Abstract
PURPOSE
Pleuroparenchymal fibroelastosis (PPFE) is a rare form of interstitial pneumonia, characterized by elastotic fibrosis involving the pleura and subpleural parenchyma, predominantly in the upper lobes. PPFE can be either idiopathic or secondary and mostly occurs as a late complication of lung or hematopoietic stem cell transplantation (HSCT). The aim of this study was to evaluate the prevalence of secondary forms in transplant recipients.
METHODS
An expert thoracic radiologist retrospectively reviewed high-resolution computed tomography exams of 700 HSCT recipients and 53 lung transplant recipients from the database of the Radiology Department of S. Orsola-Malpighi Hospital dating back from 2007. For each case that radiologically fulfilled PPFE criteria, the following details were retrieved: clinical characteristics, laboratory and functional data, pathologic findings (obtained from one patient) and metabolic data (obtained from three patients).
RESULTS
Six cases clinically and radiologically consistent with PPFE were identified: two HSCT recipients (0.28%) and four lung transplant recipients (7.54%).
CONCLUSION
In this study, PPFE was strongly associated with lung transplants as a late complication, with a prevalence of 7.54%.
Pleuroparenchymal fibroelastosis (PPFE) is a rare interstitial pneumonia, recently included in the updated classification of idiopathic interstitial pneumonia (1), first described by Amitani et al. (2) as a fibrosis of the upper lobes and then recognized as a novel clinicopathologic entity by Frankel et al. (3), who coined the term “pleuroparenchymal fibroelastosis.”
Although the etiology is unknown, amongst possible causative factors such as infections, chemotherapy, genetic predisposition, solid tumors, inhalatory exposure, gastrointestinal reflux (4), PPFE mostly occurs as a late complication of lung or hematopoietic stem cell transplantation (HSCT) (5).
The histology of PPFE is characterized by an intense elastic fibrosis of the upper lobes involving visceral pleura and subpleural parenchyma with intra-alveolar involvement. There is a marked difference between the affected lung and the adjacent normal lung (3, 5–11). A biopsy of the lung is not recommended in advanced cases of the disease and patients with poor ventilatory reserve, as is the case with lung transplant recipients and HSCT patients (9, 12). Thus, in these cases, it has been already widely recognized that a definite diagnosis of PPFE could be made based on radiologic criteria: pleuroparenchymal thickening associated with subpleural fibrosis (traction bronchiectasis, moderate reticular abnormalities, superior hilar retraction) in the upper lobes, with involvement of the lower lobes being less marked or absent (9, 12). Pneumothorax (7), plathythorax (13), blotchy parenchymal consolidations and ground glass areas might be present, mainly in the upper zones (3, 9).
The aim of this study was to evaluate the prevalence of secondary PPFE, retrospectively reviewing all high-resolution computed tomography (HRCT) exams from HSCT and lung transplants. An evaluation of the prevalence of secondary PPFE based on HRCT exams has not been found in literature to date.
Methods
An expert thoracic radiologist (M.Z.) retrospectively reviewed HRCT exams of 53 lung transplant recipients and 700 HSCT recipients from the transplants database of S. Orsola-Malpighi Hospital, dating back from 2007, in order to select cases that radiologically fulfilled PPFE criteria.
All selected cases were evaluated in a multidisciplinary team discussion and the following details were retrieved: clinical characteristics, laboratory and functional data, pathologic findings (obtained from one patient) and metabolic data (18F-FDG positron emission tomography/computed tomography ([PET/CT]) obtained from three patients).
Approval was obtained from the institution to use the patients’ records for retrospective analysis and patient confidentiality was maintained.
During the process of case selection, on the basis of clinical and radiologic evidence, the “mimickers” of PPFE such as sarcoidosis, hypersensitivity pneumonitis, pneumoconiosis (14, 15), apical cap (8, 12), tuberculosis and tuberculosis pneumothorax treatment, aspergillosis (12, 16), radiation therapy, hemothorax, connective tissue disease with lupus, rheumatoid arthritis, and spondylitis were excluded (14).
A radiologic grading system was created, identifying four grades of PPFE, based on the extension of pleuroparenchymal and blotchy opacities, traction bronchiectasis (cylindrical or cystic) and volume reduction:
Grade 1: cylindrical bronchiectasis, pleuroparenchymal and blotchy opacities distributed in the upper zones, sparing the middle and lower zones, without consistent volume reduction and with blotchy ground glass areas possibly present.
Grade 2: cylindrical bronchiectasis, pleuroparenchymal and blotchy opacities distributed in the upper and middle zones, sparing the lower zones, without consistent volume reduction and with blotchy ground glass areas possibly present.
Grade 3: cystic bronchiectasis, pleuroparenchymal and blotchy opacities distributed in the upper or in both the upper and middle zones, sparing the lower zones, with consistent volume reduction because of the upper lobe collapse and with blotchy ground glass areas possibly present.
Grade 4: cystic bronchiectasis, pleuroparenchymal and blotchy opacities involving the entire lung, including the lower zones, with the collapse of the entire lung and without blotchy ground glass areas.
Results
Six cases clinically and radiologically consistent with PPFE were identified: two HSCT recipients (Case 5 who underwent allogeneic HSCT and Case 6 who underwent autologous HSCT) and four lung transplant recipients (Case 1 and 2 who underwent a left lung transplant; Case 3 and 4 who underwent a double lung transplant) of which, one case (Case 3) was confirmed as PPFE postbiopsy. These results showed a PPFE prevalence of 7.54% (95% confidence interval [CI], 0.43%–14.6%) in lung transplant recipients and 0.28% (95% CI, 0%–0.68%) in HSCT recipients.
Patients with secondary PPFE were mainly males (male:female ratio, 5:1) between 33 and 61 years of age (mean age 51 years, median 54 years), developing fibrosis after an average of 5.3 years (within 2–13 years) post-transplant (Table 1). Recurrent respiratory infections after the transplant occurred both in the autologous HSCT (once caused by cytomegalovirus [CMV] in Case 6) and in all lung transplants (in Cases 1, 2, 3 due to CMV) (Tables 1 and 2). Regarding the exposure to chemotherapeutic drugs, all lung transplant recipients underwent triple immunosuppressive therapy (corticosteroid, mycophenolate or azathioprine, cyclosporine or tacrolimus); HSCT recipients were treated with ICE (ifosfamide, carboplatin, and etoposide) for acute myeloid leukemia and with cyclophosphamide during the conditioning regime (Table 1).
Table 1.
Clinical characteristics
| Sex | Age (yrs) at PPFE diagnosis | Presenting symptoms | Pneumothorax | Smoking history | Allergies | ILD family history | Transplantation (age at transplantation) | Diagnosis before transplantation | PPFE time delay (yrs) after transplantation | CLAD/GVHD | Acute infections | Chemotherapy | Survival after the diagnosis of PPFE/death cause | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lung transplant | ||||||||||||||
| Case 1 | M | 60 | Dyspnea | No | Ex-smoker (40 packs/yr) | No | No | Left lung transplant (56 yrs) | IPF | 4 | CLAD mixed pattern (obstructive prevalence) | CMV recurrent infections | Mycophenolate, azathioprina, cyclosporine, tacrolimus | Dead/2 yrs/lung squamous cell carcinoma and maxillary sinus carcinoma |
| Case 2 | M | 56 | Dyspnea | No | Ex-smoker (40 packs/yr) | No | No | Left lung transplant (54 yrs) | IPF | 2 | RAS | CMV acute infection | Mycophenolate, azathioprina, cyclosporine | Dead/1 yr/respiratory failure |
| Case 3 | M | 52 | Dyspnea and dry cough | No | No | No | No | Double-lung transplant (48 yrs) | Idiopathic pulmonary hypertension | 4 | CLAD mixed pattern | CMV recurrent infections | Mycophenolate, cyclosporine, tacrolimus, rituximab | Dead/2 yrs/respiratory failure |
| Case 4 | M | 43 | None | No | No | Grass pollen allergy | No | Double-lung transplant (39 yrs) | Postembolic pulmonary hypertension | 4 | CLAD mixed pattern | Acute respiratory infection | Mycophenolate, tacrolimus | Alive/6 yrs |
| HSCT | ||||||||||||||
| Case 5 | M | 33 | Dyspnea | No | No | No | No | Allogeneic HSCT (20 yrs) | AML | 13 | Acute and chronic GVHD | No | ICE, cyclophosphamide | Dead/8 yrs/respiratory failure |
| Case 6 | F | 61 | Dyspnea, dry cough, and weight loss | Yes | No | No | No | Autologous HSCT (56 yrs) | AML | 5 | - | Recurrent respiratory infection (CMV once) | ICE, cyclophosphamide, busulfan | Alive/15 yrs |
PPFE, pleuroparenchymal fibroelastosis; ILD, interstitial lung disease; CLAD, chronic lung allograft dysfunction; GVHD, acute or chronic graft versus host disease; M, male; IPF, idiopathic pulmonary fibrosis; CMV, cytomegalovirus; RAS, restrictive allograft syndrome; HSCT, hematopoietic stem cell transplantation; AML, acute myeloid leukemia; ICE, ifosfamide-carboplatin-etopside; F, female; -, no data.
Table 2.
Laboratory data
| Autoantibodies | BAL | Aspergillus | CMV | Tuberculosis | Pulmonary physiology pattern | DLCO | Histology | |
|---|---|---|---|---|---|---|---|---|
| Lung transplant | ||||||||
| Case 1 | - | Increase in neutrophils and IL-8. Pseudomonas aeruginosa, Aspergillus terreus, Klebsiella pneumoniae, Corynebacterium striatum, Staphylococcus aureus | Aspergillus terreus | Positive | Negative | Mixed pattern | - | - |
| Case 2 | - | Increase in neutrophils, IL-6, IL-8, and TNF-α | Negative | Positive | Negative | Restrictive | - | - |
| Case 3 | Anti-HLA versus the transplanted lung | Increase in neutrophils; Haemophilus | Negative | Positive | Negative | Mixed pattern firstly, then a predominant restrictive pattern | - | PPFE |
| Case 4 | Negative | Increase in neutrophils and IL-8 | Negative | Negative | Negative | Mixed pattern, initially obstructive then also restrictive | - | - |
| HSCT | ||||||||
| Case 5 | Negative | - | - | - | Negative | Mixed pattern, initially | Decreased obstructive then also restrictive | - |
| Case 6 | Negative | - | - | Positive | Negative | Restrictive | - | - |
BAL, bronchoalveolar lavage; CMV, cytomegalovirus; DLCO, diffusing capacity of the lung for carbonmonoxide; -, no data; IL-6/8, interleukin 6/8; TNF-α, tumor necrosis factor-alpha; HLA, human leukocyte antigen; PPFE, pleuroparenchymal fibroelastosis; HSCT, hematopoietic stem cell transplantation.
The pulmonary physiology demonstrated a progressive loss of allograft function in all lung transplant recipients (Table 2): Case 2 showed a restrictive pattern and the others a mixed pattern, because fibrosis established itself alongside an airway disease (chronic obstructive pulmonary disease [COPD] in Case 1, bronchial anastomotic stenosis in Case 3, tracheomalacia in Case 4). The HSCT recipients demonstrated progressive lung decline after the transplantation; one case (Case 5) presented first with a mixed defect due to concomitant COPD, then with a predominant restrictive pattern. The last HRCT for each selected patient revealed asymmetric involvement of PPFE in three patients (Cases 4, 5, 6), two of which presented significantly different severity of disease between the two lungs (Tables 3 and 4, Fig. 1).
Table 3.
Imaging findings
| PPFE severity (last control) | ILD pattern in other zones | Progressive thorax cage flattening | Air trapping (exp) | PAH (mm) | Asbestos-related calcified pleural plaques | Other features | Imaging of the native lung | 18F-FDG PET/CT | Evolution | |
|---|---|---|---|---|---|---|---|---|---|---|
| Lung transplant | ||||||||||
| Case 1 | 1 | 0 | No | - | No | No | Apical paraseptal emphysema | Lung tumor and UIP | Uptake of the pleuroparenchymal opacities | Slow |
| Case 2 | 3 | 0 | No | - | No | No | Tracheobronchial diverticula | UIP | - | Rapid |
| Case 3 | 2 | 0 | No | Right middle lobe air trapping (bronchomalacia) | Pulmonary trunk: 32; RPA: 29; LPA: 28 | No | Apical paraseptal emphysema, left superior lobar bronchus stenosis with apical atelectasia. | Pulmonary hypertension (Eisenmenger’s syndrome) | Uptake of the pleuroparenchymal opacities | Rapid |
| Case 4 | R: 3 L: 1 |
0 | No | Peripheral air trapping and tracheomalacia | Pulmonary trunk: 39; RPA: 25; LPA: 26 | No | Bilateral upper lobes bronchovascular cysts | Postembolic pulmonary hypertension | - | Slow |
| HSCT | ||||||||||
| Case 5 | R: 4 L: 1 |
0 | No | No | Pulmonary trunk: 32; RPA: 21; LPA: 20 | No | No | X | - | Slow |
| Case 6 | R: 2 L: 3 |
0 | Yes | - | Pulmonary trunk: 3; RPA: 25; LPA: 25 | No | Left pulmonary artery trombosis; “tree in bud” in the lower left lobe; thin anterior pneumothorax | X | Uptake of the pleuroparenchymal opacities | Slow |
PPFE, pleuroparenchymal fibroelastosis; ILD, interstitial lung disease; exp, expiratory CT; PAH, pulmonary arterial hypertension; 18F-FDG PET/CT: fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography; UIP, usual interstitial pneumonia; RPA, right pulmonary artery; LPA, left pulmonary artery; L, left lung; R, right lung; HSCT, hematopoietic stem cell transplantation.
Table 4.
PPFE severity at the last control
| Case | Pleuroparenchymal opacities | Maximum pleuroparenchymal thickening (mm) | Bronchiectasis | Blotchy opacities | Blotchy GGO | Note | PPFE severity |
|---|---|---|---|---|---|---|---|
| 1 | Upper zones | 22 | Upper zones (cylindrical bronchiectasis) | Upper zones | None | - | 1 |
| 2 | Upper and mid-zones | 29 | Diffuse (cystic bronchiectasis in the upper lobes) | Diffuse and peribronchial | Peribronchial | Upper lobes volume reduction | 3 |
| 3 | Upper and mid-zones | 12 | Diffuse (cylindrical bronchiectasis with apical prevalence) | Upper zones | Upper zones | - | 2 |
| 4 | R: upper and mid-zones L: upper lobe |
R: 27 L: 6 |
R: diffuse (cystic bronchiectasis in the upper lobe) L: upper lobe (cylindrical bronchiectasis) |
R: upper and mid-zones L: none |
Diffuse, peribrochial bilaterally | R: upper lobe volume reduction | R: 3 L: 1 |
| 5 | R: diffuse L: upper lobe |
R: 23 L: 14 |
R: diffuse (cystic bronchiectasis) L: upper lobe (cylindrical bronchiectasis) |
R: lower zones L: diffuse |
R: no L: lower lobe |
R: entire lung volume reduction | R: 4 L: 1 |
| 6 | R: upper and mid-zones L: diffuse |
R: 30 L: 35 |
R: upper lobe (cylindrical bronchiectasis) L: diffuse (cystic bronchiectasis in the upper lobe) |
Diffuse and peribronchial | None | L: upper lobe volume reduction | R: 2 L: 3 |
PPFE, pleuroparenchymal fibroelastosis; GGO, ground glass opacity; R, right lung; L, left lung.
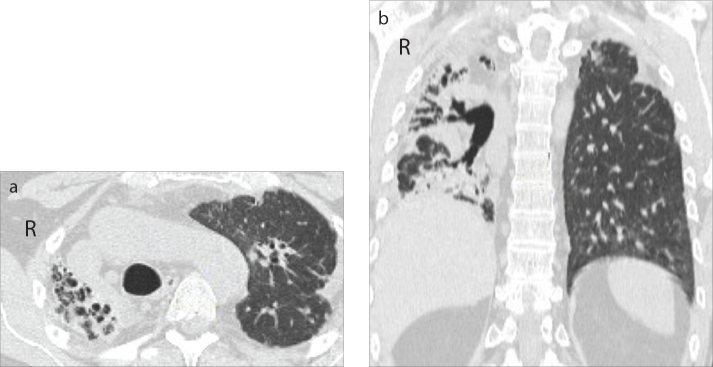
Figure 1.
a, b. Case 5: PPFE secondary to an allogeneic bone marrow transplant with asymmetric distribution on the right and left lungs. Axial (a) and coronal (b) CT images show cystic bronchiectasis, pleuroparenchymal and blotchy opacities involving the entire lung including the lower zone, together with the superior hilar retraction and the volume reduction of the entire right lung (grade 4). In the left lung, axial (a) and coronal (b) CT images show cylindrical bronchiectasis, pleuroparenchymal opacities distributed in the upper zones, without consistent volume reduction (grade 1).
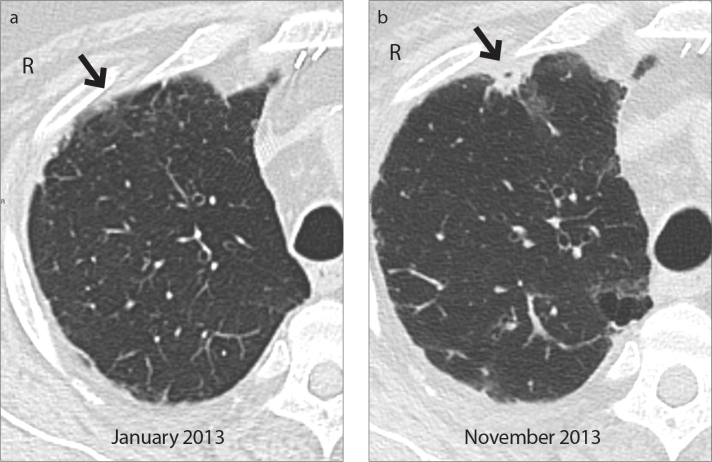
Considering PPFE severity, low (grade 1 and 2) and moderate (grade 3) stages were more prevalent; severe PPFE (grade 4) with diffuse cystic bronchiectasis and an entire collapsed lung was presented only in Case 5 (Fig. 1). Blotchy ground glass, found in four patients (from grade 1 to grade 3), were distributed in areas close to PPFE fibrotic changes (mid- and lower zones) and, during the follow-up, ground glass gradually transformed into blotchy parenchymal opacities (Fig. 2).
Figure 2.
a, b. Case 3: PPFE (grade 2) secondary to a double-lung transplantation. Axial CT image (a) shows subpleural ground glass opacity (arrow) in the anterior segment of the right upper lobe; it rapidly progressed into a consolidation, as shown in the axial CT scan (b, arrow) taken after nine months. Also note new reticular abnormalities and ground glass opacity in the posterior segment.
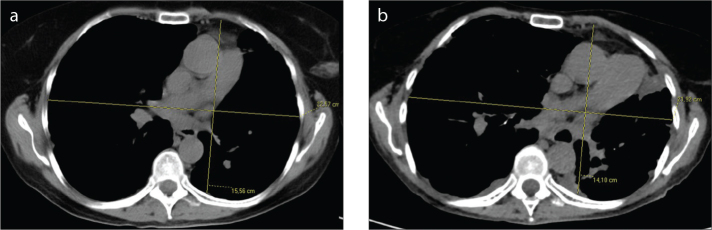
Different patterns of lower lobe fibrosis were not found in transplant lungs or HSCT recipients. Only the autologous HSCT recipient (Case 6) showed spontaneous recurrent bilateral pneumothoraces and progressive chest flattening (Fig. 3). 18F-FDG PET/CT imaging obtained from three patients (Cases 1, 3, 6), showed hypermetabolic areas corresponding to pleuroparenchymal opacities (Fig. 4). Four patients died: three (Cases 2, 3, 5) secondary to respiratory failure between one and eight years after diagnosis and Case 1 died after two years due to pulmonary cancer. Case 4 was still alive after six years and Case 6 after 15 years.
Figure 3.
a, b. Case 6: PPFE (grade 2–3) secondary to an autologous HSCT with progressive flattening of the chest. Axial CT scan at the level of the 6th thoracic vertebra on admission (a) and after nine years (b): the ratio of the anteroposterior diameter of the thoracic cage (APDT) to the transverse diameter of the thoracic cage (TDT) decreased from 0.69 (a) to 0.63 (b) (13).
Figure 4.
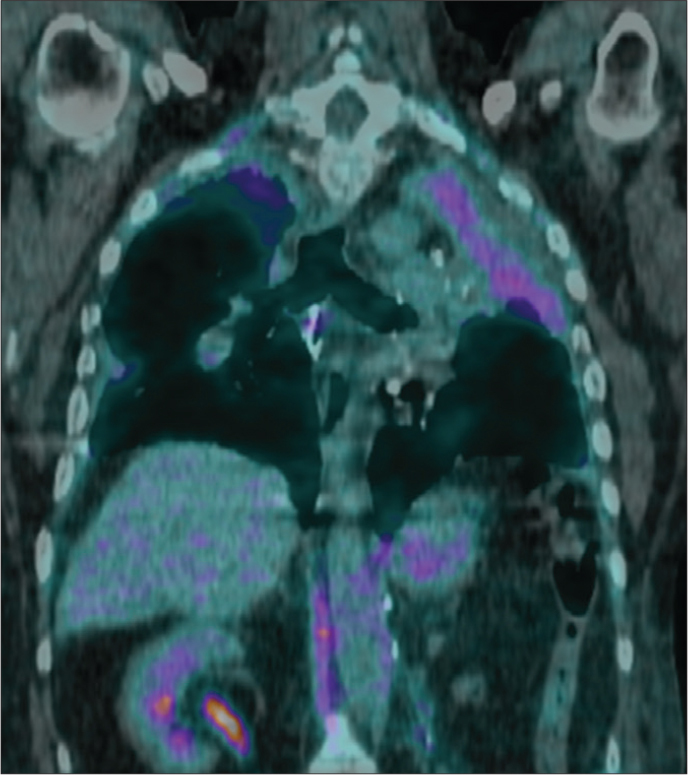
Case 3: PPFE (grade 2) secondary to a double-lung transplant. Coronal 18F-FDG PET/CT scan shows hypermetabolic areas corresponding to pleuroparenchymal upper lobes opacities (maximum standardized uptake value of 6.1, in the context of the upper left lobe atelectasis).
Two trends in the clinical and radiologic progression of PPFE were observed: slow disease progression in three patients with different grades of PPFE (Cases 4, 5, 6, not considering Case 1 who died of cancer), with a probability of survival greater than five years post-diagnosis; rapid disease progression in two patients (Case 2 and 3, Fig. 2) with low and moderate grades that rapidly developed respiratory failure and died after one and two years, respectively.
Discussion
This study demonstrates that PPFE represents a rare late post-transplant complication and it shows a higher prevalence among lung transplant recipients (7.54%) than among HSCT recipients (0.28%). Knowing the prevalence of secondary PPFE might be worth of interest because late onset noninfectious complications related to chronic lung allograft dysfunction (CLAD) (17, 18) or chronic graft-versus-host-disease (GVHD) (19) represent the major limitation of transplantation success. CLAD is defined as an irreversible decline in forced expiratory volume in 1 s (FEV1) to 80% of the baseline and includes bronchiolitis obliterans syndrome (FEV1<90% of stable baseline), the most common late complication in lung transplantation, and other two new entities: restrictive allograft syndrome (total lung capacity <90% of stable baseline and/or FEV1/forced vital capacity normal or increased) and neutrophilic reversible allograft dysfunction (it responds to azythromycin with an increase in FEV1 of at least >10%) (17–20).
The correlation between PPFE and transplants was first reported by Von der Thussen et al. (8), who described PPFE in some HSCT recipients; then Ofek et al. (10) correlated PPFE with restrictive allograft syndrome in lung transplant recipients. It has not yet been clarified whether the leading cause is chemotherapy, recurrent lung infections or cell-mediated immunity reaction as in bronchiolitis obliterans syndrome (8, 10, 17–22).
Although it was already known that secondary PPFE was prevalent (50% of cases of PPFE) (7, 8), the prevalence in lung transplant patients (7.54%, 4/53) was much higher than previously reported prevalence of 2% (13/686) (P = 0.03) (23). In the previous study, the detection of PPFE in lung transplantation was based on a review of the chest X-rays of lung transplant recipients; hence, patients who had milder forms of the disease might have been missed from that series, underestimating the prevalence of PPFE (23).
There appears to be no data on the prevalence of post-HSCT PPFE in the literature to date. In the following study, despite the wide sample range of HSCT recipients, PPFE was found only in two cases, as a late complication after an allogeneic and an autologous bone marrow transplant, respectively. Some authors demonstrated at biopsy that diffuse alveolar damage preceded the development of PPFE both in lung transplants (10) and HSCT recipients (8), suggesting that PPFE might represent a late complication of multiple factors (drugs/radiation, infections) that result in acute lung injury/diffuse alveolar damage (3, 4, 8, 9, 24). In our study, the difference between the prevalence of PPFE in HSCT recipients and lung transplant recipients implies that some risks factors, although common to both transplants, are more frequent in lung recipients.
A recent study (12) underlined the potential role of alkylating agents in the development of PPFE, in particular cyclophosphamide and carmustine. In this particular study however, a strong correlation between PPFE development and chemotherapy was not found, because, although all HSCT recipients received alkylating agents, only two out of the overall recipients (n=700) developed PPFE; while in lung transplant recipients, exposed to lower doses of chemotherapeutic agents, more cases of PPFE were identified. Further investigations are required to fully clarify the role of chemotherapeutic agents.
Recurrent lung infections, recognized as a risk factor for CLAD development (25), often caused by CMV were found in all lung transplant recipients and in the autologous HSCT recipient. Acute-GVHD was observed in the allogeneic HSCT recipient. However, a direct cause-effect relationship could not be established between these two risk factors and PPFE, since the frequency of lung infections and acute-GVHD was not known in transplant recipients who did not develop the disease. Although PPFE was described mainly in allogeneic HSCT (8, 19, 26), some autologous cases were also reported in the literature (6, 8). PPFE was observed in both cases in this particular study, hence the disease should not be considered as a form of chronic-GVHD.
A progressive flattening of the thorax cage and spontaneous pneumothorax were seldom found in this study. In particular, spontaneous pneumothorax, reported in the literature in 30% of overall patients with PPFE (7, 26, 27), was observed only in the autologous HSCT recipient. Suggested mechanisms leading to pneumothorax include: cysts in the apical fibrotic area, air trapping upstream to obliterative bronchiolitis and altered resistance of the pleura to the shear stress (6). The absence of pneumothorax in lung transplant recipients in this study might be linked to iatrogenic pleural adherences that prevent the pleura separating from the thorax cage. This data was in accordance with the literature where pneumothorax complicated fibroelastosis occurs more often in HSCT recipients (8, 26, 28) than in lung transplant recipients (23).
The mosaic attenuation pattern and air trapping consistent with constrictive obliterative bronchiolitis was not observed in this study, as other authors had previously identified in some patients with fibroelastosis secondary to HSCT (8) and lung transplantation (10). The majority of those secondary PPFE confirmed the coexistence between bronchiolitis obliterans and PPFE, postbiopsy (8, 10).
A radiologic grading system for PPFE (Table 4) was created, based on the extension of fibrosis changes, that enabled the following to be considered: PPFE progression and the relationship between the radiologic stage and disease outcome. First, concerning PPFE progression in the most advanced stages, fibrosis showed an asymmetric distribution between the two lungs as if the disease tended to progress more rapidly in one lung then in the other; moreover, ground glass areas usually appeared next to PPFE fibrosis changes, eventually becoming blotchy opacities that showed, especially if recently developed, 18F-FDG uptake at PET/CT, as other authors had already described (29). Second, regarding the relationship between the radiologic stage and disease outcome, it was interesting to note that stages (1 to 4) were not related to the prognosis: cases that slowly progressed presented with various grades of PPFE severity, including the most severe (grade 4), while those that progressed rapidly had lower grades (2, 3).
In terms of outcome, in accordance with the literature (5), the prognosis was poor and the progression variable: while the majority of cases progressed slowly, few others (lung transplant recipients) rapidly developed acute respiratory failure. Further investigations might help to identify different phenotypes of PPFE in order to better determine the prognosis.
The limitations of this study include its retrospective nature and incomplete data collection; risk factor exposure was not evaluated in lung transplant recipients and HSCT recipients who did not develop PPFE; histologic diagnosis was available only in one patient, although lung biopsy should be performed only in selected cases.
In conclusion, six cases clinically and radiologically consistent with PPFE have been described, presenting with different severity and showing a prevalence of 7.54% (95% CI, 0.43%–14.6%) in lung transplant recipients and 0.28% (95% CI, 0–0.68%) in HSCT recipients. The high prevalence of PPFE among lung transplant recipients was previously unknown and it could suggest that recurrent lung infections or immune damage might play a major role in the pathogenesis. PPFE in the autologous HSCT recipient excludes PPFE as a form of GVHD. As stated by other authors, a genetic predisposition might lead to PPFE development as a nonspecific response to various insults, such as infections, chemotherapy, and lung injury (8–10). Two trends of disease progression, regardless of fibrosis extension, were observed, suggesting the possible existence of two different PPFE phenotypes. Further investigations will be necessary in order to clarify the pathogenesis and the variable prognosis of the disease.
Main points.
Pleuroparenchymal fibroelastosis (PPFE) is a rare form of interstitial pneumonia, characterized by elastotic fibrosis involving the pleura and subpleural parenchyma, predominantly in the upper lobes.
PPFE can be either idiopathic or secondary and mostly occurs as a late complication of lung or hematopoietic stem cell transplantation (HSCT).
The prevalence of PPFE was higher in lung transplant compared with HSCT recipients.
Our results could suggest that recurrent lung infections or immune damage might play a major role in the pathogenesis.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. http://dx.doi.org/10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amitani R, Niimi A, Kuse F. Idiopathic pulmonary upper lobe fibrosis (IPUF) Kokyu. 1992;11:693–699. [Google Scholar]
- 3.Frankel SK, Cool CD, Lynch DA, et al. Idiopathic pleuroparenchymal fibroelastosis: description of a novel clinicopathologic entity. Chest. 2004;126:2007–2013. doi: 10.1378/chest.126.6.2007. http://dx.doi.org/10.1378/chest.126.6.2007. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe K. Pleuroparenchymal fibroelastosis: its clinical characteristics. Curr Respir Med Rev. 2013;9:299–237. doi: 10.2174/1573398X0904140129125307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camus P, von der Thusen J, et al. Pleuroparenchymal fibroelastosis: one more walk on the wild side of drugs? Eur Respir J. 2014;44:289–296. doi: 10.1183/09031936.00088414. http://dx.doi.org/10.1183/09031936.00088414. [DOI] [PubMed] [Google Scholar]
- 6.Becker CD, Gil J, Padilla ML. Idiopathic pleuroparenchymal fibroelastosis: an unrecognized or misdiagnosed entity? Mod Pathol. 2008;21:784–787. doi: 10.1038/modpathol.2008.56. http://dx.doi.org/10.1038/modpathol.2008.56. [DOI] [PubMed] [Google Scholar]
- 7.Von Der Thusen JH. Pleuroparenchymal fibroelastosis: its pathological characteristics. Curr Respir Med Rev. 2013;9:238–247. doi: 10.2174/1573398X113096660025. http://dx.doi.org/10.2174/1573398X113096660025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Von Der Thusen JH, Hansell DM, Tominaga M, et al. Pleuroparenchymal fibroelastosis in patients with pulmonary disease secondary to bone marrow transplantation. Mod Pathol. 2011;24:1633–1639. doi: 10.1038/modpathol.2011.114. http://dx.doi.org/10.1038/modpathol.2011.114. [DOI] [PubMed] [Google Scholar]
- 9.Reddy TL, Tominaga M, Hansell DM, et al. Pleuroparenchymal fibroelastosis: a spectrum of histopathological and imaging phenotypes. Eur Respir J. 2012;40:377–385. doi: 10.1183/09031936.00165111. http://dx.doi.org/10.1183/09031936.00165111. [DOI] [PubMed] [Google Scholar]
- 10.Ofek E, Sato M, Saito T, et al. Restrictive allograft syndrome post lung transplantation is characterized by pleuroparenchymal fibroelastosis. Mod Pathol. 2013;26:350–356. doi: 10.1038/modpathol.2012.171. http://dx.doi.org/10.1038/modpathol.2012.171. [DOI] [PubMed] [Google Scholar]
- 11.Larsen BT, Colby TV. Update for pathologists on idiopathic interstitial pneumonias. Arch Pathol Lab Med. 2012;136:1234–1241. doi: 10.5858/arpa.2012-0225-RA. http://dx.doi.org/10.5858/arpa.2012-0225-RA. [DOI] [PubMed] [Google Scholar]
- 12.Beynat-Mouterde C, Beltramo G, Lezmi G, et al. Pleuroparenchymal fibroelastosis as a late complication of chemotherapy agents. Eur Respir J. 2014;44:523–527. doi: 10.1183/09031936.00214713. http://dx.doi.org/10.1183/09031936.00214713. [DOI] [PubMed] [Google Scholar]
- 13.Harada T, Yoshida Y, Kitasato Y, et al. The thoracic cage becomes flattened in the progression of pleuroparenchymal fibroelastosis. Eur Respir Rev. 2014;23:263–266. doi: 10.1183/09059180.00006713. http://dx.doi.org/10.1183/09059180.00006713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huggins JT, Sahn SA. Causes and management of pleural fibrosis. Respirology. 2004;9:441–447. doi: 10.1111/j.1440-1843.2004.00630.x. http://dx.doi.org/10.1111/j.1440-1843.2004.00630.x. [DOI] [PubMed] [Google Scholar]
- 15.Walker CM, Takasugi JE, Chung JH, et al. Tumor-like conditions of the pleura. Radiographics. 2012;32:971–985. doi: 10.1148/rg.324115184. http://dx.doi.org/10.1148/rg.324115184. [DOI] [PubMed] [Google Scholar]
- 16.Kurosaki F, Bando M, Nakayama M, et al. Clinical features of pulmonary aspergillosis associated with interstitial pneumonia. Intern Med. 2014;53:1299–1306. doi: 10.2169/internalmedicine.53.1578. http://dx.doi.org/10.2169/internalmedicine.53.1578. [DOI] [PubMed] [Google Scholar]
- 17.Knoop C, Estenne M. Chronic allograft dysfunction. Clin Chest Med. 2011;32:311–326. doi: 10.1016/j.ccm.2011.02.009. http://dx.doi.org/10.1016/j.ccm.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Todd JL, Christie JD, Palmer SM, et al. Update in lung transplantation 2013. Am J Respir Crit Care Med. 2014;190:19–24. doi: 10.1164/rccm.201402-0384UP. http://dx.doi.org/10.1164/rccm.201402-0384UP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pena E, Souza CA, Escuissato DL, et al. Noninfectious pulmonary complications after hematopoietic stem cell transplantation: practical approach to imaging diagnosis. Radiographics. 2014;34:663–683. doi: 10.1148/rg.343135080. http://dx.doi.org/10.1148/rg.343135080. [DOI] [PubMed] [Google Scholar]
- 20.Verleden SE, Vandermeulen E, Ruttens D, et al. Neutrophilic reversible allograft dysfunction (NRAD) and restrictive allograft syndrome (RAS) Semin Respir Crit Care Med. 2013;34:352–360. doi: 10.1055/s-0033-1348463. http://dx.doi.org/10.1055/s-0033-1348463. [DOI] [PubMed] [Google Scholar]
- 21.Sato M, Waddell TK, Wagnetz U, et al. Restrictive allograft syndrome (RAS): a novel form of chronic lung allograft dysfunction. J Heart Lung Transplant. 2011;30:735–742. doi: 10.1016/j.healun.2011.01.712. http://dx.doi.org/10.1016/j.healun.2011.01.712. [DOI] [PubMed] [Google Scholar]
- 22.Verleden SE, de Jong PA, Ruttens D, et al. Functional and computed tomographic evolution and survival of restrictive allograft syndrome after lung transplantation. J Heart Lung Transplant. 2014;33:270–277. doi: 10.1016/j.healun.2013.12.011. http://dx.doi.org/10.1016/j.healun.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Pakhale SS, Hadjiliadis D, Howell DN, et al. Upper lobe fibrosis: a novel manifestation of chronic allograft dysfunction in lung transplantation. J Heart Lung Transplant. 2005;24:1260–1268. doi: 10.1016/j.healun.2004.08.026. http://dx.doi.org/10.1016/j.healun.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 24.Piciucchi S, Tomassetti S, Casoni G, et al. High resolution CT and histological findings in idiopathic pleuroparenchymal fibroelastosis: features and differential diagnosis. Respir Res. 2011;12:111. doi: 10.1186/1465-9921-12-111. http://dx.doi.org/10.1186/1465-9921-12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verleden SE, Ruttens D, Vandermeulen E, et al. Bronchiolitis obliterans syndrome and restrictive allograft syndrome: do risk factors differ? Transplantation. 2013;95:1167–1172. doi: 10.1097/TP.0b013e318286e076. http://dx.doi.org/10.1097/TP.0b013e318286e076. [DOI] [PubMed] [Google Scholar]
- 26.Sverzellati N, Zompatori M, Poletti V, et al. Small chronic pneumothoraces and pulmonary parenchymal abnormalities after bone marrow transplantation. J Thorac Imaging. 2007;22:230–234. doi: 10.1097/RTI.0b013e31802bddca. http://dx.doi.org/10.1097/RTI.0b013e31802bddca. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe K, Nagata N, Kitasato Y, et al. Rapid decrease in forced vital capacity in patients with idiopathic pulmonary upper lobe fibrosis. Respir Investig. 2012;50:88–97. doi: 10.1016/j.resinv.2012.06.003. http://dx.doi.org/10.1016/j.resinv.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Fujikura Y, Kanoh S, Kouzaki Y, Hara Y, Matsubara O, Kawana A. Pleuroparenchymal fibroelastosis as a series of airway complications associated with chronic graft-versus-host disease following allogeneic bone marrow transplantation. Intern Med. 2014;53:43–46. doi: 10.2169/internalmedicine.53.1124. http://dx.doi.org/10.2169/internalmedicine.53.1124. [DOI] [PubMed] [Google Scholar]
- 29.Machuca S, Niazi M, Diaz-Fuentes G, et al. Pleuroparenchymal fibroelastosis presenting as a hypermetabolic lung nodule. J Bronchology Interv Pulmonol. 2011;18:65–68. doi: 10.1097/LBR.0b013e318207b396. http://dx.doi.org/10.1097/LBR.0b013e318207b396. [DOI] [PubMed] [Google Scholar]