Abstract
Purpose
Cancer cells can exploit the programmed death-1 (PD-1) immune checkpoint pathway to avoid immune surveillance by modulating T-lymphocyte activity. In part, this may occur through overexpression of PD-1 and PD-1 pathway ligands (PD-L1 and PD-L2) in the tumor microenvironment. PD-1 blockade has produced significant antitumor activity in solid tumors, and similar evidence has emerged in hematologic malignancies.
Methods
In this phase I, open-label, dose-escalation, cohort-expansion study, patients with relapsed or refractory B-cell lymphoma, T-cell lymphoma, and multiple myeloma received the anti–PD-1 monoclonal antibody nivolumab at doses of 1 or 3 mg/kg every 2 weeks. This study aimed to evaluate the safety and efficacy of nivolumab and to assess PD-L1/PD-L2 locus integrity and protein expression.
Results
Eighty-one patients were treated (follicular lymphoma, n = 10; diffuse large B-cell lymphoma, n = 11; other B-cell lymphomas, n = 10; mycosis fungoides, n = 13; peripheral T-cell lymphoma, n = 5; other T-cell lymphomas, n = 5; multiple myeloma, n = 27). Patients had received a median of three (range, one to 12) prior systemic treatments. Drug-related adverse events occurred in 51 (63%) patients, and most were grade 1 or 2. Objective response rates were 40%, 36%, 15%, and 40% among patients with follicular lymphoma, diffuse large B-cell lymphoma, mycosis fungoides, and peripheral T-cell lymphoma, respectively. Median time of follow-up observation was 66.6 weeks (range, 1.6 to 132.0+ weeks). Durations of response in individual patients ranged from 6.0 to 81.6+ weeks.
Conclusion
Nivolumab was well tolerated and exhibited antitumor activity in extensively pretreated patients with relapsed or refractory B- and T-cell lymphomas. Additional studies of nivolumab in these diseases are ongoing.
INTRODUCTION
The programmed death-1 (PD-1) pathway is an immune checkpoint to attenuate T-cell–mediated immune responses and may be exploited by tumors to avoid immune surveillance.1 PD-1 and its ligands PD-L1 and PD-L2 are commonly expressed in tumor or neoplastic microenvironments, including epithelial malignancies, classic Hodgkin lymphoma (HL), and non-Hodgkin lymphoma (NHL), although the expression of PD-L1 seems to vary significantly among lymphoma subtypes.1-3 There is more evidence for the role of PD-L1 in follicular lymphoma (FL),4 but its role in other types of NHL has not been as clearly described.3 Tumor cell expression of PD-L1 and PD-L2 can be upregulated due to genetic alterations of the 9p24.1 locus.5-7 This is particularly true in HL, where amplification of 9p24.1 is frequently observed and is associated with positive staining for the ligands.8 Inflammatory signaling facilitates PD-1 ligand overexpression on multiple immune and nonimmune cell types.1 Multiple myeloma (MM) plasma cells exposed to inflammatory mediators, such as interferon-gamma and Toll-like receptor ligands, demonstrate increased expression of PD-L1; however, upregulation is not present in cells from patients with monoclonal gammopathy of uncertain significance.9 PD-L1 interaction with PD-1 on tumor-infiltrating lymphocytes limits T-cell proliferation and cytotoxic functions.10 The role of PD-L2 is less clear.
Immune blockade of the PD-1/PD-L1 interaction by monoclonal antibodies can restore the antitumor activity of cytotoxic T cells.11 PD-1–blocking antibodies (nivolumab and pembrolizumab) produced durable objective responses and improved overall survival (OS) in patients with solid tumors12-15 and hematologic malignancies, including HL.8
NHL and MM are characterized by the neoplastic transformation and proliferation of normal lymphocytes and plasma cells, respectively. Patients with these diseases who experience a relapse after initial treatment or autologous stem cell transplantation (SCT) generally have a poor prognosis. Nivolumab (OPDIVO; Bristol-Myers Squibb, Princeton, NJ) is a fully human immunoglobulin G4 monoclonal antibody that targets the membrane PD-1 receptor on T cells and potentiates T-cell activity. This study evaluated the safety of nivolumab in patients with various hematologic malignancies. The integrity of the PD-L1 and PD-L2 loci and the expression of PD-L1 and PD-L2 proteins were also examined among a subset of patients with biopsy tissue available for analyses.
METHODS
Patients
This study was conducted in accordance with International Conference on Harmonisation Good Clinical Practice guidelines16 and approved by the institutional review board of an independent ethics committee at each center. All patients provided written informed consent before enrollment on the basis of the ethical principles outlined in the Declaration of Helsinki.17 For this phase I, open-label, dose-escalation, cohort-expansion study, patients with relapsed or refractory HL, NHL, and MM were eligible if they were age 18 years or older, had an Eastern Cooperative Oncology Group performance status of 0 or 1 with detectable or measurable disease, and had received at least one prior chemotherapy regimen. Patients with CNS involvement, concomitant secondary malignancies, active autoimmune disease, prior organ allograft or allogeneic SCT, prior treatment with immune checkpoint antibodies or concurrent treatment with receptor activator of nuclear factor-κB ligand inhibitors were excluded. Results for the patients with HL have been reported separately.8
Treatment and Monitoring
During the dose-escalation portion of the study, eligible patients were treated with nivolumab 1 or 3 mg/kg administered as a 1-h infusion at weeks 1 and 4 and then every 2 weeks for up to 2 years, unless patients experienced disease progression or excessive toxicity or achieved a complete response (CR). Patients who discontinued treatment with ongoing response or stable disease (SD) could be retreated with nivolumab for confirmed disease progression that occurred within 1 year. The dose for the cohort expansion was selected on the basis of the safety determination from the dose-escalation portion of the trial. Because the maximum tolerated dose of nivolumab was not reached at the highest protocol-specified dose, patients in the cohort expansion were treated with nivolumab 3 mg/kg.
The primary objective of the study was safety. Secondary objectives included evaluation of antitumor activity and detection of alterations of the PD-L1 (CD274) and PD-L2 (CD273; PDCD1LG2) loci and protein expression of the encoded ligands. Safety outcomes were evaluated by assessment of adverse events (AEs) throughout the study and for 100 days after the last dose was administered. AEs were categorized in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4).18 The safety evaluation included assessment of inflammatory AEs, such as pneumonitis, colitis, hypophysitis, thyroiditis, and skin disorders. These immune-mediated AEs are consistent with the mechanism of action of nivolumab and are known to occur with immune checkpoint inhibitors. Antitumor efficacy was assessed by tabulations of best overall response and analyses of objective response rate (ORR), duration of response (DOR), and progression-free survival.
Biomarker Assessment
Before initiating treatment with nivolumab, biopsies were performed to evaluate the tumor cells and immune-infiltrating cells for expression of immune-modulating proteins, including PD-L1 and PD-L2. Pretreatment bone marrow biopsies were mandatory for all patients and could be used as the baseline sample for biomarker assessment.
Fluorescent in situ hybridization (FISH) was performed on lymphoma tissue sections to analyze PD-L1 and PD-L2 loci as previously described.8 The bacterial artificial chromosome probes (Children’s Hospital of Oakland Research Institute, Oakland, CA) RP11-599H2O, which maps to 9p24.1 and includes CD274 (encoding PD-L1, labeled with Spectrum Orange [Abbott Molecular, Abbott Park, IL]), and RP11-635N21, which also maps to 9p24.1 and includes PDCD1LG2 (encoding PD-L2, labeled with Spectrum Green [Abbott Molecular]), were cohybridized. A control centromeric probe (Spectrum Aqua-labeled CEP9 [Abbott Molecular]) was hybridized according to the manufacturer’s recommendations.8
Immunohistochemical (IHC) staining was performed by means of an automated staining system (BOND-III; Leica Biosystems, Buffalo Grove, IL) by using a double-staining technique for PD-L1 (405.9A11)19 and PAX5 (24/Pax-5; BD Biosciences, Franklin Lakes, NJ), and for PD-L2 (366C.9E5)7 and phosphorylated signal transducer and activator of transcription (STAT) 3 (D3A7; Cell Signaling Technology, Beverly, MA).8 IHC staining was reviewed and scored by a hematopathologist who collected the following data: percentage of malignant cells with positive staining (0% to 100%), intensity of staining of malignant and nonmalignant cells (0 = no staining, 1+ = weak/equivocal staining, 2+ = moderate staining, and 3+ = strong staining), and primary subcellular localization of positive staining (nuclear, cytoplasmic, or cell membrane). Previously published criteria for categorizing cases as positive for PD-L1 or PD-L2 expression in malignant cells were used. Malignant cells needed to exhibit 2+ or 3+ membrane staining in ≥ 5% of malignant cells for PD-L1 and 2+ or 3+ cytoplasmic or membrane staining in ≥ 5% of malignant cells for PD-L2. For the tumor microenvironment, ≥ 20% of nonmalignant cells needed to exhibit positive staining for PD-L1 or PD-L2 to be categorized as positive.1,7,8
Statistical Analysis
All patients with NHL and MM who received at least one dose of nivolumab monotherapy were included in the safety and antitumor activity analysis. AEs were coded by using the Medical Dictionary for Regulatory Activities (version 17.0) and tabulated by system organ class and preferred term. The causal relationship between study medication and AEs was determined by the investigator at each site.
Efficacy assessments of individual best objective response were performed by the principal investigator at each site who used the revised Response Criteria for Malignant Lymphoma for patients with NHL,20 the International Myeloma Working Group Uniform Response Criteria for patients with MM,21 and the Clinical End Points and Response Criteria in Mycosis Fungoides and Sézary syndrome22 for patients with cutaneous T-cell lymphoma (CTCL). The ORR was the proportion of patients with a CR or a partial response (PR) as the best overall response. Median DOR, progression-free survival, and OS were estimated by Kaplan-Meier methodology.
RESULTS
Patients
Eighty-one patients with NHL or MM began treatment with nivolumab monotherapy between August 2012 and April 2015 when the database was locked for an interim analysis. One patient with chronic myelogenous leukemia was enrolled, and safety results for that patient are included in this analysis. Patients’ baseline characteristics are shown in Table 1. The 27 patients with MM had a median age of 63 years (range, 32 to 81 years). Median ages for 31 patients with B-cell lymphomas and 23 patients with T-cell lymphomas were 65 years (range, 23 to 74 years) and 61 years (range, 30 to 81 years), respectively. In the B-cell lymphoma group, 11 patients had DLBCL; 10 had FL; and 10 had other B-cell lymphomas, including mantle cell (n = 4), small lymphocytic (n = 2), primary mediastinal B cell (n = 2), marginal zone (n = 1), and B-cell NHL not otherwise specified (n = 1). The T-cell lymphoma group included 13 patients with mycosis fungoides (MF), five with peripheral T-cell lymphoma (PTCL), two with Sézary syndrome CTCL, and three with other non-CTCL. The median number of prior systemic treatment regimens in all patients was three (range, one to 12). Of patients with MM, DLBCL, T-cell lymphoma, and FL, 96%, 91%, 87%, and 70%, respectively, had received two or more prior systemic treatment regimens and 56%, 18%, 9%, and 20%, respectively, had a history of autologous SCT. Of 27 patients with MM, 24 experienced disease progression after prior immunomodulator and proteasome inhibitor therapy.
Table 1.
Baseline Characteristics
| Characteristic | B-Cell Lymphoma, No. (%) | T-Cell Lymphoma, No. (%) | Multiple Myeloma, No. (%) |
|---|---|---|---|
| No. of patients | 31 | 23 | 27 |
| Age, years | |||
| Median | 65 | 61 | 63 |
| Range | 23-74 | 30-81 | 32-81 |
| Sex | |||
| Female | 11 (35) | 8 (35) | 15 (56) |
| Male | 20 (65) | 15 (65) | 12 (44) |
| Race | |||
| White | 29 (94) | 17 (74) | 22 (81) |
| Black | 1 (3) | 3 (13) | 5 (19) |
| Asian | 1 (3) | 1 (4) | 0 |
| Other | 0 | 2 (9) | 0 |
| ECOG performance status | |||
| 0 | 16 (52) | 4 (17) | 13 (48) |
| 1 | 12 (39) | 18 (78) | 13 (48) |
| 2 | 0 | 0 | 1 (4) |
| Not reported | 3 (10) | 1 (4) | 0 |
| Extranodal involvement | 8 (26) | 4 (17) | NA |
| Prior systemic therapies | |||
| 2-3 | 15 (48) | 6 (26) | 12 (44) |
| 4-5 | 7 (23) | 9 (39) | 8 (30) |
| ≥ 6 | 5 (16) | 5 (22) | 6 (22) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; NA, not applicable.
Safety
Seventy-nine (96%) patients experienced at least one AE. Drug-related AEs of any grade were reported in 53 (65%) patients (Table 2). Fifteen (18%) patients had grade 3 drug-related AEs, two had grade 4 AEs, and one had a grade 5 AE. Immune-mediated AEs, potentially related to enhancements in immune response in the form of T-cell activation and proliferation, occurred in 28 (34%) patients and were predominantly grade 1 or 2. In 13 (46%) patients, the immune-mediated AEs resolved without treatment or interruption of nivolumab. Fifteen patients required treatment of immune-mediated AEs; of these, five had to discontinue nivolumab. With the exclusion of one patient whose AE outcome was unknown, immune-mediated AEs resolved spontaneously or after protocol-specified treatment in 82% of patients. Overall, 12 (15%) patients discontinued treatment because of drug-related AEs, including one episode each of grade 1 myositis and conjunctivitis; grade 2 enteritis and pneumonitis; grade 3 pneumonitis, stomatitis, neutropenia, diplopia, creatine phosphokinase increase, and rash; and grade 4 pneumonitis, pustular rash, and sepsis.
Table 2.
Drug-Related AEs
| AEs | Any Grade, No. (%) | Grade ≥ 3, No. (%) |
|---|---|---|
| Summary of AEs by tumor type | ||
| B-cell NHL* (n = 31) | 22 (71) | 8 (26) |
| T-cell NHL (n = 23) | 17 (74) | 5 (22) |
| Multiple myeloma (n = 27) | 14 (52) | 5 (19) |
| Chronic myelogenous leukemia (n = 1) | 0 | 0 |
| Any grade AE in ≥ 5% of patients and all grade ≥ 3 AEs | ||
| Nonhematologic | ||
| Fatigue | 14 (17) | 0 |
| Pneumonitis | 9 (11) | 3 (4) |
| Decreased appetite | 7 (9) | 0 |
| Pruritus | 7 (9) | 0 |
| Rash | 7 (9) | 1 (1) |
| Diarrhea | 6 (7) | 0 |
| Pyrexia | 6 (7) | 0 |
| Hypocalcemia | 5 (6) | 0 |
| Blood creatine phosphokinase increased | 3 (4) | 1 (1) |
| Lipase increased | 3 (4) | 1 (1) |
| Mucosal inflammation | 3 (4) | 1 (1) |
| Stomatitis | 2 (2) | 1 (1) |
| Diplopia | 1 (1) | 1 (1) |
| Pneumonia | 1 (1) | 1 (1) |
| Pulmonary embolism | 1 (1) | 1 (1) |
| Rash pustular | 1 (1) | 1 (1) |
| Sepsis | 1 (1) | 1 (1) |
| ARDS† | 1 (1) | 1 (1) |
| Hematologic | ||
| Anemia | 5 (6) | 3 (4) |
| Leukopenia | 4 (5) | 3 (4) |
| Lymphopenia | 3 (4) | 1 (1) |
| Neutropenia | 3 (4) | 1 (1) |
| Eosinophilia | 1 (1) | 1 (1) |
| Lymphocyte decrease | 1 (1) | 1 (1) |
| Select AEs‡ | ||
| Skin (pruritus, rash) | 15 (18) | 1 (1) |
| Pulmonary (pneumonitis) | 9 (11) | 3 (4) |
| Gastrointestinal (diarrhea, enteritis) | 6 (7) | 0 |
| Hypersensitivity (hypersensitivity, infusion reactions) | 3 (4) | 0 |
| Hepatic (ALT increased, AST increased) | 2 (2) | 0 |
| Renal (blood creatinine increased) | 2 (2) | 0 |
Abbreviations: AE, adverse event; ARDS, acute respiratory distress syndrome; NHL, non-Hodgkin lymphoma.
One grade 5 event was observed (pneumonitis/ARDS).
Event was grade 5.
Select AEs have potential immunologic etiology that require frequent monitoring and intervention.
Fatal pneumonitis occurred in one patient, a 51-year-old woman with small lymphocytic B-cell lymphoma. She had nine prior systemic treatment regimens, including rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone, bendamustine, ibritumomab tiuxetan radioimmunotherapy, panobinostat, ofatumumab, everolimus, and pentostatin. Active conditions at enrollment were exercise-induced asthma, tobacco use, chronic bronchitis, and sinusitis. Pretreatment symptoms included a chronic dry cough. Approximately 2 weeks after the first dose of nivolumab 3 mg/kg, pneumonitis developed. Despite treatment with corticosteroids and anti-infective medications, the patient experienced progressive deterioration complicated by respiratory failure and died 19 days after receiving one dose of nivolumab 6 days after pneumonitis onset.
Of the remaining eight patients with drug-related pneumonitis, two discontinued nivolumab due to pneumonitis (grade 2, n = 1; grade 3, n = 1). For five patients who continued nivolumab, pneumonitis (grade 1, n = 3; grade 2, n = 2) resolved in < 40 days without treatment or with corticosteroids. For one patient, grade 1 pneumonitis resolved with antibiotic and corticosteroid treatment but required > 40 days for resolution. None of the patients with drug-related pneumonitis had prior gemcitabine treatment; however, one patient received radiation to the right-side mediastinum and superior vena cava approximately 1 month before onset of grade 2 pneumonitis.
Clinical Outcomes
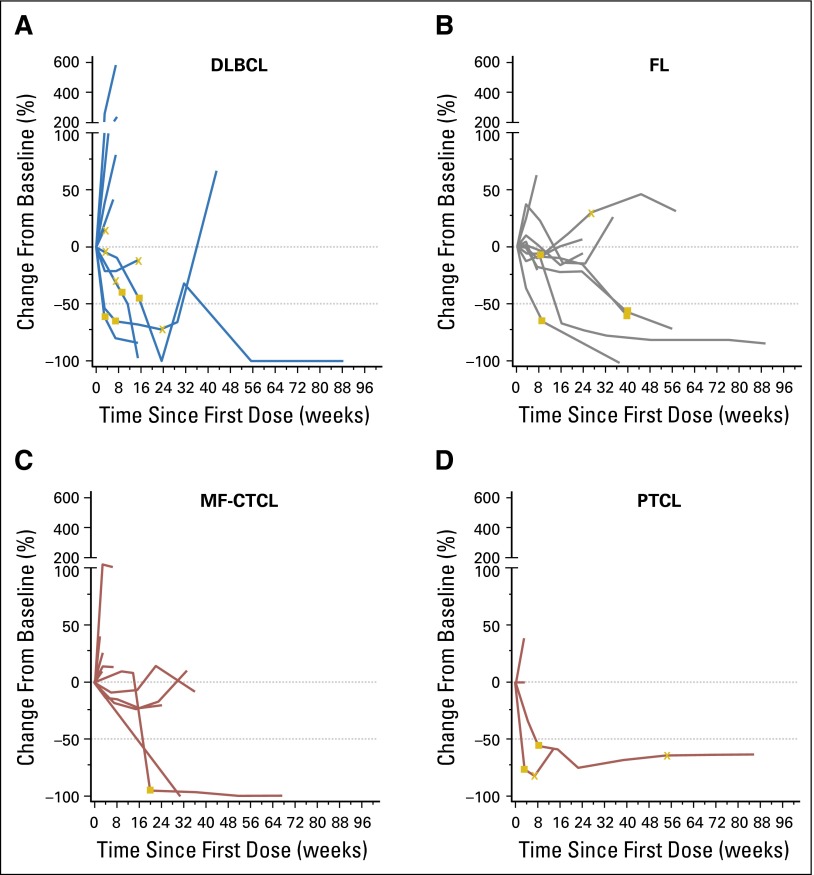
Among the 10 patients with FL and 11 patients with DLBCL, the ORRs were 40% (CR, n = 1; PR, n = 3) and 36% (CR, n = 2; PR, n = 2), respectively (Table 3; Figs 1A and 1B). Objective responses were not observed among the 10 patients with other B-cell lymphomas. With a median observation time of 91.4 weeks for FL, three of four patients have had ongoing responses (all four continue to be followed); individual response durations were 81.6+ weeks for the patient with a CR and 27.1+, 28.1+, and 32.1+ weeks for the patients with PRs. The median follow-up duration for patients with DLBCL was 22.7 weeks; one of four patients in this group has had an ongoing response, and two continue to be followed. Individual response durations for the patients with CRs were 6.0 and 77.3+ weeks. For the patients with PRs, individual response durations were 12.1+ and 22.1 weeks. Sixteen patients with B-cell lymphoma had SD, with a median duration of 23.1 weeks (range, 6.9 to 44.0+ weeks).
Table 3.
Efficacy Results
| Tumor | OR, No. (%) | CR, No. (%) | PR, No. (%) | SD, No. (%) | Median PFS, Weeks (95% CI) |
|---|---|---|---|---|---|
| B-cell lymphoma (n = 31) | 8 (26) | 3 (10) | 5 (16) | 16 (52) | 23 (7 to 44) |
| DLBCL (n = 11) | 4 (36) | 2 (18) | 2 (18) | 3 (27) | 7 (6 to 29) |
| FL (n = 10) | 4 (40) | 1 (10) | 3 (30) | 6 (60) | NR (7 to NR) |
| Other B-cell lymphoma (n = 10) | 0 | 0 | 0 | 7 (70) | 11 (3 to 39) |
| T-cell lymphoma (n = 23) | 4 (17) | 0 | 4 (17) | 10 (43) | 10 (7 to 33) |
| MF (n = 13) | 2 (15) | 0 | 2 (15) | 9 (69) | 10 (7 to 35) |
| PTCL (n = 5) | 2 (40) | 0 | 2 (40) | 0 | 14 (3 to NR) |
| Other CTCL (n = 3) | 0 | 0 | 0 | 0 | 7 (6 to NR) |
| Other non-CTCL (n = 2) | 0 | 0 | 0 | 1 (50) | 10 (2 to 18) |
| Multiple myeloma (n = 27) | 1 (4) | 1 (4)* | 0 | 17 (63) | 10 (5 to 15) |
Abbreviations: CR, complete response; CTCL, cutaneous T-cell lymphoma; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; MF, mycosis fungoides; NR, not reported; OR, objective response; PFS, progression-free survival; PR, partial response; PTCL, peripheral T-cell lymphoma; SD, stable disease.
CR was obtained after radiotherapy. SD was the best response to nivolumab.
Fig 1.
Kinetics of response for individual patients with (A) diffuse large B-cell lymphoma (DLBCL), (B) follicular lymphoma (FL), (C) mycosis fungoides cutaneous T-cell lymphoma (MF-CTCL), and (D) peripheral T-cell lymphoma (PTCL) who were treated with nivolumab 3 mg/kg. The horizontal line at –50 indicates the threshold for defining an objective response in DLBCL, FL, MF-CTCL, and PTCL. Most objective responses were sustained. The DLBCL and FL groups had individuals with responses maintained beyond 88 weeks. (closed square) first response; (X) first occurrence of new lesion.
Among the patients with T-cell lymphomas (n = 23), the response rate was 17% (PR, n = 4; Table 3; Figs 1C and 1D). Responses occurred for two patients with MF and two patients with PTCL. With median follow-up times of 42.9 and 44.0 weeks for MF and PTCL, respectively, responses are ongoing for both patients with MF, with response durations of 24.3+ and 50.0+ weeks. Response is also ongoing for one of the patients with PTCL. Response durations for the two patients with PTCL were 10.6 and 78.6+ weeks. The median duration of SD was 11.0 weeks (range, 7.1 to 42.9+ weeks) for 10 patients with T-cell lymphomas.
The median follow-up duration for patients with MM was 65.6 weeks (range, 1.6 to 126 weeks). SD was the best response for 17 (63%) patients with MM, which lasted a median of 11.4 weeks (range, 3.1 to 46.1 weeks). A patient with MM achieved a best response of SD and then had an increase in κ:λ free light chain ratio and a rib plasmacytoma that became increasingly symptomatic. There was a month-long interruption of nivolumab while the patient underwent radiation for the rib lesion. After completion of radiation, nivolumab was restarted for 2 months then discontinued because the patient had been treated for approximately 2 years and was assessed as having a CR on the basis of the rib plasmacytoma having become asymptomatic. The patient is alive and has been in remission approximately 14 months since discontinuation of nivolumab treatment.
Biomarker Assessment
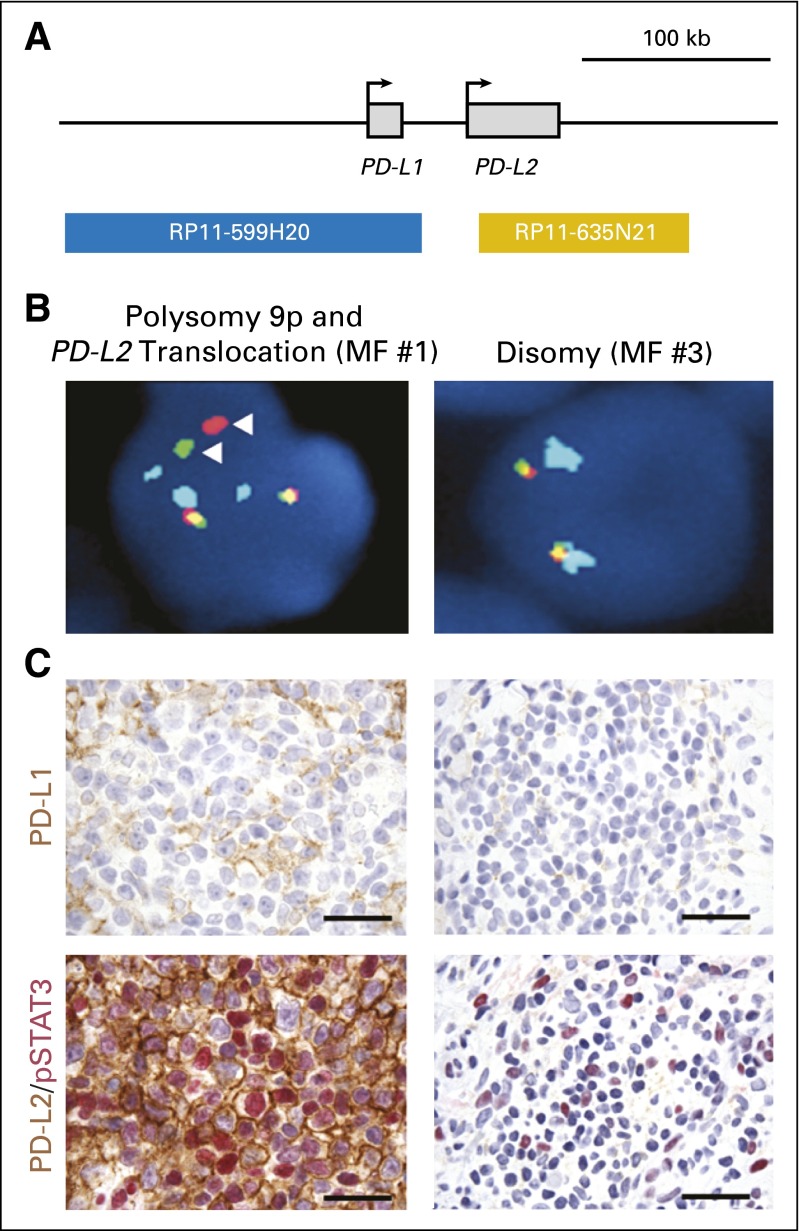
Genetic alterations within the 9p24 locus detected by FISH were present in three of 27 patients from whom samples were obtained for analyses. FISH analysis of the biopsy sample MF #1 revealed an abnormal split signal for probes targeting PD-L1 and PD-L2 consistent with a breakpoint that maps 3′ of the PD-L1 locus and 5′ of the PD-L2 transcriptional start site on one of three copies of chromosome 9 (Fig 2; Appendix Table A1, online only). IHC staining for PD-L2 revealed intense (3+) and membranous protein expression in 70% of malignant cells (Fig 2; Appendix Table A1). Positive nuclear staining for phosphorylated STAT3 consistent with active Janus kinase-STAT signaling in the malignant cells was also observed (Fig 2). In contrast, IHC for PD-L1 revealed scattered positive staining in < 20% of nonmalignant cells and virtually no staining of malignant cells (Fig 2; Appendix Table A1). The morphologic features were consistent with large-cell transformation of MF. The patient achieved a PR for 47 weeks but discontinued therapy due to toxicity.
Fig 2.
Immunohistochemical staining for PD-L1 in patient biopsy samples. (A) Orientation of chromosomal probes for fluorescent in situ hybridization. (B) Fluorescent in situ hybridization analysis of MF #1 (left) and disruption of chromosome 9 between PD-L1 and PD-L2 and three copies of chromosome 9 (arrowheads) and of MF #3 (right), which shows disomy 9. (C) Immunohistochemical staining of MF #1 (top and bottom left) and MF #3 (top and bottom right) for PD-L1 (brown = positive staining [top]) and for PD-L2 (brown) and for pSTAT3 (red [bottom]). Images in (B) and (C), magnification ×1,000. Scale bars = 50 μm. MF, mycosis fungoides; PD-L1, programmed death ligand 1; PD-L2, programmed death ligand 2; pSTAT3, phosphorylated signal transducer and activator of transcription 3.
Two patients with DLBCL exhibited low-level polysomy of PD-L1/PD-L2. One had a best response of PR. The other patient had moderate intensity (2+) PD-L1 expression in a cell membranous pattern in 2% of malignant cells and had a best response of SD.
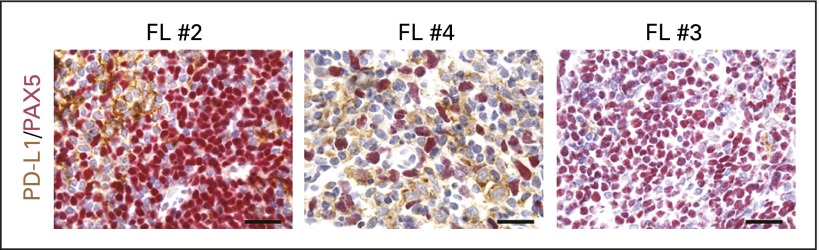
IHC analyses for PD-L1 and PD-L2 were largely negative in the malignant cells of the remaining cases, which lacked genetic alterations of PD-L1 and PD-L2 (Appendix Table A1). One of the B-cell lymphomas not otherwise specified exhibited PD-L2 expression (2+) in 20% of malignant cells, and one MF (MF #2) had PD-L1 expression (2+) in 20% of malignant cells (Appendix Table A1). In most cases, including certain FLs (Fig 3; Appendix Table A1), IHC staining revealed PD-L1 and, to a lesser extent, PD-L2 expression in variable percentages of nonmalignant immune cells within the tumor microenvironment (data not shown).
Fig 3.
Immunohistochemical staining for programmed death ligand 1 (PD-L1; brown) and the B-cell lineage marker PAX5 (red) in three cases of follicular lymphoma (FL) show positive cell membrane expression of PD-L1 among the nonmalignant (PAX5-negative) cells within the tumor microenvironment. Magnification, ×1,000. Scale bars = 50 μm.
DISCUSSION
In patients with advanced hematologic malignancies, nivolumab had a safety profile similar to that seen in studies that involved patients with solid tumors12 and HL.8 The majority of drug-related AEs were grade 1 or 2, and the incidence of severe or life-threatening drug-related AEs was low across the various disease cohorts. Pneumonitis was observed in a small proportion of patients; most cases were grade 1 or 2 and were uniformly distributed across the spectrum of tumors in the study.
Nivolumab therapy resulted in ORRs of 36% and 40% among patients with DLBCL and FL, respectively. With continued nivolumab therapy, the depth of objective responses may improve as demonstrated by one patient with DLBCL with an initial PR (at 16 weeks) that converted to a CR (at 72 weeks) with extended treatment. Response durations exceeded 1 year for two (one each with FL and DLBCL) of three patients who achieved a CR and ≥ 6 months for patients with FL who achieved a PR.
Although less frequent than in B-cell lymphomas, durable objective responses were also seen in some PTCLs and CTCLs—diseases that are resistant to most standard chemotherapies. Thus, meaningful clinical activity was seen in both B-cell and T-cell lymphoma subtypes.
Tumor regression responses were not seen among patients with MM in this study, with the exception of one CR occurring after local radiation therapy, which suggests a possible abscopal effect.23 The reason for the lack of objective responses in MM, compared with other tumors, is unclear and may relate to the immunosuppressive nature of the MM tumor microenvironment.24
As previously reported, immunotherapy with ipilimumab produced objective responses in two (11%) of 18 patients (DLBCL, n = 1; FL, n = 1) with relapsed/refractory NHL.25 Studies that involved combinations of nivolumab with rituximab, ipilimumab, and other immuno-oncology therapies are ongoing to determine whether response rates and DOR achieved with nivolumab monotherapy can be improved, particularly in these B-cell lymphoma subtypes.
Genetic alterations of PD-L1 and PD-L2 were rare among the NHLs evaluated in this study—a stark contrast to the frequent 9p24.1 alterations in HLs.8 Among the evaluated NHLs, the most significant finding was a chromosomal rearrangement that involved PD-L2 and associated high-level PD-L2 protein expression in malignant cells of a transformed MF (MF #1; Appendix Table A1). PD-L1 and, to a lesser extent, PD-L2 proteins were expressed by nonmalignant cells within the tumor microenvironment in NHLs, including certain FLs. These data highlight the likely differences in the frequency of 9p24.1 alterations and associated expression of the PD-1 ligands in specific lymphoid malignancies. The roles of these biomarkers as predictors of response to nivolumab are under current evaluation in phase II trials of DLBCL and FL.
In conclusion, PD-1 blockade with nivolumab is associated with a favorable safety profile and objective responses in patients with various types of NHL. Accordingly, phase II trials with nivolumab are under way in patients with relapsed or refractory FL and DLBCL. These studies and future combination trials will help to define the role of PD-1 blockade in hematologic malignancies.
Acknowledgment
Michelle Daniels of inScience Communications, Springer Healthcare, provided medical writing support funded by Bristol-Myers Squibb.
Appendix
Table A1.
PD-L1 and PD-L2 Genetic and IHC Analysis
| Diagnosis | Genetic Analysis | IHC | ||||
|---|---|---|---|---|---|---|
| Polysomy | Gain | Amplification | Translocation | Tumor PD-L1 (%, Int) | Tumor PD-L2 (%, Int) | |
| B-cell lymphoma (n = 20) | ||||||
| DLBCL (n = 6) | ||||||
| DLBCL #1 | + | – | – | – | –* | – |
| DLBCL #2 | + | – | – | – | – | – |
| DLBCL #3 | – | – | – | – | – | – |
| DLBCL #4 | – | – | – | – | – | – |
| DLBCL #5 | – | – | – | – | – | – |
| DLBCL #6 | – | – | – | – | – | – |
| FL (n = 6) | ||||||
| FL #1 | – | – | – | – | – | – |
| FL #2† | – | – | – | – | – | – |
| FL #3 | – | – | – | – | – | – |
| FL #4† | – | – | – | – | – | – |
| FL #5 | – | – | – | – | – | – |
| FL #6 | – | – | – | – | NA | NA |
| Other B-cell lymphoma (n = 8) | ||||||
| MCL #1 | – | – | – | – | 5%, 3+ | – |
| MCL #2 | – | – | – | – | – | – |
| MCL #3 | – | – | – | – | – | – |
| MCL #4 | – | – | – | – | – | – |
| MZL | – | – | – | – | – | – |
| SLL #1 | – | – | – | – | – | – |
| SLL #2 | – | – | – | – | – | NA |
| B-cell NOS | – | – | – | – | – | 20%, 2+ (c) |
| T-cell lymphoma (n = 8) | ||||||
| MF (n = 4) | ||||||
| MF #1 | + | – | – | + | – | 70%, 3+ (m) |
| MF #2 | – | – | – | – | 20%, 2+ | – |
| MF #3 | – | – | – | – | – | – |
| MF #4 | – | – | – | – | – | – |
| PTCL (n = 3) | ||||||
| PTCL #1 | – | – | – | – | – | – |
| PTCL #2 | – | – | – | – | – | – |
| PTCL #3 | – | – | – | – | – | – |
| Other T-cell lymphoma (n = 1) | ||||||
| Other #1 | – | – | – | – | – | – |
NOTE. Previously published criteria for the genetic (Ansell et al8) and PD-L1 IHC (Chen et al1) analyses were used. Tumor cells needed to exhibit 2+ or 3+ membrane staining (Int) in ≥ 5% of malignant cells to be considered positive for PD-L1. For PD-L2, 2+ to 3+ cytoplasmic or membrane staining in ≥ 5% of malignant cells was termed positive. Abbreviations: c, cytoplasmic staining; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; IHC, immunohistochemical; Int, intensity; m, membrane staining; MCL, mantle cell lymphoma; MF, mycosis fungoides; MZL, marginal zone lymphoma; NA, not available; NOS, not otherwise specified; PD-L1, programmed death ligand 1; PD-L2, programmed death ligand 2; PTCL, peripheral T-cell lymphoma; SLL, small lymphocytic lymphoma.
Two percent of malignant cells exhibited 2+ membrane PD-L1 staining.
In FL #2 and FL #4, 40% of nonmalignant cells were PD-L1 positive.
Footnotes
Listen to the podcast by Dr Westin at www.jco.org/podcasts
Supported by Bristol-Myers Squibb, grants from the National Institutes of Health (U54CA163125 and P01AI056299 to G.J.F. and R01CA161026 to M.A.S.), and a grant from the Miller Fund (to M.A.S.).
Presented at the 56th Annual Meeting of the American Society of Hematology, San Francisco, CA, December 6-9, 2014; 20th Congress of the European Hematology Association, Vienna, Austria, June 11-14, 2015; and 13th International Conference on Malignant Lymphoma, Lugano, Switzerland, June 17-20, 2015.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT01592370.
AUTHOR CONTRIBUTIONS
Conception and design: Alexander M. Lesokhin, Philippe Armand, Bjoern Chapuy, Azra H. Ligon, Gordon J. Freeman, Scott J. Rodig, Margaret A. Shipp
Collection and assembly of data: Alexander M. Lesokhin, Philippe Armand, Bjoern Chapuy, Azra H. Ligon, Scott J. Rodig, Margaret A. Shipp, John Timmerman
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Nivolumab in Patients With Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Alexander M. Lesokhin
Stock or Other Ownership: Exelixis, Enumeral
Honoraria: Bristol-Myers Squibb, Janssen Pharmaceuticals (a Johnson & Johnson co.), Gilead Sciences (I), Novartis
Consulting or Advisory Role: Bristol-Myers Squibb, Foundation Medicine (Inst), Janssen Pharmaceuticals (a Johnson & Johnson co.), Novartis
Research Funding: Bristol-Myers Squibb (Inst), Janssen Pharmaceuticals (a Johnson & Johnson co.) (Inst)
Patents, Royalties, Other Intellectual Property: Serametrix
Stephen M. Ansell
Honoraria: WebMD, Research To Practice
Research Funding: Bristol-Myers Squibb (Inst), Celldex Therapeutics (Inst), Seattle Genetics (Inst)
Philippe Armand
Consulting or Advisory Role: Merck, Infinity Pharmaceuticals, Bristol-Myers Squibb
Research Funding: Bristol-Myers Squibb, Merck, Sequenta, Otsuka, Sigma Tau Pharmaceuticals
Emma C. Scott
No relationship to disclose
Ahmad Halwani
Research Funding: Bristol-Myers Squibb (Inst), Seattle Genetics (Inst), Pharmacyclics, Takeda Pharmaceuticals (Inst), Millennium Pharmaceuticals (Inst), Genentech, AbbVie, Kyowa Hakko Kirin (Inst), Amgen, miRagen Therapeutics (Inst), Immune Design (Inst)
Travel, Accommodations, Expenses: Seattle Genetics, Pharmacyclics, Millennium Pharmaceuticals, AbbVie, Kyowa Hakko Kirin
Martin Gutierrez
Stock or Other Ownership: COTA
Consulting or Advisory Role: Bayer AG, Eli Lilly
Speakers’ Bureau: Bristol-Myers Squibb, Merck
Research Funding: Novartis (Inst), AbbVie (Inst), MedImmune (Inst), Bristol-Myers Squibb (Inst), Rexahn Pharmaceuticals (Inst), Esanex (Inst), Karyopharm Therapeutics (Inst), TG Therapeutics (Inst), Daiichi Sankyo (Inst), EMD Serono (Inst), Eli Lilly (Inst), Acceleron Pharma (Inst), Gilead Sciences (Inst), Incyte (Inst)
Michael M. Millenson
Employment: Janssen Pharmaceuticals (a Johnson & Johnson co.) (I)
Adam D. Cohen
Consulting or Advisory Role: Celgene, Bristol-Myers Squibb, Janssen Pharmaceuticals (a Johnson & Johnson co.)
Research Funding: Bristol-Myers Squibb, Novartis (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Janssen Pharmaceuticals (a Johnson & Johnson co.), Celgene
Stephen J. Schuster
Consulting or Advisory Role: Celgene, Pharmacyclics, Gilead Sciences, Nordic Nanovector, Novartis
Research Funding: Novartis (Inst), Celgene (Inst), Gilead Sciences (Inst), Pharmacyclics (Inst), Johnson & Johnson (Inst)
Daniel Lebovic
Speakers’ Bureau: Celgene, Takeda Pharmaceuticals
Research Funding: Celgene (Inst), Onyx Pharmaceuticals (Inst), Sanofi (Inst)
Madhav Dhodapkar
Research Funding: Celgene (Inst)
David Avigan
Employment: PAREXEL International
Honoraria: Takeda Pharmaceuticals
Consulting or Advisory Role: Celgene, Seattle Genetics, Bristol-Myers Squibb, Synta Pharmaceuticals
Research Funding: Genus Oncology, Astex Pharmaceuticals
Bjoern Chapuy
No relationship to disclose
Azra H. Ligon
No relationship to disclose
Gordon J. Freeman
Stock or Other Ownership: CoStim Pharmaceuticals, CoStim Pharmaceuticals (I)
Consulting or Advisory Role: Novartis, Novartis (I), Eli Lilly, Surface Oncology (I), Genentech, Bristol-Myers Squibb
Patents, Royalties, Other Intellectual Property: Genentech, Genentech (I), Pfizer (I), Bristol-Myers Squibb, Medarex, Amplimmune, AstraZeneca, Merck, EMD Serono, Boehringer Ingelheim, Novartis, Novartis (I)
Scott J. Rodig
Honoraria: Perkin Elmer, Bristol-Myers Squibb
Consulting or Advisory Role: AstraZeneca, Perkin Elmer
Speakers’ Bureau: Bristol-Myers Squibb
Research Funding: Bristol-Myers Squibb
Patents, Royalties, Other Intellectual Property: Patent pending for use of anti-galectin1 antibodies for diagnostic use
Travel, Accommodations, Expenses: Roche
Deepika Cattry
Honoraria: Bristol-Myers Squibb
Speakers’ Bureau: Bristol-Myers Squibb
Lili Zhu
Employment: Bristol-Myers Squibb
Stock or Other Ownership: Bristol-Myers Squibb
Joseph F. Grosso
Employment: Bristol-Myers Squibb
Stock or Other Ownership: Bristol-Myers Squibb, Celgene
Consulting or Advisory Role: Kyowa Hakko Kirin (I), GlaxoSmithKline (I), AstraZeneca (I), MedImmune (I)
Research Funding: Bristol-Myers Squibb (I)
Travel, Accommodations, Expenses: Bristol-Myers Squibb
M. Brigid Bradley Garelik
Employment: Bristol-Myers Squibb
Stock or Other Ownership: Bristol-Myers Squibb
Margaret A. Shipp
Honoraria: Bristol-Myers Squibb, Merck, Gilead Sciences, Takeda Pharmaceuticals
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Gilead Sciences, Takeda Pharmaceuticals
Research Funding: Bristol-Myers Squibb (Inst), Bayer AG (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Ivan Borrello
Speakers’ Bureau: Bristol-Myers Squibb, Celgene
Research Funding: Celgene, Bristol-Myers Squibb
John Timmerman
Stock or Other Ownership: BioMarin, Medivation
Honoraria: Ono Pharmaceutical
Research Funding: Bristol-Myers Squibb, ImmunGene, Janssen Pharmaceuticals (a Johnson & Johnson co.)
Travel, Accommodations, Expenses: Bristol-Myers Squibb
REFERENCES
- 1.Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19:3462–3473. doi: 10.1158/1078-0432.CCR-13-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andorsky DJ, Yamada RE, Said J, et al. Programmed death ligand 1 is expressed by non-Hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin Cancer Res. 2011;17:4232–4244. doi: 10.1158/1078-0432.CCR-10-2660. [DOI] [PubMed] [Google Scholar]
- 3.Wilcox RA, Feldman AL, Wada DA, et al. B7-H1 (PD-L1, CD274) suppresses host immunity in T-cell lymphoproliferative disorders. Blood. 2009;114:2149–2158. doi: 10.1182/blood-2009-04-216671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang ZZ, Grote DM, Ziesmer SC, et al. PD-1 expression defines two distinct T-cell sub-populations in follicular lymphoma that differentially impact patient survival. Blood Cancer J. 2015;5:e281. doi: 10.1038/bcj.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116:3268–3277. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Twa DD, Chan FC, Ben-Neriah S, et al. Genomic rearrangements involving programmed death ligands are recurrent in primary mediastinal large B-cell lymphoma. Blood. 2014;123:2062–2065. doi: 10.1182/blood-2013-10-535443. [DOI] [PubMed] [Google Scholar]
- 7.Shi M, Roemer MG, Chapuy B, et al. Expression of programmed cell death 1 ligand 2 (PD-L2) is a distinguishing feature of primary mediastinal (thymic) large B-cell lymphoma and associated with PDCD1LG2 copy gain. Am J Surg Pathol. 2014;38:1715–1723. doi: 10.1097/PAS.0000000000000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Hamrouni A, Wolowiec D, et al. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-gamma and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110:296–304. doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- 10.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiss KA, Forde PM, Brahmer JR. Harnessing the power of the immune system via blockade of PD-1 and PD-L1: A promising new anticancer strategy. Immunotherapy. 2014;6:459–475. doi: 10.2217/imt.14.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: A randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 16. International Conference on Harmonisation Expert Working Group: Guidance for Industry: E6 Good Clinical Practice: Consolidated Guidance. Rockville, MD, Center for Drug Evaluation and Research, 1996.
- 17. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. 59th WMA General Assembly, Seoul, Korea, October 2008.
- 18.National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0; US Department of Health and Human Services. Bethesda, MD: National Institutes of Health; 2009. [Google Scholar]
- 19.Mahoney KM, Sun H, Liao X, et al. PD-L1 antibodies to its cytoplasmic domain most clearly delineate cell membranes in immunohistochemical staining of tumor cells. Cancer Immunol Res. 2015;3:1308–1315. doi: 10.1158/2326-6066.CIR-15-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 21.Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: Report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–4695. doi: 10.1182/blood-2010-10-299487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olsen EA, Whittaker S, Kim YH, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: A consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598–2607. doi: 10.1200/JCO.2010.32.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawano Y, Moschetta M, Manier S, et al. Targeting the bone marrow microenvironment in multiple myeloma. Immunol Rev. 2015;263:160–172. doi: 10.1111/imr.12233. [DOI] [PubMed] [Google Scholar]
- 25.Ansell SM, Hurvitz SA, Koenig PA, et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2009;15:6446–6453. doi: 10.1158/1078-0432.CCR-09-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]