Abstract
Pulmonary arteriovenous malformations (PAVMs) are vascular anomalies of the lung and carry the risk of cerebral thromboembolism, brain abscess, or pulmonary hemorrhage. We describe a 64-year-old male with hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu syndrome) who presented with a five-year history of progressive effort dyspnea and a PAVM in the right upper lobe successfully treated by transcatheter embolization of feeding arteries using a new occlusion device, the ArtVentive Endoluminal Occlusion System™.
Pulmonary arteriovenous malformations (PAVMs) are abnormal direct communications between pulmonary arteries and pulmonary veins without interpositions of a capillary bed (1). PAVMs are associated with hereditary hemorrhagic telangiectasia (HHT) or Rendu-Osler-Weber disease in up to 90% of cases, whereas the remaining 5%–10% are sporadic fistulas. Conversely, 15%–35% of HHT patients will present PAVMs (2).
The PAVMs consist of three different anatomical components: one or more feeding arteries, an aneurismal sac, and one or more draining veins. In about 80% of cases, PAVMs are simple, meaning there is only one feeding artery into the arteriovenous fistula site; in about 20% of cases they are complex, meaning there are multiple feeding arteries (3). The absence of interpositions of a capillary bed prevents the functionality of lungs putting the patients at high risk for pulmonary hemorrhage, right-left shunt, and paradoxical embolism with a rate that can reach almost 50%, increasing even more during pregnancy; thus PAVMs should be treated as early as possible (4).
Until the seventies the treatment of choice has been surgery, including pneumonectomy, lobectomy, wedge resection, segmentectomy, or vascular ligation (5). Currently, transcatheter embolization is the gold-standard therapy for PAVMs, avoiding surgery in the majority of cases. Embolic devices consist of detachable occlusion balloon, vascular coils, and/or Amplatzer vascular plugs (6). The ArtVentive Endoluminal Occlusion SystemTM (EOS; ArtVentive Medical Groups Inc.) device has been developed for percutaneous occlusion of the peripheral arterial and venous vasculature. The experience about this occlusion device is limited to varicoceles (7).
In this case report, we discuss transcatheter embolization treatment with ArtVentive EOSTM and subsequent resolution of a complex right upper lobe PAVM in a 64-year-old man who presented with a history of severe and continuous dyspnea. To the best of our knowledge there are no articles in the literature describing the use of this new device in the treatment of PAVMs.
Technique
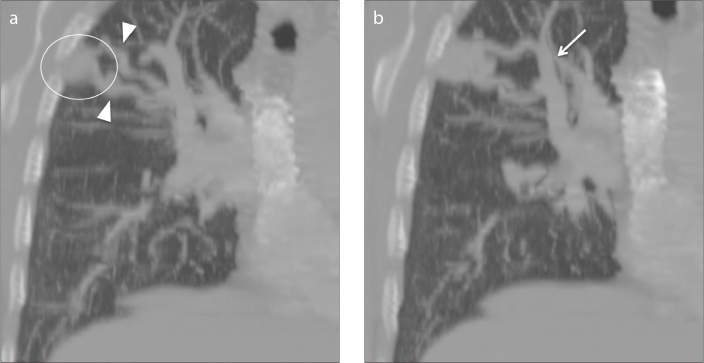
A 64-year-old male with Osler-Weber-Rendu syndrome presented with a five-year history of progressive effort dyspnea. He also had recurrent epistaxis and rectal bleeding. There was no family history of bleeding disorders. Clinical examination showed central cyanosis, pan-digital clubbing, normal heart sounds, and no murmur over the precordium. There was a short systolic murmur audible in the right infra-axillary area increasing with deep inspiration and Muller’s maneuver. Chest x-ray showed a homogeneous opacity in the right upper zone. Computed tomography (CT) performed during the current admission demonstrated a PAVM in the right upper lobe with two feeding arteries and draining veins (Fig. 1).
Figure 1.
a, b. Coronal multiplanar reconstruction CT angiography images. Panel (a) shows pulmonary arteriovenous malformation (PAVM) in the upper right lobe (circle) appearing as a rounded well-circumscribed lesion with two branching afferent feeding vessels (arrowheads). Panel (b) shows a dilated efferent draining vessel (arrow).
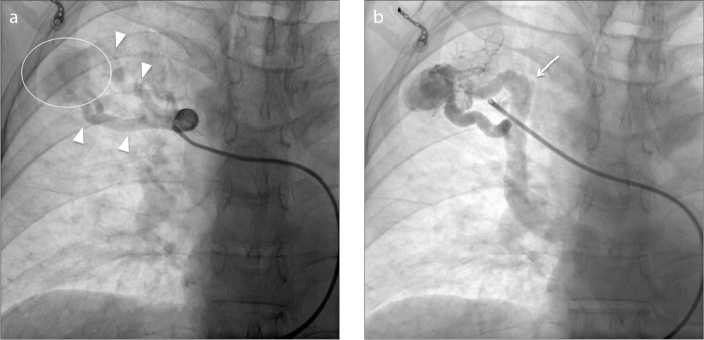
Transcatheter embolization procedure was planned to treat the lesion and written consent was obtained. Local anesthesia was administered around the right femoral vein. Selective catheterization of the upper right pulmonary artery was obtained by a 8F vascular sheath into right common femoral vein, a 7F multipurpose guiding catheter Mach one (Boston Scientific), a 5F HeadHunter H1 catheter (Cook Inc.), and hydrophilic coated guidewire (Glidewire, Terumo). Anteroposterior and oblique views were obtained demonstrating the presence of two feeding arteries that supply a high-flow large pulmonary arteriovenous fistula with rapid opacification of the draining vein in the right upper lobe (Fig. 2).
Figure 2.
a, b. Anteroposterior digital spot film images. Panel (a) shows superselective catheterization of the right upper lobe PAVM (circle) feeding arteries (arrowheads) through coaxial system. Panel (b) shows enlarged draining veins (arrow); this draining vein pattern is presumably related to the high flow output within the PAVM.
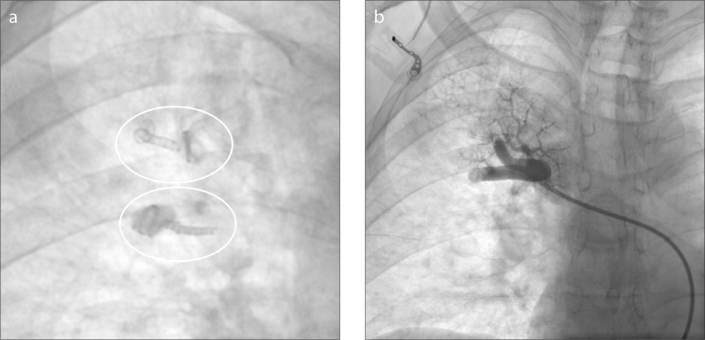
Considering the presence of two large feeding arteries and the high flow rate of the fistula, embolization of the PAVM using the new ArtVentive EOSTM was planned. This implant is made of a Nitinol scaffold with an impermeable expanded polytetrafluoroethylene (ePTFE) membrane and is designed with radial force sufficient to provide radial stiffness and strength against the vessel wall, and to minimize postdeployment migration. The implant carrier catheter contains one implant loaded on the distal end and a deployment handle on the proximal end. The catheter has a stiff proximal section for pushing and a flexible distal section for tracking. The catheter is packaged with a protective sheath over the implant, which should be removed before the delivery. The deployment handle has a side port to accommodate syringe attachment. The handle has two retaining tabs, one blue and one yellow, which prevent premature deployment of the implant, and two pulls for releasing the implant. Deployment is a two-stage process; first, the proximal end is released to check the device position and then the entire device is released if the position is correct. Soon after deployment, the ePTFE membrane results in immediate vessel occlusion. The implant carrier catheter, adequately flushed to remove air, is advanced along the guiding catheter into the distal part of the two feeding arteries. The device diameter is chosen according to the measurement of the afferent feeding artery diameter on CT and angiography examinations and manufacturer’s recommendations that states the ArtVentive EOSTM should exceed the diameter of the vessel to be embolized by 20%. In our case, the two feeding arteries were 7 mm in diameter. The size of the EOSTM implant was chosen as 8 mm, which is recommended for vessel diameters of 4.5–8.0 mm. The patient was heparinized to keep the activated clotting time around 180–200 s. The guiding catheter was then pulled backwards, allowing the device to expand. This procedure was done on both feeding arteries. At the final angiographic control there was immediate technical success with correct positioning of the device, complete occlusion of the two target arteries and no further inflow into the arteriovenous fistula (Fig. 3).
Figure 3.
a, b. Anteroposterior digital spot film (a) after deployment of two ArtVentive EOS™ (circles) devices. Contrast fluoroscopy (b) documents complete occlusion of the two feeding arteries with preserved flow of the upper pulmonary artery.
After this procedure, the patient’s ensuing hospital stay was uneventful and he was discharged after two days.
Discussion
In our case, the utilization of ArtVentive EOSTM allowed us to obtain a complete and immediate occlusion of a large PAVM of the right upper zone.
The treatment of reference for symptomatic PAVM patients is transcatheter embolization with vascular coils, detachable occlusion balloon, and/or Amplatzer vascular plugs; this minimally invasive procedure avoids risks associated with a surgical operation and general anesthesia and prevents the loss of pulmonary parenchyma in lung resection surgery. The aim of transcatheter embolization is to occlude all PAVM feeding arteries by selective catheterization of pulmonary arteries using a coaxial system, via a percutaneous femoral venous approach (1, 2). Endovascular embolization of PAVMs is an effective and durable procedure in the majority of patients (83% of patients in the study of Mager et al.) (8).
Coil embolization is achieved using the anchor technique, which consists of coil blockage within a small collateral branch of the main feeding artery immediately upstream the PAVM, allowing cross-sectional optimal occlusion and preventing further accidental device mobilization and distal migration to systemic circulation. The most feared complication of coil embolization is device migration, which occurs in 2% to 4% of cases in association with high-flow PAVMs and large feeding arteries. Other complications of coil embolization are rare in experienced hands and include gaseous embolism, stroke, pulmonary infarctions, and hemoptysis (1–5). Detachable balloons are no longer used in PAVMs treatment (2).
Endovascular treatment of PAVMs with Amplatzer vascular plug is an effective method for obtaining good long-term results. Because PAVMs have lower pressure and slower flow than medium-sized arteries (e.g., visceral and peripheral arteries), the incidence of recanalization in PAVM appears to be higher than in those vessels. One of the disadvantages of using the Amplatzer plug is the need for catheter exchanges for delivering and deploying the device in smaller, remote, and extremely tortuous vessels. Moreover, the device cannot stop the flow instantly, because there are small interstices in the mesh of the device, taking time to achieve complete occlusion (5–9).
The innovation of our report lies in the use of ArtVentive EOSTM device, developed for percutaneous, peripheral occlusion of the peripheral arterial and venous vasculature. The initial experience with this device was only in varicocele treatment, where it was described as a safe and effective new device for occlusion of vessels. The utilization of this device has some potential advantages such as reduced cost of the procedure and lower radiation exposure for patients and operating physicians; moreover, the ability to deploy in a range of vessels diameters, “oversizing” the device to the target vein by 20% to accommodate high flow rate and unpredictable wall elasticity, reduces the possibility of device migration (7).
In conclusion, ArtVentive EOSTM appears to be a useful device for endovascular treatment of PAVMs, particularly in cases of large feeding and draining vessels. This technology may provide a significant reduction in complication rates (reducing the risk of device migration), shorter procedural and inpatient time, lower procedural and device costs and shorter exposure to radiation for patients and the operating physicians. Moreover, this device can instantly seal the target vessel to stop the flow so that complete occlusion after successful deployment is proven immediately. However, future studies on the utilization of other available sizes and long-term results are necessary to demonstrate the safety and effectiveness of ArtVentive EOSTM in PAVM treatment.
Main points.
Pulmonary arteriovenous malformations (PAVMs) are high-flow, low-pressure shunts consisting of feeding arteries, intervening aneurysmal sacs and draining veins, with up to 90% of the cases occurring in patients affected by hereditary hemorrhagic telangiectasia.
Percutaneous endovascular embolotherapy performed by selective occlusion of PAMV feeding arteries using a coaxial system is currently the treatment of choice.
ArtVentive EOSTM would seem to be a useful device for endovascular treatment of PAVMs reducing complication rates, particularly device migration and paradoxical embolism, and providing a shorter procedural time with lower exposure to radiation for patients and the operating physicians.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Lacombe P, Lacout A, Marcy PY, et al. Diagnosis and treatment of pulmonary arteriovenous malformations in hereditary hemorrhagic telangiectasia: an overview. Diagn Interv Imaging. 2013;94:835–848. doi: 10.1016/j.diii.2013.03.014. http://dx.doi.org/10.1016/j.diii.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Cartin-Ceba R, Swanson KL, Krowka MJ. Pulmonary arteriovenous malformations. Chest. 2013;144:1033–1044. doi: 10.1378/chest.12-0924. http://dx.doi.org/10.1378/chest.12-0924. [DOI] [PubMed] [Google Scholar]
- 3.Ando K, Mochizuki A, Kurimoto N, et al. Coil embolization for pulmonary arteriovenous malformation as an organ-sparing therapy: Outcome of long-term follow-up. Ann Thorac Cardiovasc Surg. 2011;17:118–123. doi: 10.5761/atcs.oa.10.01536. http://dx.doi.org/10.5761/atcs.oa.10.01536. [DOI] [PubMed] [Google Scholar]
- 4.Pierucci P, Murphy J, Henderson KJ, Chyun DA, White RI., Jr New definition and natural history of patients with diffuse pulmonary arteriovenous malformations: twenty-seven-year experience. Chest. 2008;133:653–661. doi: 10.1378/chest.07-1949. http://dx.doi.org/10.1378/chest.07-1949. [DOI] [PubMed] [Google Scholar]
- 5.Swischuk JL, Castaneda F, Smouse HB, Fox PF, Brady TM. Embolization of pulmonary arteriovenous malformations. Semin Interv Radiol. 2000;17:171–183. http://dx.doi.org/10.1055/s-2000-9444. [Google Scholar]
- 6.Greben CR, Setton A, Putterman D, Caplin D, Lenner R, Gandras EJ. Pulmonary arteriovenous malformation embolization: How we do it. Tech Vasc Interv Rad. 2013;16:39–44. doi: 10.1053/j.tvir.2013.01.005. http://dx.doi.org/10.1053/j.tvir.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Venbrux AC, Rudakov L, Plass A, Emmert MY, Ebner A. A new occlusion device: application of the ArtVentive enoluminal occlusion system (EOS) - first in human clinical trial. Cardiovasc Interv Radiol. 2014;37:85–93. doi: 10.1007/s00270-013-0626-y. http://dx.doi.org/10.1007/s00270-013-0626-y. [DOI] [PubMed] [Google Scholar]
- 8.Mager JJ, Overtoom TT, Blauw H, Lammers JW, Westermann CJ. Embolotherapy of pulmonary arteriovenous malformations: long-term results in 112 patients. J Vasc Interv Radiol. 2004;15:451–456. doi: 10.1097/01.rvi.0000126811.05229.b6. http://dx.doi.org/10.1097/01.RVI.0000126811.05229.B6. [DOI] [PubMed] [Google Scholar]
- 9.Rossi M, Rebonato A, Greco L, et al. A new device for vascular embolization: report on case of two pulmonary arteriovenous fistulas embolization using the Amplatzer vascular plug. Cardiovasc Interv Radiol. 2006;29:902–906. doi: 10.1007/s00270-005-0160-7. http://dx.doi.org/10.1007/s00270-005-0160-7. [DOI] [PubMed] [Google Scholar]