Summary
Background
Toll‐like receptor 2 (TLR2) is a widely expressed pattern recognition receptor critical for innate immunity. TLR2 is also a key regulator of mucosal immunity implicated in the development of allergic disease. TLR2 activators are found in many common foods, but the role of TLR2 in oral tolerance and allergic sensitization to foods is not well understood.
Objective
The purpose of this study was to evaluate the impacts of TLR2 expression and TLR2 activation on oral tolerance to food antigens in a murine model.
Methods
Mice were fed ovalbumin (OVA) or peanut butter with or without the addition of low doses of TLR2 activators Pam3 CSK 4 or FSL‐1. Oral tolerance was assessed by analysing antibody responses after a systemic antigen challenge. OVA‐specific Tregs were assessed in the Peyer's patches, mesenteric lymph nodes, and spleen in wild‐type and TLR2−/− mice. Low‐dose Pam3 CSK 4 was also tested as an oral adjuvant.
Results
Oral tolerance was successfully induced in both wild‐type and TLR2−/− recipient mice, with an associated regulatory T‐cell response. Oral TLR2 activation, with low‐dose Pam3 CSK 4 or FSL‐1, during oral antigen exposure was found to alter oral tolerance and was associated with the development of substantial IgE and IgA responses to foods upon systemic challenge. Low‐dose oral Pam3 CSK 4 treatment also selectively enhanced antigen‐specific IgA responses to oral antigen exposure.
Conclusions and Clinical Relevance
TLR2 is not necessary for oral tolerance induction, but oral TLR2 activation modulates humoral IgE and IgA responses during tolerance development. Low‐dose Pam3 CSK 4 is also an effective oral adjuvant that selectively enhances IgA production. These observations are pertinent to the optimization of oral allergen immunotherapy and oral vaccine development.
Keywords: Allergy, oral tolerance, TLR2, IgA, IgE, Treg, murine, ovalbumin, peanut
Introduction
Oral tolerance can be defined as antigen‐specific humoral and cellular hypo‐responsiveness following oral antigen exposure 1, 2. Tolerance is readily induced upon oral exposure to food antigens, especially early in life, and is critical for the prevention of food allergy. It is not clear what impact TLR activators have on regulating the balance between oral tolerance and sensitization.
TLR2 is important for maintaining a regulatory intestinal environment and epithelial barrier function in the context of colitis 3, 4, 5, 6. TLR2 polymorphisms are also associated with deficits in immune regulation such as those observed in allergic asthma, other atopic disease, and inflammatory bowel disease 7, 8, 9, 10. Dysregulated immune responses to environmental microbial stimuli may be involved in food allergy 11, 12. Intestinal bacteria present an ongoing source of TLR2 activators in the intestinal environment, and many common foods such as processed meats, chocolate, yoghurt, and cheese include TLR2 activators 13. Increasingly, evidence suggests that microflora and probiotics can regulate the intestinal environment via TLR2 activation 14, 15.
Regulatory T cells (Tregs) are involved in the induction and maintenance of oral tolerance to foods 16. TLR2 activation has been shown to impair suppressive capacity through direct actions on Tregs, T effector cells, and dendritic cells (DCs) 17, 18, 19. Systemic administration of the TLR2 activator Pam3CSK4 impairs the activity of adoptively transferred Tregs in vivo 17. The direct effects of TLR2 activators on B cells could also enhance humoral responses to oral antigen. 20, 21.
Although oral tolerance is the desired outcome of exposure to foods, it presents a substantial barrier to oral vaccines. Lipopeptides such as Pam3CSK4 have been identified as potential mucosal vaccine adjuvants when delivered at high doses via the oral or nasal route 22, 23. The relationships between oral immunization, oral tolerance, and TLR2 activation are not well understood.
There are currently no studies directly investigating the impact of TLR2 expression and activation on the development of oral tolerance and humoral responses to food antigens. Exploring this relationship is critical to our understanding of food allergy.
Materials and methods
Antibodies and reagents
Anti‐mouse IgG1 (clone: RMG1‐1), IgG2a (clone: RMG2a‐62), IgA (clone: RMA‐1), and CD4‐PerCP (clone: RM4‐5‐APC) antibodies were purchased from BioLegend (San Diego, CA, USA). Anti‐mouse IgE was purchased from eBiosciences (San Diego, CA, USA) and Southern Biotech (Birmingham, AL, USA). All other antibodies were from eBiosciences. Magnetic activated cell sorting (MACS) was performed with ‘CD4+ T‐cell isolation kit II’ and ‘CD62L (L‐selectin) microbeads’ from Miltenyi Biotec Inc. (Auburn, CA, USA). Crude peanut extract (CPE) was purchased from GREER® Laboratories, Inc. (Lenoir, NC, USA). Pam3CSK4 (Pam3CysSerLys4) and FSL‐1 (Pam2CGDPKHPKSF) were obtained from EMC Microcollections (Tübingen, Germany). Grade V OVA was obtained from Sigma‐Aldrich (Oakville, ON, Canada). When tested in vitro for the ability to stimulate IL‐6 production from wild‐type C57BL/6 or TLR2−/− splenocytes during a 24‐h incubation, OVA (4 mg/mL) did not result in IL‐6 production by cells from either strain above medium control levels, indicating low LPS and TLR2 ligand levels (data not shown). As expected, Pam3CSK4 (200 μg/mL) induced significant IL‐6 production from C57BL/6 cells, but not from TLR2−/− cells (data not shown). Furthermore, at 50 μg/mL the E.coli‐derived LPS preparation (Sigma‐Aldrich, catalogue number L4524) induced higher IL‐6 levels in C57BL/6 cultures compared to TLR2−/− cultures (approximately 37% higher), suggesting the presence of some TLR2 ligand content in this preparation but the majority of biological activity resulting from other signalling mechanisms (data not shown).
Mice
Male mice (C57BL/6 or BALB/c 6–8 weeks old) were obtained from Jackson Laboratories (Bar Harbour, ME, USA), Charles River Laboratories (Montreal, QC, Canada), or bred from such founder mice. B6.129‐Tlr2tm1Kir/J (TLR2−/−) mice were bred from stock obtained from Jackson Laboratories. BALB/c mice were the strain of choice for tolerance experiments, but some mechanistic studies with transgenic strains necessitated the use of mice on a C57BL/6 background.
B6.SJL‐Ptprca Pepcb/BoyJ (CD45.1+) mice and B6.Cg‐Tg(TcraTcrb)425Cbn/J (OT‐II+) founder mice were purchased from Jackson Laboratories. CD45.1+/OT‐II+ males were then crossed with Foxp3‐GFP females, originally acquired from Dr. Mohamed Oukka 24 and bred on site. T cells isolated from the CD45.1+/OT‐II+/Foxp3‐GFP cross mice (hereafter referred to as OT‐II T cells) were used in all adoptive transfer studies to provide several experimental advantages. The CD45.1+ marker allowed discrimination of transferred cells from endogenous CD45.2+ lymphocytes, the OT‐II+ CD4+ T cells responded exclusively to the OVA323–339 peptide fragment, and the Foxp3‐GFP marker allowed reliable identification of Foxp3+ transferred cells (Tregs) by flow cytometry. Studies comparing natural vs. inducible Tregs were performed in male Foxp3‐GFP mice.
All animals were housed in a specific pathogen‐free facility on a 12‐h light/dark cycle. Approval for all animal studies was granted by the Dalhousie University Committee on Laboratory Animals.
Induction and assessment of tolerance to ovalbumin
Mice were provided with 4 mg/mL OVA in drinking water or drinking water alone ad libitum for 7 days. Prior to immunization (day −2), all mice were returned to normal water (Fig. 1a). At this dose, BALB/c mice consumed on average 13.75 mg ± 0.79 SEM of OVA/mouse/day (n = 8).
Figure 1.
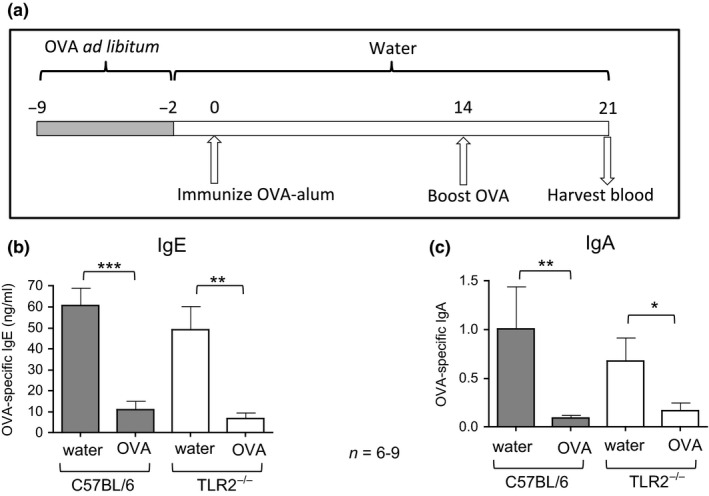
Humoral tolerance is intact in TLR2−/− mice. (a) Schematic of methods for tolerance induction and antibody assessment. Mice were provided with 4 mg/mL OVA in water ad libitum, while control mice were provided with normal water (not depicted). On day 0, all mice were immunized by i.p. injection of OVA‐alum in PBS and then boosted by i.p. injection of OVA in PBS. On day 21, blood and/or faecal samples were harvested. (b) OVA‐specific IgE antibody levels in plasma were compared between tolerized mice treated orally with OVA and control C57BL/6 or TLR2−/− mice following immunization and challenge. Bars represent mean ng/mL IgE ± SEM. (c) OVA‐specific IgA antibody levels in plasma were compared between groups. Bars represent mean A490 ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
All groups were immunized i.p. (day 0) with 50 μg (C57BL/6 mice) or 10 μg (BALB/c mice) of OVA precipitated to alum (Fig. 1a) and boosted by i.p. injection of 10 μg (C57BL/6 mice) or 1 μg (BALB/c mice) soluble OVA in PBS on day 14. Blood and faecal samples were harvested on day 21. In studies examining the role of TLR activators on tolerance, mice were additionally treated with OVA gavage (1 mg in 100 μL PBS) 3 times during the week of ad libitum OVA treatment (days −9, −6, and −3) to ensure precise, concurrent delivery of OVA and TLR activators (Fig. 3a). In some groups, OVA gavage treatments were supplemented with one of the following: 10 μg TLR2/1 activator Pam3CSK4, 5 μg TLR2/6 activator FSL‐1, or 10 μg TLR4 activator LPS derived from E. coli (Sigma‐Aldrich, catalogue number L4524). These groups were compared to tolerized mice receiving 3 gavage treatments of OVA in PBS alone, and control mice receiving 3 gavage treatments of PBS. Oral treatment with FSL‐1 was performed at a 5‐μg dose due to toxic effects observed at higher doses.
Figure 3.
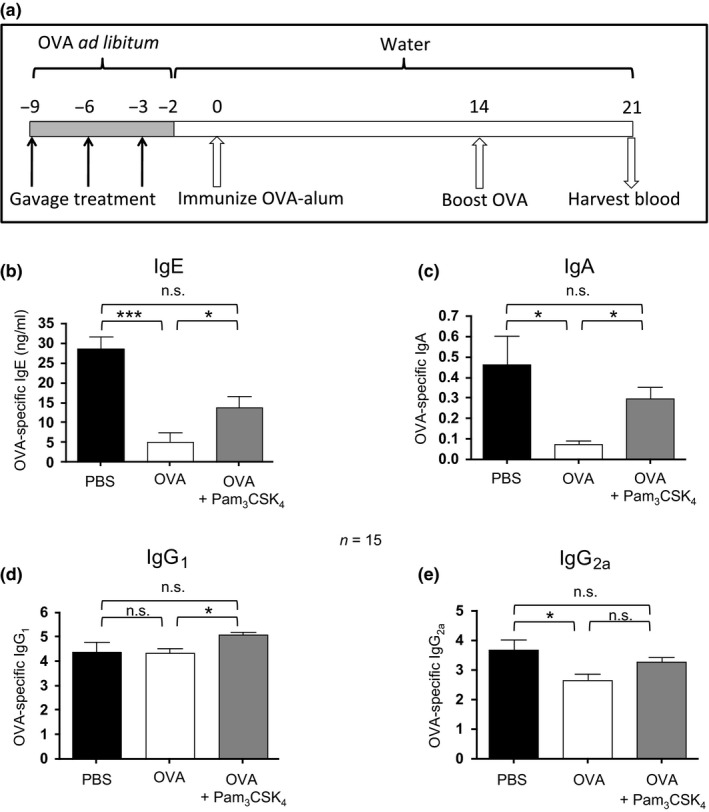
Oral Pam3 CSK 4 treatment modulates humoral tolerance to OVA upon systemic challenge. (a) Schematic of methods for tolerance induction and antibody assessment. Mice were treated for 1 week with OVA ad libitum and supplemented with 3 gavage treatments of OVA ± TLR activators. Control mice were provided with normal water and received gavage doses of PBS (not depicted). On day 0, all mice were immunized by i.p. injection of OVA‐alum in PBS and then boosted by i.p. injection of OVA in PBS. On day 21 blood was harvested. (b) OVA‐specific IgE levels in plasma were assessed by ELISA and expressed as ng/mL. (c) OVA‐specific plasma IgA levels were assessed by ELISA and expressed as standard‐adjusted A490. (d) OVA‐specific plasma IgG1 levels were assessed by ELISA, analysed by titre analysis and expressed as median with IR. (e) OVA‐specific plasma IgG2a levels were assessed by ELISA and analysed by titre analysis. IgE and IgA levels were compared between groups by anova followed by Bonferroni's multiple comparison test, whereas IgG1 and IgG2a levels were compared between groups by Kruskal–Wallis test followed by Dunn's multiple comparison test. Bars represent mean ± SEM IgE and IgA levels, or median –Log titre IgG1 and IgG2a levels with interquartile range. *P < 0.05, ***P < 0.001, n.s., not significant.
Induction and assessment of tolerance to peanut
Mice were fed peanut butter ad libitum (KRAFT® ‘All Natural Peanut Butter’; Don Mills, Canada) for 7 consecutive days (days −9 to −2), followed by 2 days of regular chow, or chow throughout as control, according to a tolerance protocol that was previously demonstrated to provide physiological protection against anaphylaxis during a systemic peanut challenge 25. BALB/c mice consumed an average equivalent to 488 mg peanut protein/day (n = 10). Mice were immunized on day 0 with 10 μg crude peanut extract (CPE)‐precipitated to alum. All groups were boosted with 1 μg CPE in 100 μL PBS on day 14.
To examine the role of TLR activators on tolerance, mice were treated with CPE by gavage (1 mg in 100 μL PBS) 3 times during the week of ad libitum peanut butter (days −9, −6, and −3) in the place of OVA according to the schedule of Fig. 3a. In one group, CPE gavage treatments were supplemented with 10 μg Pam3CSK4. This group was compared to tolerized mice receiving 3 gavage treatments of CPE in PBS alone and control mice receiving 3 gavage treatments of PBS.
ELISA assays
Ovalbumin‐specific and peanut‐specific antibodies were measured by an antigen capture ELISA assay as previously described 25. OVA‐specific IgG1 and IgG2a antibody levels were determined by titre threshold. Samples that failed to reach the titre threshold were designated as non‐responders and assigned a ‐Log titre value of 0.01. OVA‐specific and peanut‐specific IgE and IgA levels were assessed by comparisons of final absorbance at 490 nm (A490 value) adjusted to standard, as the low levels of antibody observed were not appropriate for titre analysis. Similarly, peanut‐specific IgG1 and IgG2a levels were compared by A490. Samples with values below background were designated as non‐responders and assigned an A490 value of 0.01. OVA‐specific IgE A490 values were standardized relative to a commercial OVA‐specific IgE standard (Chondrex Inc.; Redmond, WA, USA) and reported as ng/mL. Total IgA was expressed as μg/mL for plasma or μg/100 mg (wet weight) faeces, based on a standard curve of murine IgA (eBioscience).
ELISPOT assay of lymphoid tissues
For analysis by ELISPOT, LP lymphocytes were isolated from BALB/c mice as described by Hadis et al. 16. ELISPOT plates were coated with 100 μL of 1 mg/mL OVA (for OVA‐specific IgA assays) or 10 μg/mL of purified anti‐IgA antibody (BioLegend) (for total IgA assay) at 4°C overnight. The following day plates were washed and blocked for 2 h with RPMI‐1640, 10% FBS, 1% nonessential amino acids, penicillin, streptomycin, and glutamine. Isolated cells were then plated at 1 × 106 cells/well and incubated at 37°C for 24 h. Plates were washed, and HRP‐labelled anti‐IgA (Southern Biotech) was added for 1 h at 37°C. Spots were detected with AEC staining kit (Sigma‐Aldrich) and counted with Immunospot reader and software (Cellular Technology Limited, Cleveland, OH, USA) and then expressed as spots/1 × 106 cells.
Adoptive transfer and antigen‐specific Treg assessment
Naïve CD4+ T cells were isolated from OT‐II/CD45.1+/Foxp3‐GFP+ mice as previously described 25. Cells were treated with Cell Proliferation Dye eFluor® 670 (eBioscience), and 1 × 106 of these cells were injected i.v. into recipient wild‐type or TLR2−/− male mice. One day later, mice were provided with OVA at 4 mg/mL ad libitum in drinking water; controls received normal drinking water (Fig. 2a). In some studies, ad libitum OVA treatment was supplemented with 3 gavage treatments (Fig. 5a). After 7 days, tissues were harvested as previously described 25 and analysed by flow cytometry.
Figure 2.
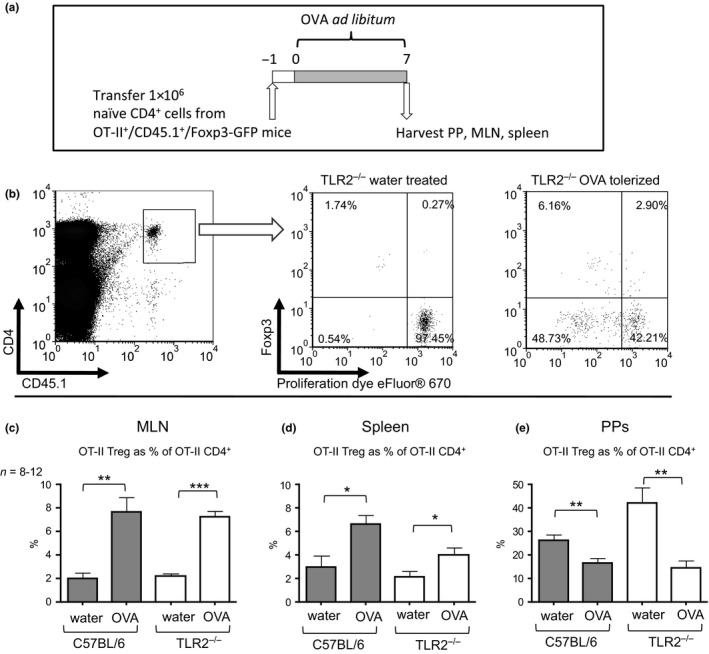
OVA‐specific transferred T cells are responsive to oral tolerance in TLR2−/− recipient mice. (a) Schematic of methods for adoptive transfer and assessment of Tregs in OVA tolerance. 1 × 106 naïve CD62L+/CD4+ cells were purified by MACS from OT‐II +/CD45.1+/Foxp3‐GFP mice and adoptively transferred into naïve C57BL/6 or TLR2−/− recipient mice by i.v. injection. Mice were provided with 4 mg/mL OVA in water ad libitum, while control mice were provided with normal water (not depicted). On day 7, the PPs, MLN, and spleen were harvested and assessed for recovered OT‐II +/CD4+/CD45.1+ cells by flow cytometry. (b) Representative plots of transferred cells recovered from the MLN outlining gating strategy for analysis of flow cytometry. (c), Transferred OT‐II Treg cells recovered from the MLNs, spleens (d), and PPs (e) were compared between OVA‐tolerized and untreated C57BL/6 or TLR2−/− mice by t‐tests. Bars represent mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 5.
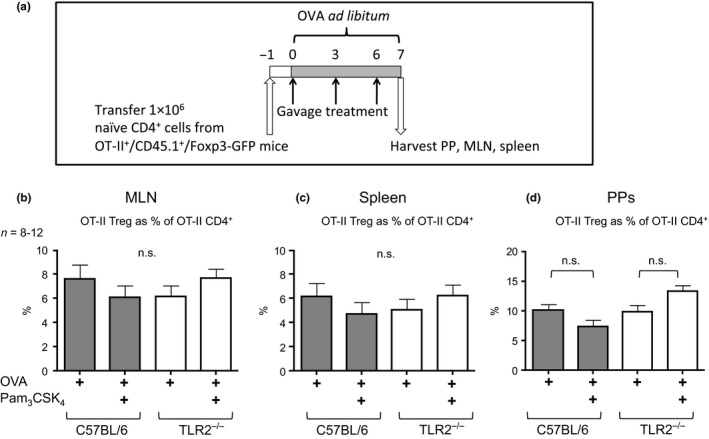
Antigen‐specific Treg levels are not altered in vivo by oral Pam3 CSK 4 treatment. (a) Schematic of methods for adoptive transfer and assessment of Tregs in OVA tolerance. 1 × 106 naïve CD62L+/CD4+ cells were purified by MACS from OT‐II +/CD45.1+/Foxp3‐GFP mice and adoptively transferred into naïve C57BL/6 or TLR2−/− recipient mice by i.v. injection. Mice were treated with OVA ad libitum and supplemented with 3 gavage treatments of OVA ± Pam3 CSK 4. On day 7, the PPs, MLN, and spleen were harvested and assessed for recovered OT‐II +/CD4+/CD45.1+ cells by flow cytometry. (b) Transferred OT‐II Treg cells recovered from the MLNs, spleens (c), and PPs (d) were compared by anova followed by Bonferroni's multiple comparison test. Bars represent mean ± SEM. n.s., not significant.
Flow cytometry
Staining was performed in 1% v/v rat and/or murine serum with primary anti‐mouse antibodies: CD62L‐PE (clone: MEL‐14), CD4‐biotin (clone: L3T4), CD4‐APC (clone: GK1.5), CD4‐PerCP (clone: RM4‐5), CD45.1‐PE (clone: A20), CD3Ɛ‐PE (clone: 145‐2C11), CD304‐APC (clone: 3DS304M), or appropriate isotype controls. Secondary staining was performed for 15–20 min with streptavidin‐PerCP (BioLegend) in conditions with biotinylated primary antibodies. In some studies, cells were treated with Cell Proliferation Dye eFluor® 670 (eBioscience) prior to adoptive transfer. Data were collected on a FACSCalibur from BD Biosciences (San Jose, CA, USA). Analysis of flow cytometry was performed with FCS Express 3 software (De Novo Software, Los Angeles, CA, USA). For analysis of adoptively transferred populations, cells were analysed first through a gate of live lymphocytes. These cells were then gated on CD45.1 expression and CD4 expression. Cells were then gated on Foxp3 expression and/or proliferation dye. For analysis of in vivo cell proliferation, ‘undivided cells’ were gated based on levels of Cell Proliferation Dye eFluor® 670 in CD4+/CD45.1+ cells recovered from control mice that had not been treated with OVA (Fig. 2b).
Statistical analysis
All data sets were tested for normality. OVA‐specific IgG1 and IgG2a antibody titres and ELISPOT data sets were compared by Mann–Whitney test or between multiple groups by Kruskal–Wallis nonparametric analysis followed by Dunn's multiple comparison test. Where normally distributed, results were compared between two groups by Student's t‐test or between multiple groups by one‐way anova followed by Dunnett's multiple comparison test. Where not normally distributed, IgE and IgA levels were compared between two groups by Mann–Whitney test. Peanut‐specific IgG1 and IgG2a were compared by Student's t‐test. Total IgA levels and Treg numbers were compared between multiple groups by one‐way anova and Bonferroni's multiple comparison test or Dunnett's multiple comparison test.
Results
TLR2 is not required for successful oral tolerance
There is strong evidence to suggest that TLR2 can modulate Treg function 17, 19 and the intestinal regulatory environment 5, 6. To assess whether TLR2 was critical for oral tolerance induction, TLR2−/− or wild‐type mice were provided with OVA in drinking water for 1 week or untreated water as a control. Mice were immunized and boosted with OVA as indicated (Fig. 1a). OVA‐specific IgE levels following immunization were significantly suppressed in plasma of both wild‐type and TLR2−/− mice treated orally with OVA compared to controls (P < 0.001, P < 0.01, respectively) (Fig. 1b). OVA‐specific IgA levels were similarly suppressed in both groups (P < 0.01, P < 0.05, respectively) (Fig. 1c).
The development and distribution of OVA‐specific Tregs were compared in TLR2−/− recipient and wild‐type mice in response to oral antigen. Mice received 1 × 106 naïve OT‐II CD4+ T cells. Test ‘tolerized’ groups were then provided with OVA in water ad libitum for 1 week (Fig. 2). The proportion of OVA‐specific Tregs was significantly increased in the MLN of both tolerized wild‐type and TLR2−/− mice when compared to untolerized controls (P < 0.01, P < 0.001, respectively) (Fig. 2c). Similarly, systemic OVA‐specific Tregs in the spleen of tolerized wild‐type and TLR2−/− mice were also increased following OVA treatment (P < 0.05, P < 0.05, respectively) (Fig. 2d). The PPs of both wild‐type and TLR2−/− mice were found to contain significantly lower proportions of OVA‐specific Tregs following oral tolerance induction (P < 0.01 and P < 0.01, respectively) (Fig. 2e). These Treg response and antibody profiles are consistent with oral tolerance induction that provides significant physiological protection from a systemic allergen challenge 25. Taken together, these results demonstrate that TLR2 is not required for the development of oral tolerance.
Oral TLR2 activation modulates humoral responses during tolerance induction
Although TLR2 expression was not required for successful oral tolerance induction, we assessed whether transient TLR2 activation concurrent with oral antigen exposure could alter the progression of oral tolerance and the humoral response to OVA. Two groups of BALB/c mice were fed OVA in drinking water ad libitum for 1 week. During this week, one group received 3 gavage treatments of 1 mg OVA + Pam3CSK4 (10 μg), while the second group received 1 mg OVA by gavage (Fig. 3a). Antibody responses to OVA immunization of these groups were compared to controls that received no OVA in drinking water and PBS by gavage. Oral treatment with OVA resulted in suppression of the specific IgE, IgA, and IgG2a responses (P < 0.001, P < 0.05, P < 0.05, respectively) (Fig. 3). Pam3CSK4 treatment prevented the suppression of IgE, IgA, and IgG2a responses. OVA‐specific IgG1 was elevated following OVA + Pam3CSK4 treatment compared to mice treated with OVA alone (P < 0.05) (Fig. 3d). Oral treatment with three separate 5 μg doses of FSL‐1, a TLR2/6 activator, yielded similar results (Table 1A). However, treatment with a similar dose of a potent TLR4 activator (LPS) selectively prevented oral tolerance in the IgA compartment (P < 0.05) but had no significant impact on IgE responses (Table 1B).
Table 1.
Comparison of oral tolerance induction to OVA or peanut butter with different innate activators
| OVA‐specific antibody levels in plasma 1 week after boost | IgE | IgA | IgG1 | IgG2a |
|---|---|---|---|---|
| (A) Comparison between control, OVA‐tolerized, and FSL‐1‐treated tolerized groups (n = 10) | ||||
| Control | 59.23 ± 6.70 | 0.293 ± 0.067 | 5.12 ± 1.25 | 3.30 ± 1.32 |
| OVA tolerized | 11.59 ± 3.59*** | 0.186 ± 0.040 | 4.88 ± 1.84 | 2.65 ± 0.99 |
| OVA tolerized + FSL‐1 | 30.79 ± 6.38 | 0.453 ± 0.073 † | 5.30 ± 0.69 † | 3.38 ± 0.95 |
| (B) Comparison between control, OVA tolerized, and LPS‐treated tolerized groups (n = 14–15) | ||||
| Control | 28.87 ± 4.66 | 0.138 ± 0.045 | 4.23 ± 1.98 | 2.94 ± 2.26 |
| OVA tolerized | 9.59 ± 2.06*** | 0.038 ± 0.008 | 4.58 ± 0.88 | 2.93 ± 0.96 |
| OVA tolerized + LPS | 11.91 ± 2.60** | 0.197 ± 0.062 † | 4.80 ± 1.35 | 3.15 ± 1.31 |
| Peanut‐specific antibody levels in plasma 1 week after boost | ||||
|---|---|---|---|---|
| (C) Comparison between control, peanut butter tolerized, and Pam3CSK4‐treated tolerized groups (n = 10) | ||||
| Control | 0.241 ± 0.083 | 0.077 ± 0.023 | 0.199 ± 0.024 | 0.616 ± 0.075 |
| Peanut butter tolerized | 0.107 ± 0.093* | 0.010 ± 0.003* | 0.022 ± 0.009*** | 0.119 ± 0.049*** |
| Peanut butter tolerized + Pam3CSK4 | 0.011 ± 0.003** | 0.038 ± 0.012 | 0.115 ± 0.037 | 0.173 ± 0.049*** |
OVA‐specific antibody levels are expressed as mean ng/mL ± SEM (IgE), mean A490 ± SEM (IgA), or median –Log titre ± IR (IgG1, IgG2a) (A and B). IgE and IgA levels were compared between groups by anova followed by Bonferroni's Multiple Comparison test. IgG1 and IgG2a levels were compared between groups by Kruskal–Wallis test followed by Dunn's multiple comparison test. (C) Peanut‐specific antibody levels are expressed as mean A490 ± SEM. Antibody levels were compared between groups by anova followed by Bonferroni's multiple comparison test.
Significant differences following post‐test comparisons between tolerized groups and the corresponding control group are identified by ‘*’. *P < 0.05, **P < 0.01, ***P < 0.001.
Significant differences following post‐test comparisons between tolerized groups and tolerized + innate activator groups are represented by ‘†’. † P < 0.05.
The impact of TLR2 activation via Pam3CSK4 on oral tolerance induction to the common food allergen peanut was examined. Peanut‐specific antibody levels were significantly reduced in peanut butter‐fed mice compared to control mice (IgE P < 0.05, IgA P < 0.05, IgG1 P < 0.001, IgG2a P < 0.001) (Table 1C). When mice were treated with Pam3CSK4 by gavage concurrent with peanut butter, levels of peanut‐specific IgA and IgG1 were no longer significantly reduced upon systemic peanut immunization compared to control peanut‐sensitized mice (Table 1C). However, peanut‐specific IgE and IgG2a responses remained suppressed.
The impact of oral Pam3CSK4 treatment on immunization was also assessed in the absence of oral OVA, as an additional control. BALB/c mice were treated with Pam3CSK4 or PBS by gavage according to Fig. 3a, without the addition of OVA by gavage or ad libitum. Following OVA immunization, there was no difference between mean OVA‐specific IgE levels in BALB/c mice treated with PBS (64.94 ng/mL ± 4.233, n = 10) or Pam3CSK4 alone (49.43 ng/mL ± 9.243, n = 11) (P = 0.16). Similarly, OVA‐specific IgA levels in BALB/c mice receiving PBS (0.1353 A490 ± 0.03423, n = 10) were not significantly different following OVA immunization compared to mice receiving Pam3CSK4 alone (0.1720 A490 ± 0.04156, n = 11) (P = 0.51). Similar results were obtained in C57BL/6 mice (data not shown), confirming that oral Pam3CSK4 must therefore be administered concurrently with OVA in order to alter later responses to OVA immunization.
Oral Pam3CSK4 treatment augments antigen‐specific IgA‐producing B‐cell levels
Following the observation that oral Pam3CSK4 treatment reliably impacts systemic IgA production, IgA‐producing B cells were assessed in the spleen by ELISPOT. Mice were fed OVA in water and additionally treated by gavage with OVA alone or OVA and Pam3CSK4 for 1 week (Fig. 4a). Controls were provided with water ad libitum and treated by gavage with PBS. After a week without OVA for all groups, the spleens and LPs were analysed for OVA‐specific IgA‐producing B cells and total IgA‐producing cells. ELISPOT analysis revealed a significant increase in the median number of OVA‐specific IgA‐producing cells/1 × 106 cells from the spleens of mice treated in vivo by gavage with OVA + Pam3CSK4 compared to mice treated with OVA by gavage without Pam3CSK4 or compared to controls treated with PBS by gavage (P < 0.001, P < 0.01 respectively) (Fig. 4b). B cells were also assessed locally in the LP, which revealed a significant increase in the median number of OVA‐specific IgA‐producing cells/1 × 106 cells from the LP of mice treated with OVA + Pam3CSK4 compared to PBS gavage controls (P < 0.05), although not significantly greater compared to OVA‐treated mice (Fig. 4d). Total IgA‐producing cells were also assessed in the spleen and LP. No significant difference in the number of total IgA‐producing cells was observed between any treatment groups (Figs 4c and e).
Figure 4.
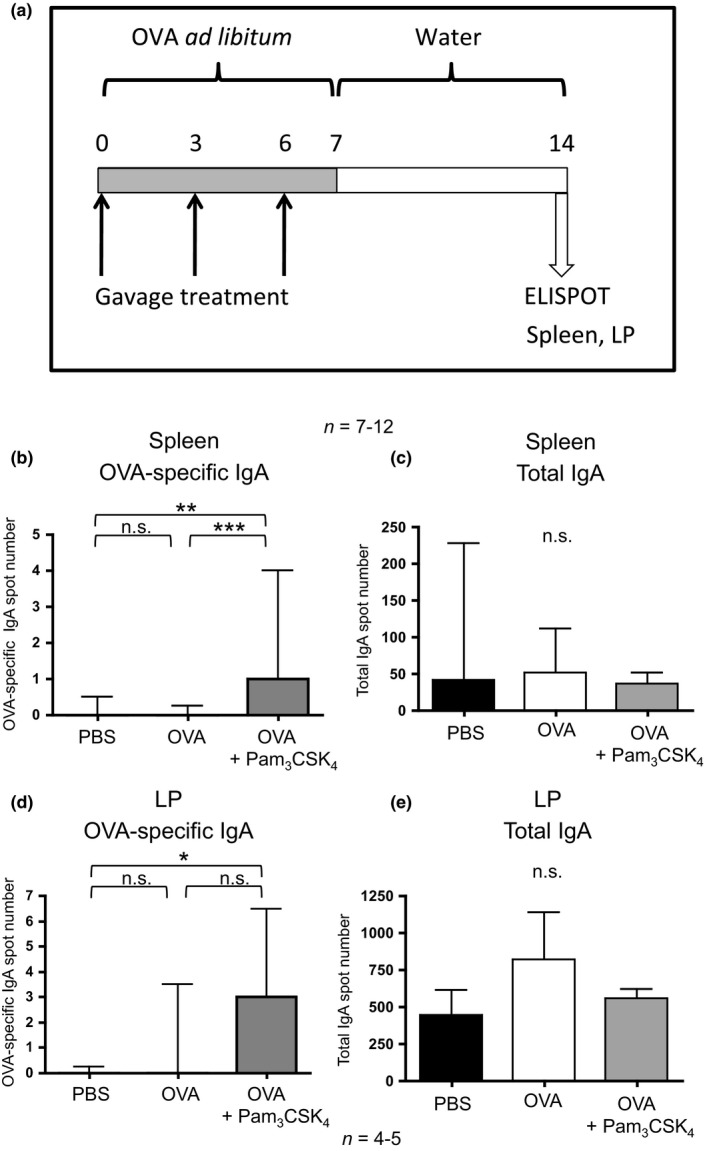
Antigen‐specific IgA‐producing B cells are enhanced by oral Pam3 CSK 4 treatment. (a) BALB/c mice were treated by gavage with OVA ± Pam3 CSK 4 with OVA provided ad libitum over 1 week. Control mice were treated with PBS by gavage and water ad libitum (not depicted). On day 14, spleens and LP were harvested and cultured for ELISPOT analysis. Spot number was compared between groups on the basis of OVA‐specific IgA‐producing cells in the spleen (b) and LP (d), and non‐specific total IgA‐producing cells in the spleen (c) and LP (e). Spot numbers were compared between groups by Kruskal–Wallis test followed by Dunn's multiple comparison test. Bars represent median spot number/1 × 106 cells with interquartile range. *P < 0.05, **P < 0.01, ***P < 0.001, n.s., not significant.
TLR2 activation does not alter antigen‐specific Treg levels during oral tolerance induction
Having observed a potent immunomodulatory effect of oral Pam3CSK4 treatment on the induction of oral tolerance, the involvement of Tregs was investigated. Antigen‐specific Treg levels were assessed at mucosal and systemic sites. Wild‐type and TLR2−/− mice underwent adoptive transfer of OT‐II T cells and OVA treatment with or without the further addition of Pam3CSK4, as described above (Fig. 5a). The PPs, MLNs, and spleens were harvested and analysed for OT‐II Tregs, non‐Tregs, and CD4+ T cells. The proportion of OVA‐specific Tregs was not significantly altered in the MLNs, spleens, or PPs of C57BL/6 tolerized mice upon treatment with OVA + Pam3CSK4 compared to OVA‐treated controls (Figs 5b–d).
The impact of oral Pam3CSK4 treatment on the proportion of inducible Tregs (iTregs) vs. natural Tregs (nTregs) was also assessed. Foxp3‐GFP mice were treated orally by gavage with OVA with or without Pam3CSK4 (Fig. 6a). On day 7, the spleens, MLNs, and PPs were harvested and iTreg vs. nTreg levels were assessed by flow cytometry. Tregs were grouped as CD304+ (nTregs) or CD304− (iTregs). CD304, also known as neuropilin‐1 (Nrp1), is expressed in high levels predominantly on thymus‐derived Treg cells 26, 27 and has therefore recently been recognized as a reliable marker to discriminate between thymus‐derived nTregs and peripherally induced iTregs 28, 29, 30. No significant difference in the percentage of iTregs was observed between groups treated orally with OVA alone or groups treated orally with OVA + Pam3CSK4 (Fig. 6b). The proportion of iTregs from both treatment groups was highest in lymphatic tissues close to the intestinal interface: PPs > MLN > spleen (Fig. 6b).
Figure 6.
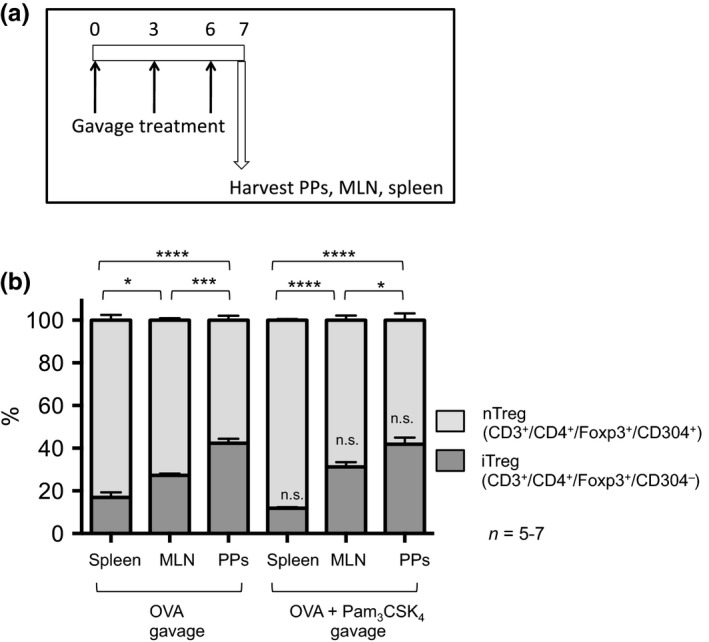
The proportion of total iTregs is not altered by oral Pam3 CSK 4 treatment. (a) Foxp3‐GFP mice were treated orally with OVA ± Pam3 CSK 4 by gavage on days 0, 3, and 6. On day 7, the spleens, MLNs, and PPs were harvested and iTreg vs. nTreg levels for each tissue were assessed by flow cytometry. (b) Cells were first gated based on CD3+/CD4+/Foxp3‐GFP + to identify total Tregs and then grouped as CD304+ (nTregs) or CD304− (iTregs). The iTreg levels were compared between groups by anova with Bonferroni's multiple comparison test. Comparisons within treatments (between tissues) are indicated with comparison lines; comparisons across treatments for each tissue are indicated above the iTreg bar. *P < 0.05, ***P < 0.001, ****P < 0.0001, n.s., not significant.
Low‐dose Pam3CSK4 is a selective oral adjuvant for IgA responses in mice
Previous animal studies with high‐dose lipopeptides (150 μg/dose) identified Pam3CSK4 as a potential oral adjuvant 22. In the light of our observations that low‐dose Pam3CSK4 modulates IgA responses, its oral adjuvant properties were similarly assessed.
BALB/c mice were treated by gavage with 3 weekly oral doses of either OVA, OVA + Pam3CSK4 (10 μg), or PBS control (Fig. 7a). One week after the final gavage treatment, OVA‐specific antibody levels were assessed and compared to mice immunized by a standard i.p. OVA‐alum injection followed by an i.p. OVA boost (Figs 7b–f). When Pam3CSK4 was delivered orally with OVA, OVA‐specific IgA levels in plasma were enhanced compared to mice treated only with oral OVA (P < 0.05). Levels of OVA‐specific IgE, IgG1, and IgG2a were not significantly altered by Pam3CSK4 treatment. OVA‐specific secreted faecal IgA levels were also significantly higher in Pam3CSK4 treated mice compared to OVA alone or OVA‐alum i.p. immunized (P < 0.001, P < 0.001, respectively). Although the use of Pam3CSK4 as an oral adjuvant with OVA significantly enhanced antigen‐specific IgA responses, it did not alter the overall production or secretion of total IgA (Fig. 7g).
Figure 7.
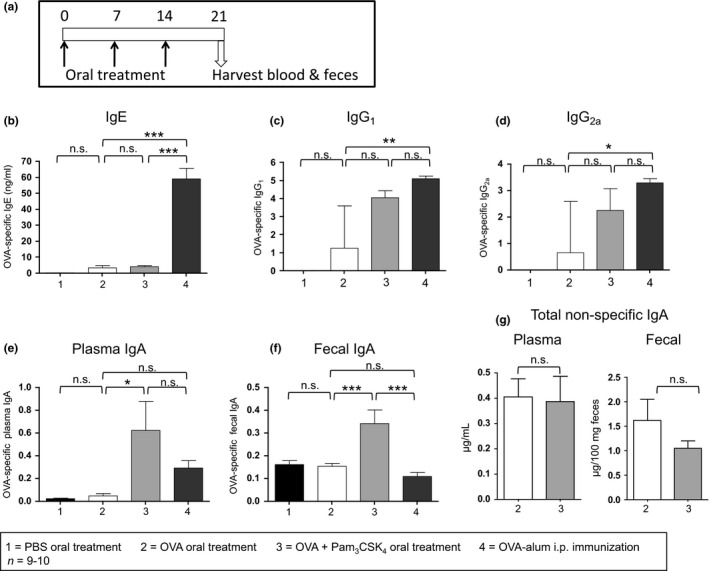
Pam3 CSK 4 can be used as an oral adjuvant to selectively enhance antigen‐specific IgA. (a) BALB/c mice were treated by gavage 3 times (days 0, 7, 14) with PBS, 1 mg OVA, or 1 mg OVA + 10 μg Pam3 CSK 4. Other mice were immunized by i.p. injection of 10 μg OVA‐alum on day 0 and then boosted by i.p. injection of 1 μg OVA on day 14. Blood and faecal samples were harvested from all mice on day 21. OVA‐specific IgE (b), IgG1 (c), IgG2a (d), and IgA (e) were measured in plasma by ELISA and compared by anova with Bonferroni's multiple comparison test (IgE, IgA) or by Kruskal–Wallis test with Dunn's multiple comparison test (IgG1, IgG2a). OVA‐specific IgE levels were expressed as mean ± SEM ng/mL, while IgG1 and IgG2a levels were analysed by titre analysis and expressed as median –Log titre with interquartile range. (f) OVA‐specific IgA was measured in faecal samples by ELISA and expressed as mean ± SEM standard‐adjusted A490 and then groups were compared by anova with Bonferroni's multiple comparison test. (g) Total non‐specific IgA was measured in plasma and faecal samples from mice treated orally 3 times with 1 mg OVA or 1 mg OVA + 10 μg Pam3 CSK 4. Plasma levels were expressed as mean ± SEM μg/mL and faecal levels were expressed as mean ± SEM μg/100 mg faeces. *P < 0.05, **P < 0.01, ***P < 0.001, n.s., not significant.
Discussion
This study demonstrates that TLR2 expression is not required for the induction of oral tolerance and generation of food‐specific Tregs in response to oral antigen. However, oral delivery of a TLR2 activator altered the progression of oral tolerance to a purified protein antigen such that IgE and IgA responses to subsequent systemic challenge were no longer suppressed. Systemic antigen‐specific IgA‐producing B‐cell levels were also enhanced by TLR2 activation during tolerance induction. An antigen‐specific and class‐selective amplification of the IgA response to antigen upon repeated oral exposure with low‐dose Pam3CSK4 was also observed. These findings have important implications for understanding the optimal immunological environment to promote tolerance and prevent allergic disease, in addition to informing potential oral vaccination strategies. Notably, when using a more complex peanut antigen, with endogenous innate immune activating properties such as complement activation 31, TLR2 activator treatment did not significantly modulate IgE responses and only altered tolerance in the IgA compartment. It is possible that the differential IgE responses to Pam3CSK4 in OVA vs. peanut is related to endogenous TLR ligand levels.
While the relationships between oral immunization, oral tolerance, and TLR2 activation have not previously been thoroughly examined, one study reported that the addition of Pam3CSK4 in sublingual immunotherapy reduced airway hyperresponsiveness and the local TH2 response in sensitized animals 32. Recent work by Stiehm et al. 33 also indicates that TLR2 activation can reduce TH2 polarization in a model of airway allergy. These findings highlight the possibility that oral TLR2 activation may have different outcomes in alternative mucosal sites. Together with our results, these findings suggest regulatory roles for enhanced IgA responses in models of allergy.
Several elegant previous studies have demonstrated that TLR2 expression is essential to maintain adequate intestinal immune regulation, barrier function 5, 6, 34, and appropriate innate responses to tissue injury 35. Our results from TLR2−/− mice suggest that, despite these factors, oral tolerance is not dependent on TLR2‐mediated tight junction barrier function, nor is it substantially modulated by cell bound or soluble TLR2 expression. It is possible that the major epithelial barrier defects reported in the absence of TLR2 are limited to the large intestine, allowing normal antigen uptake and food processing in the small intestine. Consistent with our observations, Boulard et al. 36 have demonstrated that intestinal regulation and Treg levels are unchanged in TLR2−/− mice in chronic models of inflammatory bowel disease, in contrast to the profound impact of TLR2 deficiency in acute inflammatory models such as DSS colitis 4, 5.
Multiple reports have indicated that B cells are responsive to TLR2 stimulation, which can result in intestinal homing, plasma cell differentiation, and amplified antibody production 21, 37, 38. Work by Jain et al. 38 has also shown that TLR2 activation of B cells enhanced their responses. Our data show that antigen‐specific IgA‐producing B cells were augmented in the LP and spleen following transient TLR2 activation, but total IgA‐producing B cells were not. This suggests that Pam3CSK4 acts to amplify antigen‐specific B cell responses to the new oral antigen without altering non‐specific total B‐cell responses. This antigen‐specific outcome resulting from transient low‐dose TLR2 activation could be an asset in the context of therapeutic applications.
In previous studies, the Escherichia coli‐derived heat labile enterotoxin LT‐IIa‐B5 was shown to be an effective TLR2‐dependent mucosal adjuvant, amplifying antigen‐specific CD4+ proliferation, secreted IgA, and serum IgG following intranasal delivery 39. These findings complement our observation that TLR2 activation, with low doses of oral Pam3CSK4, selectively elicits a potent serum and secreted antigen‐specific IgA response. Early studies with lipopeptides found that oral treatment with high doses of Pam3CSK4 (150 μg) promoted antigen‐specific IgA and IgG2a levels in plasma, in addition to enhancing faecal IgA 22. Our results validate the observation that oral Pam3CSK4 treatment is a potent oral adjuvant.
BALB/c mice are known to polarize towards Th2 responses and are typically a model system of choice for studies examining allergy and tolerance 40, 41, 42. For these reasons, where possible, we used BALB/c mice in our studies. However, to directly examine the necessity for TLR2 in tolerance induction, we employed TLR2−/− mice, which are on a C57BL/6 background, in selected studies. Moreover, to perform adoptive transfer studies with antigen‐specific OT‐II T cells crossed onto the Foxp3‐GFP strain, a compatible C57BL/6 recipient strain was necessary. In this model of tolerance, OVA‐specific T cell and Treg levels, and total iTreg levels, were unchanged in Pam3CSK4‐treated mice despite significant changes to antibody production. It has been previously noted that BALB/c mice express more Tregs than C57BL/6 mice, and the BALB/c T cells are reportedly more responsive to suppression 43. It may be that adoptive transfer studies to assess antigen‐specific Tregs in BALB/c mice following oral Pam3CSK4 treatment would show changes more reflective of the antibody results. Furthermore, TLR2 activation of Tregs can have differential outcomes on their expansion and suppressive function 17, 44. Therefore, although Pam3CSK4 treatment did not significantly alter antigen‐specific Treg levels in vivo during OVA tolerance in our studies, it is possible that their suppressive function was impaired.
Overall, despite its critical role in maintaining intestinal homeostasis, TLR2 expression is not required to facilitate oral tolerance to protein antigens. However, targeted local TLR2 activation can have a profound impact on the progression of oral tolerance in the IgE and IgA compartments and the selective regulation of both systemic and mucosal IgA responses to oral antigen. These findings suggest that greater care must be taken in considering the TLR2 activator content of food antigens, especially in the context of initial food exposures and oral immunotherapy.
Funding
This research was supported by AllerGen NCE Inc. and the Canadian Institutes for Health Research. M.C.T. was supported by a studentship from the Natural Sciences and Engineering Research Council of Canada, and K.R.C. was supported by a studentship from AllerGen NCE Inc.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
The authors would like to thank Yisong Wei and Nong Xu for technical assistance, Dr. Jun Wang for assistance with animal models, and Dr. Ian Haidl for helpful discussions regarding experiments and the manuscript.
Tunis M. C., Dawod B., Carson K. R., Veinotte L. L. and Marshall J. S.. Clinical & Experimental Allergy, 2015. (45) 1690–1702.
References
- 1. Faria AM, Weiner HL. Oral tolerance. Immunol Rev 2005; 206:232–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vickery BP, Burks AW. Immunotherapy in the treatment of food allergy: focus on oral tolerance. Curr Opin Allergy Clin Immunol 2009; 9:364–70. [DOI] [PubMed] [Google Scholar]
- 3. Ey B, Eyking A, Klepak M et al Loss of TLR2 worsens spontaneous colitis in MDR1A deficiency through commensally induced pyroptosis. J Immunol 2013; 190:5676–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Albert EJ, Marshall JS. Aging in the absence of TLR2 is associated with reduced IFN‐gamma responses in the large intestine and increased severity of induced colitis. J Leukoc Biol 2008; 83:833–42. [DOI] [PubMed] [Google Scholar]
- 5. Cario E. Barrier‐protective function of intestinal epithelial Toll‐like receptor 2. Mucosal Immunol 2008; 1(Suppl 1):S62–6. [DOI] [PubMed] [Google Scholar]
- 6. Cario E, Gerken G, Podolsky DK. Toll‐like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 2007; 132:1359–74. [DOI] [PubMed] [Google Scholar]
- 7. Pierik M, Joossens S, Van Steen K et al Toll‐like receptor‐1, ‐2, and ‐6 polymorphisms influence disease extension in inflammatory bowel diseases. Inflamm Bowel Dis 2006; 12:1–8. [DOI] [PubMed] [Google Scholar]
- 8. Oh DY, Schumann RR, Hamann L, Neumann K, Worm M, Heine G. Association of the toll‐like receptor 2 A‐16934T promoter polymorphism with severe atopic dermatitis. Allergy 2009; 64:1608–15. [DOI] [PubMed] [Google Scholar]
- 9. Eder W, Klimecki W, Yu L et al Toll‐like receptor 2 as a major gene for asthma in children of European farmers. J Allergy Clin Immunol 2004; 113:482–8. [DOI] [PubMed] [Google Scholar]
- 10. Bjornvold M, Munthe‐Kaas MC, Egeland T et al A TLR2 polymorphism is associated with type 1 diabetes and allergic asthma. Genes Immun 2009; 10:181–7. [DOI] [PubMed] [Google Scholar]
- 11. Okada H, Kuhn C, Feillet H, Bach JF. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol 2010; 160:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berin MC, Sampson HA. Mucosal immunology of food allergy. Curr Biol 2013; 23:R389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Erridge C. The capacity of foodstuffs to induce innate immune activation of human monocytes in vitro is dependent on food content of stimulants of Toll‐like receptors 2 and 4. Br J Nutr 2011; 105:15–23. [DOI] [PubMed] [Google Scholar]
- 14. Sakai F, Hosoya T, Ono‐Ohmachi A et al Lactobacillus gasseri SBT2055 induces TGF‐beta expression in dendritic cells and activates TLR2 signal to produce IgA in the small intestine. PLoS ONE 2014; 9:e105370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tunis MC, Marshall JS. Toll‐like receptor 2 as a regulator of oral tolerance in the gastrointestinal tract. Mediators Inflamm 2014; 2014:606383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hadis U, Wahl B, Schulz O et al Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity 2011; 34:237–46. [DOI] [PubMed] [Google Scholar]
- 17. Sutmuller RP, den Brok MH, Kramer M et al Toll‐like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest 2006; 116:485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Maren WW, Nierkens S, Toonen LW, Bolscher JM, Sutmuller RP, Adema GJ. Multifaceted effects of synthetic TLR2 ligand and Legionella pneumophilia on Treg‐mediated suppression of T cell activation. BMC Immunol 2011; 12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lal G, Yin N, Xu J et al Distinct inflammatory signals have physiologically divergent effects on epigenetic regulation of Foxp3 expression and Treg function. Am J Transplant 2011; 11:203–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gururajan M, Jacob J, Pulendran B. Toll‐like receptor expression and responsiveness of distinct murine splenic and mucosal B‐cell subsets. PLoS ONE 2007; 2:e863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liang Y, Hasturk H, Elliot J et al Toll‐like receptor 2 induces mucosal homing receptor expression and IgA production by human B cells. Clin Immunol 2011; 138:33–40. [DOI] [PubMed] [Google Scholar]
- 22. Huber M, Baier W, Bessler WG, Heinevetter L. Modulation of the Th1/Th2 bias by lipopeptide and saponin adjuvants in orally immunized mice. Immunobiology 2002; 205:61–73. [DOI] [PubMed] [Google Scholar]
- 23. Baier W, Masihi N, Huber M, Hoffmann P, Bessler WG. Lipopeptides as immunoadjuvants and immunostimulants in mucosal immunization. Immunobiology 2000; 201:391–405. [DOI] [PubMed] [Google Scholar]
- 24. Bettelli E, Carrier Y, Gao W et al Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006; 441:235–8. [DOI] [PubMed] [Google Scholar]
- 25. Tunis MC, Dawicki W, Carson KR, Wang J, Marshall JS. Mast cells and IgE activation do not alter the development of oral tolerance in a murine model. J Allergy Clin Immunol 2012; 130:705–15 e1. [DOI] [PubMed] [Google Scholar]
- 26. Yadav M, Louvet C, Davini D et al Neuropilin‐1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med 2012; 209:1713–22, S1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weiss JM, Bilate AM, Gobert M et al Neuropilin 1 is expressed on thymus‐derived natural regulatory T cells, but not mucosa‐generated induced Foxp3+ T reg cells. J Exp Med 2012; 209:1723–42, S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin X, Chen M, Liu Y et al Advances in distinguishing natural from induced Foxp3(+) regulatory T cells. Int J Clin Exp Pathol 2013; 6:116–23. [PMC free article] [PubMed] [Google Scholar]
- 29. Huang YJ, Haist V, Baumgartner W et al Induced and thymus‐derived Foxp3(+) regulatory T cells share a common niche. Eur J Immunol 2014; 44:460–8. [DOI] [PubMed] [Google Scholar]
- 30. Dhamne C, Chung Y, Alousi AM, Cooper LJ, Tran DQ. Peripheral and thymic foxp3(+) regulatory T cells in search of origin, distinction, and function. Front Immunol 2013; 4:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kodama T, Sekine H, Takahashi M et al Role of complement in a murine model of peanut‐induced anaphylaxis. Immunobiology 2013; 218:844–50. [DOI] [PubMed] [Google Scholar]
- 32. Lombardi V, Van Overtvelt L, Horiot S et al Toll‐like receptor 2 agonist Pam3CSK4 enhances the induction of antigen‐specific tolerance via the sublingual route. Clin Exp Allergy 2008; 38:1819–29. [DOI] [PubMed] [Google Scholar]
- 33. Stiehm M, Peters K, Wiesmuller KH, Bufe A, Peters M. A novel synthetic lipopeptide is allergy‐protective by the induction of LPS‐tolerance. Clin Exp Allergy 2013; 43:785–97. [DOI] [PubMed] [Google Scholar]
- 34. Cario E, Gerken G, Podolsky DK. Toll‐like receptor 2 enhances ZO‐1‐associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology 2004; 127:224–38. [DOI] [PubMed] [Google Scholar]
- 35. Aprahamian CJ, Lorenz RG, Harmon CM, Dimmit RA. Toll‐like receptor 2 is protective of ischemia‐reperfusion‐mediated small‐bowel injury in a murine model. Pediatr Crit Care Med 2008; 9:105–9. [DOI] [PubMed] [Google Scholar]
- 36. Boulard O, Asquith MJ, Powrie F, Maloy KJ. TLR2‐independent induction and regulation of chronic intestinal inflammation. Eur J Immunol 2010; 40:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boeglin E, Smulski CR, Brun S, Milosevic S, Schneider P, Fournel S. Toll‐like receptor agonists synergize with CD40L to induce either proliferation or plasma cell differentiation of mouse B cells. PLoS ONE 2011; 6:e25542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jain S, Chodisetti SB, Agrewala JN. CD40 signaling synergizes with TLR‐2 in the BCR independent activation of resting B cells. PLoS ONE 2011; 6:e20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee CH, Masso‐Welch P, Hajishengallis G, Connell TD. TLR2‐dependent modulation of dendritic cells by LT‐IIa‐B5, a novel mucosal adjuvant derived from a type II heat‐labile enterotoxin. J Leukoc Biol 2011; 90:911–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Van Gramberg JL, de Veer MJ, O'Hehir RE, Meeusen EN, Bischof RJ. Use of animal models to investigate major allergens associated with food allergy. J Allergy (Cairo) 2013; 2013:635695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gueders MM, Paulissen G, Crahay C et al Mouse models of asthma: a comparison between C57BL/6 and BALB/c strains regarding bronchial responsiveness, inflammation, and cytokine production. Inflamm Res 2009; 58:845–54. [DOI] [PubMed] [Google Scholar]
- 42. Zhang Y, Lamm WJ, Albert RK, Chi EY, Henderson WR Jr, Lewis DB. Influence of the route of allergen administration and genetic background on the murine allergic pulmonary response. Am J Respir Crit Care Med 1997; 155:661–9. [DOI] [PubMed] [Google Scholar]
- 43. Chen X, Oppenheim JJ, Howard OM. BALB/c mice have more CD4+CD25+ T regulatory cells and show greater susceptibility to suppression of their CD4+CD25‐ responder T cells than C57BL/6 mice. J Leukoc Biol 2005; 78:114–21. [DOI] [PubMed] [Google Scholar]
- 44. Liu H, Komai‐Koma M, Xu D, Liew FY. Toll‐like receptor 2 signaling modulates the functions of CD4+ CD25+ regulatory T cells. Proc Natl Acad Sci USA 2006; 103:7048–53. [DOI] [PMC free article] [PubMed] [Google Scholar]


