and ischemic heart disease (IHD, n = 1). Eight (30%) patients had no systemic conditions. None of the patients had previous history of taking any treatment for BRVO i.e. patients were treatment naïve. BCVA at presentation ranged from Snellen’s acuity 6/7.5 to counting fingers (CF). At presentation, 41% eyes were phakic without cataract, 44% had nuclear sclerosis I (NS I), 7% had NS II, and 4% had posterior subcapsular cataract. One eye (4%) was pseudophakic. Mean intraocular pressure (IOP) was 14.1(+/-2.1) mmHg. Optic disc was normal in all eyes. Most common fundus quadrant affected by BRVO was superotemporal quadrant (STQ, 59%); followed by inferotemporal quadrant (ITQ, 41%). Fundus quadrant opposite to BRVO-affected quadrant was studied as the unaffected quadrant. Hence, most common unaffected quadrant studied was ITQ. All subjects were treated with same dose of 1.25 mg/0.05ml IVB. First follow-up (scheduled 1 month post IVB) was at an average of 1.3(+/-0.6) months. Second follow-up (scheduled 3 months post IVB) was at an average of 4.52 (+/-4.4) months. All changes on OCT, FFA, BCVA and mf-ERG parameters during follow-up visits were compared to the baseline using the paired t-test. The demographic details, clinical parameters, and imaging results are summarised in S1 File. Details regarding OCT, FFA, and mf-ERG of study subjects are available at http://dx.doi.org/10.5061/dryad.3cp54.
OCT Parameters
Inter-observer agreements for measurements of CFT in affected eye at presentation, on 1st follow-up and on 2nd follow-up were kappa value = 0.96, 0.93 and 0.95, respectively. Mean CFT at presentation was 499.5 (+/-229.7) microns. It reduced significantly to 258.3 (+/-150.3) microns on 1st follow-up (P value <0.001), (95% CI [148.41, 333.96]) and 267.9 (+/-159.3) microns (P value <0.001), (95% CI [127.18, 422.32]) on 2nd follow-up (Table 1).
Table 1. Measured values for central foveal thickness (CFT) in affected eyes.
| Visit | Mean | Standard Deviation | Kappa value | P value for paired t-test in comparison to the values ‘at presentation’ | 95% Confidence Interval |
|---|---|---|---|---|---|
| At presentation | 499.5 | 229.7 | 0.96 | - | |
| 1st follow-up | 258.3 | 150.3 | 0.93 | 0.00 | (148.41, 333.96) |
| 2nd follow-up | 267.9 | 159.3 | 0.95 | 0.00 | (127.18, 422.32) |
Maximum Retinal Thickness (MRT) in affected eyes
Inter-observer agreements for the measurements at presentation, on 1st follow-up and 2nd follow-up were kappa value = 0.94, 0.96 and 0.96, respectively. Mean MRT at presentation was 664 (+/-149.5) microns. It reduced significantly to 462.3 (+/-188.7) microns on 1st follow-up (P value <0.001), (95% CI [132.25, 271.23]) and 408.1 (+/-193.6) microns (P value <0.001), (95% CI [164.89, 374.06]) on 2nd follow-up (Table 2).
Table 2. Measured values for maximum retinal thickness (MRT) in affected eyes.
| Visit | Mean | Standard Deviation | Kappa value | P value for paired t-test in comparison to the values ‘at presentation’ | 95% Confidence Interval |
|---|---|---|---|---|---|
| At presentation | 664 | 149.5 | 0.94 | Not applicable | |
| 1st Follow-up | 462.3 | 188.7 | 0.96 | 0.00 | (132.25, 271.23) |
| 2nd Follow-up | 408.1 | 193.6 | 0.96 | 0.00 | (164.89, 374.06) |
Normal Retinal Thickness (NRT) of the affected eyes
Inter-observer agreements for the measurements at presentation, on 1st follow-up and on 2nd Follow-up were kappa value = 0.97, 0.96 and 0.98 respectively. Mean NRT at presentation was 241.9 (+/-18.9) microns. It changed to 241 (+/-19.9) microns on 1st follow-up (P value = 0.49), (95% CI [-1.5, 4.24]) and 235.7 (+/-21.7) microns (P value = 0.34), (95% CI [-3.31, 10.19]) on 2nd follow-up (Table 3).
Table 3. Measured values for normal retinal thickness (NRT) in affected eyes.
| Visit | Mean | Standard Deviation | Kappa value | P value for paired t-test in comparison to the values ‘at presentation’ | 95% Confidence Interval |
|---|---|---|---|---|---|
| At presentation | 241.9 | 18.9 | 0.97 | - | |
| 1st follow-up | 241 | 19.9 | 0.96 | 0.49 | (-1.5, 4.24) |
| 2nd follow-up | 235.7 | 21.7 | 0.98 | 0.34 | (-3.31, 10.19) |
Signs of acute ischemia
None of the OCT scan images showed either development of hyper-reflectivity of inner retina or appearance of ‘Middle Limiting Membrane (MLM)’ sign in either of the follow-up scans.
CFT of contralateral eyes
Inter-observer agreement for measurements at presentation, on 1st follow-up and on 2nd follow-up were kappa value = 0.97, 0.98 and 0.99, respectively. Mean CFT of contralateral eyes at presentation was 175.6 (+/-18.8) microns. It changed to 177.9 (+/-19.8) microns (P value = 0.06), (95% CI [-5.56, 0.16)]) on 1st follow-up and 187.1 (+/-18.2) microns (P value = 0.28), (95% CI [-8.61, 2.14]) on 2nd follow-up (Table 4).
Table 4. Measured values for central foveal thickness (CFT) in contralateral eyes.
| Visit | Mean | Standard Deviation | Kappa value | P value for paired t-test in comparison to the values ‘at presentation’ | 95% Confidence Interval |
|---|---|---|---|---|---|
| At presentation | 175.2 | 18.8 | 0.97 | - | |
| 1st follow-up | 177.9 | 19.8 | 0.98 | 0.06 | (-5.56, 0.16) |
| On 2nd Follow-up | 187.1 | 18.2 | 0.99 | 0.28 | (-8.61, 2.14) |
FFA Parameters
Outline of Foveal Avascular Zone (FAZ)
Inter-observer agreements for the findings at presentation, on 1st follow-up and on 2nd follow-up were kappa value = 0.94, 0.98 and 0.94, respectively. The outline of FAZ as a whole was found to be intact in 6 eyes (22%) and distorted in 21 eyes (78%) at presentation. On 1st follow-up; it remained same in 22 (92%), improved from ‘distorted’ to ‘intact’ FAZ in 2 (8%) whereas none of them worsened from ‘intact’ to ‘distorted’. On 2nd follow-up, it remained same in 100% eyes. There was neither improvement from ‘distorted’ to ‘intact’ nor worsening from ‘intact’ to ‘distorted’ in any of the eyes.
Area of FAZ
Inter-observer agreements for the measurements at presentation, on 1st follow-up and on 2nd follow-up were kappa value = 0.86, 0.90 and 0.84, respectively. Mean FAZ area at presentation was 0.65 (+/-0.34) mm2 and changed to 0.61 (+/-0.21) mm2 on 1st follow-up and to 0.57(+/-0.29) mm2 on 2nd follow-up. These changes were not statistically significant (P values 0.953 and 0.213, 95% CIs [-0.05, 0.27] and [-0.07, 0.27] respectively). (see Table 5).
Table 5. Measured values for area of foveal avascular zone (FAZ) in affected eyes.
| Visit | Mean | Standard Deviation | Kappa value | P value for paired t-test in comparison to the values ‘at presentation’ | 95% Confidence Interval |
|---|---|---|---|---|---|
| At presentation | 0.65 | 0.34 | 0.96 | - | |
| 1st follow-up | 0.61 | 0.21 | 0.93 | 0.953 | (-0.05, 0.27) |
| 2nd follow-up | 0.57 | 0.29 | 0.95 | 0.213 | (-0.07, 0.27) |
Outline of half of FAZ on the unaffected side of retina (hemi-circumference)
Inter-observer agreements for the findings at presentation, on 1st follow-up and on 2nd follow-up were kappa value = 0.96, 0.96 and 0.94, respectively. Distortion of hemi-circumference of FAZ was classified into three grades. Grade I: no distortion, grade II: distortion <50% of normal outline, and Grade III: distortion >50% of normal outline. At presentation, 22 eyes (82%) were classified into Grade I, 3 eyes (11%) as Grade II, and 2 eyes (7%) as grade III. On 1st follow-up; it remained same in 22 (92%), improved in 2 (8%) and worsened in none. On 2nd follow-up (n = 9); it remained same in 5 (56%), worsened in 4 (44%) and improved in none.
CNP areas in the unaffected side of retina
Inter-observer agreements for the findings at presentation, on 1st follow-up and on 2nd follow-up was kappa value = 1 at each visit. None of the eyes showed presence of CNP areas on the unaffected side of the retina at presentation. There were no new development of CNP areas post IVB, both on 1st follow-up and 2nd follow-up.
CNP areas in the contralateral eyes
Inter-observer agreements for the findings at presentation, on 1st follow-up and on 2nd follow-up was kappa value = 1 at each visit. None of the patients showed presence of any leakage or CNP areas in the contralateral eyes at presentation. There were no new development of CNP areas post IVB, both on 1st follow-up and 2nd follow-up.
BCVA
Mean BCVA at presentation was 0.64 (+/-0.41) logMAR which improved to 0.32 (+/-0.26) logMAR on 1st follow-up and further improved to 0.28 (+/-0.24) logMAR on 2nd follow-up. Individually; BCVA on 1st follow-up improved in 21 (78%), remained same in 3 (11%) and worsened in 3 (11%) eyes. On 2nd Follow-up, compared to the BCVA at presentation; BCVA improved in 12 (81%), remained same in 3 (12%) and worsened in 1 (6%) eyes.
mf-ERG Parameters
‘Affected’ quadrant
Measured values of mean N1 and P1 amplitudes in affected eyes at presentation, on 1st follow-up and 2nd follow-up are listed in Table 6. Interval changes were not significant. The mean N1 and P1 implicit times at presentation were 18.64 (+/-2.21) ms and 33.95 (+/-2.91) ms, respectively. On 1st follow-up, the mean values changed to 17.28 (+/-2.20) ms and 33.65 (+/-3.44) ms, respectively (P values = 0.016 and 0.438, 95% CIs [3.27, 9.69] and [-0.79, 1.75]). The reduction in N1 implicit time was statistically significant but changes in P1 implicit time were not statistically significant. On 2nd follow-up, the mean values changed to 18.23 (+/-2.78) ms and 33.7 (+/-4.01) ms, respectively (P values = 0.328 and 0.147, 95% CIs [-1.52, 3.86] and [-1, 5.26]). These changes were not statistically significant (Table 7).
Table 6. Measured values of N1 and P1 amplitudes in the 'affected' quadrant.
| Visit | N1 Amplitude | P1 Amplitude | ||||
|---|---|---|---|---|---|---|
| Mean (nV/deg2) | Standard Deviation | P value for paired t-test in comparison to values ‘at presentation’ (95% Confidence Interval) | Mean (nV/deg2) | Standard Deviation | P value for paired t-test in comparison to values ‘at presentation’ (95% Confidence Interval) | |
| At presentation | -3.88 | 1.25 | - | 10.14 | 3.09 | - |
| 1st follow-up | -3.85 | 1.09 | 0.69 (-2.11, -0.23) | 10.45 | 3.38 | 0.20 (-2.01, 0.45) |
| 2nd follow-up | -3.8 | 0.99 | 0.19 (-0.48, 1.94) | 10.2 | 3.14 | 0.11 (-5.11, 0.71) |
Table 7. Measured values of N1 and P1 implicit times in the 'affected' quadrant.
| Visit | N1 Implicit Time | P1 Implicit Time | ||||
|---|---|---|---|---|---|---|
| Mean (milli-seconds) | Standard Deviation | P value for paired t-test in comparison to the values ‘at presentation’ | Mean (milli-seconds) | Standard Deviation | P value for paired t-test in comparison to the values ‘at presentation’ | |
| At presentation | 18.64 | 2.21 | - | 33.95 | 2.91 | - |
| 1st follow-up | 17.28 | 2.20 | 0.016 (3.27, 9.69) | 33.65 | 3.44 | 0.438 (-0.79, 1.75) |
| 2nd follow-up | 18.23 | 2.78 | 0.328 (-1.52, 3.86) | 33.7 | 4.01 | 0.147 (-1, 5.26) |
‘Unaffected’ quadrant
Measured values of mean N1 and P1 amplitudes in unaffected quadrant of affected eyes at presentation, on 1st follow-up and 2nd follow-up are listed in Table 8. Interval changes were not significant. Measured values of mean N1 and P1 implicit times in unaffected quadrant of affected eyes at presentation, on 1st follow-up and 2nd follow-up are listed in Table 9. Interval changes were not significant.
Table 8. Measured values of N1 and P1 amplitudes in the 'unaffected' quadrant.
| Visit | N1 Amplitude | P1 Amplitude | ||||
|---|---|---|---|---|---|---|
| Mean (nV/deg2) | Standard Deviation | P value for paired t-test in comparison to the values ‘at presentation’ | Mean (nV/deg2) | Standard Deviation | P value for paired t-test in comparison to the values ‘at presentation’ | |
| At presentation | -6.10 | 4.00 | - | 17.17 | 11.54 | - |
| 1st follow-up | -5.06 | 1.80 | 0.337 (-4.46, -0.67) | 14.15 | 4.22 | 0.369 (-2.33, 5.97) |
| 2nd follow-up | -5.33 | 1.30 | 0.631 (-0.93, 1.42) | 15.29 | 4.69 | 0.197 (-4.22, 1.08) |
Table 9. Measured values of N1 and P1 implicit times in the 'unaffected' quadrant of affected eyes.
| Visit | N1 Implicit Time | P1 Implicit Time | ||||
|---|---|---|---|---|---|---|
| Mean (milli-seconds) | Standard Deviation | P value for paired t-test in comparison to the values ‘at presentation’ | Mean (milli-seconds) | Standard Deviation | P value for paired t-test in comparison to the values ‘at presentation’ | |
| At presentation | 16.61 | 1.39 | - | 30.80 | 2.03 | - |
| 1st follow-up | 16.24 | 2.08 | 0.321 (2.22, 8.15) | 30.31 | 2.31 | 0.090 (-0.16, 2.02) |
| 2nd follow-up | 16.06 | 1.05 | 0.213 (-0.66, 1.83) | 29.29 | 1.49 | 0.050 (0.02, 4.53) |
Contralateral eyes
Measured values of mean N1 and P1 amplitudes in corresponding quadrant of unaffected eyes at presentation, on 1st follow-up and 2nd follow-up are listed in Table 10. Interval changes were not significant. Measured values of mean N1 and P1 implicit times in corresponding quadrant of unaffected eyes at presentation, on 1st follow-up and 2nd follow-up are listed in Table 11. Interval changes were not significant.
Table 10. Measured values of N1 and P1 amplitudes in the corresponding quadrant of 'unaffected' contralateral eyes.
| Visit | N1 Amplitude | P1 Amplitude | ||||
|---|---|---|---|---|---|---|
| Mean (nV/deg2) | Standard Deviation | P value for paired t-test in comparison to the values ‘at presentation’ | Mean (nV/deg2) | Standard Deviation | P value for paired t-test in comparison to the values ‘at presentation’ | |
| At presentation | -5.39 | 1.56 | - | 15.89 | 3.89 | - |
| 1st follow-up | -5.14 | 1.71 | 0.793 (-3.06, -0.47) | 15.29 | 4.15 | 0.782 (-1.51, 1.16) |
| 2nd follow-up | -5.39 | 1.90 | 0.380 (-0.57, 1.28) | 15.9 | 5.52 | 0.208 (-4.1, 1.1) |
Table 11. Measured values of N1 and P1 implicit times in the corresponding quadrant in contralateral eyes.
| Visit | N1 Implicit Time | P1 Implicit Time | ||||
|---|---|---|---|---|---|---|
| Mean (milli-seconds) | Standard Deviation | P value for paired t-test in comparison to the values ‘at presentation’ | Mean (milli-seconds) | Standard Deviation | P value for paired t-test in comparison to the values ‘at presentation’ | |
| At presentation | 16.16 | 1.25 | - | 29.47 | 1.56 | - |
| 1st follow-up | 16.31 | 1.61 | 0.800 (-1.78, 7.59) | 29.78 | 1.88 | 0.902 (-0.84, 0.75) |
| 2nd follow-up | 15.96 | 1.02 | 0.114 (-0.23, 1.63) | 29.4 | 1.51 | 0.797 (-0.93, 1.16) |
Discussion
Of late, there have been several animal studies, case reports and theories that suggest possible ischemic effect of anti-VEGF agents on ocular structures. Considering the vast usage of anti-VEGF agents in today’s ophthalmic practices it becomes imperative to identify the potential ischemic effects of these therapeutic agents on normal retina, if any. There is little description about fluorescein angiographic patterns in the BRAVO and CRUISE studies although vision and OCT results were very encouraging. [27, 28] The rationale of this prospective study was to gather evidence of any potential ischemic effects of IVB on the retina using anatomic and functional investigations like OCT, FFA and mf-ERG. The vitreous half-life of IVB being 6.7 days, the elimination period of the drug ranges from 33.5 to 46.9 days (i.e. 5 to 7 half-lives). Hence, we followed up patients at 1 month and 3 months after administration of single dose of IVB which ensured complete elimination of drug from the eye. This duration of follow-up appears to be adequate to study the potential adverse effects associated with one-time administration of IVB. In a recent study, Campbell et al analysed the systemic adverse events with intravitreal injection of vascular endothelial growth factor inhibitors in a nested case-control study. They found that intravitreal injections of bevacizumab and ranibizumab were not associated with significant risks of ischaemic stroke, acute myocardial infarction, congestive heart failure, or venous thromboembolism. [29]
In our study, improvement in BCVA corresponded to significant reduction in CFT and macular edema as evident by OCT measurements. There was no significant change in thickness of unaffected retina following IVB. Thus, OCT changes were not suggestive of any structural changes caused due to any potential ischemic effects of IVB. Changes in FFA did show an improvement in distortion of the outline of FAZ after receiving IVB. The hemi-circumference of FAZ in the unaffected retinal side also did not show adverse changes in any of the eyes at 1 month and 56% eyes at 3 months after IVB. This worsening of the hemi-circumference of FAZ outline 3 months after IVB could be partially attributed to chronicity of macular edema. There was no new development of any CNP areas either after 1 month or 3 months of IVB that could provide any evidence of retinal ischemia. mf-ERG findings in affected quadrant showed significant reduction in N1 implicit time suggestive of an improved functional status of affected quadrant of retina 3 months after IVB. N1, P1 amplitudes and P1 implicit time showed no significant changes at either 1 month after IVB or 3 months after IVB in the affected quadrant of retina. In the unaffected part of retina, none of the readings i.e. N1, P1 amplitudes or N1, P1 implicit time showed any significant change either after 1 month or after 3 months of IVB. Thus mf-ERG did not provide any evidence of deterioration of functional status of retina due to any potential ischemic effect of IVB. The measurements, findings and interpretations drawn from the investigations of the contralateral eye also did not show any evidence of potential ischemic effect in the contralateral eye due to systemic absorption of IVB.
Hence, no evidence of progressive ischaemia attributable to bevacizumab treatment was observed in this series of patients. However, whether ischemic adverse effects will emerge with repeated IVB injections as a consequence of cumulative dosing will need further investigation. The setting of our study being a tertiary care centre, the numbers of fresh BRVO cases without prior intervention were limited. Thus, the limitations of our study include a small sample size with a small follow-up period. A larger prospective study with a longer follow-up period would provide better statistical significance to our results.
Supporting Information
Flowsheet listing the demographic, clinical and investigational details of all study subjects.
(XLSX)
Acknowledgments
We acknowledge the help of our colleagues in the Vitreoretinal department for referring cases for this study. This paper was presented at the ‘Vitreoretina Society of India’ annual meeting at Agra, India in December 2014. None of the authors have any conflict of interest to declare.
Data Availability
Most of the data from this study are available with the paper and Supporting Information files. Details regarding OCT, FFA, and mf-ERG of study subjects are available at http://dx.doi.org/10.5061/dryad.3cp54.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Saxena S. Laser photocoagulation in retinal vein occlusion: branch vein occlusion study and central vein occlusion study recommendations. Indian J Ophthalmol 1997;45:125–128. [PubMed] [Google Scholar]
- 2.Pai SA, Shetty R, Vijayan PB, Venkatasubramaniam G, Yadav NK, Shetty BK, et al. Clinical, anatomic, and electrophysiologic evaluation following intravitreal bevacizumab for macular edema in retinal vein occlusion. Am J Ophthalmol 2007;143:601–606. [DOI] [PubMed] [Google Scholar]
- 3.Rabena MD, Pieramici DJ, Castellarin AA, Nasir MA, Avery RL. Intravitreal bevacizumab (Avastin) in the treatment of macular edema secondary to branch retinal vein occlusion. Retina 2007;27:419–425. [DOI] [PubMed] [Google Scholar]
- 4.Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, Rundle AC, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 2010;117:1102–1112.e1. 10.1016/j.ophtha.2010.02.021 [DOI] [PubMed] [Google Scholar]
- 5.Isola V, Pece A, Massironi C, Reposi S, Dimastrogiovanni F. Accelerated ischemic vascular retinopathy after intravitreally injected bevacizumab for central retinal vein occlusion in elderly patients. Clin Ophthalmol 2013;7:455–460. 10.2147/OPTH.S30156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen E. Hsu J, Park CH. Acute visual acuity loss following intravitreal bevacizumab for diabetic macular edema. Ophthalmic Surg Lasers 2009;40:68–70. [DOI] [PubMed] [Google Scholar]
- 7.Kim KS, Chang HR, Song S. Ischaemic change after intravitreal bevacizumab (Avastin) injection for macular oedema secondary to non-ischaemic central retinal vein occlusion. Acta Ophthalmol 2008;86:925–927. 10.1111/j.1755-3768.2008.01175.x [DOI] [PubMed] [Google Scholar]
- 8.Goel N, Kumar V, Ghosh B. Ischemic maculopathy following intravitreal bevacizumab for refractory diabetic macular edema. Int Ophthalmol 2011;31:39–42. 10.1007/s10792-010-9390-z [DOI] [PubMed] [Google Scholar]
- 9.Pece A, Isola V, Piermarocchi S, Calori G. Efficacy and safety of anti-vascular endothelial growth factor (VEGF) therapy with intravitreal ranibizumab (Lucentis) for naive retinal vein occlusion: 1-year follow-up. Br J Ophthalmol 2011;95:56–68. 10.1136/bjo.2009.174060 [DOI] [PubMed] [Google Scholar]
- 10.Ameri H, Chader GJ, Kim JG, Sadda SR, Rao NA, Humayun MS. The effects of intravitreous bevacizumab on retinal neovascular membrane and normal capillaries in rabbits. Invest Ophthalmol Vis Sci 2007;48:5708–5715. [DOI] [PubMed] [Google Scholar]
- 11.Ganssauge M, Wilhelm H, Bartz-Schmidt KU, Aisenbrey S. Non-arteritic anterior ischemic optic neuropathy (NA-AION) after intravitreal injection of bevacizumab (Avastin) for treatment of angioid streaks in pseudoxanthoma elasticum. Graefes Arch Clin Exp Ophthalmol. 2009;247:1707–1710. [DOI] [PubMed] [Google Scholar]
- 12.Hosseini H, Razeghinejad MR. Anterior ischemic optic neuropathy after intravitreal injection of bevacizumab. J Neuroophthalmol 2009;29:160–161. 10.1097/WNO.0b013e3181a58fd1 [DOI] [PubMed] [Google Scholar]
- 13.D'Amore PA. Vascular endothelial cell growth factor-a: not just for endothelial cells anymore. Am J Pathol 2007;171:14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rouvas AA, Papakostas TD, Ladas ID, Vergados I. Enlargement of the hypofluorescent post photodynamic therapy treatment spot after a combination of photodynamic therapy with an intravitreal injection of bevacizumab for retinal angiomatous proliferation. Graefe's Arch Clin Exp Ophthalmol 2008;247:1707–1710. [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama K, Choshi T, Kimoto K, Shinoda K, Nakatsuka K. Retinal circulatory disturbances following intracameral injection of bevacizumab for neovascular glaucoma. Acta Ophthalmol 2008;86:927–928. 10.1111/j.1755-3768.2008.01187.x [DOI] [PubMed] [Google Scholar]
- 16.Pieramici DJ, Rabena M, Castellarin AA, Nasir M, See R, Norton T, et al. Ranibizumab for the treatment of macular edema associated with perfused central retinal vein occlusions. Ophthalmology 2008;115:e47–54. 10.1016/j.ophtha.2008.06.021 [DOI] [PubMed] [Google Scholar]
- 17.Ikeda F, Kishi S. Inner neural retina loss in central retinal artery occlusion. Jpn J Ophthalmol 2010;54:423–429. 10.1007/s10384-010-0841-x [DOI] [PubMed] [Google Scholar]
- 18.Chu YK, Hong YT, Byeon SH, Kwon OW. In vivo detection of acute ischemic damages in retinal arterial occlusion with optical coherence tomography: A "Prominent Middle Limiting Membrane Sign". Retina 2013;33:2110–2117. 10.1097/IAE.0b013e3182899205 [DOI] [PubMed] [Google Scholar]
- 19.Mathew R, Papavasileiou E, Sivaprasad S. Autofluorescence and high-definition optical coherence tomography of retinal artery occlusions. Clin Ophthalmol 2010;4:1159–1163. 10.2147/OPTH.S13592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parodi MB, Visintin F, Della Rupe P, Ravalico G. Foveal avascular zone in macular branch retinal vein occlusion. Int Ophthalmol 1995;19:25–28. [DOI] [PubMed] [Google Scholar]
- 21.Early Treatment Diabetic Retinopathy Study Research Group Classification of diabetic retinopathy from fluorescein angiograms. ETDRS report number 11. Ophthalmology 1991;98 (5 Suppl):807–822. [PubMed] [Google Scholar]
- 22.Hasegawa S, Ohshima A, Hayakawa Y, Takagi M, Abe H. Multifocal electroretinograms in patients with branch retinal artery occlusion. Invest Ophthalmol Vis Sci 2001;42:298–304. [PubMed] [Google Scholar]
- 23.Ohshima A, Hasegawa S, Takada R, Takagi M, Abe H. Multifocal electroretinograms in patients with branch retinal artery occlusion. Jpn J Ophthalmol 45:516–522. [DOI] [PubMed] [Google Scholar]
- 24.Hvarfner C, Andreasson S, Larsson J. Multifocal electroretinography and fluorescein angiography in retinal vein. Retina 2006;26:292–296. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda J, Hasegawa S, Suzuki K, Ichibe M, Tanimoto N, Usui T, et al. [Multifocal electroretinograms in patients with retinal vein occlusion]. Nippon Ganka Gakkai Zasshi 2004;108:84–91. [PubMed] [Google Scholar]
- 26.Wildberger H, Junghardt A. Local visual field defects correlate with the multifocal electroretinogram (mfERG) in retinal vascular branch occlusion. Klinische Monatsblatter fur Augenheilkunde 2002;219:254–258. [DOI] [PubMed] [Google Scholar]
- 27.Brown DM, Campochiaro PA, Bhisitkul RB, Ho AC, Gray S, Saroj N, et al. Sustained Benefits from Ranibizumab for Macular Edema Following Branch Retinal Vein Occlusion: 12-Month Outcomes of a Phase III Study. Ophthalmology 2011;18:1594–1602. [DOI] [PubMed] [Google Scholar]
- 28.Brown DM, Campochiaro PA, Singh RP, Li Z, Gray S, Saroj N, et al. Ranibizumab for Macular Edema following Central Retinal Vein Occlusion Six-Month Primary End Point Results of a Phase III Study. Ophthalmology 2010;117:1124–1133. 10.1016/j.ophtha.2010.02.022 [DOI] [PubMed] [Google Scholar]
- 29.Campbell RJ, Gill SS, Bronskill SE, Paterson JM, Whitehead M, Bell CM. Adverse events with intravitreal injection of vascular endothelial growth factor inhibitors: nested case-control study. BMJ 2012;345:e4203 10.1136/bmj.e4203 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flowsheet listing the demographic, clinical and investigational details of all study subjects.
(XLSX)
Data Availability Statement
Most of the data from this study are available with the paper and Supporting Information files. Details regarding OCT, FFA, and mf-ERG of study subjects are available at http://dx.doi.org/10.5061/dryad.3cp54.