Abstract
Purpose
Cisplatin is widely used but highly ototoxic. Effects of cumulative cisplatin dose on hearing loss have not been comprehensively evaluated in survivors of adult-onset cancer.
Patients and Methods
Comprehensive audiological measures were conducted on 488 North American male germ cell tumor (GCT) survivors in relation to cumulative cisplatin dose, including audiograms (0.25 to 12 kHz), tests of middle ear function, and tinnitus. American Speech-Language-Hearing Association criteria defined hearing loss severity. The geometric mean of hearing thresholds (0.25 to 12 kHz) summarized overall hearing status consistent with audiometric guidelines. Patients were sorted into quartiles of hearing thresholds of age- and sex-matched controls.
Results
Increasing cumulative cisplatin dose (median, 400 mg/m2; range, 200 to 800 mg/m2) was significantly related to hearing loss at 4, 6, 8, 10, and 12 kHz (P trends, .021 to < .001): every 100 mg/m2 increase resulted in a 3.2-dB impairment in age-adjusted overall hearing threshold (4 to 12 kHz; P < .001). Cumulative cisplatin doses > 300 mg/m2 were associated with greater American Speech-Language-Hearing Association–defined hearing loss severity (odds ratio, 1.59; P = .0066) and worse normative-matched quartiles (odds ratio, 1.33; P = .093) compared with smaller doses. Almost one in five (18%) patients had severe to profound hearing loss. Tinnitus (40% patients) was significantly correlated with reduced hearing at each frequency (P < .001). Noise-induced damage (10% patients) was unaffected by cisplatin dose (P = .59). Hypertension was significantly related (P = .0066) to overall hearing threshold (4 to 12 kHz) in age- and cisplatin dose–adjusted analyses. Middle ear deficits occurred in 22.3% of patients but, as expected, were not related to cytotoxic drug dosage.
Conclusion
Follow-up of adult-onset cancer survivors given cisplatin should include routine inquiry for hearing status and tinnitus, referral to audiologists as clinically indicated, and hypertension control. Patients should be urged to avoid noise exposure, ototoxic drugs, and other factors that further damage hearing.
INTRODUCTION
The current 5-year relative survival rate for all cancers taken together approximates 66%.1 As a result, there are 14.5 million cancer survivors in the United States. This number will increase to 19 million by 2024,2 with 97% representing survivors of adult-onset cancer. Given these increasing numbers, in-depth investigations of treatment toxicities that affect functional status, such as cisplatin-related hearing loss and tinnitus, are increasingly important.3 Cisplatin is one of the most ototoxic drugs in clinical use, causing permanent, bilateral sensorineural hearing loss in substantial numbers of patients, with many experiencing permanent tinnitus.4-6 Nonetheless, few comprehensive audiometric data exist for cisplatin-associated hearing loss in adult-onset cancer survivors. Several investigations of patients with head and neck cancer were confounded by cranial radiotherapy7-9; limitations of other studies included small numbers,6 concomitant vincristine,6,10,11 and restriction of audiometric testing to just a few frequencies.10,11 To our knowledge, only one series evaluated hearing loss in terms of cumulative cisplatin dose,11 and none included audiometric assessments of noise-induced damage, middle ear function, or evaluation of speech processing.4,6-11
To fill important gaps in these areas, we conducted comprehensive audiometric testing in relation to cumulative cisplatin dose in 488 men with adult-onset germ cell tumors (GCT), testing all frequencies between 0.25 and 12 kHz and evaluating audiologically defined noise-induced damage, speech processing, tinnitus, patient-reported outcomes, and middle ear function.
PATIENTS AND METHODS
Patients
All patients were enrolled in the Platinum Study, which includes eight cancer centers in the United States and Canada.12-15 Eligibility criteria included: men with a diagnosis of histologically or serologically confirmed GCT, age younger than 50 years at diagnosis and age 18 years or older at study consent, treatment with cisplatin-based chemotherapy, and no subsequent salvage chemotherapy. Study procedures were approved by the Human Subjects Review Board at each institution. This report covers all 488 patients who completed audiometric testing through April 16, 2015.
Data Abstracted From Medical Records
For each patient, standardized forms were used to collect demographic and clinical data, including treatment information.16,17 Dose data were collected for cisplatin, etoposide, and bleomycin, with cumulative dose available for 95% of patients included in dose-response analyses. For the remaining 5%, cumulative dose was imputed from the median dose administered to all patients given the same regimen. Of all patients, 88% received either three to four cycles of bleomycin, etoposide, and cisplatin (BEP), or four cycles of etoposide and cisplatin (EP) at currently recommended standard doses.
Audiometric Testing
Pure-tone air conduction thresholds were obtained bilaterally for each patient at frequencies of 0.25 to 12 kHz as in prior studies,18-20 covering the speech frequency range, including those important for perceiving vowels and consonants. Frequencies of 10 and 12 kHz were included, given their importance in the early diagnosis of pediatric cisplatin-induced hearing loss.21-23 Otoscopy and bone-conduction thresholds (0.25 to 4 kHz) evaluated middle ear function. Speech reception thresholds (SRTs), which use speech stimuli consisting of two-syllable words, quantified speech processing.
Classification of hearing loss and assessment of severity.
International American Speech-Language-Hearing Association (ASHA) criteria defined hearing loss as a hearing threshold at any frequency (0.25 to 12 kHz) that exceeded 20 dB for either ear.24-26 ASHA criteria defined hearing loss severity as follows: mild: 21 to 40 dB; moderate: 41 to 55 dB; moderately severe: 56 to 70 dB; severe: 71 to 90 dB; and profound: > 90 dB; for at least one tested frequency for either ear.24
Statistical analysis.
Cumulative cisplatin dose (mg/m2) was compared with the air conduction threshold at each frequency in the 0.25 to 12 kHz range, with statistical significance defined as P < .05 for dose in the linear regression model: hearing threshold = dose + age at audiometry. No evidence for nonlinearity of the cisplatin dose-response relationship was observed, as evaluated by comparisons of the linear model with cubic and quadratic models at each frequency (P > .05 in all cases).
To derive a summary measure of each patient’s overall hearing status across all frequencies taken together (0.25 to 12 kHz), we calculated the geometric mean of air conduction hearing thresholds, consistent with previous audiometric guidelines.27 For each patient i, the geometric mean Yi was calculated using standard methods28 by taking the arithmetic mean of the natural log-transformed hearing threshold, di, from n frequencies and then using exponentiation to return the computation to the original dB scale (ie, log-average):
This summary measure Yi (overall hearing threshold [0.25 to 12 kHz]) was normally distributed on the basis of histogram shape, and the Shapiro-Wilk test29 for departure from normality was not significant (P = .24). The summary measure Yi was tested for association with age at diagnosis or age at audiometry using a linear regression model: overall hearing threshold = age. Asymmetry was defined as a > 20-dB difference in geometric means between ears.
The effect of cumulative etoposide dose (mg/m2) and bleomycin dose (units) on the geometric mean of air conduction thresholds (4 to 12 kHz) was evaluated with and without cumulative cisplatin dose as a covariate. Statistical significance was defined as P < .05 for the dose term in the following linear regression model: hearing threshold = dose + age at audiometry (+ doseCisplatin). Standard audiometric definitions30 defined noise-induced hearing loss as hearing thresholds at 6 and 8 kHz at least 5 dB less (better hearing) than thresholds at middle frequencies (3 to 4 kHz).
Comparisons with normative population.
Given the relationship between changes in hearing thresholds with increasing age,31 results were compared with a large unscreened male reference population32 as applied in a previous study.10,33 Although several investigations described audiometrically defined hearing patterns in male populations,34,35 only Engdahl et al32 provided the quartiles of hearing thresholds (4, 6, 8 kHz) for a broad age range (eg, 20 to 29, 30 to 39, 40 to 49, 50 to 59, and 60 to 69 years). For frequencies for which we observed statistically significant relationships between increasing cumulative cisplatin dose and increasing (worse) hearing thresholds (4, 6, 8, 10, 12 kHz), normative data were available for 4, 6, and 8 kHz.32
Statistical analysis.
For each patient, we compared the geometric mean of hearing thresholds (4, 6, 8 kHz) to the expected geometric mean in the age-specific normative sample for 25th, 50th, and 75th percentiles.32 Each patient was allocated to the respective quartile (1 to 4) of the reference population, with quartile 4 representing the most severe hearing impairment. Cumulative cisplatin dose groups (≤ 300 mg/m2 and > 300 mg/m2) were tested for association with age-matched normative quartiles (quartile = dose group) and with ASHA-defined severity classes (severity class = dose group + age at audiometry) by ordinal regression. The 300 mg/m2 cut point was chosen, given its correspondence to the most commonly used study regimen (BEP), with the standard three cycles resulting in a cumulative cisplatin dose of 300 mg/m2.
Patient-Reported Outcomes
Patients completed questionnaires concerning neurotoxic symptoms, lifestyle habits, comorbidities, and medication use.36 The Scale for Chemotherapy-Induced Neurotoxicity (SCIN), validated in testicular cancer survivors (TCS) given cisplatin-based chemotherapy,37 was used to query tinnitus and hearing; this was supplemented with validated hearing questions from Ventry and Weinstein38 regarding noise exposure, hearing aid use, and problems hearing words or language in crowds.
Statistical analyses.
SCIN results, smoking status (current smoker, ever smoker), and hypertension (defined as prescription medication for hypertension) were compared with the overall hearing threshold (4 to 12 kHz) and SRT. To test whether each variable (response) was associated with hearing thresholds, we fit the following linear regression model: overall hearing threshold = response + age at audiometry. All statistical models were fit using R version 3.1.1 (http://www.R-project.org/). Plots were constructed using the R package ggplot2.39 SCIN results and supplemental hearing-related questions were also tested for association with normative age-defined quartiles. For all analyses, statistical significance was defined as P < .05 (two-sided).
RESULTS
Median age at diagnosis was 31 years (range, 15 to 49 years), and median interval between chemotherapy and audiometry was 4.25 years (range, 1 to 30.3 years; Table 1). Chemotherapy consisted largely of BEP (60.5%) or EP (32.0%). Median cumulative cisplatin dose was 400 mg/m2 (range, 198 to 800 mg/m2). Hypertension (with prescription medication) was reported by 12.3% of patients; 30.5% and 5.9% of patients were former and current smokers, respectively.
Table 1.
Demographic Features, Clinical Characteristics, and Patient-Reported Outcomes for 488 Male Germ Cell Tumor Survivors at the Time of Enrollment Onto the Platinum Study
| Characteristic | No. (%) |
|---|---|
| Total patients | 488 |
| Age at GCT diagnosis, years | |
| Median (range) | 31 (15-49) |
| < 20 | 24 (4.9) |
| 20-29 | 187 (38.3) |
| 30-39 | 182 (37.3) |
| 40-49 | 95 (19.5) |
| Age at audiometry, years | |
| Median (range) | 38 (20-68) |
| 20-29 | 88 (18.0) |
| 30-39 | 180 (36.9) |
| 40-49 | 138 (28.3) |
| 50-59 | 75 (15.4) |
| 60-69 | 7 (1.4) |
| Time from chemotherapy to audiometry, months | |
| Median (range) | 51 (5-364) |
| < 24 | 105 (21.8) |
| 24-47 | 121 (25.2) |
| 48-71 | 72 (15.0) |
| 72-119 | 81 (16.8) |
| ≥ 120 | 102 (21.2) |
| Race | |
| White | 428 (87.7) |
| Nonwhite | 54 (11.1) |
| Not available | 6 (1.2) |
| Calendar year of diagnosis | |
| 1980-1989 | 5 (1.0) |
| 1990-1999 | 57 (11.7) |
| 2000-2009 | 207 (42.4) |
| 2010-2015 | 219 (44.9) |
| Histology | |
| Seminoma | 139 (28.5) |
| Nonseminoma/mixed GCT | 344 (70.5) |
| GCT, not otherwise specified | 5 (1.0) |
| Clinical stage | |
| I | 138 (28.3) |
| II | 184 (37.7) |
| IIIa | 102 (20.9) |
| Otherb | 63 (12.9) |
| Not specified | 1 (0.2) |
| Site of GCT | |
| Testis | 435 (89.1) |
| Extragonadal | 52 (10.7) |
| Not specified | 1 (0.2) |
| Chemotherapy regimen | |
| BEP: total cyclesc | 295 (60.5) |
| 2 | 8 (1.6) |
| 3 | 183 (37.5) |
| 4 | 99 (20.3) |
| 5+ | 5 (1.0) |
| EP: total cyclesd | 156 (32.0) |
| ≤ 3 | 1 (0.2) |
| 4 | 150 (30.7) |
| 5+ | 5 (1.0) |
| Cisplatin, etoposide, ifosfamide: total cyclese | 21 (4.3) |
| 3 | 1 (0.2) |
| 4 | 17 (3.5) |
| 5+ | 3 (0.6) |
| Other cisplatin-based regimens: total cyclesf | 16 (3.3) |
| 3 | 2 (0.4) |
| 4 | 14 (2.9) |
| Cumulative dose of cisplatin, all patients, mg/m2g | |
| < 300 | 25 (5.1) |
| 300 | 165 (33.8) |
| 301-399 | 22 (5.0) |
| 400 | 254 (52.1) |
| > 400 | 22 (4.5) |
| Education | |
| High school or less | 48 (9.8) |
| After high school but not collegeh | 96 (19.7) |
| College/university graduate | 222 (45.5) |
| Postgraduate level | 112 (23.0) |
| Other/prefer not to say/not answered | 10 (2.0) |
| Smoking status | |
| Current smoker | 29 (5.9) |
| Former smoker | 149 (30.5) |
| Never smoker | 294 (60.2) |
| Not answered | 16 (3.3) |
| Hypertension and on prescription medication | 60 (12.3) |
| Noise exposure | |
| None | 271 (55.5) |
| Work-related only | 83 (17.0) |
| Non–work-related only | 53 (11.1) |
| Both | 73 (15.0) |
| Otheri | 7 (1.4) |
| Tinnitus | |
| Not at all | 287 (58.8) |
| A little | 115 (23.6) |
| Quite a bit | 38 (7.8) |
| Very much | 39 (8.0) |
| Not answered | 9 (1.8) |
| Reduced hearing | |
| Not at all | 335 (68.7) |
| A little | 108 (22.1) |
| Quite a bit | 24 (4.9) |
| Very much | 12 (2.5) |
| Not answered | 9 (1.8) |
| Problems hearing in crowds | |
| Yes | 145 (29.7) |
| No | 316 (64.8) |
| Don’t know/not sure | 20 (4.1) |
| Not answered | 7 (1.4) |
| Require hearing aid | |
| No | 476 (97.5) |
| In one ear | 1 (0.2) |
| In both ears | 5 (1.0) |
| Not answered | 6 (1.2) |
Abbreviations: BEP, bleomycin, etoposide, and cisplatin; EP, etoposide, and cisplatin; GCT, germ cell tumor; IV, intravenously.
Includes IIIc (n = 16).
Includes 59 patients for whom data were indicated as unavailable/not applicable and eight for whom this variable was not available/blank.
Median cumulative cisplatin doses among patients given three and four cycles of BEP were 300 mg/m2 (range, 272 to 400 mg/m2) and 400 mg/m2 (range, 198 to 600 mg/m2), respectively. For all BEP-treated patients, the median cumulative cisplatin dose was 300 mg/m2 (range, 198 to 800 mg/m2). Corresponding median doses of etoposide were 1,500 mg/m2 (range, 1,014 to 1,677 mg/m2), 2,000 mg/m2 (range, 500 to 4,645 mg/m2), and 1,500 mg/m2 (range, 500 to 4,645 mg/m2), respectively, with median doses of bleomycin 270 units (range, 64 to 330 units), 360 units (range, 60 to 360 units), and 270 units (range, 60 to 540 units), respectively. Of all men treated with BEP, 215 received standard dosages for each cycle (bleomycin 30 units IV weekly; etoposide 100 mg/m2 IV once per day × 5 days; cisplatin 20 mg/m2 IV once per day × 5 days), and 80 received a modified dose for at least one cycle.
Median cumulative dose of cisplatin among patients given four cycles of EP was 400 mg/m2 (range, 400 to 600 mg/m2), and among all patients given EP it was 400 mg/m2 (range, 200 to 600 mg/m2). Corresponding median doses of etoposide were 2,000 mg/m2 (range, 500 to 3,000 mg/m2) and 2,000 mg/m2 (range, 500 to 3,000 mg/m2), respectively. Of men receiving EP, 99 received standard dose (etoposide 100 mg/m2 IV once per day × 5 days, cisplatin 20 mg/m2 IV once per day × 5 days), and 57 received a modified dose for at least one cycle.
Median cumulative dose of cisplatin among all men receiving this regimen was 400 mg/m2 (range, 300 to 800 mg/m2). Corresponding median doses of etoposide and ifosfamide were 1,500 mg/m2 (range, 1,000 to 2,000 mg/m2) and 24 g/m2 (range, 6 to 30 g/m2), respectively. Of men receiving cisplatin, etoposide, and ifosfamide, 14 received standard doses (cisplatin 20 mg/m2 IV once per day × 5 days, etoposide 75 mg/m2 IV once per day × 5 days, ifosfamide 1.2 g/m2 IV once per day × 5 days), and seven received a modified dose for at least one cycle.
Of the 16 patients, 11 received combination chemotherapy consisting of cisplatin and ifosfamide; one received cisplatin, etoposide, bleomycin, and ifosfamide. For the remaining four, other combinations of cisplatin-based chemotherapy were applied.
Median cumulative dose of cisplatin among all patients was 400 mg/m2 (range, 198 to 800 mg/m2).
This includes some college/university but without conferral of a degree (n = 76).
Includes one patient who answered “no” to job-related noise exposure but did not reply to question regarding other noise sources and six patients who answered “no” to other sources of noise exposure but did not reply to question regarding job-related noise.
Overall Hearing Loss
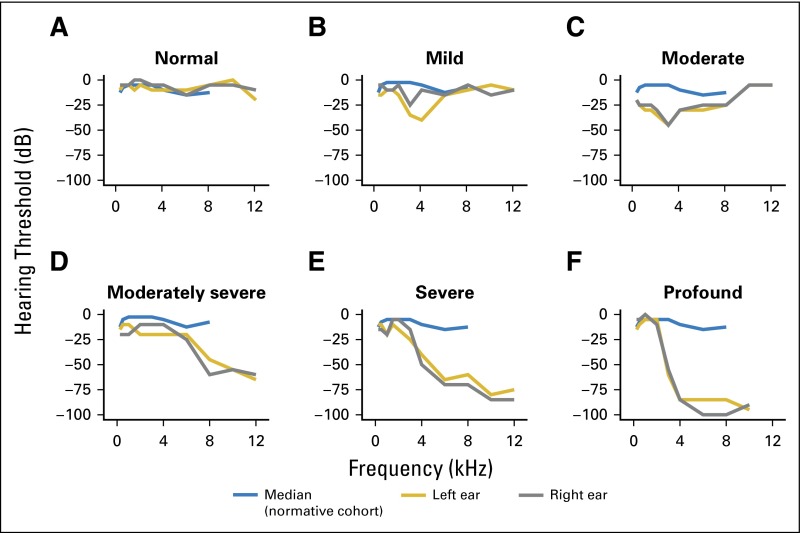
Audiogram shapes showed a largely high-frequency, sloping hearing loss, with substantial interindividual variation (Fig 1). Only 20% of patients had normal hearing (ie, flat audiogram; Fig 1A), whereas others had differing degrees of hearing loss, with ASHA-defined mild, moderate, moderately severe, or severe/profound hearing loss, in 25%, 16%, 21%, and 18%, respectively (Figs 1B to 1F).24 For two patients with ear asymmetry, data were averaged.
Fig 1.
Representative left- and right-ear audiograms with varying levels of severity defined by American Speech-Language-Hearing Association criteria24 in the Platinum Study cohort. Results shown here are derived from patients age 28 to 35 years. The median (blue line) is derived from the normative cohort of men in each patient's respective age group (20 to 29 or 30 to 39 years; Table 4 in Engdahl et al 200532). Hearing loss classification: (A) normal hearing, (B) sensorineural, (C) mixed (sensorineural and conductive), (D) sensorineural, (E) sensorineural, (F) sensorineural.
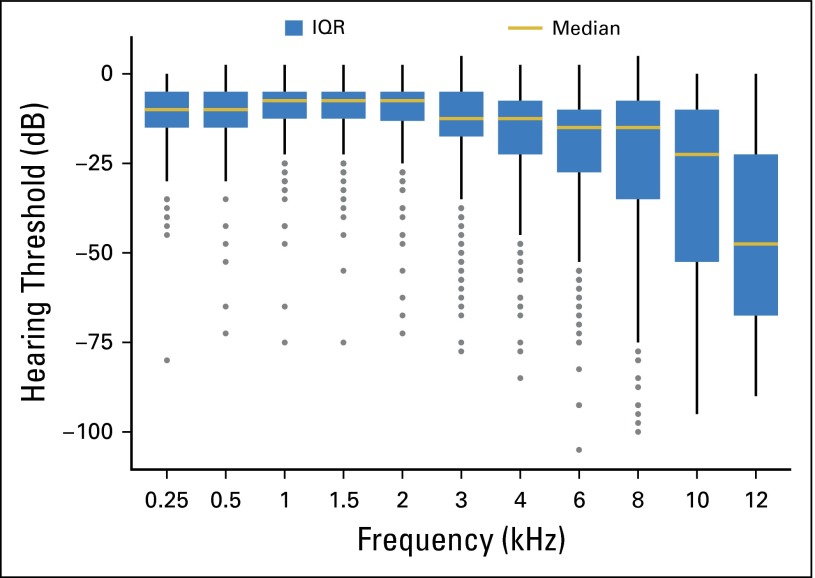
Most patients (388 of 488, 80%) had a hearing loss of > 20 dB (Fig 2).24 The overall pattern of kHz-specific hearing thresholds accentuated its high-frequency nature, with the largest reductions at 12 kHz. Age at either audiometry (P < .001) or GCT diagnosis (P < .001) strongly associated with impaired overall hearing threshold (0.25 to 12 kHz). Because these variables were highly correlated (R = 0.79), only the former was subsequently applied.
Fig 2.
Distribution of hearing threshold levels (decibels of hearing loss) at each audiometric frequency (Hz; N = 488 patients). The threshold level for each patient represents the mean of left- and right-ear audiogram thresholds. Boxes define the interquartile range (IQR), and the gold horizontal line represents the median. The upper whisker extends from the third quartile to the highest value that is within 1.5 × IQR. The lower whisker extends from the first quartile to the lowest value within 1.5 × IQR. Outliers beyond 1.5 × IQR are plotted as individual data points.
Cumulative Cisplatin Dose
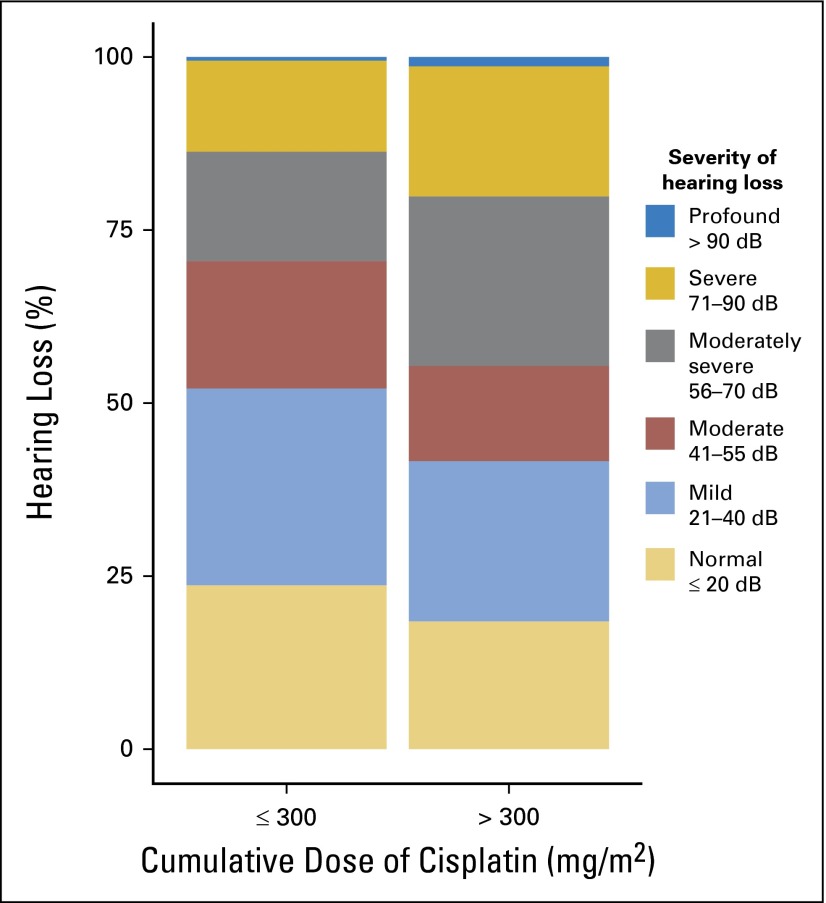
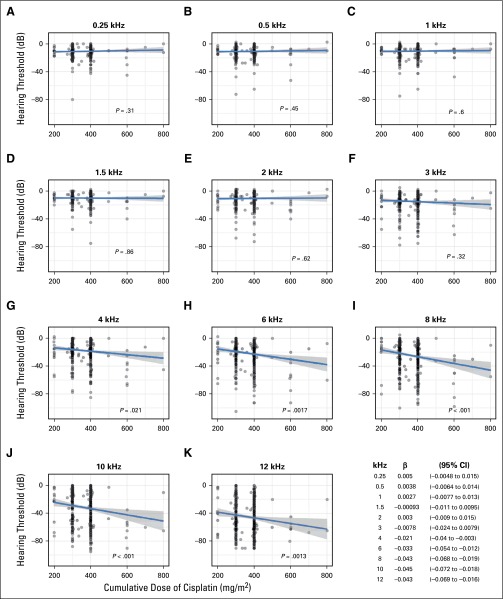
Statistically significant age-adjusted relationships between increasing cumulative cisplatin dose and increasing (worse) hearing thresholds (dB) existed for 4 kHz (P = .021), 6 kHz (P = .0017), 8 kHz (P < .001), 10 kHz (P < .001), and 12 kHz (P = .0013), but not for other frequencies (Appendix Fig A1, online only). Cumulative cisplatin doses > 300 mg/m2 were associated with increased ASHA severity classes24 compared with ≤ 300 mg/m2 after age adjustment (Fig 3; odds ratio [OR], 1.59; 95% CI, 1.14 to 2.21; P = .0066). Moderately severe to profound hearing loss occurred in 29.5% and 44.6% of patients, respectively, after ≤ 300 mg/m2 and > 300 mg/m2 of cisplatin. Dose group did not correlate with age (P = .24).
Fig 3.
Severity of American Speech-Language-Hearing Association–defined hearing loss24 according to cumulative cisplatin dose group (mg/m2).
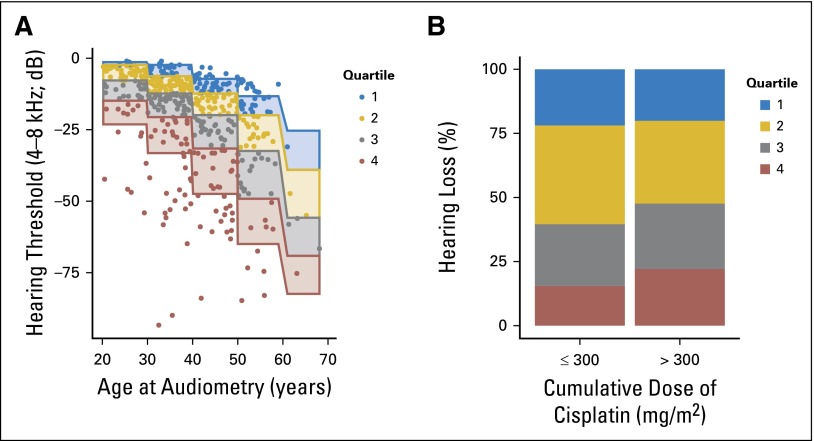
Comparisons to Normative Population
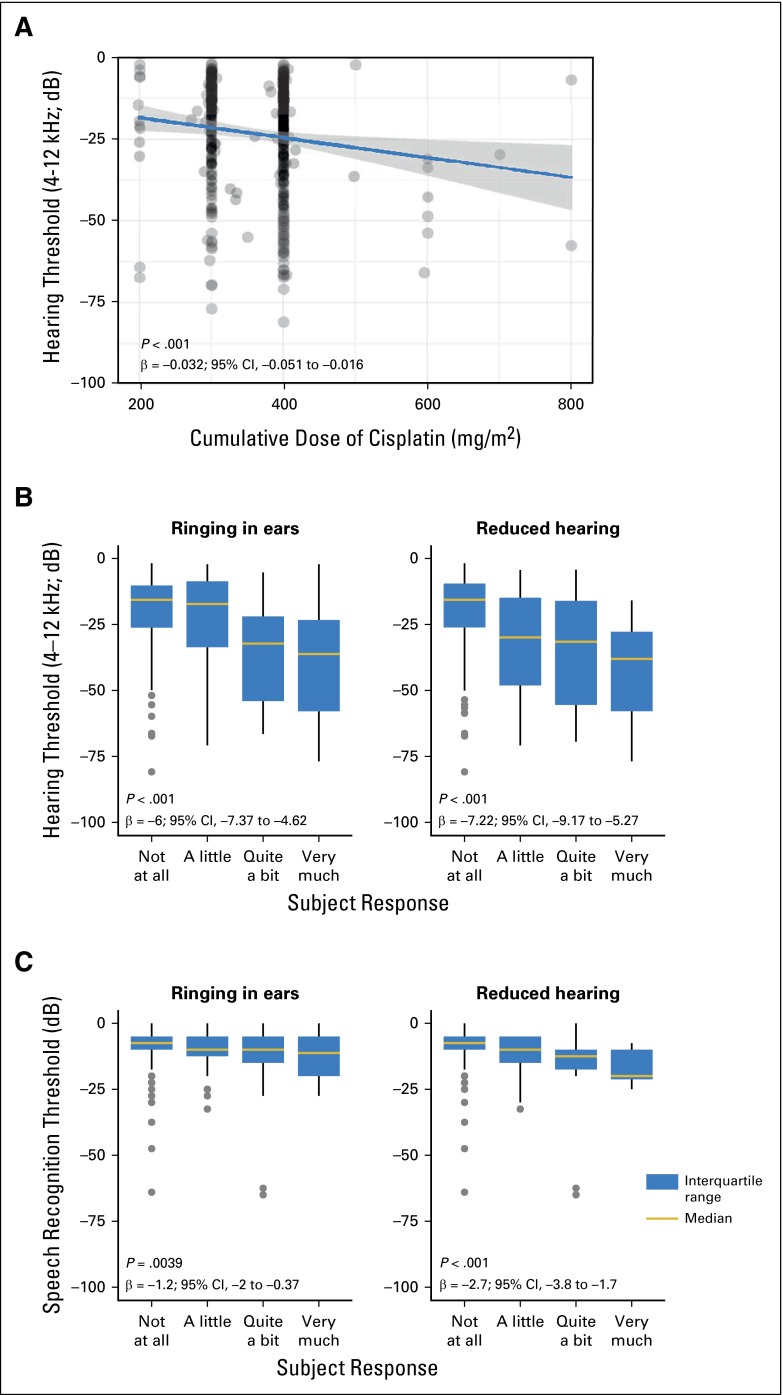
Figure 4A shows subject distribution by age and normative hearing quartile. Patients administered > 300 mg/m2 cisplatin were more likely to be in a higher quartile (worse hearing) than those given ≤ 300 mg/m2 (Fig 4B; OR, 1.33; 95% CI, 0.95 to 1.85; P = .093). For every 100-mg/m2 increase in cumulative cisplatin dose, a 3.2-dB decline in overall hearing threshold (4 to 12 kHz) occurred after age adjustment (Fig 5A; P < .001). Time from chemotherapy to audiometry correlated with worse overall hearing (R = 0.23; 95% CI, 0.14 to 0.32), likely a result of the strong positive correlation with age (R = 0.48; 95% CI, 0.41 to 0.55). On adjustment for both age and time since chemotherapy, we still observed for every 100-mg/m2 increase in cumulative cisplatin dose a 3.3-dB decline in overall hearing threshold (4 to 12 kHz; P < .001). In this multivariate model, time from chemotherapy was not significant (P = .42), whereas age was highly significant (P < .001).
Fig 4.
(A) Comparison of overall hearing threshold (4 to 8 kHz) among study subjects with quartiles of age-specific normative data. Quartiles 1-4 represent the following normative percentile ranges, respectively: 10% to 24.9%, 25% to 49.9%, 50% to 74.9%, and 75% to 90%. (B) Comparison of the two cumulative cisplatin dose groups with quartiles of age-specific normative data.
Fig 5.
(A) Comparison of overall hearing threshold (4 to 12 kHz) to cumulative cisplatin dose. The P value is for the dose term in the following linear regression model: dB = dose + age at audiometry. (B) Comparison of overall hearing threshold (4 to 12 kHz) to the Scale for Chemotherapy-Induced Long-Term Neurotoxicity items for ringing in ears and reduced hearing.37 The P value is for the response term in the following linear regression model: dB = response + age at audiometry. (C) Comparison of speech recognition threshold to the Scale for Chemotherapy-Induced Long-term Neurotoxicity items for ringing in ears and reduced hearing.37 The P value is for the response term in the following linear regression model: dB = response + age at audiometry.
Noise-Induced Hearing Loss
Of 388 patients with hearing loss (> 20 dB) at any frequency, 39 (10%) also displayed audiometrically defined noise damage. Risk did not differ for patients given a cumulative cisplatin dose of > 300 mg/m2 or ≤ 300 mg/m2 (P = .59), nor did noise damage affect normative quartile assignment (P = .42). Work-related noise exposure was associated with a noise notch in the audiogram (OR, 1.89; 95% CI, 0.96 to 3.69; P = .062), as observed by others.38
Conductive Hearing Loss (middle ear deficit)
Of all 488 patients, 58% (283) had pure sensorineural hearing loss, 0.4% (two) had pure conductive hearing loss, and 21% (103) had mixed hearing loss. As expected, no association with cumulative dose of cisplatin, etoposide, or bleomycin was evident for the 105 patients with conductive hearing loss (P = .78, 0.69, 0.42, respectively).
Other Risk Factors
In analyses adjusted for age and cisplatin dose, impaired overall hearing threshold (4 to 12 kHz) was significantly associated with hypertension (n = 60; P = .0066). No significant association was evident for current smoking (n = 29; P = .079) or ever smoking (current plus former; n = 178; P = .095).
Associations With Patient-Reported Outcomes
Approximately 30% and 40% of patients, respectively, reported reduced hearing or tinnitus (Table 1). Impaired overall hearing threshold (4 to 12 kHz) was strongly associated with degree of self-reported hearing loss (P < .001) and with tinnitus (P < .001) after age adjustment (Fig 5B). Statistically significant relationships between tinnitus and impaired hearing thresholds were observed at each frequency: 0.25 kHz (P = .012), 0.5 kHz (P = .0058), 1 kHz (P = .0019), 1.5 kHz (P < .001), 2 kHz (P < .001), 3 kHz (P < .001), 4 kHz (P < .001), 6 kHz (P < .001), 8 kHz (P < .001), 10 kHz (P < .001), and 12 kHz (P < .001). Similar results were obtained for SRT and tinnitus (P = .0039) and reduced hearing (P < .001; Fig 5C). Tinnitus did not correlate with time since chemotherapy (P = .17)
DISCUSSION
To our knowledge, this is the largest and most comprehensive study of cisplatin-associated ototoxicity in survivors of adult-onset cancer, including quantitative comparisons of frequency-specific audiometric findings with patient-reported outcomes. New findings include statistically significant associations between increasing cumulative cisplatin dose and hearing loss at each of 4, 6, 8, 10, and 12 kHz and with severity of hearing loss defined by ASHA criteria.24 Highly significant associations between hearing loss at each kHz frequency and tinnitus were observed, in addition to significant correlations between SRT and tinnitus and self-reported hearing loss. Cumulative cisplatin dose was not related to audiometrically defined noise-induced hearing loss. Impaired overall hearing threshold (4 to 12 kHz) was significantly associated with hypertension but not smoking status. Middle ear deficits were observed.
There are few audiometric data in survivors of adult-onset cancer treated with cisplatin-based chemotherapy without cranial radiotherapy. Among 86 TCS, Bokemeyer et al6 tested frequencies of 0.5 to 8 kHz at a median of 4.8 years after cisplatin-based chemotherapy. Hearing loss was reported in 66% of patients, but frequency-specific associations with cumulative cisplatin dose were not examined. Glendenning et al11 evaluated frequencies of 1, 2, 4, and 8 kHz among 260 TCS, with cumulative cisplatin dose associated with hearing loss only at 8 kHz. An investigation of several hundred cisplatin-treated TCS in Norway restricted audiometric testing to 4 kHz and compared hearing loss with a normative population.10 Although TCS given larger numbers of cycles of cisplatin-based chemotherapy were assigned to greater hearing-impaired normative quartiles, the effect of cumulative cisplatin dose was not addressed.10
More than 90% of our 488 patients received modern cisplatin-based chemotherapy consisting largely of BEP or EP; thus, results are relevant to current practice. Hearing deficits were observed throughout the speech perception range, including the higher frequencies reported in pediatric studies,21,23,40,41 likely accounting for the strong correlation we observed for overall hearing threshold (4 to 12 kHz) and SRT and patient-reported hearing deficits (Fig 5B).
One in five patients had severe or profound hearing loss, a level at which hearing aids are typically recommended, but few used them, likely because of high cost, lack of insurance coverage, low performance/price ratio, or appearance.42 It is noteworthy that awareness of this problem and the patient’s acceptance of hearing loss typically increases hearing aid use.42 An additional 37% of patients with moderate or moderately severe ASHA-defined hearing loss would benefit from additional audiological follow-up, as clinically indicated. Worsening of audiometrically assessed cisplatin-associated hearing loss with time has been reported in childhood cancer survivors43,44 but has not been longitudinally studied in survivors of adult-onset cancer to our knowledge. The effect of cumulative cisplatin dose on hearing loss in our cross-sectional investigation remained statistically significant for many decades (P < .001), although the effect of increasing age was even stronger. Because higher frequencies are also disproportionately affected by age-related hearing loss, it will be critical to evaluate the extent to which survivors of adult-onset cancer given cisplatin-based chemotherapy may experience accelerated age-related sensory deficits.
Tinnitus
Tinnitus was significantly associated with impaired hearing at all frequencies, including 10 and 12 kHz, and reflects neural changes in the brain’s central auditory system.45,46 Hearing loss perturbs normal input to this area, a mismatch between excitatory and inhibitory networks occurs, and neurons underlying sound perception become abnormally activated, even without external stimulation. In some patients, tinnitus becomes debilitating as a sensory, anxiety-producing, and communication deficit.47 Few treatments are effective, with most relying on behavioral modification.47
Noise Damage
The presence of noise notches in audiograms has not been previously evaluated in cancer survivors given cisplatin6,10,11 or cranial radiotherapy.4,7-9 The proportion (10%) of our patients with audiograms suggestive of noise damage is comparable to, or slightly less than, normative data assembled by the US Centers for Disease Control and Prevention, which reports that 17% of adults (20 to 69 years) demonstrate permanent noise-induced hearing damage.48 Our slightly lower prevalence may reflect the younger age distribution compared with population norms.
Conductive Hearing Loss (middle ear deficits)
The prevalence of conductive hearing loss is approximately 8% in adults without cancer31,32,49 and generally attributed to infection- or treatment- or age-related damage to sound conduction mechanisms.50 An explanation for the increased prevalence we observed is not readily apparent and, as expected, was not related to cytotoxic drug exposure dosage levels.
Comment
Strengths of our study include large numbers of patients, detailed treatment data, thorough hearing evaluations, adjustments for age-related hearing loss, and consideration of covariates. Our quantitative assessment of cumulative cisplatin dose and frequency-specific hearing loss is not confounded by vincristine6,10,11 or cranial radiotherapy.4,7-9 Although pediatric investigations of cisplatin-induced hearing loss21-23,51,52 have yielded valuable perspective on classification approaches, pediatric scales do not take into account age-related hearing loss. To adjust for this effect, we not only included age in multivariate analyses but also compared hearing thresholds with age-matched normative data, because as in previous studies of ototoxicity in adult-onset cancer survivors, baseline hearing measures were not available.6,8,9,53 Because suitable data were unavailable for a North American cohort, we used normative data from a National Institutes of Health–sponsored study of 40,541 Norwegian men (97% white).32 Although we found expected relationships between higher cisplatin dose and assignment to worse age-matched hearing quartiles, we were not able to adjust for potentially differing distributions of hypertension, smoking, and other potential confounders.54 Any differences, however, are unlikely to materially affect our conclusions.
Cancer Survivor Plans and Future Research
Although follow-up hearing assessment guidelines exist for children given cisplatin-based chemotherapy,51 we found no similar recommendations for adult-onset cancer survivors. For children, a complete audiological evaluation at entry into long-term follow-up is recommended by the Children’s Oncology Group,51 with annual testing if hearing loss is detected or as recommended by an audiologist, as well as yearly questioning about hearing status. Similarly, for adult-onset cancer survivors after cisplatin-based chemotherapy, our findings suggest that health care providers should at a minimum annually query patients about hearing status, consulting with audiologists as indicated. Patients should also be urged to avoid noise exposure, ototoxic drugs, and other factors that may further damage hearing. As recommended by national regulatory agencies for the general population,55,56 patients should be advised to wear hearing protection in noisy environments and take advantage of new, digital hearing aids and other innovative auditory therapies that are emerging.57 Given the significant relationship we found between hypertension and hearing loss and reported in other studies for tobacco use,10,11 health care providers should monitor blood pressure and encourage smoking cessation.
Because alterations in the highly successful GCT regimens are unlikely, our results point to the importance of ongoing research aimed at the identification of genetic variants associated with cisplatin-related ototoxicity.58 It is possible that genomic analysis may be able to eventually identify patients with newly diagnosed testicular cancer susceptible to ototoxicity. These findings could affect decisions with regard to the administration of adjuvant chemotherapy for high-risk clinical stage I disease or adjuvant chemotherapy after initial orchiectomy and retroperitoneal lymph node dissection with positive lymph nodes to prevent recurrence.59,60
Acknowledgment
Presented in part at the 51st Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 29-June 2, 2015. We thank Shannon Salvog, MA (University of South Florida) and M. Lisa Miller (Indiana University) for administrative support.
Appendix
The Platinum Study Group consists of Howard D. Sesso (Brigham and Women’s Hospital, Boston, MA); Clair J. Beard and Stephanie Curreri (Dana-Farber Cancer Institute); Lawrence H. Einhorn, Lois B. Travis, Mary Jacqueline Brames, Somer Case-Eads, and Shirin Ardeshir-Rouhani-Fard (Indiana University); Jeri Kim (MD Anderson Cancer Center); Darren R. Feldman, Erin Jacobsen, and Deborah Silber (Memorial Sloan Kettering Cancer Center); Lynn Anson-Cartwright and Robert Hamilton (Princess Margaret Hospital); Nancy J. Cox (Vanderbilt University); M. Eileen Dolan (University of Chicago); David J. Vaughn, Linda Jacobs, Sarah Lena Panzer, and Donna Pucci (University of Pennsylvania); Debbie Baker, Cindy Casaceli, Chunkit Fung, and Eileen Johnson (University of Rochester); Heather E. Wheeler (Loyola University Chicago); and Robert D. Frisina (University of South Florida). The Platinum Study Group Advisory Committee consists of George Bosl (Memorial Sloan Kettering Cancer Center); Sophie D. Fossa (Norwegian Radium Hospital); Mary Gospodarowicz (Princess Margaret Hospital); and Leslie L. Robison (St. Jude Children’s Research Hospital). Enrolling sites for the Platinum Study as of April 16, 2015 were Memorial Sloan Kettering Cancer Center, Indiana University, Princess Margaret Hospital, University of Pennsylvania, University of Rochester, and Dana-Farber Cancer Institute.
Fig A1.
Comparison of hearing thresholds at each audiometric frequency to cumulative dose of cisplatin (mg/m2). The P value represents the dose term in the following linear regression model: hearing threshold = dose + age at audiometry. The dose term effect size (β) and 95% CIs for each frequency are shown at lower right.
Footnotes
For the Platinum Study Group. The members and affiliations of the Study Group are listed in the online-only Appendix.
Supported by National Cancer Institute Grant No. 1 R01 CA157823 (L.B.T.).
The views expressed in this article are the authors’ own and not an official position of the institutions or funder.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
See accompanying editorial on page 2687
AUTHOR CONTRIBUTIONS
Conception and design: Robert D. Frisina, Heather E. Wheeler, Sophie D. Fossa, Howard D. Sesso, Clair J. Beard, Lawrence H. Einhorn, M. Eileen Dolan, Lois B. Travis
Financial support: Lois B. Travis
Administrative support: Robert D. Frisina, Lois B. Travis
Provision of study materials or patients: Chunkit Fung, Darren R. Feldman, Robert Hamilton, David J. Vaughn, Clair J. Beard, Lawrence H. Einhorn
Collection and assembly of data: Heather E. Wheeler, Robert Hamilton, David J. Vaughn, Clair J. Beard, Amy Budnick, Eileen M. Johnson, Lois B. Travis
Data analysis and interpretation: Robert D. Frisina, Heather E. Wheeler, Sophie D. Fossa, Sarah L. Kerns, Chunkit Fung, Howard D. Sesso, Patrick O. Monahan, Darren R. Feldman, Clair J. Beard, Shirin Ardeshir-Rouhani-Fard, Steven E. Lipshultz, M. Eileen Dolan, Lois B. Travis
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Comprehensive Audiometric Analysis of Hearing Impairment and Tinnitus after Cisplatin-Based Chemotherapy in Survivors of Adult-Onset Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Robert D. Frisina
Patents, Royalties, Other Intellectual Property: University of South Florida-Tampa: biomedical engineering patents related to hearing loss; not related to cancer
Heather E. Wheeler
No relationship to disclose.
Sophie D. Fossa
No relationship to disclose.
Sarah L. Kerns
No relationship to disclose.
Chunkit Fung
Stock or Other Ownership: GlaxoSmithKline
Consulting or Advisory Role: Janssen Pharmaceuticals, Dendreon, Bayer HealthCare Pharmaceuticals, Novartis
Research Funding: Astellas Pharma (Inst)
Howard D. Sesso
No relationship to disclose.
Patrick O. Monahan
No relationship to disclose.
Darren R. Feldman
Consulting or Advisory Role: Bayer, Gilead Sciences (I), Seattle Genetics
Research Funding: Novartis
Robert Hamilton
Honoraria: Janssen Pharmaceuticals, AbbVie, Bayer HealthCare Pharmaceuticals, Astellas Pharma
Consulting or Advisory Role: Bayer HealthCare Pharmaceuticals
Research Funding: Janssen Pharmaceuticals
David J. Vaughn
Consulting or Advisory Role: Astellas Pharma
Clair J. Beard
No relationship to disclose.
Amy Budnick
No relationship to disclose.
Eileen M. Johnson
No relationship to disclose.
Shirin Ardeshir-Rouhani-Fard
No relationship to disclose.
Lawrence H. Einhorn
No relationship to disclose.
Steven E. Lipshultz
Leadership: Tenet Health Care: board member for clinically integrated network
Honoraria: EuroMeds (honorarium for 2014 Transatlantique En Oncologie Meeting)
Consulting or Advisory Role: Axio Research
Research Funding: Pfizer
Travel, Accommodations, Expenses: Regeneron Pharmaceuticals, Clinigen Group
M. Eileen Dolan
Research Funding: Insys Therapeutics
Expert Testimony: Pfizer
Travel, Accommodations, Expenses: Axiogenesis
Lois B. Travis
No relationship to disclose.
REFERENCES
- 1.Altekruse S, Kosary C, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2007. Bethesda, MD, National Cancer Institute, 2010. [Google Scholar]
- 2.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 3.Elena JW, Travis LB, Simonds NI, et al. Leveraging epidemiology and clinical studies of cancer outcomes: Recommendations and opportunities for translational research. J Natl Cancer Inst. 2013;105:85–94. doi: 10.1093/jnci/djs473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schell MJ, McHaney VA, Green AA, et al. Hearing loss in children and young adults receiving cisplatin with or without prior cranial irradiation. J Clin Oncol. 1989;7:754–760. doi: 10.1200/JCO.1989.7.6.754. [DOI] [PubMed] [Google Scholar]
- 5.Osanto S, Bukman A, Van Hoek F, et al. Long-term effects of chemotherapy in patients with testicular cancer. J Clin Oncol. 1992;10:574–579. doi: 10.1200/JCO.1992.10.4.574. [DOI] [PubMed] [Google Scholar]
- 6.Bokemeyer C, Berger CC, Hartmann JT, et al. Analysis of risk factors for cisplatin-induced ototoxicity in patients with testicular cancer. Br J Cancer. 1998;77:1355–1362. doi: 10.1038/bjc.1998.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theunissen EA, Zuur CL, Józwiak K, et al. Prediction of hearing loss due to cisplatin chemoradiotherapy. JAMA Otolaryngol Head Neck Surg. 2015;141:810–815. doi: 10.1001/jamaoto.2015.1515. [DOI] [PubMed] [Google Scholar]
- 8.Chen WC, Jackson A, Budnick AS, et al. Sensorineural hearing loss in combined modality treatment of nasopharyngeal carcinoma. Cancer. 2006;106:820–829. doi: 10.1002/cncr.21683. [DOI] [PubMed] [Google Scholar]
- 9.Zuur CL, Simis YJ, Lansdaal PE, et al. Ototoxicity in a randomized phase III trial of intra-arterial compared with intravenous cisplatin chemoradiation in patients with locally advanced head and neck cancer. J Clin Oncol. 2007;25:3759–3765. doi: 10.1200/JCO.2006.08.9540. [DOI] [PubMed] [Google Scholar]
- 10.Brydøy M, Oldenburg J, Klepp O, et al. Observational study of prevalence of long-term Raynaud-like phenomena and neurological side effects in testicular cancer survivors. J Natl Cancer Inst. 2009;101:1682–1695. doi: 10.1093/jnci/djp413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glendenning JL, Barbachano Y, Norman AR, et al. Long-term neurologic and peripheral vascular toxicity after chemotherapy treatment of testicular cancer. Cancer. 2010;116:2322–2331. doi: 10.1002/cncr.24981. [DOI] [PubMed] [Google Scholar]
- 12. Case-Eads SL, Travis LB, Fung C, et al: Psychotropic and stimulant medication (PSM) use among testicular cancer survivors (TCS): A multi-institutional clinical study of 680 patients given cisplatin-based chemotherapy. J Clin Oncol 34, 2015 (suppl; abstr 242)
- 13. Fung C, Feldman DR, Hamilton RJ, et al: Cardiovascular disease risk factors among cisplatin-treated testicular cancer survivors (TCS): A multicenter clinical study of U.S. and Canadian patients. J Clin Oncol 33, 2015 (suppl 7; abstr 391) [Google Scholar]
- 14. Wheeler HE, Travis LB, Budnick A, et al: Comprehensive characterization of cisplatin-related hearing loss in U.S. and Canadian testicular cancer survivors (TCS). J Clin Oncol 33, 2015 (suppl; abstr 9570) [Google Scholar]
- 15. Zaid MA, Sesso HD, Fung C, et al: Chronic health conditions following cisplatin-based chemotherapy: A multi-institutional study of 680 testicular cancer survivors (TCS). J Clin Oncol 33, 2015 (suppl; abstr 9519) [Google Scholar]
- 16.Travis LB, Andersson M, Gospodarowicz M, et al. Treatment-associated leukemia following testicular cancer. J Natl Cancer Inst. 2000;92:1165–1171. doi: 10.1093/jnci/92.14.1165. [DOI] [PubMed] [Google Scholar]
- 17.Travis LB, Holowaty EJ, Bergfeldt K, et al. Risk of leukemia after platinum-based chemotherapy for ovarian cancer. N Engl J Med. 1999;340:351–357. doi: 10.1056/NEJM199902043400504. [DOI] [PubMed] [Google Scholar]
- 18.Frisina ST, Mapes F, Kim S, et al. Characterization of hearing loss in aged type II diabetics. Hear Res. 2006;211:103–113. doi: 10.1016/j.heares.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guimaraes P, Frisina ST, Mapes F, et al: Progestin negatively affects hearing in aged women. Proc Natl Acad Sci U S A 103:14246-14249, 2006 . [DOI] [PMC free article] [PubMed]
- 20.Newman DL, Fisher LM, Ohmen J, et al. GRM7 variants associated with age-related hearing loss based on auditory perception. Hear Res. 2012;294:125–132. doi: 10.1016/j.heares.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knight KR, Kraemer DF, Winter C, et al. Early changes in auditory function as a result of platinum chemotherapy: Use of extended high-frequency audiometry and evoked distortion product otoacoustic emissions. J Clin Oncol. 2007;25:1190–1195. doi: 10.1200/JCO.2006.07.9723. [DOI] [PubMed] [Google Scholar]
- 22.Chang KW, Chinosornvatana N. Practical grading system for evaluating cisplatin ototoxicity in children. J Clin Oncol. 2010;28:1788–1795. doi: 10.1200/JCO.2009.24.4228. [DOI] [PubMed] [Google Scholar]
- 23.Abujamra AL, Escosteguy JR, Dall’Igna C, et al. The use of high-frequency audiometry increases the diagnosis of asymptomatic hearing loss in pediatric patients treated with cisplatin-based chemotherapy. Pediatr Blood Cancer. 2013;60:474–478. doi: 10.1002/pbc.24236. [DOI] [PubMed] [Google Scholar]
- 24. American Speech-Language-Hearing Association: Degree of Hearing Loss. www.asha.org/public/hearing/Degree-of-Hearing-Loss/
- 25.Clark JG. Uses and abuses of hearing loss classification. ASHA. 1981;23:493–500. [PubMed] [Google Scholar]
- 26. Le Prell CG, Hensley BN, Campbell KC, et al: Evidence of hearing loss in a “normally-hearing” college-student population. Int J Audiol 50:S21-S31, 2011 (suppl 21) [DOI] [PMC free article] [PubMed]
- 27.Casali JG, Robinson GS. Noise in Industry: Auditory Effects, Measurement, Regulation and Management. New York, NY, CRC Press, 2003 doi: 10.1201/9780203010457.pt2. [Google Scholar]
- 28.Crawley MJ. Statistics: An Introduction. New York, NY, Wiley, 2007. [Google Scholar]
- 29.Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965;52:591–611. [Google Scholar]
- 30.McBride DI, Williams S. Audiometric notch as a sign of noise induced hearing loss. Occup Environ Med. 2001;58:46–51. doi: 10.1136/oem.58.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cruickshanks KJ, Tweed TS, Wiley TL, et al. The 5-year incidence and progression of hearing loss: The epidemiology of hearing loss study. Arch Otolaryngol Head Neck Surg. 2003;129:1041–1046. doi: 10.1001/archotol.129.10.1041. [DOI] [PubMed] [Google Scholar]
- 32.Engdahl B, Tambs K, Borchgrevink HM, et al. Screened and unscreened hearing threshold levels for the adult population: Results from the Nord-Trøndelag Hearing Loss Study. Int J Audiol. 2005;44:213–230. doi: 10.1080/14992020500057731. [DOI] [PubMed] [Google Scholar]
- 33.Oldenburg J, Kraggerud SM, Brydøy M, et al. Association between long-term neuro-toxicities in testicular cancer survivors and polymorphisms in glutathione-s-transferase-P1 and -M1, a retrospective cross sectional study. J Transl Med. 2007;5:70. doi: 10.1186/1479-5876-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urben SL, Benninger MS, Gibbens ND. Asymmetric sensorineural hearing loss in a community-based population. Otolaryngol Head Neck Surg. 1999;120:809–814. doi: 10.1016/S0194-5998(99)70318-9. [DOI] [PubMed] [Google Scholar]
- 35.Allen PD, Eddins DA. Presbycusis phenotypes form a heterogeneous continuum when ordered by degree and configuration of hearing loss. Hear Res. 2010;264:10–20. doi: 10.1016/j.heares.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palomar García V, Abdulghani Martínez F, Bodet Agustí E, et al. Drug-induced otoxicity: Current status. Acta Otolaryngol. 2001;121:569–572. doi: 10.1080/00016480121545. [DOI] [PubMed] [Google Scholar]
- 37.Oldenburg J, Fosså SD, Dahl AA. Scale for chemotherapy-induced long-term neurotoxicity (SCIN): Psychometrics, validation, and findings in a large sample of testicular cancer survivors. Qual Life Res. 2006;15:791–800. doi: 10.1007/s11136-005-5370-6. [DOI] [PubMed] [Google Scholar]
- 38.Ventry IM, Weinstein BE. The hearing handicap inventory for the elderly: A new tool. Ear Hear. 1982;3:128–134. doi: 10.1097/00003446-198205000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Wickham H. Ggplot2 Elegant Graphics for Data Analysis. New York, NY: Springer Science; 2009. [Google Scholar]
- 40. Knight KR, Kraemer DF, Winter C, et al: Early changes in auditory function as a result of platinum chemotherapy: Use of extended high-frequency audiometry and evoked distortion product otoacoustic emissions. J Clin Oncol 25: 1190-1195, 2007. [DOI] [PubMed]
- 41.Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: Underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol. 2005;23:8588–8596. doi: 10.1200/JCO.2004.00.5355. [DOI] [PubMed] [Google Scholar]
- 42.Knudsen LV, Oberg M, Nielsen C, et al. Factors influencing help seeking, hearing aid uptake, hearing aid use and satisfaction with hearing aids: A review of the literature. Trends Amplif. 2010;14:127–154. doi: 10.1177/1084713810385712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertolini P, Lassalle M, Mercier G, et al. Platinum compound-related ototoxicity in children: Long-term follow-up reveals continuous worsening of hearing loss. J Pediatr Hematol Oncol. 2004;26:649–655. doi: 10.1097/01.mph.0000141348.62532.73. [DOI] [PubMed] [Google Scholar]
- 44.Al-Khatib T, Cohen N, Carret AS, et al. Cisplatinum ototoxicity in children, long-term follow up. Int J Pediatr Otorhinolaryngol. 2010;74:913–919. doi: 10.1016/j.ijporl.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 45.Kaltenbach JA. Tinnitus: Models and mechanisms. Hear Res. 2011;276:52–60. doi: 10.1016/j.heares.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeng FG. An active loudness model suggesting tinnitus as increased central noise and hyperacusis as increased nonlinear gain. Hear Res. 2013;295:172–179. doi: 10.1016/j.heares.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lockwood AH, Salvi RJ, Burkard RF. Tinnitus. N Engl J Med. 2002;347:904–910. doi: 10.1056/NEJMra013395. [DOI] [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention . Noise Induced Hearing Loss. Atlanta, GA, CDC, 2015. [Google Scholar]
- 49.Demeester K, van Wieringen A, Hendrickx J J, et al. Audiometric shape and presbycusis. Int J Audiol. 2009;48:222–232. doi: 10.1080/14992020802441799. [DOI] [PubMed] [Google Scholar]
- 50. Chole RA: Chronic otitis media, mastoiditis, and petrositis, in Flint PW Haughey BH, Lund VJ, et al (eds): Cummings Otolaryngology: Head and Neck Surgery (ed 6). Philadelphia, PA, Elsevier Mosby, 2015, pp 2139-2155. [Google Scholar]
- 51.Children’s Oncology Group . Long-Term Follow-up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancer. www.survivorshipguidelines.org/ [Google Scholar]
- 52.Brock PR, Knight KR, Freyer DR, et al. Platinum-induced ototoxicity in children: A consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J Clin Oncol. 2012;30:2408–2417. doi: 10.1200/JCO.2011.39.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Biro K, Noszek L, Prekopp P, et al. Characteristics and risk factors of cisplatin-induced ototoxicity in testicular cancer patients detected by distortion product otoacoustic emission. Oncology. 2006;70:177–184. doi: 10.1159/000093776. [DOI] [PubMed] [Google Scholar]
- 54.Cruickshanks KJ, Klein R, Klein BE, et al. Cigarette smoking and hearing loss: The epidemiology of hearing loss study. JAMA. 1998;279:1715–1719. doi: 10.1001/jama.279.21.1715. [DOI] [PubMed] [Google Scholar]
- 55.National Institute of Occupational Safety and Health Noise and Hearing Loss Prevention. www.cdc.gov/niosh/topics/noise/toolbox.html.
- 56.Occupational Safety & Health Administration Occupational Noise Exposure. https://www.osha.gov/SLTC/noisehearingconservation/index.html.
- 57. National Institute on Deafness and Communication Disorders: Hearing Aids. Bethesda, MD, National Institute on Deafness and Other Communication Disorders, 2016.
- 58.Travis LB, Fossa SD, Sesso HD, et al. Chemotherapy-induced peripheral neurotoxicity and ototoxicity: New paradigms for translational genomics. J Natl Cancer Inst. 2014;106:1–11. doi: 10.1093/jnci/dju044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Albers P, Albrecht W, Algaba F, et al. Guidelines on testicular cancer: 2015 Update. Eur Urol. 2015;68:1054–1068. doi: 10.1016/j.eururo.2015.07.044. [DOI] [PubMed] [Google Scholar]
- 60.Hanna NH, Einhorn LH. Testicular cancer--discoveries and updates. N Engl J Med. 2014;371:2005–2016. doi: 10.1056/NEJMra1407550. [DOI] [PubMed] [Google Scholar]