Abstract
Purpose
Quality of life is a key component of cancer care; however, the factors that determine quality of life are not well understood. The aim of this study was to examine the relationship between quality of life parameters, performance status (PS), and the systemic inflammatory response in patients with advanced cancer.
Methods
An international biobank of patients with advanced cancer was analyzed. Quality of life was assessed at a single time point by using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C-30 (EORTC QLQ-C30). PS was assessed by using the Eastern Cooperative Oncology Group (ECOG) classification. Systemic inflammation was assessed by using the modified Glasgow Prognostic Score (mGPS), which combines C-reactive protein and albumin. The relationship between quality of life parameters, ECOG PS, and the mGPS was examined.
Results
Data were available for 2,520 patients, and the most common cancers were GI (585 patients [22.2%]) and pulmonary (443 patients [17.6%]). The median survival was 4.25 months (interquartile range, 1.36 to 12.9 months). Increasing mGPS (systemic inflammation) and deteriorating PS were associated with deterioration in quality-of-life parameters (P < .001). Increasing systemic inflammation was associated with deterioration in quality-of-life parameters independent of PS.
Conclusion
Systemic inflammation was associated with quality-of-life parameters independent of PS in patients with advanced cancer. Further investigation of these relationships in longitudinal studies and investigations of possible effects of attenuating systemic inflammation are now warranted.
INTRODUCTION
Clinical trials in oncology have changed in the past 30 years. The initial evaluation criteria for clinical trials focused on tumor size, time to progression, and survival. However, over the past three decades, there has been an evolution toward the inclusion of quality-of-life parameters, and these are now recognized as a key component in clinical trial evaluations.1,2 Indeed, the vast majority of clinical trials now report quality-of-life parameters as either primary or secondary end points, and trials that improve quality of life in patients with cancer are of increasing interest, particularly in patients with advanced cancer in whom the scope for improving survival has proved to be limited.
One of the most common instruments used to assess quality of life is the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30),3 which has been used in more than 9,000 clinical trials.2 The EORTC QLQ-C30 assesses various patient-reported outcomes, including level of functioning, overall or global quality of life, and symptoms such as pain and depression.
Physical functioning is clearly a key component of quality of life and is usually assessed objectively by using performance status (PS) scales.4,5 Moreover, because of its clear prognostic value for survival, PS is used to guide the clinician regarding the most appropriate treatment for patients with advanced cancer. As early as the first century, Celsus’ De Medicina described the cardinal signs of inflammation as a key component of pain and disease.6 These early observations are now supported by recent consistent evidence that pain and other symptoms are related to systemic inflammation in the cancer setting.7,8
The presence of systemic inflammation is also recognized to have independent prognostic value in patients with cancer. In particular, the combination of C-reactive protein (CRP) and albumin (termed the modified Glasgow Prognostic Score [mGPS]) has been shown to have prognostic value complementary to PS in patients with advanced cancer.9-12 In patients near the end of life, both PS and the systemic inflammatory response are affected, and therefore deteriorating quality of life may be affected by decreasing PS, systemic inflammation, or both. The question is: How do PS and the systemic inflammatory response interact with patient-reported outcome measures (PROMs) of quality of life? If these PROMs were shown to be associated specifically with PS and/or systemic inflammation, then this would provide valuable insight into their etiology. Furthermore, it would enable key components of quality of life to be targeted by using interventions that improve PS and/or attenuate systemic inflammation.
The aim of this study was therefore to evaluate key components of quality of life (by using PROMs) and to examine their relationship to PS and the systemic inflammatory response. The hypothesis being examined was that systemic inflammation is associated with quality of life independent of PS.
METHODS
Study Sample
An international biobank of adult patients with cancer was analyzed. These data were collected prospectively at a single time point from multiple centers across Europe.13,14 Patients were age 18 years or older, had metastatic or locoregional cancer, and were recruited from both inpatient and outpatient settings by using a convenience sampling approach. Patients who could not communicate in the primary language at the study center and/or had a psychiatric diagnosis with cognitive impairment were excluded. All patients provided written informed consent for their data to be stored and assessed, with ethical approval accordingly.
Procedure and Assessment
All patients had age, sex, primary cancer type, presence of metastatic disease, and PS recorded. Data were collected on quality-of-life variables by using PROMs from the EORTC QLQ-C30 version 3.3 The following were assessed: global health status, role function, cognitive function, physical function, emotional function, social function, fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite, constipation, and diarrhea.
Systemic inflammation was measured by using CRP and albumin and was taken by venous blood sampling at point of consent. The limit of detection of CRP was < 5 mg/L, and all samples (CRP and albumin) were analyzed at a central laboratory. The mGPS was calculated as follows:
CRP ≤ 10 mg/L = 0
CRP > 10 mg/L = 1
CRP > 10 mg/L and albumin < 35 g/L = 2
Increasing mGPS score is related to increasing systemic inflammation, and these cutoffs are based on previous studies that examined the mGPS.9
Statistical Analysis
Key patient demographic characteristics were described. If a variable was considered binary and/or categorical, then the number and percentage across the patient population are presented. The normality of the data of the EORTC-QLQ-C30 scales was examined by using Q-Q plots (Data Supplement). The plots indicated that it was reasonable to present parametric statistics.
If a variable was considered continuous, then the mean and standard deviation are presented (unless otherwise specified). All statistical testing was conducted at the 5% significance level and 95% CIs are reported throughout. Because of the large number of comparisons depicted in Tables 1 and 2, the Bonferroni correction was applied, and statistical significance was taken as P < .001.
Table 1.
Relationship Between EORTC QLQ-C30 Global Health Status/Functional Scales (discrete categories) and ECOG PS and mGPS
| ECOG PS | mGPS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | All | P | |||||||||
| Total No. | Mean | SD | Total No. | Mean | SD | Total No. | Mean | SD | Total No. | Mean | SD | ||
| Global health | |||||||||||||
| 0-1 | 313 | 56.3 | 23.7 | 148 | 51.1 | 23.1 | 197 | 45.3 | 21.6 | 658 | 51.8 | 23.4 | < .001 |
| 2 | 295 | 44.9 | 23.6 | 211 | 40.4 | 20.3 | 398 | 37.2 | 23.2 | 904 | 40.5 | 22.9 | < .001 |
| 3 | 119 | 40.1 | 24.4 | 65 | 33.2 | 21.5 | 326 | 34.8 | 23.5 | 510 | 35.8 | 23.6 | .062 |
| 4 | 26 | 31.4 | 25.4 | 6 | 30.6 | 16.4 | 80 | 31.5 | 24.4 | 112 | 31.4 | 24.1 | .983 |
| All | 753 | 48.4 | 24.9 | 430 | 42.9 | 22.4 | 1,010 | 37.6 | 23.5 | 2,184 | 42.3 | 24.2 | < .001 |
| P | < .001 | < .001 | < .001 | < .001 | |||||||||
| Role | |||||||||||||
| 0-1 | 315 | 56.5 | 32.7 | 148 | 47.0 | 31.0 | 198 | 38.4 | 32.1 | 661 | 48.9 | 33.0 | < .001 |
| 2 | 297 | 32.1 | 30.4 | 211 | 30.4 | 27.6 | 398 | 24.7 | 28.0 | 906 | 28.5 | 28.9 | .001 |
| 3 | 120 | 19.7 | 26.0 | 69 | 15.9 | 25.3 | 333 | 15.3 | 23.6 | 522 | 16.4 | 24.4 | .099 |
| 4 | 27 | 7.4 | 16.2 | 6 | 8.3 | 20.4 | 80 | 8.1 | 15.9 | 113 | 8.0 | 16.1 | .848 |
| All | 759 | 39.3 | 34.0 | 434 | 33.4 | 30.4 | 1,009 | 23.0 | 28.3 | 2,202 | 30.7 | 31.7 | < .001 |
| P | < .001 | < .001 | < .001 | < .001 | |||||||||
| Cognitive | |||||||||||||
| 0-1 | 314 | 78.9 | 22.9 | 149 | 78.2 | 22.7 | 199 | 74.6 | 24.0 | 662 | 77.4 | 23.2 | .050 |
| 2 | 296 | 69.3 | 25.5 | 212 | 70.9 | 23.7 | 400 | 64.6 | 27.1 | 908 | 67.6 | 25.9 | .013 |
| 3 | 119 | 61.6 | 27.0 | 68 | 63.2 | 28.0 | 332 | 58.7 | 28.1 | 519 | 60.0 | 27.8 | .242 |
| 4 | 26 | 61.5 | 27.8 | 6 | 72.2 | 25.1 | 80 | 49.4 | 28.0 | 112 | 53.4 | 28.4 | .035 |
| All | 755 | 71.8 | 25.6 | 435 | 72.2 | 24.5 | 1,011 | 63.4 | 27.8 | 2,201 | 68.0 | 26.7 | < .001 |
| P | < .001 | < .001 | < .001 | < .001 | |||||||||
| Physical | |||||||||||||
| 0-1 | 315 | 69.2 | 20.3 | 149 | 63.7 | 23.1 | 205 | 58.7 | 24.0 | 663 | 64.8 | 22.5 | < .001 |
| 2 | 298 | 48.7 | 22.5 | 216 | 46.1 | 20.1 | 439 | 43.2 | 21.5 | 914 | 45.7 | 21.6 | .001 |
| 3 | 119 | 24.0 | 21.4 | 69 | 26.3 | 24.0 | 415 | 22.5 | 19.8 | 520 | 23.3 | 20.8 | .345 |
| 4 | 27 | 9.4 | 17.2 | 6 | 14.4 | 32.2 | 153 | 13.2 | 16.8 | 113 | 12.4 | 17.8 | .353 |
| All | 759 | 51.9 | 27.6 | 440 | 48.5 | 25.6 | 1,212 | 37.0 | 25.8 | 2,210 | 44.5 | 27.3 | < .001 |
| P | < .001 | < .001 | < .001 | < .001 | |||||||||
| Emotional | |||||||||||||
| 0-1 | 314 | 73.4 | 23.4 | 149 | 72.1 | 22.8 | 199 | 69.6 | 24.0 | 662 | 71.9 | 23.5 | .080 |
| 2 | 296 | 67.9 | 23.9 | 212 | 71.0 | 20.9 | 400 | 66.5 | 26.4 | 908 | 68.0 | 24.5 | .371 |
| 3 | 119 | 68.3 | 26.4 | 67 | 60.2 | 24.4 | 332 | 64.9 | 26.7 | 518 | 65.1 | 26.4 | .374 |
| 4 | 26 | 66.8 | 23.8 | 6 | 66.7 | 24.2 | 80 | 59.1 | 25.9 | 112 | 61.3 | 25.4 | .160 |
| All | 755 | 70.2 | 24.3 | 434 | 69.7 | 22.5 | 1,011 | 65.9 | 26.1 | 2,200 | 68.2 | 24.9 | < .001 |
| P | .011 | .002 | .003 | < .001 | |||||||||
| Social | |||||||||||||
| 0-1 | 314 | 65.5 | 29.9 | 149 | 57.6 | 30.4 | 198 | 50.5 | 32.6 | 661 | 59.3 | 31.5 | < .001 |
| 2 | 296 | 46.6 | 31.3 | 211 | 49.8 | 30.2 | 400 | 41.0 | 31.7 | 907 | 44.9 | 31.4 | .012 |
| 3 | 119 | 49.0 | 35.7 | 67 | 43.3 | 35.8 | 330 | 40.2 | 32.7 | 516 | 42.6 | 33.9 | .016 |
| 4 | 26 | 44.9 | 35.5 | 6 | 19.4 | 22.2 | 80 | 38.7 | 32.0 | 112 | 39.1 | 32.6 | .537 |
| All | 755 | 54.8 | 32.9 | 433 | 51.0 | 31.6 | 1,008 | 42.4 | 32.4 | 2,196 | 48.3 | 32.9 | < .001 |
| P | < .001 | < .001 | .001 | < .001 | |||||||||
NOTE. The columns of P values show the χ2 test for trend results examining the relationship between modified Glasgow Prognostic Score (mGPS) and the global health/patient-reported outcome measures (PROMs) discrete categories for each performance status group separately. The rows of P values show the χ2 test for trend results examining the relationship between performance status and global health/PROMs discrete categories for each mGPS group separately.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C-30; SD, standard deviation.
Table 2.
Relationship Between EORTC QLQ-C30 Symptom Scales (discrete categories) and ECOG PS and mGPS
| ECOG PS | mGPS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | All | P | |||||||||
| Total No. | Mean | SD | Total No. | Mean | SD | Total No. | Mean | SD | Total No. | Mean | SD | ||
| Fatigue | |||||||||||||
| 0-1 | 315 | 44.5 | 25.8 | 149 | 51.6 | 24.0 | 199 | 57.2 | 25.8 | 663 | 49.9 | 25.9 | < .001 |
| 2 | 296 | 59.3 | 23.7 | 212 | 62.7 | 23.5 | 401 | 68.7 | 24.4 | 909 | 64.2 | 24.3 | < .001 |
| 3 | 120 | 65.2 | 24.7 | 68 | 70.3 | 23.2 | 333 | 68.2 | 24.4 | 521 | 67.8 | 24.3 | .330 |
| 4 | 26 | 68.4 | 23.5 | 6 | 74.1 | 25.0 | 80 | 71.7 | 22.2 | 112 | 71.0 | 22.5 | .551 |
| All | 757 | 54.4 | 26.2 | 435 | 60.2 | 24.5 | 1,013 | 66.5 | 24.9 | 2,205 | 61.1 | 25.8 | < .001 |
| P | < .001 | < .001 | < .001 | < .001 | |||||||||
| Nausea | |||||||||||||
| 0-1 | 314 | 15.5 | 23.1 | 149 | 14.5 | 20.4 | 199 | 20.9 | 24.6 | 662 | 16.9 | 23.1 | .017 |
| 2 | 297 | 22.2 | 27.2 | 215 | 21.4 | 24.5 | 401 | 29.5 | 31.3 | 913 | 25.2 | 28.7 | < .001 |
| 3 | 120 | 18.3 | 25.0 | 68 | 27.0 | 29.4 | 334 | 23.3 | 27.0 | 522 | 22.6 | 26.9 | .152 |
| 4 | 26 | 17.9 | 24.0 | 6 | 33.3 | 36.5 | 81 | 24.5 | 29.8 | 113 | 23.5 | 28.9 | .381 |
| All | 757 | 18.6 | 25.2 | 438 | 20.1 | 24.5 | 1,015 | 25.4 | 28.7 | 2,210 | 22.0 | 26.9 | < .001 |
| P | .109 | < .001 | .935 | < .001 | |||||||||
| Pain | |||||||||||||
| 0-1 | 315 | 37.1 | 30.3 | 149 | 51.1 | 31.2 | 199 | 52.0 | 32.2 | 663 | 44.7 | 31.9 | < .001 |
| 2 | 297 | 54.1 | 30.5 | 215 | 60.1 | 28.2 | 401 | 61.5 | 30.5 | 913 | 58.8 | 30.1 | .002 |
| 3 | 120 | 56.5 | 31.3 | 69 | 68.8 | 26.8 | 333 | 62.9 | 28.3 | 522 | 62.2 | 29.0 | .100 |
| 4 | 26 | 55.8 | 35.9 | 6 | 77.8 | 32.8 | 81 | 65.4 | 27.7 | 113 | 63.9 | 30.2 | .212 |
| All | 758 | 47.5 | 31.9 | 439 | 58.7 | 29.7 | 1,014 | 60.4 | 30.2 | 2,211 | 55.6 | 31.2 | < .001 |
| P | < .001 | < .001 | < .001 | < .001 | |||||||||
| Dyspnea | |||||||||||||
| 0-1 | 314 | 23.0 | 27.6 | 149 | 27.7 | 30.9 | 199 | 28.5 | 32.4 | 662 | 25.7 | 29.9 | .036 |
| 2 | 295 | 28.7 | 32.3 | 215 | 33.2 | 34.2 | 400 | 37.8 | 35.6 | 910 | 33.7 | 34.4 | .001 |
| 3 | 119 | 34.5 | 33.0 | 69 | 41.5 | 36.8 | 331 | 37.5 | 34.2 | 519 | 37.3 | 34.3 | .552 |
| 4 | 26 | 30.8 | 31.2 | 6 | 44.4 | 45.5 | 81 | 45.7 | 39.2 | 113 | 42.2 | 38.1 | .089 |
| All | 754 | 27.3 | 30.7 | 439 | 32.8 | 33.9 | 1,011 | 36.5 | 35.1 | 2,204 | 32.6 | 33.6 | < .001 |
| P | .001 | .004 | < .001 | < .001 | |||||||||
| Sleep | |||||||||||||
| 0-1 | 315 | 28.8 | 30.7 | 149 | 31.1 | 32.0 | 199 | 34.5 | 33.7 | 663 | 31.0 | 31.6 | .046 |
| 2 | 296 | 36.5 | 33.1 | 214 | 35.8 | 33.8 | 401 | 36.7 | 34.7 | 911 | 36.4 | 34.0 | .931 |
| 3 | 120 | 35.6 | 34.5 | 66 | 34.8 | 32.3 | 331 | 31.7 | 33.0 | 517 | 33.0 | 33.2 | .249 |
| 4 | 26 | 30.8 | 35.2 | 6 | 16.7 | 27.9 | 81 | 28.8 | 33.2 | 113 | 28.6 | 33.3 | .892 |
| All | 757 | 32.9 | 32.6 | 435 | 33.8 | 32.3 | 1,012 | 34.0 | 33.9 | 2,204 | 33.6 | 33.1 | .514 |
| P | .041 | .585 | .072 | .834 | |||||||||
| Appetite | |||||||||||||
| 0-1 | 314 | 28.7 | 31.9 | 149 | 32.9 | 34.2 | 199 | 47.4 | 35.3 | 662 | 35.2 | 34.4 | < .001 |
| 2 | 297 | 39.8 | 36.9 | 213 | 44.0 | 35.8 | 398 | 55.9 | 37.4 | 908 | 47.9 | 37.5 | < .001 |
| 3 | 120 | 36.1 | 34.7 | 68 | 56.4 | 34.7 | 333 | 54.3 | 36.5 | 521 | 50.4 | 36.6 | < .001 |
| 4 | 26 | 43.6 | 36.2 | 6 | 44.4 | 45.5 | 81 | 55.6 | 36.1 | 113 | 52.2 | 36.7 | .132 |
| All | 757 | 34.7 | 34.9 | 436 | 42.1 | 36.0 | 1,011 | 53.7 | 36.7 | 2,204 | 44.9 | 36.9 | < .001 |
| P | .001 | < .001 | .086 | < .001 | |||||||||
| Constipation | |||||||||||||
| 0-1 | 313 | 29.1 | 33.8 | 149 | 35.6 | 34.1 | 199 | 36.3 | 36.0 | 661 | 32.7 | 34.7 | .015 |
| 2 | 297 | 41.2 | 37.3 | 213 | 42.7 | 35.8 | 400 | 46.3 | 39.2 | 910 | 43.8 | 37.9 | .071 |
| 3 | 118 | 46.3 | 36.7 | 68 | 56.9 | 36.0 | 329 | 41.9 | 37.5 | 515 | 44.9 | 37.4 | .107 |
| 4 | 26 | 53.8 | 41.2 | 6 | 38.9 | 49.1 | 79 | 44.7 | 38.8 | 111 | 46.5 | 39.8 | .347 |
| All | 754 | 37.4 | 36.7 | 436 | 42.4 | 36.0 | 1,007 | 42.8 | 38.1 | 2,197 | 40.9 | 37.3 | .003 |
| P | < .001 | < .001 | .214 | < .001 | |||||||||
| Diarrhea | |||||||||||||
| 0-1 | 314 | 16.7 | 26.5 | 149 | 13.2 | 23.5 | 199 | 26.3 | 33.3 | 662 | 18.8 | 28.5 | .001 |
| 2 | 297 | 19.5 | 31.2 | 212 | 16.7 | 28.0 | 398 | 22.2 | 30.7 | 907 | 20.0 | 30.3 | .203 |
| 3 | 117 | 17.1 | 28.6 | 67 | 13.4 | 27.3 | 329 | 20.6 | 33.3 | 513 | 18.8 | 31.6 | .197 |
| 4 | 26 | 14.1 | 23.4 | 6 | 5.6 | 13.6 | 80 | 16.3 | 27.6 | 112 | 15.2 | 26.0 | .625 |
| All | 754 | 17.8 | 28.7 | 434 | 14.8 | 26.3 | 1,006 | 22.0 | 31.9 | 2,194 | 19.1 | 29.9 | .002 |
| P | .905 | .925 | .011 | .506 | |||||||||
NOTE. The columns of P values show the χ2 test for trend results examining the relationship between modified Glasgow Prognostic Score (mGPS) and the global health/patient-reported outcome measures (PROMs) discrete categories for each performance status group separately. The rows of P values show the χ2 test for trend results examining the relationship between performance status and global health/PROMs discrete categories for each mGPS group separately.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C-30; SD, standard deviation.
Assessment of PROMs was performed by using the EORTC QLQ-C30 scales calculated by the scoring procedures as described by Aaronson et al.3,15 EORTC QLQ-C30 scales were analyzed as discrete categories representing underlying continuous constructs. A high score on a global health or functional scale represents a high or healthy level of functioning; a high score on the symptom scale represents a high level of symptoms. Differences in EORTC scales were interpreted as having little, moderate, or substantial effect clinically, on the basis of a change of > 5 to 10, 10 to 20, or > 20 points, respectively.16,17 To facilitate analysis, Karnofsky PS was transformed as described by Ma et al18 into Eastern Cooperative Oncology Group (ECOG) PS groupings. The mGPSs were grouped by using thresholds described previously.
To examine the relationship between PROMs and ECOG PS, a series of χ2 tests for trend were used. Similar analysis was performed to examine the relationship between PROMs and mGPS. A multivariate logistic regression analysis was also performed by using selected functional and symptom scales separately to help predict mGPS in the ECOG PS 0-1 cohort.
To examine whether any of the statistically significant EORTC QLQ-C30 parameters were individually associated with mGPS in the ECOG PS 0-1 cohort, multivariate logistic regression analysis was carried out for functional and symptom scales separately. Backward elimination regression was performed in which the significance level to stay in the model was set at 0.1. All analyses were performed in SPSS Version 21.0 (SPSS, Chicago, IL).
RESULTS
Of the complete biobank data (N = 3,311), data on systemic inflammation and PS were available for 2,520 patients. EORTC QLQ-C30 data were available for approximately 2,200 patients. Patient demographic characteristics are provided in Table 3. The most common cancer types were GI (585 patients [23.2%]) and pulmonary (443 patients [17.6%]). The mean age was 62.4 years (standard deviation, 12.21 years), and the median survival was 4.25 months (interquartile range, 1.36 to 12.9 months). The majority of patients were inpatients (1,947 [77.3%]), and the median PS was 2 (interquartile range, 1 to 3). In all, 1,830 patients (72.6%) had metastatic disease.
Table 3.
Patient Demographic Characteristics (N = 2,520)
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| < 65 | 1,382 | 54.8 |
| 65-74 | 704 | 27.9 |
| ≥ 75 | 434 | 17.2 |
| Sex | ||
| Male | 1,304 | 51.7 |
| Female | 1,216 | 48.3 |
| Primary cancer site | ||
| Breast | 341 | 13.5 |
| Urologic | 423 | 16.8 |
| Gynecologic | 154 | 6.1 |
| GI | 585 | 23.2 |
| Hematologic | 133 | 5.3 |
| Head and neck | 106 | 4.2 |
| Pulmonary | 443 | 17.6 |
| Other | 335 | 13.3 |
| Spread | ||
| None | 302 | 12.0 |
| Locoregional | 388 | 15.4 |
| Metastases | 1,830 | 72.6 |
| Place of care | ||
| Inpatient | 1,947 | 77.3 |
| Outpatient | 573 | 22.8 |
| ECOG performance score | ||
| 0-1 | 682 | 27.1 |
| 2 | 994 | 39.4 |
| 3 | 646 | 25.6 |
| 4 | 198 | 7.9 |
| mGPS | ||
| 0 | 807 | 32.0 |
| 1 | 501 | 19.9 |
| 2 | 1,212 | 48.1 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; mGPS, modified Glasgow Prognostic Score.
The relationship between PROMs (global health, role function, cognitive function, physical function, emotional function, and social function), PS, and systemic inflammation is depicted in Table 1. Deteriorating PS and increasing mGPS (systemic inflammation) were individually associated with a worsening in all EORTC PROMs (P < .001 and P < .001, respectively, for all PROMs). In particular, there was a deterioration in global health outcomes across mGPSs independent of PS (ie, PS 0-1 category: mGPS 0, 56.3; mGPS 1, 51.1; mGPS 2, 45.3; P < .001). A similar pattern was also seen with role, physical, and social outcome measures (P < .001).
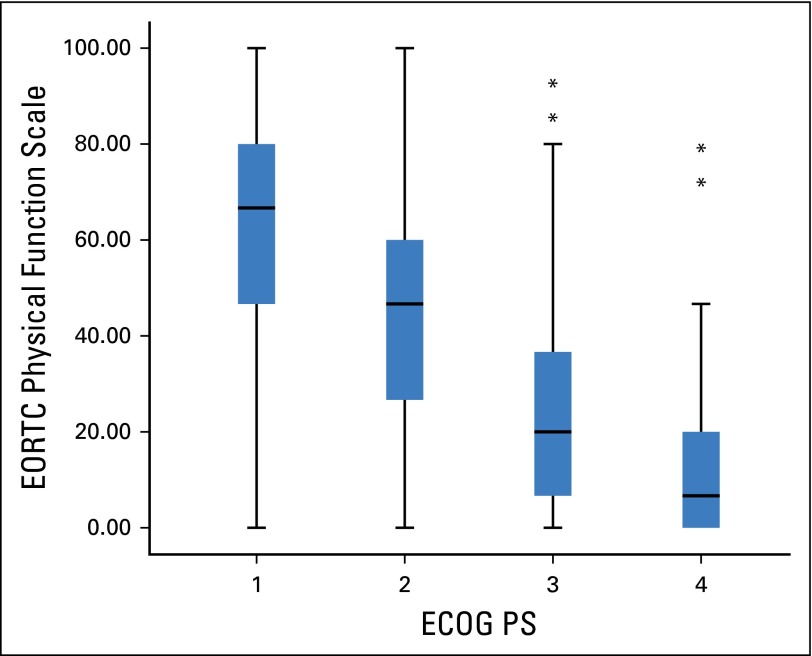
Because it was anticipated that patient-assessed physical function (EORTC) and clinician-assessed PS (ECOG) both reflect activity level, the relationship between them was examined and is shown in Figure 1. The Spearman rank correlation was –0.61 (P < .001). This shows that as PS deteriorated (ie, increasing ECOG score), patient-assessed physical function score decreased.
Fig 1.
Box and whisker plot showing the relationship between European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire C-30 physical function scale and Eastern Cooperative Oncology Group performance status (ECOG PS). Spearman rank correlation was −0.61 (P < .001). (*) Outlier values that are greater than 1.5 × interquartile range.
The relationship between symptom components of quality of life (fatigue, nausea, pain, dyspnea, sleep, appetite, constipation, and diarrhea), PS, and systemic inflammation is depicted in Table 2. Deteriorating PS and increasing mGPS (systemic inflammation) were individually associated with a worsening of almost all symptoms: fatigue (P < .001 and P < .001, respectively), nausea (P < .001 and P < .001, respectively), pain (P < .001 and P < .001, respectively), dyspnea (P < .001 and P < .001, respectively), and appetite (P < .001 and P < .001, respectively). However, a worsening in constipation symptoms was associated with deteriorating PS only (P < .001). In particular, there was a deterioration in fatigue across mGPS categories, despite having good PS (ie, PS 0-1 category: mGPS 0, 44.5; mGPS 1, 51.6; mGPS 2, 57.2; P < .001). A similar pattern was also seen with pain, appetite, and diarrhea (P < .001).
Of the significant function scales (ie, role function, physical function, and social function) on mGPS, only role function was independently associated with the mGPS (odds ratio [OR], 0.986; 95% CI, 0.980 to 0.991; P < .001). Of the significant symptom scales (ie, fatigue, pain, and appetite) on mGPS, only fatigue (OR, 1.009; 95% CI, 1.002 to 1.017; P = .018) and appetite (OR, 1.012; 95% CI, 1.006 to 1.017; P < .001) were independently associated with the mGPS.
Of those independently associated (eg, in ECOG PS 0-1 category) with mGPS, role function, fatigue, and appetite had differences of 18.1 points (32.0%), 12.7 points (22.2%), and 18.7 points (39.4%) across mGPS categories, consistent with clinically meaningful deterioration in quality of life.
DISCUSSION
To the best of our knowledge, this is the first study to examine how the combination of PS (ECOG) and systemic inflammation (mGPS) relates to functional and symptom scales of quality of life in patients with advanced cancer. In particular, the results of this study show a clear and consistent relationship between worsening of quality-of-life parameters, such as global health, role, physical and social functioning, and fatigue, pain, appetite symptoms with increased systemic inflammation, independent of good PS. Taken together, the results of this large cohort study suggest that the presence of a systemic inflammatory response is associated with functional decline and may be a factor associated with the deterioration of quality of life in patients with advanced cancer.
The implications of these results are several and profound. First, given that the same combination of ECOG PS and the mGPS has previously been shown to effectively predict survival rates in patients with advanced cancer,10 this objectively ties the deterioration of different PROMs to the deterioration of likely survival. Therefore, for the first time, the combination of ECOG PS and mGPS provides a simple framework for the study of quality of life and the impact of symptom control on survival in patients with advanced cancer.
Second, the findings support the rationale that attenuation of the systemic inflammatory response may improve quality of life in patients with cancer.19 The systemic inflammatory response in patients with cancer primarily reflects an upregulation of the innate immune/inflammatory response. Whether this results primarily from proinflammatory cytokines from tumor cells, stromal cells, inflammatory cells, or a combination is not yet clear. Given these results and their role in elaborating the systemic inflammatory response and changes in tissue metabolism, the interleukin-6 (IL-6)/Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway is a plausible mediator of the deterioration of PS and quality of life in patients with advanced cancer.20,21 This approach is the subject of ongoing clinical trials and will provide new insight into whether the relationship between the systemic inflammatory response and quality of life is causal in nature.20 Indeed, it is our hope that our results will stimulate further studies to examine the nature of the relationship between the components of the EORTC QLQ-C30, ECOG PS, and the mGPS in clinical trial data.
These persuasive arguments challenge the traditional paradigm in advanced cancer of treating symptoms once they have developed. This is in contrast to other disease groups (eg, cardiovascular disease, diabetes, and rheumatology) in which treatment is aimed at prevention or risk reduction of disease sequelae.22,23 Because there is an attitude of therapeutic nihilism that refractory cancer symptoms (eg, difficult-to-control pain, fatigue, and cachexia) are an inevitable consequence of advanced disease, there has been a failure to advance the research agenda in symptom management. Now there is a sound rationale for a proactive or preventative approach to treating symptoms in advanced cancer and the systemic inflammatory response is a feasible target for this.
Emerging evidence seems to support this rationale. To illustrate, the beneficial effects on cancer cachexia in non–small-cell lung cancer have been reported by using tocilizumab (an IL-6 receptor antibody), IL-6 having a seminal role in tumorigenesis.24 Further downstream in the innate immunity pathway, JAK1 inhibition by using ruxolitinib improved symptoms in patients with myelofibrosis.25 Future clinical trials that target inflammation through both specific and broad immunomodulatory approaches and that have quality-of-life parameters as primary end points are now warranted.
In patients near the end of life, both PS and the systemic inflammatory response are altered, and therefore poorer quality of life may be a result of poorer PS, systemic inflammation, or both. A possible alternative explanation is that systemic inflammation merely reflects the tumor burden and that it is the tumor-specific molecular processes that drive both the alterations in PS and the systemic inflammatory response. However, over the last 15 years, there has been an evolution in the thinking of how tumors grow and disseminate: from the earlier work in which the intrinsic characteristics of the tumor were considered to largely determined the process26 to an understanding that local27 and systemic inflammatory responses28 play a key role in disease progression and survival in patients with cancer.
This concept is consistent with recent progress in the area of immuno-oncology and suggests a more complex tumor-host interaction. The systemic inflammatory response is now recognized to have prognostic value, independent of tumor stage, in patients with cancer.9 In particular, the combination of CRP and albumin to form a cumulative systemic inflammation–based prognostic score (mGPS) has been shown to have prognostic value complementary to PS in patients with advanced cancer in whom tumor stage has less value.9-12 That the systemic inflammatory response is associated with a poorer quality of life, even in patients with good PS, suggests an inflammatory basis, at least in part, to changes in quality of life and PROMs in patients with advanced cancer. Furthermore, it suggests that immune interventions that attenuate systemic inflammation may improve key components of quality of life.
The main limitation of this study is that other factors (eg, oncology treatment) may have affected the quality-of-life parameters reported. However, the size of the population studied combined with the heterogeneity of the primary cancer types suggest that they would have been unlikely to affect the quality-of-life parameters. In addition, the convenience sampling approach in the primary data collection resulted in small numbers of patients with ECOG PS 4 category, but this may reflect that such patients are too unwell to participate in clinical trials. In this study, the associations reported cannot be interpreted as having causal relationship.
Another interesting aspect is whether patient-assessed measures are comparable to physician-assessed measures: EORTC QLQ-C30 Physical Function versus ECOG PS. In this study, there was a strong negative correlation (rs = –0.61; P < .001) between the patient-assessed physical function (EORTC) and clinician-assessed PS (ECOG), that is, as physical function score decreases (worsens) PS increases (gets worse).29 This seems to confirm that the patient and physician are both assessing a similar quality-of-life domain.
Several function scales (role function, physical function, and social function) were associated with the mGPS independent of good ECOG PS. However, on multiple regression analysis, only role function was independently associated with the mGPS. Similarly, several symptom scales (fatigue, pain, and appetite) were associated with the mGPS independent of ECOG PS. However, on multiple regression analysis, only fatigue and appetite symptoms were independently associated with the mGPS. Taken together, our results suggest that the systemic inflammatory response plays a role in the deterioration of quality of life in patients with advanced cancer. In addition, the findings suggest that, independent of good PS, the systemic inflammatory response has its main effect on appetite, fatigue, and role functioning of patients with advanced cancer. Further longitudinal and interventional studies are required to tease out the nature of these independent relationships.
In conclusion, the results of this study in a large international cohort highlight the relationship between systemic inflammatory response and deterioration of quality of life, independent of PS, in patients with advanced cancer. These results have significant implications for better understanding and improving treatment of the deterioration of quality of life commonly experienced in patients with advanced cancer. The increasing implementation of immunotherapies in the treatment of advanced cancer provide a fertile ground for expanding the role of these therapies in improving patients’ quality of life. After all, in patients with advanced cancer, optimizing quality of life is the central tenet of cancer care.
Supplementary Material
Footnotes
Supported by Contract No. 037777 from the Norwegian Research Council and the European Union’s 6th Framework (biobank data collection).
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Barry J.A. Laird, Donald C. McMillan
Administrative support: Stein Kaasa, Pål Klepstad
Provision of study materials or patients: Marianne J. Hjermstad, Stein Kaasa, Pål Klepstad
Collection and assembly of data: Barry J.A. Laird, Marianne J. Hjermstad, Stein Kaasa, Pål Klepstad
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Quality of Life in Patients With Advanced Cancer: Differential Association With Performance Status and Systemic Inflammatory Response
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Barry J.A. Laird
No relationship to disclose
Marie Fallon
Honoraria: GW Pharmaceuticals
Speakers’ Bureau: Mundipharma
Research Funding: Pfizer, Mundipharma, GW Pharmaceuticals
Travel, Accommodations, Expenses: Mundipharma, Astellas Pharma
Marianne J. Hjermstad
No relationship to disclose
Sharon Tuck
No relationship to disclose
Stein Kaasa
No relationship to disclose
Pål Klepstad
No relationship to disclose
Donald C. McMillan
No relationship to disclose
REFERENCES
- 1. Fayers PM, Machin D: Quality of Life: The Assessment, Analysis and Interpretation of Patient-Reported Outcomes (ed 2). Chichester, United Kingdom, John Wiley & Sons, 2007. [Google Scholar]
- 2.Velikova G, Coens C, Efficace F, et al. Health-related quality of life in EORTC clinical trials: 30 years of progress from methodological developments to making a real impact on oncology practice. Eur J Cancer. 2012;10:141–149. [Google Scholar]
- 3.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 4. MacLeod C (ed): The clinical evaluation of chemotherapeutic agents in cancer, in Evaluation of Chemotherapeutic Agents. New York, NY, Columbia University Press, 1949, pp 196. [Google Scholar]
- 5.Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: A prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer. 1996;32A:1135–1141. doi: 10.1016/0959-8049(95)00664-8. [DOI] [PubMed] [Google Scholar]
- 6.Wallach D, Kang TB, Kovalenko A. Concepts of tissue injury and cell death in inflammation: A historical perspective. Nat Rev Immunol. 2014;14:51–59. doi: 10.1038/nri3561. [DOI] [PubMed] [Google Scholar]
- 7.Laird BJ, Scott AC, Colvin LA, et al. Cancer pain and its relationship to systemic inflammation: An exploratory study. Pain. 2011;152:460–463. doi: 10.1016/j.pain.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 8.Laird BJ, McMillan DC, Fayers P, et al. The systemic inflammatory response and its relationship to pain and other symptoms in advanced cancer. Oncologist. 2013;18:1050–1055. doi: 10.1634/theoncologist.2013-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: A decade of experience in patients with cancer. Cancer Treat Rev. 2013;39:534–540. doi: 10.1016/j.ctrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Laird BJ, Kaasa S, McMillan DC, et al. Prognostic factors in patients with advanced cancer: A comparison of clinicopathological factors and the development of an inflammation-based prognostic system. Clin Cancer Res. 2013;19:5456–5464. doi: 10.1158/1078-0432.CCR-13-1066. [DOI] [PubMed] [Google Scholar]
- 11.Miura T, Matsumoto Y, Hama T, et al. Glasgow prognostic score predicts prognosis for cancer patients in palliative settings: A subanalysis of the Japan-prognostic assessment tools validation (J-ProVal) study. Support Care Cancer. 2015;23:3149–3156. doi: 10.1007/s00520-015-2693-x. [DOI] [PubMed] [Google Scholar]
- 12.de Paula Pantano N, Paiva BS, Hui D, et al. Validation of the Modified Glasgow Prognostic Score in advanced cancer patients receiving palliative care. J Pain Symptom Manage. 2016;51:270–277. doi: 10.1016/j.jpainsymman.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Klepstad P, Fladvad T, Skorpen F, et al. Influence from genetic variability on opioid use for cancer pain: A European genetic association study of 2294 cancer pain patients. Pain. 2011;152:1139–1145. doi: 10.1016/j.pain.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 14.Hjermstad MJ, Lie HC, Caraceni A, et al. Computer-based symptom assessment is feasible in patients with advanced cancer: Results from an international multicenter study, the EPCRC-CSA. J Pain Symptom Manage. 2012;44:639–654. doi: 10.1016/j.jpainsymman.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 15.Johnson C, Aaronson N, Blazeby J, et al. Guidelines for Developing Questionnaire Modules. ed 4. Brussels, Belgium, EORTC Quality of Life Group; 2011. [Google Scholar]
- 16. Fayers PM, Aaronson NK, Bjordal K, et al: EORTC QLQ-C30 Scoring Manual (ed 3). Brussels, Belgium, European Organisation for Research and Treatment of Cancer, 2001. [Google Scholar]
- 17.Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 18.Ma C, Bandukwala S, Burman D, et al. Interconversion of three measures of performance status: An empirical analysis. Eur J Cancer. 2010;46:3175–3183. doi: 10.1016/j.ejca.2010.06.126. [DOI] [PubMed] [Google Scholar]
- 19.Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493–e503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 20.Roxburgh CS, McMillan DC. Therapeutics targeting innate immune/inflammatory responses through the interleukin-6/JAK/STAT signal transduction pathway in patients with cancer. Transl Res. 2016;167:61–66. doi: 10.1016/j.trsl.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Sala D, Sacco A. Signal transducer and activator of transcription 3 signaling as a potential target to treat muscle wasting diseases. Curr Opin Clin Nutr Metab Care. 2016;19:171–176. doi: 10.1097/MCO.0000000000000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss U. Inflammation. Nature. 2008;454:427. doi: 10.1038/454427a. [DOI] [PubMed] [Google Scholar]
- 23.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 24.Ando K, Takahashi F, Kato M, et al. Tocilizumab, a proposed therapy for the cachexia of Interleukin6-expressing lung cancer. PLoS One. 2014;9:e102436. doi: 10.1371/journal.pone.0102436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 27.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 28.McAllister SS, Weinberg RA. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol. 2014;16:717–727. doi: 10.1038/ncb3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans JD. Straightforward Statistics for the Behavioral Sciences. Pacific Grove, CA, Brooks/Cole Publishing, 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.