Abstract
PURPOSE
We aimed to conduct a network meta-analysis of mixed treatments for the infrapopliteal artery occlusive disease.
METHODS
We searched randomized controlled trials (RCTs) regarding balloon angioplasty (BA), nondrug metal stent (NDMS), drug-eluting balloon (DEB), or drug-eluting stent (DES) in PubMed, Embase, CENTRAL, Ovid, Sinomed, and other relevant websites. We selected and assessed the trials that met the inclusion criteria and conducted a network meta-analysis using the ADDIS software.
RESULTS
We included 11 relevant trials. We analyzed data of 1322 patients with infrapopliteal artery occlusive disease, of which 351 were in the NDMS vs. DES trials, 231 in the NDMS vs. BA trials, 490 in the BA vs. DEB trials, 50 in the DEB vs. DES trials, and 200 in the BA vs. DES trials. The network meta-analysis indicated that with NDMS as the reference, DES had a better result with respect to restenosis (odds ratio [OR], 5.16; 95% credible interval [CI], 1.58–18.41; probability of the best treatment, 84%) and amputation (OR, 2.50; 95% CI, 0.81–7.11; probability of the best treatment, 61%) and DEB had a better result with respect to target lesion revascularization (TLR; OR, 3.74; 95% CI, 0.78–17.05; probability of the best treatment, 57%). Moreover, with BA as the reference, NDMS had a better result with respect to technical success (OR, 0.10; 95% CI, 0.00–1.15; probability of the best treatment, 86%).
CONCLUSION
Our meta-analysis revealed that DES is a better treatment with respect to short-term patency and limb salvage rate, NMDS may provide a better technical success, and DEB and DES are good choices for reducing revascularization.
Infrapopliteal arterial occlusive disease is a kind of atherosclerotic disease that affects one or more blood vessels of the anterior tibial artery, posterior tibial artery, tibioperoneal trunk, and peroneal artery, which cause luminal stenosis or occlusion. The disease always leads to pain, limping, ulcers, and gangrene of the limb below the knee. The limbs of patients whose Rutherford class is greater than four may require urgent revascularization. The methods to treat infrapopliteal arterial occlusive disease include drug therapy, stem cell therapy, and surgery. Infrapopliteal arterial bypass is an effective method to revascularize the ischemic limb, but the surgery is complex and has high trauma (1).
With the emergence of endovascular treatment and the development of endovascular equipment, techniques such as balloon angioplasty (BA) have become indispensable for treating infrapopliteal arterial occlusive disease; however, the low long-term patency has become a hindrance for administering endovascular treatment for long lesions and small lumen in the infrapopliteal artery (2). There is an ongoing search for new equipment and techniques, such as drug-eluting balloon (DEB) and drug-eluting stent (DES), to improve the long-term patency. Some randomized controlled trials (RCTs) found the new techniques to be obviously better than BA, but these trials were limited to comparisons between two treatments only and they included a small number of patients (3), which decreased their reliability.
Moreover, previous meta-analyses have been confined to comparisons between two treatments (4), and there were no RCTs available. While traditional meta-analytical methods pertain to pair-wise comparisons between two interventions, network meta-analysis can be used for all possible comparisons in a body of evidence regardless of whether there have been direct head-to-head comparisons in clinical trials (5). Network meta-analysis can statistically compare the mixed treatments and obtain relative scientific comparison results (6). Currently, there is no network meta-analysis for comparing mixed treatments for the infrapopliteal arterial occlusive disease. Hence, in this study we compared four kinds of regular endovascular treatments and aimed to determine the best endovascular treatment through network meta-analysis of RCTs.
Methods
Eligibility criteria
A feasible protocol was drawn up for the meta-analysis in advance. Based on the PICOST principle, the inclusion criteria were as follows (7): trials including patients with infrapopliteal arterial occlusive disease; interventions included any two of BA, nondrug metal stent (NDMS, with the exception of absorbable metal stent), DEB, and DES; trials with outcomes of technical success, amputation, restenosis, and target lesion revascularization (TLR); prospective RCTs; and articles published in English or Chinese before December 2014. Trials were excluded if: they examined other vascular diseases at the same time, contained nonendovascular treatment, data could not be extracted, the sample population included <20 patients, or follow-up time was <6 months.
Search strategy and study selection
We performed a systematic search, according to the PRISMA guidelines (8), in PubMed, Embase, CENTRAL, Ovid, Sinomed, and other relevant websites; in addition, we searched the relevant previous meta-analyses and used their references as a supplement. Using infrapopliteal and randomized as keywords, we searched for articles in English and Chinese with a time restriction of December 2014. One author screened the studies with respect to title and abstract for inclusion. Identified articles were independently evaluated by another author to confirm their eligibility. Those studies that qualified for full-text analysis were examined by two independent reviewers for inclusion in the analysis.
Data extraction
The data extraction form was designed according to the Cochrane guidelines (9), was randomly tested in one trial, and modified according to the requirements of the analysis. Two authors independently extracted data, double entered the data using the EpiData software (ver. 3.1), and checked for consistency; the data were then discussed with a third party to settle discrepancies. The major information extracted included: article information (first author, study types, year of publication, and country); characteristics of participants (gender, age, renal insufficiency, diabetes mellitus, dyslipidemia, hypertension, smoker, critical limb ischemia [CLI], target lesion length, occlusive lesion, and follow-up time); outcomes (technical success, restenosis, amputation, and TLR); and study quality (risk of bias).
Quality assessment and statistical analysis
The study quality was assessed using the Cochrane Collaboration’s tool for assessing the risk of bias (10). Seven aspects, i.e., random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias, were independently assessed and illustrated by two reviewers, and the data was negotiated with the third party in case of discrepancies. The RevMan software (v 5.2) was used only for the risk of bias summary.
In our network meta-analysis, the ADDIS software (v 1.16.5) was used for testing consistency with the node split method (11). When the P value was >0.05, the consistency model was selected for the outcome network meta-analysis; otherwise, the inconsistency model was used (12). The software is based on Bayesian hierarchical model and Markov chain Monte Carlo (MCMC) method, and has good operability and repeatability, but narrow applicability. We selected odds ratio (OR) and 95% credible interval (CI) as the effect magnitude.
Results
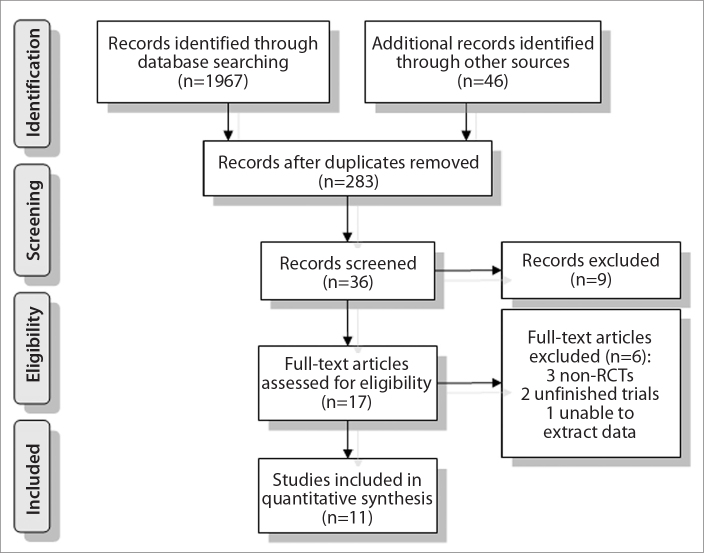
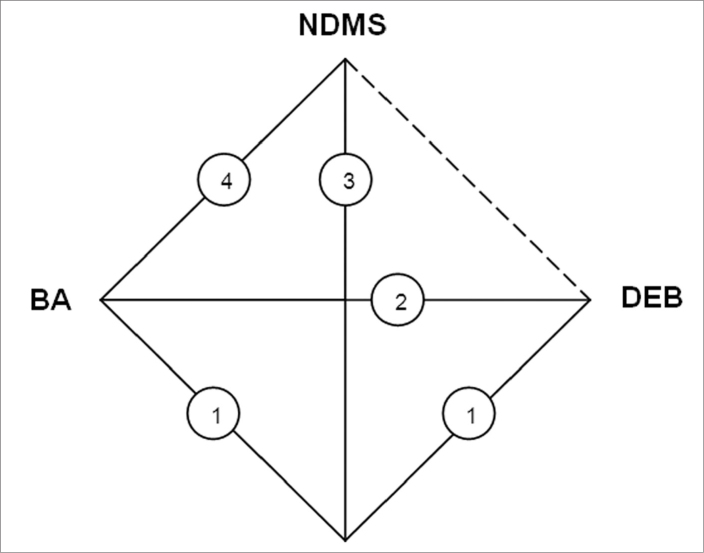
A total of 2013 articles matched the keywords. After the duplicates were removed, records were screened, and full-text articles were assessed, 11 RCTs finally fulfilled the inclusion criteria and were included in the meta-analysis (3, 13–22) as shown in Fig. 1. The earliest RCT was published in 2006, and the latest in September 2014; the distribution of the 11 RCTs are presented in Fig. 2.
Figure 1.
Flow diagram for selection of studies.
Figure 2.
Network diagram of the treatments. BA, balloon angioplasty; DEB, drug-eluting balloon; DES, drug-eluting stent; NDMS, nondrug metal stent. The straight lines denote the direct head-to-head comparisons, and the dotted lines denote the indirect comparisons in which direct comparison data are missing.
A total of 1322 participants were included in the analysis, of which 351 were in the NDMS vs. DES trials, 231 in the NDMS vs. BA trials, 490 in the BA vs. DEB trials, 50 in the DEB vs. DES trials, and 200 in the BA vs. DES trials. Majority of patients were elderly males, and more than half the patients had diabetes mellitus, dyslipidemia, and hypertension. Most patients’ symptom severity was greater than the Rutherford Class 4, indicating CLI. Anterior tibial artery and tibioperoneal trunk were the most impacted vessels, occlusion lesions partially occurred, and follow-up time was from 6 to 12 months; detailed data are shown in Table 1.
Table 1.
Clinical characteristics
| Quantity n | Male, % | Age (years)* | Renal insufficiency, % | Diabetes mellitus, % | Dyslipidemia % | Hypertension % | Smoker % | CLI % | Lesion length (mm)* | Occlusion % | Target vessel, % | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AT | PT | TT | PA | |||||||||||||
| Rand et al. 2006 | BA | 27 | N/A | 72.0 (47–80) | 11.0 | 70.0 | N/A | N/A | 63.0 | 70.4 | 24.0 (5–30) | 14.8 | N/A | N/A | N/A | N/A |
| NDMS | 24 | N/A | 68.3 (53–85) | 21.0 | 67.0 | N/A | N/A | 58.0 | 83.3 | 17.4±3.2 | 8.3 | N/A | N/A | N/A | N/A | |
| Follow-up | 6 months | |||||||||||||||
|
| ||||||||||||||||
| Falkowski et al. 2009 | DES | 25 | 56.0 | 70.5 (55–86) | N/A | 40.0 | 32.0 | 64.0 | 48.0 | 36.0 | 18.2±3.5 | N/A | 28.0 | 12.0 | 32.0 | 28.0 |
| NDMS | 25 | 60.0 | 72±10 | N/A | 40.0 | 40.0 | 60.0 | 40.0 | 28.0 | N/A | N/A | 24.0 | 8.0 | 36.0 | 32.0 | |
| Follow-up | 6 months | |||||||||||||||
|
| ||||||||||||||||
| Randon et al. 2010 | BA | 22 | 63.6 | 72±9 | 36.4 | 54.5 | 40.9 | 100.0 | 18.2 | 100.0 | N/A | N/A | 28.1 | 21.9 | 28.1 | 21.9 |
| NDMS | 16 | 37.5 | 12.5 | 62.5 | 43.7 | 100.0 | 6.2 | 100.0 | N/A | 29.2 | 25.0 | 16.6 | 29.2 | |||
| Follow-up | 12 months | |||||||||||||||
|
| ||||||||||||||||
| Brodmann et al. 2011 | BA | 33 | 39.4 | 74.9±1.3 | N/A | 72.7 | 18.2 | 81.8 | 24.2 | N/A | 78.5 (60.0–96.9) | 30.3 | N/A | N/A | N/A | N/A |
| NDMS | 21 | 57.1 | 68.9±2.9 | N/A | 76.2 | 66.7 | 90.5 | 33.3 | N/A | 27.9 (21.6–31.1) | 30.0 | N/A | N/A | N/A | N/A | |
| Follow-up | 12 months | |||||||||||||||
|
| ||||||||||||||||
| Rastan et al. 2011 | DES | 82 | 67.9 | 73.4±8 | 35.8 | 56.8 | 76.5 | 91.4 | 28.4 | 51.2 | 30.0±8.0 | 23.2 | 22 | 17 | 42 | 19 |
| NDMS | 79 | 64.9 | 72.3±9 | 35.1 | 50.6 | 76.6 | 88.3 | 28.6 | 41.8 | 31.0±9.0 | 21.5 | 31 | 13 | 33 | 23 | |
| Follow-up | 12 months | |||||||||||||||
|
| ||||||||||||||||
| Rand et al. 2011 | BA | 44 | 62.2 | 72.1±9.5 | N/A | 75.6 | N/A | N/A | N/A | N/A | 20.7±20.1 | 27.5 | N/A | N/A | N/A | N/A |
| NDMS | 44 | 68.2 | 71.4±8.0 | N/A | 79.5 | N/A | N/A | N/A | N/A | 21.1±12.2 | 19.4 | N/A | N/A | N/A | N/A | |
| Follow-up | 9 months | |||||||||||||||
|
| ||||||||||||||||
| Bosiers et al. 2012 | DES | 74 | 61.0 | 75.0±8.0 | 30.0 | 60.0 | 38.0 | 64.0 | 16.0 | 100 | 15.9±10.2 | 15.0 | 37.0 | 12.0 | 28.0 | 23.0 |
| NDMS | 66 | 67.0 | 76.0±8.4 | 33.0 | 50.0 | 38.0 | 74.0 | 11.0 | 100 | 18.9±10.0 | 17.0 | 29.0 | 13.0 | 30.0 | 28.0 | |
| Follow-up | 12 months | |||||||||||||||
|
| ||||||||||||||||
| Scheinert et al. 2012 | DES | 99 | 67.7 | 72.4±9.4 | N/A | 64.6 | 77.6 | 89.9 | 38.4 | N/A | 26.9±20.9 | 81.3 | N/A | N/A | N/A | N/A |
| BA | 101 | 75.2 | 74.3±8.2 | N/A | 64.4 | 68.3 | 91.1 | 26.3 | N/A | 26.8±21.3 | 75.4 | N/A | N/A | N/A | N/A | |
| Follow-up | 12 months | |||||||||||||||
|
| ||||||||||||||||
| Liistro et al. 2013 | DEB | 65 | 83.1 | 74.0±9.4 | 10.8 | 100 | 35.4 | 70.8 | 20.0 | 100 | 129±83 | 77.5 | 46.3 | 16.3 | 20.0 | 17.5 |
| BA | 67 | 77.6 | 75.0±9.6 | 10.4 | 100 | 23.9 | 77.6 | 10.4 | 100 | 131±79 | 82.1 | 41.0 | 23.1 | 9.0 | 26.9 | |
| Follow-up | 12 months | |||||||||||||||
|
| ||||||||||||||||
| Siablis et al. 2014 | DEB | 25 | 80.0 | 67.6±11.3 | 44.0 | 76.0 | 40.0 | 56.0 | 36.0 | N/A | 148.0±56.7 | 12.0 | 52.0 | 24.0 | N/A | 24.0 |
| DES | 25 | 72.0 | 75.3±8.0 | 32.0 | 64.0 | 52.0 | 44.0 | 24.0 | 100 | 127.0±46.5 | 23.0 | 50.0 | 27.0 | N/A | 23.0 | |
| Follow-up | 6 months | |||||||||||||||
|
| ||||||||||||||||
| Zeller et al. 2014 | DEB | 239 | 76.2 | 73.3±8.2 | 8.6 | 75.7 | 73.2 | 89.5 | 15.1 | 100.0 | 101.5±91.0 | 38.6 | N/A | N/A | N/A | N/A |
| BA | 119 | 70.6 | 71.7±9.9 | 12.5 | 68.9 | 67.2 | 89.1 | 13.4 | 99.0 | 128.6±94.6 | 45.9 | N/A | N/A | N/A | N/A | |
| Follow-up | 12 months | |||||||||||||||
RCT, randomized controlled trial; BA, balloon angioplasty; NDMS, nondrug metal stent; DES, drug-eluting stent; DEB, drug-eluting balloon; N/A, not available; CLI, critical limb ischemia; AT, anterior tibial artery; PT, posterior tibial artery; TT, tibioperoneal trunk; PA, peroneal artery.
Data are presented as mean±standard deviation or median (range).
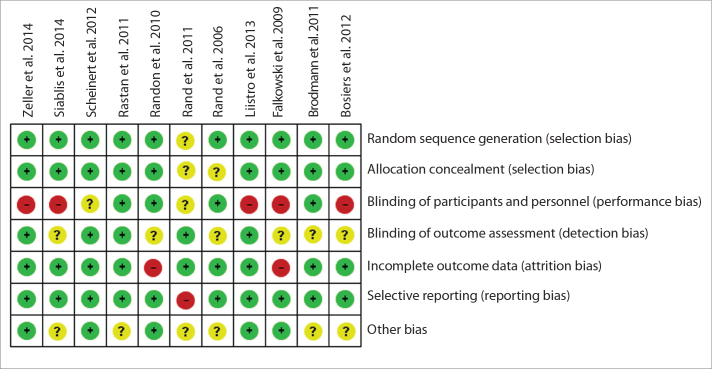
We used the Cochrane Collaboration’s tool to assess the risk of bias for the 11 trials. All of the trials were designed as prospective, randomized and controlled studies. The majority of the studies used random sequence generation and allocation concealment; incomplete outcome data and selective reporting were noticed in the majority of the studies. Some studies had no detailed information about blinding of participants and personnel, blinding of outcome assessment, and other bias. Particular examples are shown in Fig. 3.
Figure 3.
Detailed analysis of the risk of bias for each included randomized controlled trial (RCT) according to the Cochrane Collaboration’s Tool.
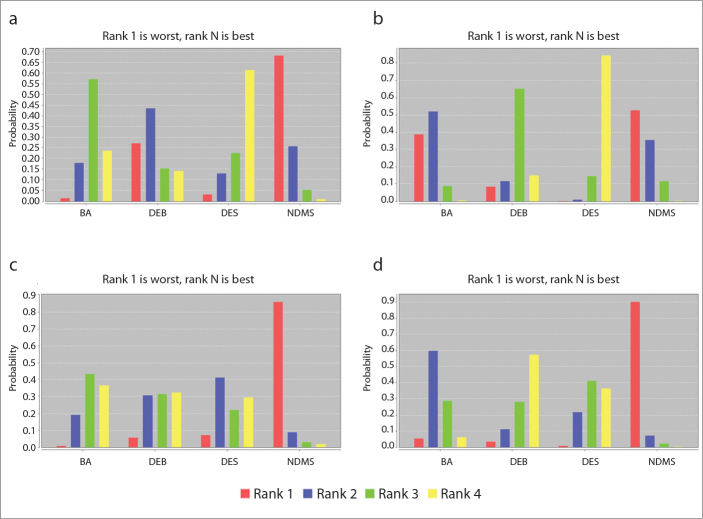
Most studies reported the outcome of amputation rate (n=10), except one study. With NDMS as the reference, OR and 95% CI for amputation were as follows: BA, 1.94 (0.85–4.75); DEB, 1.37 (0.40–6.83); and DES, 2.50 (0.81–7.11), with the result favoring other interventions (Table 2). In terms of amputation, the probabilities of DES, BA, DEB, and NDMS to be the best treatment were 61%, 24%, 14%, and 1%, respectively.
Table 2.
Network meta-analysis of included RCTs
| Amputation | |||
|---|---|---|---|
| BA | 1.42 (0.37, 3.82) | 0.80 (0.31, 2.29) | 1.94 (0.85, 4.75) |
| 0.70 (0.26, 2.74) | DEB | 0.56 (0.17, 2.92) | 1.37 (0.40, 6.83) |
| 1.26 (0.44, 3.27) | 1.79 (0.34, 6.06) | DES | 2.50 (0.81, 7.11) |
| 0.52 (0.21, 1.17) | 0.73 (0.15, 2.52) | 0.40 (0.14, 1.23) | NDMS |
|
| |||
| Restenosis | |||
| BA | 0.46 (0.10, 2.16) | 0.21 (0.05, 0.78) | 1.08 (0.33, 3.42) |
| 2.17 (0.46, 9.55) | DEB | 0.45 (0.07, 2.33) | 2.35 (0.39, 12.88) |
| 4.77 (1.28, 20.22) | 2.21 (0.43, 13.82) | DES | 5.16 (1.58, 18.41) |
| 0.93 (0.29, 3.04) | 0.43 (0.08, 2.57) | 0.19 (0.05, 0.63) | NDMS |
|
| |||
| Technical success | |||
| BA | 1.12 (0.16, 9.18) | 1.30 (0.14, 9.98) | 10.34 (0.87, 378.59) |
| 0.89 (0.11, 6.30) | DEB | 1.14 (0.08, 12.02) | 9.22 (0.39, 575.45) |
| 0.77 (0.10, 7.11) | 0.88 (0.08, 11.80) | DES | 8.48 (0.38, 580.58) |
| 0.10 (0.00, 1.15) | 0.11 (0.00, 2.53) | 0.12 (0.00, 2.63) | NDMS |
|
| |||
| TLR | |||
| BA | 0.60 (0.19, 2.03) | 0.72 (0.20, 2.16) | 2.24 (0.72, 7.13) |
| 1.66 (0.49, 5.32) | DEB | 1.20 (0.25, 4.78) | 3.74 (0.78, 17.05) |
| 1.39 (0.46, 4.98) | 0.84 (0.21, 4.05) | DES | 3.13 (1.21, 9.36) |
| 0.45 (0.14, 1.40) | 0.27 (0.06, 1.28) | 0.32 (0.11, 0.83) | NDMS |
Data are presented as odds ratio (95% credible interval).
RCT, randomized controlled trial; BA, balloon angioplasty; DEB, drug-eluting balloon; DES, drug-eluting stent; NDMS, nondrug metal stent; TLR, target lesion revascularization.
How to read the table: Treat one as reference, then read the row of the reference; the higher the OR, the higher the aspect (e.g., in the aspect of amputation, treat NDMS as the reference; then read the row of NDMS, the OR is 0.40 for DES, which is the lowest; thus, DES has the lowest amputation rate).
All trials reported the restenosis rate (n=11). With NDMS as the reference, OR and 95% CI for restenosis were as follows: BA, 1.08 (0.33–3.42); DEB, 2.35 (0.39–12.88); and DES, 5.16 (1.58–18.41), with the results favoring other interventions (Table 2). In terms of restenosis, the probabilities of DES, DEB, BA and NMDS to be the best treatment were 84%, 15%, 0%, and 0%, respectively (Fig. 4b).
Figure 4.
a–d. Rank probability of the best treatment. BA, balloon angioplasty; DEB, drug-eluting balloon; DES, drug-eluting stent; NDMS, nondrug metal stent; TLR, target lesion revascularization. Graph (a) shows the probability analysis of the best treatment for amputation, (b) probability of the best treatment for restenosis, (c) probability of the best treatment for technical success, and (d) probability of the best treatment for TLR.
All trials reported the technical success rate (n=11). With BA as the reference, OR and 95% CI for technical success were as follows: DEB, 1.12 (0.16–9.18); DES, 1.30 (0.14–9.98); and NDMS, 10.34 (0.87–378.59), with the results favoring the reference (Table 2). In terms of technical success, the probabilities of NMDS, DES, DEB, and BA to be the best treatment were 86%, 7%, 6%, and 1%, respectively (Fig. 4c).
Two studies did not provide TLR outcomes, while others provided complete data (n=9). With NDMS as the reference, OR and 95% CI for TLR were as follows: BA, 2.24 (0.72–7.13); DEB, 3.74 (0.78–17.05); and DES, 3.13 (1.21–9.36), with the results favoring DEB and DES (Table 2). In terms of TLR, the probabilities of DEB, DES, BA, and NMDS to be the best treatment were 57%, 36%, 6%, and 0%, respectively (Fig. 4d).
In the network meta-analysis, the node split method was used to test the consistency of outcomes. P > 0.05 indicated no obvious inconsistency (Fig. 3); thus, we selected the consistency model for the network meta-analysis.
Discussion
Using the ADDIS software, our results demonstrated that with NDMS as the reference, DES was a better choice with respect to restenosis (OR, 5.16; 95% CI, 1.58–18.41; probability of the best treatment, 84%) and amputation (OR, 2.50; 95% CI, 0.81–7.11; probability of the best treatment, 61%), and DES had a higher probability (84%) to be the best treatment with respect to the restenosis rate. With NDMS as the reference, DEB was a better choice with respect to TLR (OR, 3.74; 95% CI, 0.78–17.05; probability of the best treatment, 57%). OR of DEB was higher than that of BA and NDMS, but similar with that of DES. These results are consistent with the result of another systematic review (25). With BA as the reference, NDMS was a better choice with respect to the technical success (OR, 10.34; 95% CI, 0.87–378.59; probability of the best treatment, 86%).
The extensive complications and limitations of infrapopliteal bypass for CLI have prompted the development of endovascular revascularization. Unfortunately, the major limitation of endovascular therapy remains its durability. In particular, intervention in below-the-knee arteries is challenging, for which case BA rarely results in sustained patency. In the past decade, drug-eluting devices showed good performance. The use of paclitaxel or sirolimus inhibits neointima formation and reduces the occurrence of restenosis.
In the femorapopliteal artery, paclitaxel-eluting stents and paclitaxel-coated balloons offer the best long-term results, which have already been demonstrated (23). Whether the results of the femorapopliteal artery can be applied to the infrapopliteal artery remains unclear.
Traditional meta-analytical methods pertain to pair-wise comparisons between two interventions, thus only partially providing evidence that surgeons need in order to make informed decisions regarding treatment. The network meta-analysis aggregates the results of RCTs into a single unified analysis that compares three or more interventions while completely respecting randomization (24). As far as we know, previous studies performed pair-wise comparisons between NDMS, BA, DEB, or DES (25), and our study is the first network meta-analysis to compare the mixed treatments of the infrapopliteal arterial occlusive disease.
There has been lack of studies that compared DES and NDMS. Our study included three RCTs that directly compared DES and NDMS and indirectly compared four RCTs. The benefits of DES in coronary revascularization have been well established (26), leading to the potential role of DES in infrapopliteal revascularization, given the similar sizes of the infrapopliteal and coronary vessels. Early literature regarding coronary DES for the treatment of tibial disease has been positive overall, and this has been reinforced by our study. However, a previous study considered that the use of NDMS is recommended when bailout stenting is indicated because the DES trials did not demonstrate clinical benefit for DES over NDMS (25). In our results, with NDMS as the reference, DES achieved better evaluations with respect to the restenosis, amputation, and TLR rates (OR: 0.19, 0.40, and 0.32, respectively). These results indicated that DES should be the first choice instead of NDMS. In the comparison of DEB and BA, DEB performed better than BA, in both coronary artery (27) and femorapopliteal artery (23). In our analysis of the infrapopliteal artery, with BA as the reference, DEB achieved better results with respect to the restenosis and TLR rates (OR: 0.46 and 0.60, respectively) but did not decrease the amputation rate compared with BA (OR: 1.42); thus, it still has a similar technical success rate compared with BA (OR: 1.12). These results may make surgeons hesitate when selecting balloons. DEB can maintain patency and decrease TLR, but it cannot increase the limb salvage rate according to our result. In the IN.PACT DEEP trial, Zeller et al. (22) found that a safety signal that was driven by major amputations through 12 months was observed in the DEB arm compared with the percutaneous transluminal angioplasty arm (8.8% vs. 3.6%; P = 0.080). The differences in results among the trials may be because of different patient comorbidities at baseline. Furthermore, more RCTs are required to observe a significant difference. All OR and probability data can be abstracted from Table 2 and Fig. 4.
In the femorapopliteal artery, DES and DEB performed similarly, except for technical success (23). In the infrapopliteal artery, with DEB as the reference, DES had lower amputation and restenosis rates (OR: 0.56 and 0.45, respectively), but similar TLR rate (OR: 1.20) and technical success rate (OR: 1.14). These may partially differ with results in the femorapopliteal artery; however, it still indicates that DES can be the bailout stenting and the first choice instead of DEB because it provides longer patency and higher limb salvage rate. In the comparison between NDMS and BA, NDMS was always treated as the bailout choice for BA but not the first choice in some centers; moreover, our results support this standpoint. With BA as the reference, NDMS achieved similar probability with BA with respect to restenosis (OR: 1.08), but worse than BA with respect to amputation and TLR (OR: 1.94 and 2.24, respectively). In our analysis, NDMS had a higher technical success rate. NDMS had a higher OR than DES, with high probability (86%), which is confusing as our results differ from those of another analysis (28). This may be because of differences in the baseline characteristics of the target lesion.
In addition to the abovementioned treatments, bioabsorbable stents, orbital atherectomy, and excimer laser atherectomy are alternative treatment choices for surgeons. In 2009, Bosiers et al. (29) reported an RCT for bioabsorbable metal stent that was used to treat the infrapopliteal arterial occlusive disease. The first generation magnesium alloy stent was remarkably better than BA, with a complication rate of 5.3% in 30 days; however, the six-month patency rate was lower than BA (31.8%, P = 0.013). Although there is no obvious advantage with respect to short-term patency for the magnesium alloy absorbable stent, its absorbable property provides a strong basis for the treatment of the infrapopliteal artery disease in the future. In 2012, Shammas et al. (30) performed an RCT with 50 patients and compared orbital atherectomy and BA; orbital atherectomy had high technical success (93.1%) and low TLR and death (6.7% and 0%). In 2015, Piyaskulkaew et al. (31) retrospectively evaluated the data of 726 patients who underwent laser-assisted BA (n = 395) and BA (n=331) for popliteal and infrapopliteal peripheral artery diseases at a single center. An infrapopliteal artery lesion was found in 69.6% and 84% of the groups, respectively. Laser-assisted BA was associated with a high angiographic success rate and similar ipsilateral major amputation, repeat revascularization, and long-term mortality rate. The results indicated that laser can improve the passage of guidewire in chronic total occlusive lesions.
Although the long-term outcomes are not encouraging, these new treatments are still improving and are being studied in large sample populations. Better technology and more advanced materials may be utilized to resolve pain in patients in the future.
There are some limitations in our analysis. First, the analysis included only Chinese and English literature; we did not search for Japanese, Latin-derived, and other language literature; and racial differences could lead to a result bias. Second, comparison of treatments was not comprehensive; certain treatments such as orbital atherectomy and bioabsorbable stents were not included. Third, the follow-up time of the trials appeared short; without data on long-term curative effect, no guidelines can be developed regarding the choice of treatment. Fourth, we did not include multiarm trials, which may increase the risk of publication bias. Finally, the sample size was small (n=50) for comparison of DEB and DES, which may affect the authenticity of the results. The above limitations were also impacted by some objective factors and should be prevented in future research.
In conclusion, our results confirm the dominance of DES in treating infrapopliteal arterial occlusive disease, particularly with respect to the short-term patency and limb salvage rate; furthermore, with respect to TLR, DEB and DES are both good choices.
Main points.
The network meta-analysis aggregates the results of RCTs into a single unified analysis that compares four interventions for treatment of infrapopliteal artery occlusive disease while completely respecting randomization.
Drug-eluting stent (DES) is a better treatment with respect to short-term patency and limb salvage rate.
Nondrug metal stent may provide a better technical success.
Drug-eluting balloon and DES are good choices for reducing revascularization.
Table 3.
Consistency test of included RCTs with the node-splitting method
| Amputation | ||||
|---|---|---|---|---|
| Comparison | Direct effect | Indirect effect | Overall | P |
| BA vs. DEB | 0.54 (−0.81, 1.56) | −1.30 (−4.59, 1.52) | 0.35 (−1.01, 1.34) | 0.23 |
| BA vs. DES | −0.46 (−1.85, 0.92) | 0.25 (−1.50, 1.89) | −0.23 (−1.19, 0.83) | 0.50 |
| BA vs. NDMS | 0.67 (−0.21, 1.74) | 0.71 (−1.37, 2.67) | 0.66 (−0.16, 1.56) | 0.98 |
| DEB vs. DES | 0.85 (−2.01, 4.36) | −0.91 (−2.32, 0.78) | −0.58 (−1.80, 1.07) | 0.25 |
| DES vs. NDMS | 0.87 (−0.79, 2.77) | 0.95 (−0.68, 2.45) | 0.92 (−0.21, 1.96) | 0.93 |
|
| ||||
| Restenosis | ||||
| Comparison | Direct effect | Indirect effect | Overall | P |
| BA vs. DEB | −0.98 (−2.77, 0.89) | −0.08 (−3.36, 3.04) | −0.78 (−2.26, 0.77) | 0.59 |
| BA vs. DES | −0.94 (−3.43, 1.61) | −1.90 (−3.68, −0.14) | −1.56 (−3.01, −0.25) | 0.48 |
| BA vs. NDMS | 0.01 (−1.46, 1.39) | 0.34 (−2.35, 3.04) | 0.08 (−1.11, 1.23) | 0.78 |
| DEB vs. DES | −1.32 (−4.11, 1.45) | −0.44 (−2.99, 1.92) | −0.79 (−2.63, 0.85) | 0.58 |
| DES vs. NDMS | 1.75 (0.20, 3.35) | 1.41 (−1.07, 3.96) | 1.64 (0.46, 2.91) | 0.80 |
|
| ||||
| Technical success | ||||
| Comparison | Direct effect | Indirect effect | Overall | P |
| BA vs. DEB | 0.09 (−2.50, 2.65) | 0.27 (−4.80, 4.96) | 0.12 (−1.84, 2.22) | 0.94 |
| BA vs. DES | 0.29 (−2.27, 2.96) | −0.10 (−4.87, 4.59) | 0.26 (−1.96, 2.30) | 0.91 |
| BA vs. NDMS | 2.12 (−0.19, 5.50) | 8.14 (−20.12, 37.90) | 2.34 (−0.14, 5.94) | 0.72 |
| DEB vs. DES | 0.28 (−3.99, 4.83) | 0.03 (−3.42, 3.70) | 0.13 (−2.47, 2.49) | 0.94 |
| DES vs. NDMS | 11.65 (−12.53, 46.22) | 2.03 (−1.15, 6.24) | 2.14 (−0.97, 6.36) | 0.46 |
|
| ||||
| TLR | ||||
| Comparison | Direct effect | Indirect effect | Overall | P |
| BA vs. DEB | −0.66 (−2.10, 0.72) | 0.59 (−2.50, 3.58) | −0.51 (−1.67, 0.71) | 0.42 |
| BA vs. DES | 0.39 (−1.47, 2.12) | −0.94 (−2.58, 0.50) | −0.33 (−1.61, 0.77) | 0.19 |
| BA vs. NDMS | 0.51 (−1.03, 2.10) | 1.28 (−0.96, 3.26) | 0.81 (−0.34, 1.96) | 0.49 |
| DEB vs. DES | −0.70 (−3.44, 1.97) | 0.49 (−1.60, 2.36) | 0.18 (−1.40, 1.56) | 0.42 |
| DES vs. NDMS | 1.31 (0.08, 2.67) | 0.54 (−1.59, 3.01) | 1.14 (0.19, 2.24) | 0.49 |
Data are presented as relative effect (95% credible interval).
RCT, randomized controlled trial; BA, balloon angioplasty; DEB, drug-eluting balloon; DES, drug-eluting stent; NDMS, nondrug metal stent; TLR, target lesion revascularization.
Footnotes
Financial disclosure
This study was supported by the National Nature Science Foundation of China (81070256, 81100226).
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Schamp KB, Meerwaldt R, Reijnen MM, et al. The ongoing battle between infrapopliteal angioplasty and bypass surgery for critical limb ischemia. Ann Vasc Surg. 2012;26:1145–1153. doi: 10.1016/j.avsg.2012.02.006. http://dx.doi.org/10.1016/j.avsg.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Soder HK, Manninen HI, Jaakkola P, et al. Prospective trial of infrapopliteal artery balloon angioplasty for critical limb ischemia: angiographic and clinical results. J Vasc Interv Radiol. 2000;11:1021–1031. doi: 10.1016/s1051-0443(07)61332-3. http://dx.doi.org/10.1016/S1051-0443(07)61332-3. [DOI] [PubMed] [Google Scholar]
- 3.Randon C, Jacobs B, De Ryck F, et al. Angioplasty or primary stenting for infrapopliteal lesions: results of a prospective randomized trial. Cardiovasc Intervent Radiol. 2010;33:260–269. doi: 10.1007/s00270-009-9765-6. http://dx.doi.org/10.1007/s00270-009-9765-6. [DOI] [PubMed] [Google Scholar]
- 4.Wu R, Yao C, Wang S, et al. Percutaneous transluminal angioplasty versus primary stenting in infrapopliteal arterial disease: a meta-analysis of randomized trials. J Vasc Surg. 2014;59:1711–1720. doi: 10.1016/j.jvs.2014.03.012. http://dx.doi.org/10.1016/j.jvs.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Panagiotou OA. Network meta-analysis: evidence synthesis with mixed treatment comparison. Am J Epidemiol. 2015;181:288–289. http://dx.doi.org/10.1093/aje/kwu471. [Google Scholar]
- 6.Palmerini T, Biondi-Zoccai G, Della RD, et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012;379:1393–1402. doi: 10.1016/S0140-6736(12)60324-9. http://dx.doi.org/10.1016/S0140-6736(12)60324-9. [DOI] [PubMed] [Google Scholar]
- 7.Li HJ, He LY, Liu ZS, et al. On-site quality control of acupuncture randomized controlled trial: design of content and checklist of quality control based on PICOST. Zhongguo Zhen Jiu. 2014;34:183–185. [PubMed] [Google Scholar]
- 8.Moher D, Altman DG, Liberati A, et al. PRISMA statement. Epidemiology. 2011;22:128. doi: 10.1097/EDE.0b013e3181fe7825. http://dx.doi.org/10.1097/EDE.0b013e3181fe7825. [DOI] [PubMed] [Google Scholar]
- 9.Higgins J, Green S. Secondary Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011] [updated March 2011] Available from www.cochrane-handbook.org. [Google Scholar]
- 10.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:5928. doi: 10.1136/bmj.d5928. http://dx.doi.org/10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29:932–944. doi: 10.1002/sim.3767. http://dx.doi.org/10.1002/sim.3767. [DOI] [PubMed] [Google Scholar]
- 12.Veroniki AA, Vasiliadis HS, Higgins JP, et al. Evaluation of inconsistency in networks of interventions. Int J Epidemiol. 2013;42:332–345. doi: 10.1093/ije/dys222. http://dx.doi.org/10.1093/ije/dyt106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rand T, Basile A, Cejna M, et al. PTA versus carbofilm-coated stents in infrapopliteal arteries: pilot study. Cardiovasc Intervent Radiol. 2006;29:29–38. doi: 10.1007/s00270-005-0276-9. http://dx.doi.org/10.1007/s00270-005-0276-9. [DOI] [PubMed] [Google Scholar]
- 14.Falkowski A, Poncyljusz W, Wilk G, et al. The evaluation of primary stenting of sirolimus-eluting versus bare-metal stents in the treatment of atherosclerotic lesions of crural arteries. Eur Radiol. 2009;19:966–974. doi: 10.1007/s00330-008-1225-1. http://dx.doi.org/10.1007/s00330-008-1225-1. [DOI] [PubMed] [Google Scholar]
- 15.Rand T, Lammer J, Rabbia C, et al. Percutaneous transluminal angioplasty versus turbostatic carbon-coated stents in infrapopliteal arteries: InPeria II trial. Radiology. 2011;261:634–642. doi: 10.1148/radiol.11101357. http://dx.doi.org/10.1148/radiol.11101357. [DOI] [PubMed] [Google Scholar]
- 16.Brodmann M, Froehlich H, Dorr A, et al. Percutaneous transluminal angioplasty versus primary stenting in infrapopliteal arteries in critical limb ischemia. Vasa. 2011;40:482–490. doi: 10.1024/0301-1526/a000152. http://dx.doi.org/10.1024/0301-1526/a000152. [DOI] [PubMed] [Google Scholar]
- 17.Rastan A, Tepe G, Krankenberg H, et al. Sirolimus-eluting stents vs. bare-metal stents for treatment of focal lesions in infrapopliteal arteries: a double-blind, multi-centre, randomized clinical trial. Eur Heart J. 2011;32:2274–2281. doi: 10.1093/eurheartj/ehr144. http://dx.doi.org/10.1093/eurheartj/ehr144. [DOI] [PubMed] [Google Scholar]
- 18.Scheinert D, Katsanos K, Zeller T, et al. A prospective randomized multicenter comparison of balloon angioplasty and infrapopliteal stenting with the sirolimus-eluting stent in patients with ischemic peripheral arterial disease: 1-year results from the ACHILLES trial. J Am Coll Cardiol. 2012;60:2290–2295. doi: 10.1016/j.jacc.2012.08.989. http://dx.doi.org/10.1016/j.jacc.2012.08.989. [DOI] [PubMed] [Google Scholar]
- 19.Bosiers M, Scheinert D, Peeters P, et al. Randomized comparison of everolimus-eluting versus bare-metal stents in patients with critical limb ischemia and infrapopliteal arterial occlusive disease. J Vasc Surg. 2012;55:390–398. doi: 10.1016/j.jvs.2011.07.099. http://dx.doi.org/10.1016/j.jvs.2011.07.099. [DOI] [PubMed] [Google Scholar]
- 20.Liistro F, Porto I, Angioli P, et al. Drug-eluting balloon in peripheral intervention for below the knee angioplasty evaluation (DEBATE-BTK): a randomized trial in diabetic patients with critical limb ischemia. Circulation. 2013;128:615–621. doi: 10.1161/CIRCULATIONAHA.113.001811. http://dx.doi.org/10.1161/CIRCULATIONAHA.113.001811. [DOI] [PubMed] [Google Scholar]
- 21.Siablis D, Kitrou PM, Spiliopoulos S, et al. Paclitaxel-coated balloon angioplasty versus drug-eluting stenting for the treatment of infrapopliteal long-segment arterial occlusive disease: the IDEAS randomized controlled trial. JACC Cardiovasc Interv. 2014;7:1048–1056. doi: 10.1016/j.jcin.2014.04.015. http://dx.doi.org/10.1016/j.jcin.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Zeller T, Baumgartner I, Scheinert D, et al. Drug-eluting balloon versus standard balloon angioplasty for infrapopliteal arterial revascularization in critical limb ischemia: 12-month results from the IN.PACT DEEP randomized trial. J Am Coll Cardiol. 2014;64:1568–1576. doi: 10.1016/j.jacc.2014.06.1198. http://dx.doi.org/10.1016/j.jacc.2014.06.1198. [DOI] [PubMed] [Google Scholar]
- 23.Katsanos K, Spiliopoulos S, Karunanithy N, et al. Bayesian network meta-analysis of nitinol stents, covered stents, drug-eluting stents, and drug-coated balloons in the femoropopliteal artery. J Vasc Surg. 2014;59:1123–1133. doi: 10.1016/j.jvs.2014.01.041. http://dx.doi.org/10.1016/j.jvs.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 24.Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3:80–97. doi: 10.1002/jrsm.1037. http://dx.doi.org/10.1002/jrsm.1037. [DOI] [PubMed] [Google Scholar]
- 25.Jens S, Conijn AP, Koelemay MJW, et al. Randomized trials for endovascular treatment of infrainguinal arterial disease: systematic review and meta-analysis (Part 2: Below the Knee) Eur J Vasc Endovasc Surg. 2014;47:536–544. doi: 10.1016/j.ejvs.2014.02.012. http://dx.doi.org/10.1016/j.ejvs.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Sawhney N, Moses JW, Leon MB, et al. Treatment of left anterior descending coronary artery disease with sirolimus-eluting stents. Circulation. 2004;110:374–379. doi: 10.1161/01.CIR.0000136580.34604.B8. http://dx.doi.org/10.1161/01.CIR.0000136580.34604.B8. [DOI] [PubMed] [Google Scholar]
- 27.Kwong JS, Yu CM. Drug-eluting balloons for coronary artery disease: an updated meta-analysis of randomized controlled trials. Int J Cardiol. 2013;168:2930–2932. doi: 10.1016/j.ijcard.2013.03.180. http://dx.doi.org/10.1016/j.ijcard.2013.03.180. [DOI] [PubMed] [Google Scholar]
- 28.Fusaro M, Cassese S, Ndrepepa G, et al. Drug-eluting stents for revascularization of infrapopliteal arteries: updated meta-analysis of randomized trials. JACC Cardiovasc Interv. 2013;6:1284–1293. doi: 10.1016/j.jcin.2013.08.007. http://dx.doi.org/10.1016/j.jcin.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Bosiers M, Peeters P, D’Archambeau O, et al. AMS INSIGHT--absorbable metal stent implantation for treatment of below-the-knee critical limb ischemia: 6-month analysis. Cardiovasc Intervent Radiol. 2009;32:424–435. doi: 10.1007/s00270-008-9472-8. http://dx.doi.org/10.1007/s00270-009-9530-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shammas NW, Lam R, Mustapha J, et al. Comparison of orbital atherectomy plus balloon angioplasty vs. balloon angioplasty alone in patients with critical limb ischemia: results of the CALCIUM 360 randomized pilot trial. J Endovasc Ther. 2012;19:480–488. doi: 10.1583/JEVT-12-3815MR.1. http://dx.doi.org/10.1583/JEVT-12-3815MR.1. [DOI] [PubMed] [Google Scholar]
- 31.Piyaskulkaew C, Parvataneni K, Ballout H, et al. Laser in infrapopliteal and popliteal stenosis 2 study (LIPS2): Long-term outcomes of laser-assisted balloon angioplasty versus balloon angioplasty for below knee peripheral arterial disease. Catheter Cardiovasc Interv. 2015;86:1211–1218. doi: 10.1002/ccd.26145. http://dx.doi.org/10.1002/ccd.26145. [DOI] [PMC free article] [PubMed] [Google Scholar]