Abstract
Study Design:
This research utilized a cross-sectional design with control group inclusion.
Objectives:
Preliminary evidence suggests that a portion of the patient population with chronic whiplash may have sustained spinal cord damage. Our hypothesis is that in some cases of chronic whiplash-associated disorders (WAD), observed muscle weakness in the legs will be associated with local signs of a partial spinal cord injury of the cervical spine.
Setting:
University based laboratory in Chicago, IL, USA.
Methods:
Five participants with chronic WAD were compared with five gender/age/height/weight/body mass index (BMI) control participants. For a secondary investigation, the chronic WAD group was compared with five unmatched participants with motor incomplete spinal cord injury (iSCI). Spinal cord motor tract integrity was assessed using magnetization transfer imaging. Muscle fat infiltration (MFI) was quantified using fat/water separation magnetic resonance imaging. Central volitional muscle activation of the plantarflexors was assessed using a burst superimposition technique.
Results:
We found reduced spinal cord motor tract integrity, increased MFI of the neck and lower extremity muscles and significantly impaired voluntary plantarflexor muscle activation in five participants with chronic WAD. The lower extremity structural changes and volitional weakness in chronic WAD were comparable to participants with iSCI.
Conclusion:
The results support the position that a subset of the chronic whiplash population may have sustained partial damage to the spinal cord.
Sponsorship:
NIH R01HD079076-01A1, NIH T32 HD057845 and the Foundation for Physical Therapy Promotion of Doctoral Studies program.
Subject terms: Preclinical research, Trauma
Introduction
Each year over 4 million adults seek medical care in the United States for injuries sustained in a motor vehicle collision (MVC).1 The indirect costs associated with an MVC (that is, medical treatment, rehabilitation and loss of productivity) are approximately $100 billion per year,1 representing a substantial social and economic burden. Half of all those involved in an MVC should expect full recovery within the first 2–3 months, while the other half will continue to have persistent, but mild symptoms.2 However, evidence suggests that 25% of adults injured in MVCs (~1 million per year) will present with persistent and complex signs and symptoms including, but not limited to, severe-pain-related disability,2 muscle degeneration3–6 and weakness,7 sensory and motor disturbances,8 and psychological distress.8,9
A number of psychosocial factors have been identified as prognostic of recovery from the traumatic event.10 Despite the presence and recognition of these factors, current best multimodal treatments, including physical therapy, pharmacological agents and psychological regimens, have not substantially influenced the rate of functional recovery.11,12 Unfortunately, there remains a lack of available information from diagnostic imaging studies for identifying a salient pathoanatomical lesion13,14 that is related to recovery pathways for determining who is at risk for developing chronic symptoms.15,16
However, advanced and available magnetic resonance imaging (MRI) sequences may hold some diagnostic/prognostic value.5,17 The rapid and progressive degeneration of neck muscles, with accompanying muscle fat infiltration, appears to be one pervasive sequelae of traumatic injury involving the head/neck.14,17,18 Preliminary evidence from a small sample of patients with chronic whiplash suggests similar changes that may also be evident in the lower extremity muscles, pointing to disuse atrophy, or even a potential injury involving descending white-matter pathways of the cervical cord.19
Considering the pathomechanics of a typical rear-end collision, it is plausible that, at least in some cases, a partial spinal cord injury in whiplash has occurred. It is noteworthy that both chronic whiplash and incomplete spinal cord injury (iSCI) have strikingly similar signs and symptoms, including muscle fatty infiltrates (MFI),4,5,17,20–23 reductions in volitional muscle activation,19,24,25 disturbed control of spinal nociceptive reflexes,26–28 severe pain-related disability2,8,29,30 and psychological distress.31,32 However, despite similar sequelae, it has yet to be quantitatively explored whether the signs and symptoms of chronic whiplash are truly comparable to, and thus the result of, an iSCI. Our hypothesis is that in some cases of chronic whiplash-associated disorders (WAD), distal leg weakness will be associated with local signs of a partial spinal cord injury of the cervical spine.
To test this hypothesis, we combined established MRI sequences with a quantitative assessment of the ability to generate maximal volitional motor activity (or central activation) in lower extremity muscles, and compared a sample of individuals with chronic WAD to an age/gender/height/weight/body mass index (BMI) matched group of healthy control participants. Spinal cord motor tract integrity, neck and lower extremity MFI and central activation of lower extremity muscles were investigated in both groups. In a secondary analysis, we compared our chronic WAD group with an unmatched convenience sample of participants with known iSCI.
Materials and methods
Participant characteristics
Participants were recruited from the Neuromuscular Imaging and Research Laboratory’s Whiplash database, and the Rehabilitation Institute of Chicago’s Spinal Cord Injury database, in accordance with Northwestern University IRB #STU00087983 and the Declaration of Helsinki. For the primary investigation, five individuals with chronic WAD participated (three female and two male, average age=34±8 years old). Pain-related disability was quantified using percentage scores on the Neck Disability Index (NDI). The NDI is a 10-item questionnaire that has been validated as a reliable measure of neck-related disability.33 Higher scores equate to greater pain-related disability. For this study, participants were included if they earned membership to the severe pain-related disability category (>30/100 on NDI). Five uninjured healthy control participants were matched with the chronic WAD group, in terms of age, gender, height, weight and BMI.
For the secondary investigation, five unmatched male individuals with chronic (1–5 years post injury) cervical iSCI also participated (average age=31±7 years old). Three individuals with iSCI were classified using the American Spinal Cord Injury Association Impairment Scale (AIS) as AIS D C5–8 level, while two participants were classified as AIS C C5–8 level. See Table 1 for details on the participant characteristics.
Table 1. Participant details for all groups, including gender, age, height, weight, body mass index, neck disability index score for the participants with chronic whiplash and impairment level for the participants with incomplete spinal cord injury.
| Participant # | Gender | Age (years) | Height (in) | Weight (lbs) | BMI (units) | NDI % (×/100) |
|---|---|---|---|---|---|---|
| WAD1 | M | 42 | 72 | 190 | 25.8 | 62 |
| WAD2 | F | 42 | 63 | 115 | 20.4 | 68 |
| WAD3 | F | 27 | 67 | 170 | 26.6 | 48 |
| WAD4 | M | 26 | 72 | 200 | 27.1 | 36 |
| WAD5 | F | 31 | 66 | 160 | 25.8 | 58 |
| Control1 | M | 43 | 72 | 170 | 23.1 | NA |
| Control2 | F | 44 | 63 | 135 | 23.9 | NA |
| Control3 | F | 26 | 67 | 170 | 26.6 | NA |
| Control4 | M | 25 | 72 | 185 | 25.1 | NA |
| Control5 | F | 31 | 65 | 160 | 26.6 | NA |
| iSCI1 (AIS C) | M | 28 | 77 | 260 | 30.8 | NA |
| iSCI2 (AIS D) | M | 27 | 69 | 154 | 22.7 | NA |
| iSCI3 (AIS D) | M | 44 | 70 | 150 | 21.5 | NA |
| iSCI4 (AIS D) | M | 31 | 68 | 170 | 25.8 | NA |
| iSCI5 (AIS C) | M | 26 | 75 | 195 | 24.4 | NA |
Abbreviations: AIS, American Spinal Cord Injury Association Impairment Scale; BMI, body mass index; iSCI, incomplete spinal cord injury; NA, not applicable; NDI, Neck Disability Index Score; WAD, whiplash-associated disorders.
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Magnetic resonance imaging
Spinal cord imaging
Magnetization transfer (MT) imaging of the spinal cord was performed using a 3.0 Tesla magnet (TRIO, Siemens, Erlangen, Germany). MT imaging has been shown to be a useful measure in quantifying white-matter disruption in patients with neurological disorders.34,35
Although typical MRI applications draw their signal from the protons associated with free water, there exists another pool of water protons that are bound to hydrophilic macromolecular surfaces.36 The free water has a sharp resonance peak and a long T2 (spin-spin, ~50 ms) relaxation parameter that allows delineation of signal changes in pathways of the spinal cord white matter. The bound water has a broad spectrum and a short T2 on the order of 100 μs. The short T2 makes it impossible to image this particular pool, as the two pools of protons are continuously mixing and result in cross-relaxation.36
The use of saturation transfer offers ability to probe the bound water pool while observing the free water pool. By saturating the broad resonance with a powerful off-resonance (1–2 kHz) pulse, the bound protons loose their magnetization and then exchange with the free water. The result is a decrease in the free water peak proportional to the exchange rate and concentration of the bound water.36 The amount of decrease in the free water peak, proportional to the exchange rate and concentration of the bound water, is quantified as the magnetization transfer ratio (MTR).
For our study, 2D slices were oriented parallel to the disk plane of C2-C7. The MT scan had parameters of repetition time/echo time/flip angle/averages of 300 ms/17 ms/30°/2, combining four echoes, a matrix size of 384×384, phase oversampling of 100% and an FOV of 180 mm with an in-plane resolution of 0.5 mm and a slice thickness of 4 mm, collecting 6 slices. An off-resonance MT pulse was applied 1.5 kHz with a large flip angle (540 in our implementation), and long duration (10 ms) to saturate the bound water pool. Scan acquisition time was 4 min. The non-MT scan was identical except that the MT saturation pulse was turned off and run as a separate acquisition. The MTR was calculated on a voxel-by-voxel basis using the formula:
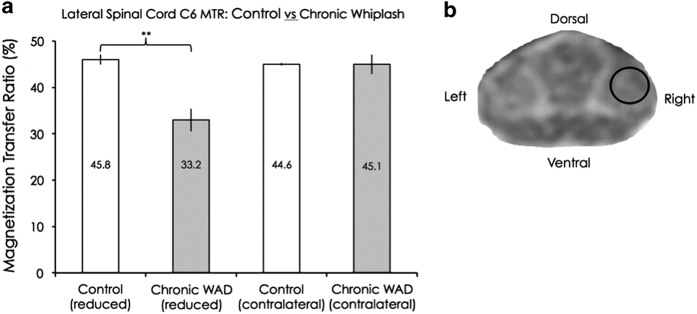
A standardized region at the dorsolateral white matter at the C6 level was chosen for investigation, making best efforts to target regions consisting of the descending corticospinal tract (see Figure 1c). We chose to target the C6 level for both the MT imaging and the fat/water imaging, as the C5–6 segment is a common injury site following whiplash.37,38 Cervical spine imaging was not performed in the iSCI group secondary to previous surgical fusion with fixation.
Figure 1.
(a) Group means and s.e.m. of the reduced and contralateral lateral spinal cord magnetization transfer ratios for the control and chronic WAD groups. A significant difference was found on one side (P<0.01), but not on the other side (P=0.80). (b) A magnetization transfer image at the C6 level with a region of interest targeting the lateral corticospinal tract area.
Muscle/fat imaging
A chemical shift based, 3D dual-echo gradient, fat-water separation MRI technique (Dixon method) was performed on all participants.39 An MR signal is primarily a composite of water and fat in the imaging voxel. As water and fat protons resonate at different frequencies, they are predictably in-phase and out-of-phase with each other as a function of time. The Dixon method takes advantage of this difference, and by acquiring the signal at specific echo times, both the in-phase and out-of-phase data can be manipulated to provide water-only and fat-only images of the muscle samples. These two images are then used for fat quantification.40 It has been previously reported that muscle cross-sectional area is decreased and muscle fat infiltration is increased in patients with iSCI.23
The 2-point Dixon examination included the cervical spine (from the cephalad portion of C3 to the caudal portion of C7) and the major muscle groups of the leg, including the soleus and the gastrocnemius. The specific Dixon MRI parameters for the two anatomic areas included: repetition time=6.59 ms, echo time 1=2.45 ms, echo time 2=3.675 ms, flip angle=12°, bandwidth 1=510 bandwidth 2=660 Hz/Px and an imaging matrix of 448×266. For the cervical spine, a standard 12-channel head coil and 4-channel neck coil was used as a receiver. A localizer scan and a T2-weighted sagittal turbo spin echo sequence were performed. A single slab was placed over the cervical spine with 36 partitions and a partition thickness of 3 mm with slab oversampling of 22% to prevent aliasing in the 3D direction. The in-plane resolution was 0.7 mm using a rectangular field of view of 320×190 mm and 6 averages resulting in an acquisition time of 4:23 min. A read field of view of 320–340 mm (FOV adjusted to fully cover the anatomy) was used on the lower extremity muscles using a 16-channel body array surface coil. For the legs, 60 slices per slab with a slice thickness of 5 mm and a slice oversampling of 6.7% were acquired. The acquisition time was 4:12 min with a rectangular view of 60%, 4 averages and an in-plane resolution of 0.5 mm. Localizer scans were used to obtain the fat/water data in the axial plane perpendicular to the tibia.
Analysis of the MR imaging was performed post hoc using customized imaging analysis programs created in MatLab (Version 2014b, MathWorks, Natick, MA, USA). Regions of interest were defined in the C6 multifidus and semispinalis cervicis in the neck, and plantarflexors (soleus and both the medial and lateral gastrocnemius) in the leg, by outlining each muscle within its fascial borders with the co-registered fat- and water-saturated sequences. The MFI percentage is a value of fat signal intensity to fat plus water signal intensity, and these MFI percentages were created using the equation:
Averages were taken over 10 consecutive slices throughout the plantarflexor muscle bulk to form the MFI percentages.
Central activation measurement
During assessments of central activation of the plantarflexor muscles, participants were secured in a seated position with hips flexed to 75°, knees flexed at 20°, and the ankle at neutral. We assessed maximum volitional torque production by requiring each participant to perform three baseline maximal isometric contractions of the plantarflexors of 3–4 s duration in an isokinetic dynamometer (Biodex Rehabilitation System v3, Shirley, NY, USA) while providing verbal encouragement to facilitate maximal torque generation.41 We applied a brief train of supramaximal electrical stimuli to the plantarflexors during maximum voluntary torque production (10 square-wave pulses, 600 μs pulse duration, 100 Hz, 135 V; Grass S48, external isolation; Grass Technologies, West Warwick, RI, USA) through 3×5” stimulation electrodes placed over the plantarflexor muscle surface, with the anode placed just distal to the popliteal fossa and the cathode placed just distal to the soleus myotendinous junction.42 Electrical stimulation was triggered manually when volitional torque appeared to reach a plateau during maximal efforts.25 The purpose of the stimulation was to compare maximum volitional torque generation to the maximum force-generating capacity of the muscle.43 We quantified central activation by then calculating the central activation ratio (CAR) for each trial. This value is the ratio of the peak volitional torque over the sum of peak volitional and electrically stimulated torque (CAR=Tv/(Tv+Ts)).43 Peak volitional torque was taken as the mean torque produced during the 100 ms immediately preceding stimulation onset.25 Subjects were given at least 2 min of rest between maximal contractions. A numeric pain rating was assessed before and after testing.
Statistical analysis
Statistical analysis of the data was performed using IBM SPSS (Version 21, Armonk, NY, USA). All data, including imaging and central activation measures, were tested for normality using Kolmogorov–Smirnov statistical analyses. Independent sample t-tests with Bonferroni correction were employed to test for significant differences between the chronic WAD and matched control groups for the primary investigation, as well as the chronic WAD and iSCI groups for the secondary investigation. Because our control group was only matched for our chronic WAD group, an analysis of variance was not utilized for statistical analysis. With the Bonferroni correction considering our two null hypotheses (chronic WAD versus control and chronic WAD versus iSCI), P-values <0.025 were considered as significant.
Results
All data met the assumptions of normality using Kolmogorov–Smirnov statistical analyses. Individual data are presented in Table 2. For the following figures, the term ‘reduced’ refers to the side of the body (whiplash versus controls) corresponding to the side of the spinal cord with reduced MTRs, while ‘contralateral’ refers to the other side.
Table 2. Individual data for all participants, including spinal cord magnetization transfer ratios, muscle fat infiltration values and central activation ratios.
| Participant # | MTR Right | MTR Left | Neck MFI Right | Neck MFI Left | Leg MFI Right | Leg MFI Left | CAR |
|---|---|---|---|---|---|---|---|
| WAD1 | 32.83 | 38.09 | 20.32 | 22.16 | 16.92 | 16.78 | 62 |
| WAD2 | 36.49 | 47.86 | 22.36 | 23.49 | 14.42 | 10.60 | 58 |
| WAD3 | 35.90 | 50.59 | 21.31 | 27.65 | 12.17 | 13.57 | 62 |
| WAD4 | 23.77 | 45.62 | 24.92 | 24.51 | 10.00 | 10.52 | 82 |
| WAD5 | 43.40 | 36.81 | 23.87 | 28.95 | 10.80 | 13.65 | 85 |
| Control1 | 43.45 | 44.01 | 21.33 | 20.62 | 11.60 | 12.43 | 99 |
| Control2 | 44.95 | 44.39 | 17.01 | 17.43 | 10.36 | 10.15 | 100 |
| Control3 | 47.98 | 45.54 | 14.10 | 16.08 | 5.89 | 6.79 | 99 |
| Control4 | 48.74 | 44.78 | 14.03 | 13.46 | 7.13 | 7.97 | 100 |
| Control5 | 44.26 | 44.00 | 19.18 | 20.16 | 11.78 | 12.74 | 99 |
| iSCI1 | NA | NA | NA | NA | 14.90 | 14.04 | 27 |
| iSCI2 | NA | NA | NA | NA | 13.94 | 12.81 | 47 |
| iSCI3 | NA | NA | NA | NA | 8.26 | 12.75 | 70 |
| iSCI4 | NA | NA | NA | NA | 8.56 | 7.79 | 66 |
| iSCI5 | NA | NA | NA | NA | 17.45 | 16.71 | 19 |
Abbreviations: CAR, central activation ratio; iSCI, incomplete spinal cord injury; MFI, muscle fat infiltration; MTR, magnetization transfer ratio; NA, not applicable; WAD, whiplash-associated disorders. All values expressed as percentages.
Spinal cord magnetization transfer imaging
Significantly reduced MTRs were found on one side of the spinal cord in the lateral corticospinal tract region, in the chronic WAD group, compared with control (P<0.01, see Figure 1).
Muscle fat infiltration
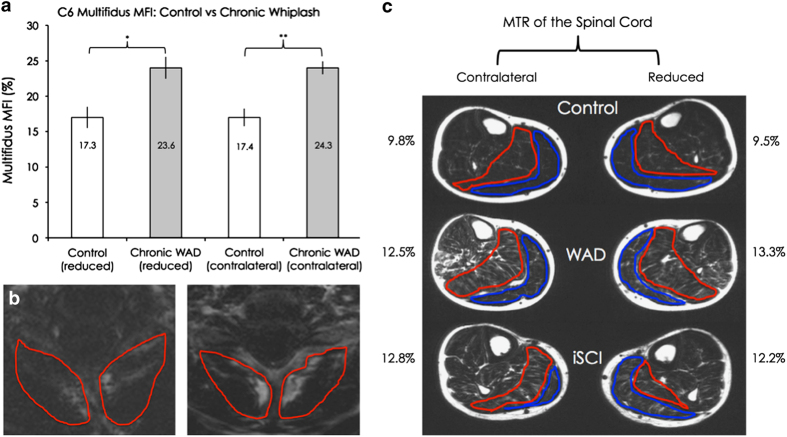
The neck multifidus-semispinalis cervicus muscles showed significantly elevated MFI percentages in the side corresponding to reduced MTR, as well as in the contralateral side, in the chronic WAD group, compared with control (P=0.02 and P<0.01, see Figure 2a).
Figure 2.
(a) Group means and s.e.m. of the multifidus-semispinalis cervicus MFI on the reduced and contralateral sides, for the control and chronic WAD groups. A significant difference was found on both the reduced (P=0.02) and contralateral sides (P<0.01). (b) Fat-saturated images of the multifidus-semispinalis cervicus MFI regions of interest, at the C6 level, for one uninjured matched-control participant (left) and a participant with chronic WAD (right). (c) Group means of the plantarflexor MFI on both the reduced and contralateral sides, for all three groups. Differences between the control and chronic WAD mean values were non-significant on the reduced side following Bonferroni correction (P=0.04), while the other comparisons were not significant (P range: 0.15–0.85).
When comparing the chronic WAD group with control, though not statistically significant following Bonferroni correction, the plantarflexor muscles yielded elevated MFI percentages on the side corresponding to reduced MTR (P=0.04) and on the contralateral side (P=0.15) (see Figure 2c). No significant differences were found between the chronic WAD group and the iSCI group for either leg (P=0.72 and 0.85 see Figure 2c).
Central activation measurement
The chronic WAD group demonstrated significant deficits in central activation of their affected plantarflexors compared with the control group, corresponding to the reduced MTR side (P<0.01, see Figure 3). In contrast, no significant difference was found between the chronic WAD group and the iSCI group with regard to mean plantarflexion CARs (P=0.07, see Figure 3). Finally, there was no change in numeric pain rating score before or after the voluntary activation testing.
Figure 3.
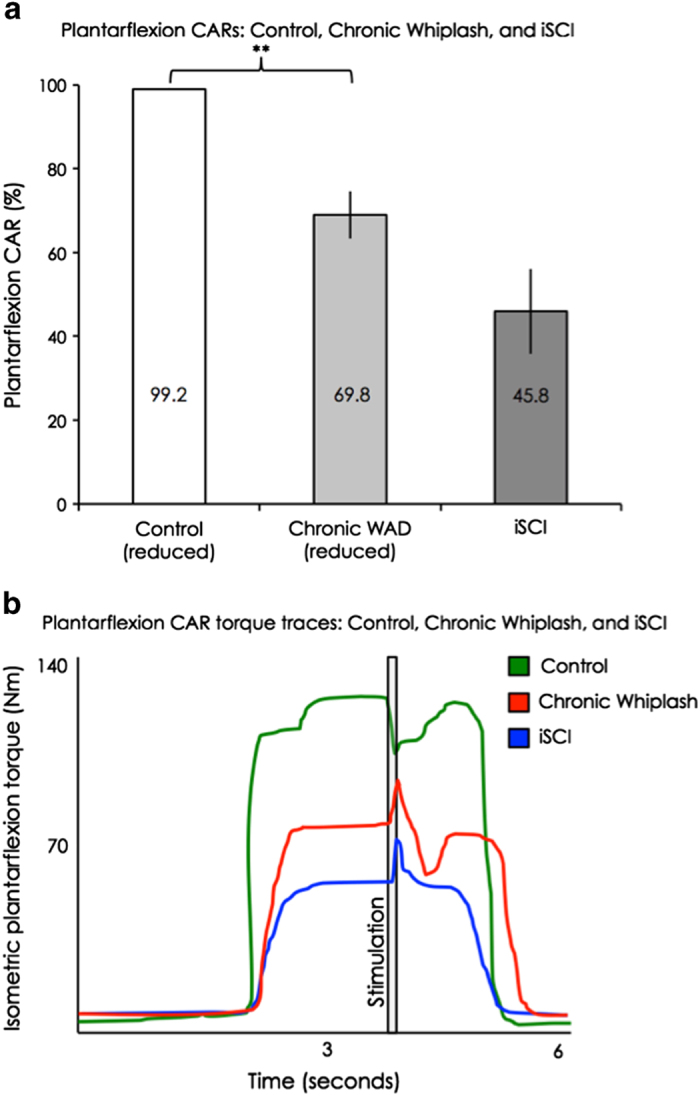
(a) Group means and s.e.m. of the plantarflexion central activation ratios on the reduced side, for the control, chronic WAD and iSCI groups. A significant difference was found comparing control with chronic WAD (P<0.01), but not comparing chronic WAD with iSCI (P=0.07). (b) Plantarflexion torque traces for central activation testing for one uninjured control participant, one representative with chronic WAD and one representative with iSCI.
Discussion
This preliminary study demonstrates reduced spinal cord motor tract integrity, structural alterations of the neck and lower extremity muscles, and significantly impaired voluntary muscle activation in five participants with chronic whiplash. The lower extremity structural changes and volitional weakness in chronic WAD were comparable to participants with iSCI.
The results support the notion that a subset of the chronic whiplash population may have sustained partial damage to the spinal cord. Reduced MTRs were found in the lateral corticospinal tract regions in chronic WAD and these were comparable to those previously found in the SCI population.44 The elevated MFI seen in the multifidus-semispinalis cervicus in the chronic WAD group is congruent with previous work including patients that develop persistent pain-related disability.5,17,18 Elevated plantarflexor fat infiltration percentages were not significantly different between chronic WAD and iSCI, and they were independent of BMI. Both the chronic WAD and iSCI groups demonstrated significant lower extremity weakness, which did not appear to be related to any pain complaints upon performance of maximally contracting the plantarflexor muscles. Reductions in CAR results for the chronic WAD and iSCI groups are in line with a previously published investigation demonstrating weakness of the quadriceps in participants with iSCI.25
Previous whiplash research suggests the spinal cord may be vulnerable to injury. Guez et al. 45 demonstrated an upregulation of neurofilament protein (an indicator of cord damage) in both the SCI and chronic WAD conditions.45 Similar to previous investigations of patients with myelopathy,46 preliminary work in chronic whiplash47 demonstrated significant reductions in N-acetylaspartate/creatine ratios with magnetic spectroscopy of the cervical cord. In addition, two independent research groups found elevated nociceptive flexor withdrawal reflexes of the lower extremities in chronic WAD, suggesting disturbed descending modulatory mechanisms.26,28
Interestingly, although laterality was observed in the spinal cord MTR measure, elevated MFI was present on both sides in the neck and lower extremities, which generates a number of competing hypotheses. These could include but are not limited to, reduced activity levels following injury, lack of central drive and/or stress system dysregulation. Further work on the investigation of these etiological factors is warranted and underway.
Limitations
Though not statistically significant, we found that the chronic WAD had elevated MFI in the plantarflexors as compared with the matched control group, with MFI values more similar to participants with iSCI. However, in this preliminary investigation we did not control for daily activity level and this will be important for current and future work as increased MFI has been shown to be associated with disuse.48
Additionally, at the beginning of establishing our methodology and investigating this lower extremity weakness phenomenon seen in some patients with chronic WAD, we did not perform CAR testing of the contralateral lower limb for the first three participants. An ongoing prospective study is currently collecting bilateral CAR measures.
Furthermore, it has been established that patients with chronic WAD often develop widespread hyperalgesia and sensory hypersensitivity.8,26,28 While we acknowledge these sensory changes, we chose to focus on the motor system and motor output for this particular study. This line of inquiry also provides foundation for investigating ascending sensory pathways in conjunction with symptoms of widespread hyperalgesia, and this research is indeed warranted.
Conclusion
In this preliminary study, we demonstrated that spinal cord motor tract integrity was reduced in a sample of participants with chronic WAD, and this corresponded to significant lower extremity weakness. Furthermore, the MFI values were similar in both patient groups (chronic WAD and iSCI), but elevated compared with controls. Further prospective cohort research on how daily physical activity and spinal cord alterations are related to MFI occurring distal to the potential injury site is warranted and underway. This study provides foundation for the exploration of potential spinal cord injury and distal changes occurring in a subset of the chronic whiplash patient population, whereby more informed assessment and management strategies could be explored and developed.
Data archiving
There were no data to deposit.
Acknowledgments
We thank all participants for their willingness to take part in this study. James M Elliott is supported by the NIH award 1 R01HD079076-01A1, entitled ‘Neuromuscular Mechanisms Underlying Poor Recovery from Whiplash Injuries’. Andrew C Smith is supported by the NIH-funded Training Program in the Neurobiology of Movement and Rehabilitation Sciences at Northwestern, supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant T32 HD057845, and by the Foundation for Physical Therapy Promotion of Doctoral Studies program.
JME and TBP—Relevant financial activities outside the submitted work: board membership, consultancy, other (Pain ID LLC), payment for lectures.
References
- Naumann R , Dellinger A , Zaloshnja E , Lawrence B , Miller T . Incidence and total lifetime costs of motor vehicle-related fatal and nonfatal injury by road user type, United States, 2005. Traffic Inj Prev 2010; 11: 353–360. [DOI] [PubMed] [Google Scholar]
- Sterling M , Hendrikz J , Kenardy J , Kristjansson E , Dumas JP , Niere K et al. Assessment and validation of prognostic models for poor functional recovery 12 months after whiplash injury: a multicentre inception cohort study. Pain 2012; 153: 1727–1734. [DOI] [PubMed] [Google Scholar]
- Elliott J . Are there implications for morphological changes in neck muscles after whiplash injury? Spine (Phila Pa 1976) 2011; 36: S205–S210. [DOI] [PubMed] [Google Scholar]
- Elliott J , Jull G , Noteboom JT , Darnell R , Galloway G , Gibbon WW . Fatty infiltration in the cervical extensor muscles in persistent whiplash-associated disorders: a magnetic resonance imaging analysis. Spine (Phila Pa 1976) 2006; 31: E847–E855. [DOI] [PubMed] [Google Scholar]
- Elliott J , Pedler A , Kenardy J , Galloway G , Jull G , Sterling M . The temporal development of fatty infiltrates in the neck muscles following whiplash injury: an association with pain and posttraumatic stress. PLoS ONE 2011; 6: e21194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J , O’Leary S , Sterling M , Hendrikz J , Pedler A , Jull G . Magnetic resonance imaging findings of fatty infiltrate in the cervical flexors in chronic whiplash. Spine (Phila Pa 1976) 2010; 35: 948–954. [DOI] [PubMed] [Google Scholar]
- Sterling M , Jull G , Vicenzino B , Kenardy J , Darnell R . Development of motor system dysfunction following whiplash injury. Pain 2003; 103: 65–73. [DOI] [PubMed] [Google Scholar]
- Sterling M , Jull G , Kenardy J . Physical and psychological factors maintain long-term predictive capacity post-whiplash injury. Pain 2006; 122: 102–108. [DOI] [PubMed] [Google Scholar]
- Sterling M , Kenardy J . Physical and psychological aspects of whiplash: Important considerations for primary care assessment. Man Ther 2008; 13: 93–102. [DOI] [PubMed] [Google Scholar]
- Carroll LJ , Holm LW , Hogg-Johnson S , Côté P , Cassidy JD , Haldeman S et al. Course and prognostic factors for neck pain in whiplash-associated disorders (WAD). Eur Spine J 2008; 17: 83–92. [DOI] [PubMed] [Google Scholar]
- Lamb S , Gates S , Williams M , Williamson EM , Mt-Isa S , Withers EJ et al. Emergency department treatments and physiotherapy for acute whiplash: a pragmatic, two-step, randomised controlled trial. Lancet 2013; 381: 546–556. [DOI] [PubMed] [Google Scholar]
- Jull G , Kenardy J , Hendrikz J , Cohen M , Sterling M . Management of acute whiplash: a randomized controlled trial of multidisciplinary stratified treatments. Pain 2013; 154: 1798–1806. [DOI] [PubMed] [Google Scholar]
- Sterling M , McLean S , Sullivan M , Elliott J , Buitenhuis J , Kamper S . Potential processes involved in the initiation and maintenance of whiplash-associated disorders: discussion paper 3. Spine (Phila Pa 1976) 2011; 36: S322–S329. [DOI] [PubMed] [Google Scholar]
- Curatolo M , Bogduk N , Ivancic P , McLean S , Siegmund G , Winkelstein B . The role of tissue damage in whiplash associated disorders: discussion paper 1. Spine (Phila Pa 1976) 2011; 36: S309–S315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie C , Hendrikz J , Kenardy J , Sterling M . Derivation of a clinical prediction rule to identify both chronic moderate/severe disability and full recovery following whiplash injury. Pain 2013; 154: 2198–2206. [DOI] [PubMed] [Google Scholar]
- Ritchie C , Hendrikz J , Jull G , Elliott J , Sterling M . External validation of a clinical prediction rule to predict full recovery and ongoing moderate/severe disability following acute whiplash injury. J Orthop Sport Phys Ther 2015; 45: 242–250. [DOI] [PubMed] [Google Scholar]
- Elliott J , Courtney D , Rademaker A , Pinto D , Sterling M , Parrish T . The rapid and progressive degeneration of the cervical multifidus in whiplash: a MRI study of fatty infiltration. Spine (Phila Pa 1976) 2015; 40: E694–E700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott R , Pedler A , Sterling M , Hides J , Murphey T , Hoggarth M et al. The geography of fatty infiltrates within the cervical multifidus and semispinalis cervicis in individuals with chronic whiplash-associated disorders. J Orthop Sports Phys Ther 2015, 1–20. [DOI] [PMC free article] [PubMed]
- Elliott JM , Dewald JP , Hornby T , Walton DM , Parrish TB . Mechanisms underlying chronic whiplash: contributions from an incomplete spinal cord injury? Pain Med 2014; 15: 1938–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J , Sterling M , Noteboom J , Darnell R , Galloway G , Jull G . Fatty infiltrate in the cervical extensor muscles is not a feature of chronic, insidious-onset neck pain. Clin Radiol 2008; 63: 681–687. [DOI] [PubMed] [Google Scholar]
- Elliott J , Jull G , Noteboom J , Galloway G . MRI study of the cross-sectional area for the cervical extensor musculature in patients with persistent whiplash associated disorders (WAD). Man Ther 2008; 13: 258–265. [DOI] [PubMed] [Google Scholar]
- Elliott J , Sterling M , Noteboom J , Treleaven J , Galloway G , Jull G . The clinical presentation of chronic whiplash and the relationship to findings of MRI fatty infiltrates in the cervical extensor musculature: a preliminary investigation. Eur Spine J 2009; 18: 1371–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgey AS , Dudley GA . Skeletal muscle atrophy and increased intramuscular fat after incomplete spinal cord injury. Spinal Cord 2007; 45: 304–309. [DOI] [PubMed] [Google Scholar]
- Thompson C , Lewek M , Jayaraman A , Hornby T . Central excitability contributes to supramaximal volitional contractions in human incomplete spinal cord injury. J Physiol 2011; 589: 3739–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornby TG , Lewek MD , Thompson CK , Heitz R . Repeated maximal volitional effort contractions in human spinal cord injury: initial torque increases and reduced fatigue. Neurorehabil Neural Rep 2009; 23: 928–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banic B , Petersen-Felix S , Andersen OK , Radanov BP , Villiger PM , Arendt-Nielsen L et al. Evidence for spinal cord hypersensitivity in chronic pain after whiplash injury and in fibromyalgia. Pain 2004; 107: 7–15. [DOI] [PubMed] [Google Scholar]
- Hornby TG , Tysseling-Mattiace VM , Benz EN , Schmit BD . Contribution of muscle afferents to prolonged flexion withdrawal reflexes in human spinal cord injury. J Neurophysiol 2004; 92: 3375–3384. [DOI] [PubMed] [Google Scholar]
- Sterling M . Differential development of sensory hypersensitivity and a measure of spinal cord hyperexcitability following whiplash injury. Pain 2010; 150: 501–506. [DOI] [PubMed] [Google Scholar]
- Sterling M , Jull G , Vincenzino B , Kenardy J , Darnell R . Physical and psychological factors predict outcome following whiplash injury. Pain 2005; 114: 141–148. [DOI] [PubMed] [Google Scholar]
- Siddall PJ , McClelland JM , Rutkowski SB , Cousins MJ . A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 2003; 103: 249–257. [DOI] [PubMed] [Google Scholar]
- Sterling M , Kenardy J , Jull G , Vincenzino B . The development of psychological changes following whiplash injury. Pain 2003; 106: 481–489. [DOI] [PubMed] [Google Scholar]
- Migliorini C , Tonge B , Taleporos G . Spinal cord injury and mental health. Aust NZ J Psychiatry 2008; 42: 309–314. [DOI] [PubMed] [Google Scholar]
- Vernon H , Mior S . The Neck Disability Index: a study of reliability and validity. J Manip Physiol Ther 1991; 14: 409–415. [PubMed] [Google Scholar]
- Sinson G , Bagley LJ , Cecil KM , Torchia M , McGowan JC , Lenkinski RE et al. Magnetization transfer imaging and proton MR spectroscopy in the evaluation of axonal injury: Correlation with clinical outcome after traumatic brain injury. Am J Neuroradiol 2001; 22: 143–151. [PMC free article] [PubMed] [Google Scholar]
- Van Buchem MA , Grossman RI , Armstrong C , Polansky M , Miki Y , Heyning FH et al. Correlation of volumetric magnetization transfer imaging with clinical data in MS. Neurology 1998; 50: 1609–1617. [DOI] [PubMed] [Google Scholar]
- Mehta RC , Pike GB , Enzmann DR . Magnetization transfer magnetic resonance imaging: a clinical review. Top Magn Reson Imaging 1996; 8: 214–230. [PubMed] [Google Scholar]
- Panjabi MM , Ito S , Pearson AM , Ivancic PC . Injury mechanisms of the cervical intervertebral disc during simulated whiplash. Spine (Phila Pa 1976) 2004; 29: 1217–1225. [DOI] [PubMed] [Google Scholar]
- Ito S , Ivancic PC , Pearson AM , Tominaga Y , Gimenez SE , Rubin W et al. Cervical intervertebral disc injury during simulated frontal impact. Eur Spine J 2005; 14: 356–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC , Parrish TB , Abbott R , Hoggarth MA , Mendoza K , Chen YF et al. Muscle-fat MRI: 1.5 tesla and 3.0 tesla versus histology. Muscle Nerve 2014; 50: 170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon WT . Simple proton spectroscopic imaging. Radiology 1984; 153: 189–194. [DOI] [PubMed] [Google Scholar]
- Gandevia SC . Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 2001; 81: 1725–1789. [DOI] [PubMed] [Google Scholar]
- Scaglioni G , Martin A . Assessment of plantar flexors activation capacity: nerve versus muscle stimulation by single versus double pulse. Eur J Appl Physiol 2009; 106: 563–572. [DOI] [PubMed] [Google Scholar]
- Kent-Braun JA , Blanc RLE . Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve 1996; 19: 861–869. [DOI] [PubMed] [Google Scholar]
- Cohen-Adad J , El Mendili M-M , Lehéricy S , Pradat PF , Blancho S , Rossignol S et al. Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. Neuroimage 2011; 55: 1024–1033. [DOI] [PubMed] [Google Scholar]
- Guéz M , Hildingsson C , Rosengren L , Karlsson K , Toolanen G . Nervous tissue damage markers in cerebrospinal fluid after cervical spine injuries and whiplash trauma. J Neurotrauma 2003; 20: 853–858. [DOI] [PubMed] [Google Scholar]
- Holly LT , Freitas B , McArthur DL , Salamon N . Proton magnetic resonance spectroscopy to evaluate spinal cord axonal injury in cervical spondylotic myelopathy. J Neurosurg Spine 2009; 10: 194–200. [DOI] [PubMed] [Google Scholar]
- Elliott JM , Pedler a R , Cowin G , Sterling M , McMahon K . Spinal cord metabolism and muscle water diffusion in whiplash. Spinal Cord 2012; 50: 474–476. [DOI] [PubMed] [Google Scholar]
- Manini TM , Clark BC , Nalls MA , Goodpaster BH , Ploutz-Snyder LL , Harris TB . Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr 2007; 85: 377–384. [DOI] [PubMed] [Google Scholar]