Abstract
PURPOSE
We aimed to evaluate the value of 18F-FDG positron emission tomography/computed tomography (PET/CT) for identifying the possible causes of secondary hemophagocytic lymphohistiocytosis (HLH).
METHODS
Forty-five cases (17 female, 28 male; age, 17–79 years) with secondary HLH were included. The standard of reference for diagnosis in all patients was a combination of histology, clinical results (medical history, physical examination, and laboratory test results), and follow-up imaging for at least 12 months. All cases underwent 18F-FDG PET/CT to identify the possible trigger in HLH.
RESULTS
Of 45 secondary HLH cases 10 (22.2%) were associated with infection, seven (15.6%) with rheumatic disease, and 28 (62.2%) with lymphoma. PET/CT images of 22 secondary HLH cases (48.9%) showed true positive results. PET/CT images demonstrated obvious tracer uptake in five of 10 secondary HLH cases with infection, one of three cases with lupus, two of two cases with rheumatoid arthritis, one of two cases with adult-onset Still disease, and 13 of 28 cases with lymphoma.
CONCLUSION
PET/CT is helpful for identifying the possible trigger (infection or malignant disease) and extent of secondary HLH. However, PET/CT alone is not sufficient to make a correct differential diagnosis.
Hemophagocytic lymphohistiocytosis (HLH) is a rare systemic inflammatory syndrome associated with numerous conditions, such as infection, tumor, and autoimmune diseases (1). HLH has been traditionally divided into primary and secondary forms. HLH is a hyperinflammatory, uncontrolled immune response triggered by different stimuli. Although control of hyperinflammation is the number one priority, the search for a medically treatable stimulus is also necessary (2). Conversely, there is much difficulty in demonstrating the underlying causes of secondary HLH.
18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) has been widely applied in the process of malignant (3–5) and benign diseases (6), such as fever of unknown origin (7, 8). However, rare studies reported the role of PET/CT in demonstrating the underlying cause of secondary HLH. The purpose of the present study is to evaluate the value of 18F-FDG PET/CT for demonstrating the possible causes of secondary HLH.
Methods
Patient population
A total of 45 cases (17 female and 28 male; mean age 46 years, age range 17–79 years) admitted to our hospital between January 2008 and December 2012 with a diagnosis of secondary HLH were included in this retrospective study. The HLH diagnostic criteria revised by HLH Investigation Group was used in this study (9). Patients with primary HLH were excluded. In addition, patients with an active malignancy, known infection or inflammation, were also excluded. All patients underwent PET/CT examination. Relevant data of the patients were retrieved and analyzed.
Clinical management
Relevant medical history was obtained and physical examination was performed, including laboratory surveys, for all HLH patients. This included routine blood tests, urinalysis, Gruber-Widal agglutination, Brucella serum agglutination, rheumatoid factor, antineutrophilic and antinuclear cytoplasmic antibodies, ultrasonography, and radiography. In some conditions, serologic tests for Epstein-Barr virus, cytomegalovirus, Brucella, Toxoplasma, herpes, and hepatitis viruses, CT, MRI, and biopsy were performed. PET/CT was used for the differential diagnosis of possible cause of HLH. The final diagnosis of all cases was based on comprehensive analysis of laboratory, imaging results, and follow-up.
18F-FDG-PET/CT imaging
Whole body PET/CT scan was performed. The mean dose of intravenous 18F-FDG was 350MBq (range, 277.5–432.9 MBq). The scan coverage was from the top of the head to the mid-thigh. After the emission scan, low-dose CT was acquired for attenuation correction and anatomic localization of PET images. PET/CT scans started 45–60 min after 18F-FDG injection. Emission scans of PET/CT were obtained in three-dimensional (3D) mode. The PET/CT images were evaluated in transverse, sagittal, and coronal planes and in rotating maximum-intensity projection series.
Interpretation of images
All patients were followed for at least 12 months. Two experienced double-board certified radiologist/nuclear physicians interpreted the 18F-FDG PET/CT images on a high-resolution computer screen and reached a consensus for each scan. 18F-FDG PET/CT images were judged to be positive if focal accumulation of the 18F-FDG was detected outside of the areas of physiologic uptake, not just based on the standardized uptake values (SUV). True-positive result was defined as a PET/CT scan showing a focal 18F-FDG positive lesion, which subsequently proved to be the trigger of the secondary HLH. PET/CT of a true-positive case contributed to identification of the cause of secondary HLH, whether it was infection or malignant disease. False-positive result was considered as hypermetabolic foci that could not be recognized as the potential cause of secondary HLH. Normal 18F-FDG accumulations were considered as true negative. If a lesion (including noninfectious inflammatory, infectious, or malignancy disease) was identified during follow-up, the negative PET/CT was recognized as a false-negative result.
Statistical analysis
All statistical tests were performed using SPSS 20.0 software (IBM Corp.). Descriptive statistics were calculated as mean ± standard deviation (SD).
Results
Secondary HLH patients comprised 10 cases (22.2%) associated with infection, seven cases (15.6%) with rheumatic disease, and 28 cases (62.2%) with lymphoma. Based on histology and follow-up data, PET/CT images of 22 of 45 secondary HLH cases (48.9%) were considered as true positive. True-positive cases comprised five secondary HLH patients associated with infection, four secondary HLH cases associated with rheumatic diseases, and 13 secondary HLH cases associated with lymphoma. In true-positive cases, PET/CT showed increased 18F-FDG distribution in the bone marrow (20/22), lungs (15/22), and joints (5/22); multiple FDG-avid lymphadenopathy in the neck, axillary, thoracic, abdominopelvic, and inguinal regions (19/22); and enlarged liver and spleen (18/22).
Ten secondary HLH cases associated with infection comprised four cases with Epstein-Barr virus (EBV), three cases with cytomegalovirus, three cases with human herpes virus. Five of ten secondary HLH patients associated with infection demonstrated obvious 18F-FDG uptake on PET/CT, comprising three cases with EBV, one case with cytomegalovirus, and one case with human herpes virus. PET/CT of the remaining five secondary HLH patients associated with infection were considered as false negative.
Seven secondary HLH cases with rheumatic diseases comprised three cases with systemic lupus erythematosus (SLE), two cases with rheumatoid arthritis, and two cases with adult-onset Still disease. In this investigation, PET/CT of one secondary HLH patient associated with SLE demonstrated obvious 18F-FDG uptake in multiple lymph nodes (Fig. 1) and enlarged spleen, which suggested active inflammation. PET/CT of one secondary HLH patient associated with adult-onset Still disease demonstrated obvious 18F-FDG uptake in multiple organs. PET/CT of two secondary HLH patients associated with rheumatoid arthritis demonstrated obvious 18F-FDG uptake in multiple joints, such as shoulder, hip, and wrist joints, which also suggested active inflammation of these joints.
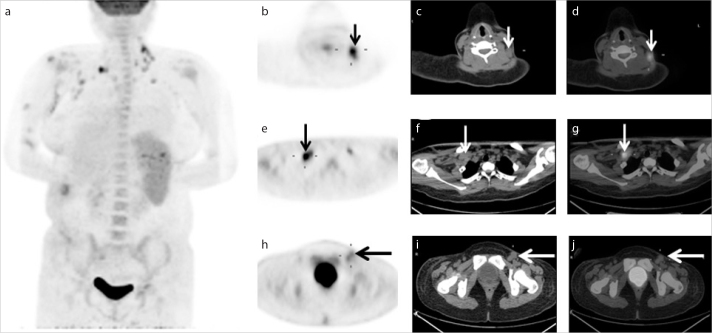
Figure 1.
a–j. A 29-year-old Chinese male presenting with fatigue and high fever lasting for two weeks. The patient had a history of systemic lupus erythematosus (SLE). According to medical history and histopathology, the patient was diagnosed with secondary hemophagocytic lymphohistiocytosis (HLH) associated with SLE. The maximum intensity projection (a), PET (b, e, h), CT (c, f, i), and fused (d, g, j) images showed significantly hypermetabolic lymph nodes in the bilateral cervical (small arrows in b–d), axillary (medium arrows in e–g), left inguinal lymph nodes (large arrows in h–j). There was significantly increased uptake of 18F-FDG in the spleen, pelvic bone, proximal location of long bones, and centrum.
Twenty-eight secondary HLH cases associated with lymphoma in this study comprised 14 cases with T-cell non-Hodgkin lymphoma (NHL), eight cases with large cell anaplastic lymphoma, four cases with diffuse large B-cell lymphoma, and two cases with Hodgkin lymphoma. In this investigation, PET/CT images of 15 of 28 secondary HLH cases associated with lymphoma did not show increased 18F-FDG uptake. These 15 cases comprised seven cases associated with T-cell NHL, six cases with large cell anaplastic lymphoma, one case with diffuse large B-cell lymphoma, and one case with Hodgkin lymphoma. PET/CT of 13 of 28 secondary HLH patients with lymphoma demonstrated obvious 18F-FDG uptake, comprising seven cases with T-cell NHL (Fig. 2), two cases with large cell anaplastic lymphoma, three cases with diffuse large B-cell lymphoma (Fig. 3), and one case with Hodgkin lymphoma (Table).
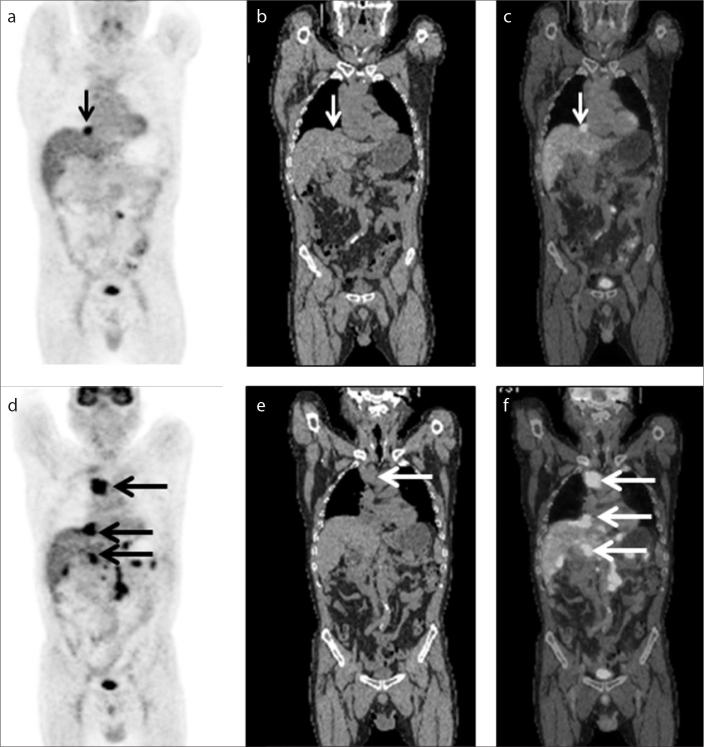
Figure 2.
a–i. A 39-year-old Chinese female presenting with weakness, fatigue, and high fever. According to medical history and histopathology, the patient was diagnosed with secondary HLH associated with T-cell non-Hodgkin lymphoma. The PET (a, d, g), CT (b, e, h), and fused (c, f, i) images showed significantly hypermetabolic lymph nodes in the axillary and bilateral cervical (small and medium arrows in a–f), axillary (large arrows in g–i), left inguinal, and retroperitoneal lymph nodes, and increased uptake of 18F-FDG in the spleen (void arrows in g–i).
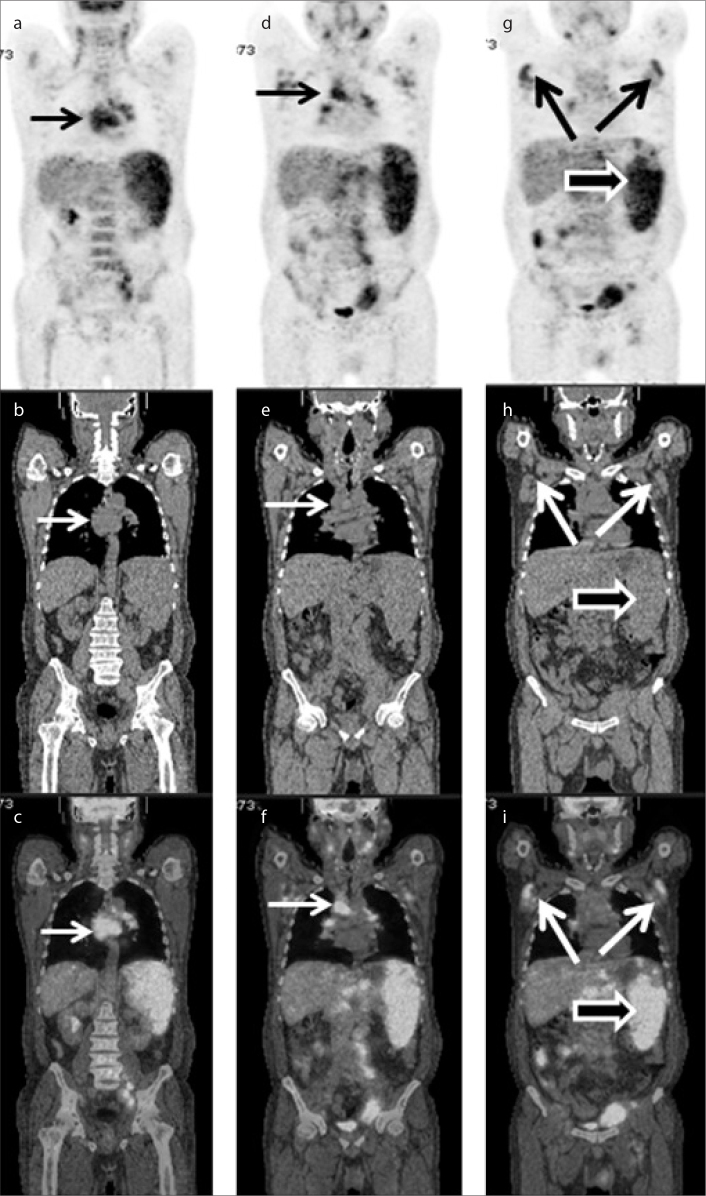
Figure 3.
a–f. A 48-year-old Chinese male presenting with fever of unknown origin. The medical history included diffuse large B-cell lymphoma. According to medical history and histopathology, the patient was diagnosed with secondary HLH associated with diffuse large B-cell lymphoma. The first coronal PET (a), CT (b) and fused (c) images showed significantly hypermetabolic lymph nodes in the retroperitoneal region (small arrows in a–c). The second coronal PET (d), CT (e) and fused (f) images showed multiple, significantly hypermetabolic lymph nodes in the thoracic, abdominal, and retroperitoneal regions (large arrows in d–f), which indicated progression of diffuse large B-cell lymphoma.
Table.
Number of cases with true positive 18F-FDG PET/CT results in different underlying causes of secondary HLH
| Cause | No. of cases | True positive cases | |
|---|---|---|---|
| Infection (n=10) | Epstein-Barr virus | 4 | 3 |
| Cytomegalovirus | 3 | 1 | |
| Human herpes virus | 3 | 1 | |
|
| |||
| Rheumatic disease (n=7) | Systemic lupus erythematosus | 3 | 1 |
| Rheumatoid arthritis | 2 | 2 | |
| Adult-onset Still disease | 2 | 1 | |
|
| |||
| Lymphoma (n=28) | T-cell non-Hodgkin lymphoma | 14 | 7 |
| Large cell anaplastic lymphoma | 8 | 2 | |
| Diffuse large B-cell lymphoma | 4 | 3 | |
| Hodgkin lymphoma | 2 | 1 | |
|
| |||
| Total | 45 | 22 | |
Discussion
HLH is characterized by overwhelming immune activation, and hyperinflammation (10–13). Secondary HLH is associated with a variety of diseases. HLH triggered by infection is the most common cause (2, 14). In our study, 18F-FDG PET/CT of five of 10 secondary HLH patients after infection demonstrated obvious FDG uptake, including three cases with EBV, one case with cytomegalovirus, and one case with human herpes virus. The 18F-FDG PET/CT of the remaining five secondary HLH patients after infection can be considered as false-negative. In our study, PET/CT was considered to be true positive if abnormal imaging tracer uptake was detected as the cause of secondary HLH, regardless of it being caused by infection or malignant disease. However, 18F-FDG PET/CT findings alone are not sufficient to make a differential diagnosis of infection versus malignant disease. Biopsy is essential to definitively distinguish malignancy from infection. Previous studies have reported EBV infection mimicking lymphoma on PET/CT. 18F-FDG PET/CT has been used in acute and chronic inflammatory diseases, since activated leukocytes uptake 18F-FDG (8). Kubota et al. (15) reported increased uptake of 18F-FDG in granulocytes and activated macrophages. Mäkitie et al. (16) reported that the nasopharyngeal carcinoma patients with normal PET scans had low EBV viremia and were in remission, while two patients with abnormal PET results had high EBV concentrations and relapsed with distant metastasis. Based on these limited data, we speculate that the false-negative result of PET/CT in secondary HLH with virus infection might be related to low virus titer. As 18F-FDG is a nonspecific tracer, it can accumulate in infectious or neoplastic tissues, and it is also observed in activated lymphocytes, monocytes, granulocytes. Therefore, the disadvantage of PET/CT in secondary HLH is the impossibility to differentiate among inflammation, infectious disease, and malignancy. However, in HLH patients this appears to be an advantage rather than a disadvantage as inflammation, infectious disease, and malignancy are the potential triggers of HLH. Further diagnostic examinations are needed in most HLH patients. Biopsy is essential to definitively distinguish malignancy from infection (17, 18).
If HLH occurs in autoinflammatory or autoimmune diseases patients, it can be also called macrophage activation syndrome (MAS). The potential trigger of MAS patients includes arthritis, SLE, or other rheumatic diseases (13,19). In our study, 18F-FDG PET/CT of one secondary HLH patient associated with SLE demonstrated obvious 18F-FDG uptake in numerous lymph nodes, which suggested active inflammation. PET/CT has become one valuable ancillary method in evaluating rheumatoid arthritis. Wang et al. (20) reported that PET/CT could be applied not only to evaluate the extent of rheumatoid arthritis of the whole body, but also to evaluate the therapy response. In this study 18F-FDG PET/CT of two secondary HLH patients associated with rheumatoid arthritis demonstrated obvious 18F-FDG uptake in multiple joints, such as shoulder, hip, wrist joints, suggesting active inflammation of these joints. Increased 18F-FDG uptake in the wrist, hip, shoulder joints in rheumatoid arthritis patients has been reported previously (21). In this paper 18F-FDG PET/CT of one secondary HLH patient associated with adult-onset Still disease demonstrated obvious imaging tracer uptake in multiple organs, including lymph node, spleen, and bone marrow. In total, 18F-FDG PET/CT plays an important role in the course of autoimmune diseases (22–26), particularly in secondary HLH associated with autoimmune diseases.
Malignant lymphomas associated with HLH are often of T-cell origin (27). In our study, PET/CT images of 15 of 28 secondary HLH cases associated with lymphoma did not show increased 18F-FDG uptake. These 15 cases comprised seven cases associated with T-cell NHL, six cases with large cell anaplastic lymphoma, one case with diffuse large B-cell lymphoma, and one case with Hodgkin lymphoma. Cheson et al. (28) reported that PET is a strongly recommended application during therapy for patients with routinely 18F-FDG avid diseases, such as Hodgkin lymphoma and diffuse large B-cell lymphoma. Karantanis et al. (29) reported PET avidity rate was 80%–100% in extranodal NK-cell lymphoma (ENKL), angioimmunoblastic T-cell lymphoma, and anaplastic large cell lymphoma.
A limitation of this study was its relative small sample size. HLH is a rare disease, and the standard diagnostic and treatment protocol does not recommend PET/CT scanning during the process of HLH. In this study, we retrospectively analyzed secondary HLH patients who underwent PET/CT scan to identify the underlying cause of HLH. Therefore, diagnostic accuracy in the current study may be influenced by referral bias or selection bias. Although the results of this study are still preliminary, the data suggest that PET/CT may be effective in diagnosing and aiding diagnoses in patients with secondary HLH.
In conclusion, this clinical research suggests that PET/CT is helpful for identifying the possible trigger and extent of secondary HLH. PET/CT can show not only the degree of HLH activity (including benign and malignant disease), but also the distribution of HLH in the whole body. However, PET/CT scans alone are not enough to make a correct differential diagnosis among different potential diseases. Biopsy is essential to definitively distinguish malignancy from infection. Further research with a larger population and multicenter data analysis should be undertaken to validate the clinical role of PET/CT in secondary HLH.
Main points.
It is very important to demonstrate the underlying cause of secondary hemophagocytic lymphohistiocytosis (HLH).
18F-FDG PET/CT is helpful in identifying the possible causes and extent of secondary HLH. 18F-FDG PET/CT can show not only the degree of HLH activity, but also the distribution of HLH in the whole body.
18F-FDG PET/CT scans alone are not enough to make a correct differential diagnosis among different potential diseases.
Footnotes
The abstract of this study was presented at Society of Nuclear Medicine and Molecular Imaging Annual Meeting 2015 56:1424.
Financial disclosure
Jigang Yang was supported by 2014 Beijing Excellent Talent Fund (No:2014000021223ZK45), Beijing Natural Science Foundation (No: 7152041), 2015 Capital Clinical Characteristics Project.
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Rosado FG, Kim AS. Hemophagocytic lymphohistiocytosis: an update on diagnosis and pathogenesis. Am J Clin Pathol. 2013;139:713–727. doi: 10.1309/AJCP4ZDKJ4ICOUAT. http://dx.doi.org/10.1309/AJCP4ZDKJ4ICOUAT. [DOI] [PubMed] [Google Scholar]
- 2.Janka GE, Lehmberg K. Hemophagocytic lymphohistiocytosis: pathogenesis and treatment. Hematology Am Soc Hematol Educ Program. 2013;2013:605–611. doi: 10.1182/asheducation-2013.1.605. [DOI] [PubMed] [Google Scholar]
- 3.Yang J, Zhen L, Zhuang H. Bone marrow metastases from alveolar rhabdomyosarcoma with impressive FDG PET/CT finding but less-revealing bone scintigraphy. Clin Nucl Med. 2013;38:988–991. doi: 10.1097/RLU.0000000000000277. http://dx.doi.org/10.1097/RLU.0000000000000277. [DOI] [PubMed] [Google Scholar]
- 4.Yang J, Codreanu I, Servaes S, Zhuang H. Earlier detection of bone metastases from pleomorphic liposarcoma in a pediatric patient by FDG PET/CT than planar 99mTc MDP bone scan. Clin Nucl Med. 2012;37:e104–107. doi: 10.1097/RLU.0b013e3182478da8. http://dx.doi.org/10.1097/RLU.0b013e3182478da8. [DOI] [PubMed] [Google Scholar]
- 5.Yang J, Codreanu I, Servaes S, Zhuang H. Metastatic embryonal rhabdomyosarcoma to the pancreas presenting as acute pancreatitis detected by FDG PET/CT. Clin Nucl Med. 2012;37:694–696. doi: 10.1097/RLU.0b013e31824c6066. http://dx.doi.org/10.1097/RLU.0b013e31824c6066. [DOI] [PubMed] [Google Scholar]
- 6.Jin H, Yuan L, Li C, Kan Y, Hao R, Yang J. Diagnostic performance of FDG PET or PET/CT in prosthetic infection after arthroplasty: a meta-analysis. Q J Nucl Med Mol Imaging. 2014;58:85–93. [PubMed] [Google Scholar]
- 7.Tokmak H, Ergonul O, Demirkol O, Cetiner M, Ferhanoglu B. Diagnostic contribution of (18)F-FDG-PET/CT in fever of unknown origin. Int J Infect Dis. 2014;19:53–58. doi: 10.1016/j.ijid.2013.10.009. http://dx.doi.org/10.1016/j.ijid.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Hao R, Yuan L, Kan Y, Li C, Yang J. Diagnostic performance of 18F-FDG PET/CT in patients with fever of unknown origin: a meta-analysis. Nucl Med Commun. 2013;34:682–688. doi: 10.1097/MNM.0b013e328361cd0e. http://dx.doi.org/10.1097/MNM.0b013e328361cd0e. [DOI] [PubMed] [Google Scholar]
- 9.Henter JI, Horne A, Arico M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–131. doi: 10.1002/pbc.21039. http://dx.doi.org/10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 10.Brisse E, Wouters CH, Matthys P. Hemophagocytic lymphohistiocytosis (HLH): A heterogeneous spectrum of cytokine-driven immune disorders. Cytokine Growth Factor Rev. 2015;26:263–280. doi: 10.1016/j.cytogfr.2014.10.001. http://dx.doi.org/10.1016/j.cytogfr.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Lane S, Andrist C, Nagarajan A. Hemophagocytic lymphohistiocytosis (HLH) in a 25-year-old presenting with multisystem organ failure. W V Med J. 2013;109:22–23. [PubMed] [Google Scholar]
- 12.Mehta RS, Smith RE. Hemophagocytic lymphohistiocytosis (HLH): a review of literature. Med Oncol. 2013;30:740. doi: 10.1007/s12032-013-0740-3. http://dx.doi.org/10.1007/s12032-013-0740-3. [DOI] [PubMed] [Google Scholar]
- 13.Atteritano M, David A, Bagnato G, et al. Haemophagocytic syndrome in rheumatic patients. A systematic review. Eur Rev Med Pharmacol Sci. 2012;16:1414–1424. [PubMed] [Google Scholar]
- 14.Yuan L, Zou L, Li C, Yang J. Intense and diffuse lung uptake of 99mTc-MDP in a patient with pneumonia associated with secondary hemophagocytic lymphohistiocytosis. Clin Nucl Med. 2014;39:1000–1002. doi: 10.1097/RLU.0000000000000483. http://dx.doi.org/10.1097/RLU.0000000000000483. [DOI] [PubMed] [Google Scholar]
- 15.Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluorodeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med. 1992;33:1972–1980. [PubMed] [Google Scholar]
- 16.Makitie AA, Reis PP, Irish J, et al. Correlation of Epstein-Barr virus DNA in cell-free plasma, functional imaging and clinical course in locally advanced nasopharyngeal cancer: a pilot study. Head Neck. 2004;26:815–822. doi: 10.1002/hed.20028. http://dx.doi.org/10.1002/hed.20028. [DOI] [PubMed] [Google Scholar]
- 17.Thomas DL, Syrbu S, Graham MM. Epstein-Barr virus mimicking lymphoma on FDG-PET/CT. Clin Nucl Med. 2009;34:891–893. doi: 10.1097/RLU.0b013e3181bed135. http://dx.doi.org/10.1097/RLU.0b013e3181bed135. [DOI] [PubMed] [Google Scholar]
- 18.Lustberg MB, Aras O, Meisenberg BR. FDG PET/CT findings in acute adult mononucleosis mimicking malignant lymphoma. Eur J Haematol. 2008;81:154–156. doi: 10.1111/j.1600-0609.2008.01088.x. http://dx.doi.org/10.1111/j.1600-0609.2008.01088.x. [DOI] [PubMed] [Google Scholar]
- 19.Melikoglu MA, Melikoglu M. The clinical importance of lymphadenopathy in systemic lupus erythematosus. Acta Reumatol Port. 2008;33:402–406. [PubMed] [Google Scholar]
- 20.Wang SC, Xie Q, Lv WF. Positron emission tomography/computed tomography imaging and rheumatoid arthritis. Int J Rheum Dis. 2014;17:248–255. doi: 10.1111/1756-185X.12316. http://dx.doi.org/10.1111/1756-185X.12316. [DOI] [PubMed] [Google Scholar]
- 21.Taniguchi Y, Arii K, Kumon Y, et al. Positron emission tomography/computed tomography: a clinical tool for evaluation of enthesitis in patients with spondyloarthritides. Rheumatology (Oxford) 2010;49:348–354. doi: 10.1093/rheumatology/kep379. http://dx.doi.org/10.1093/rheumatology/kep379. [DOI] [PubMed] [Google Scholar]
- 22.Yamashita H, Kubota K, Takahashi Y, et al. Clinical value of (1)(8)F-fluoro-dexoxyglucose positron emission tomography/computed tomography in patients with adult-onset Still’s disease: a seven-case series and review of the literature. Mod Rheumatol. 2014;24:645–650. doi: 10.3109/14397595.2013.850998. http://dx.doi.org/10.3109/14397595.2013.850998. [DOI] [PubMed] [Google Scholar]
- 23.Curiel R, Akin EA, Beaulieu G, DePalma L, Hashefi M. PET/CT imaging in systemic lupus erythematosus. Ann N Y Acad Sci. 2011;1228:71–80. doi: 10.1111/j.1749-6632.2011.06076.x. http://dx.doi.org/10.1111/j.1749-6632.2011.06076.x. [DOI] [PubMed] [Google Scholar]
- 24.Beckers C, Ribbens C, Andre B, et al. Assessment of disease activity in rheumatoid arthritis with (18)F-FDG PET. J Nucl Med. 2004;45:956–964. [PubMed] [Google Scholar]
- 25.Yamashita H, Kubota K, Mimori A. Clinical value of whole-body PET/CT in patients with active rheumatic diseases. Arthritis Res Ther. 2014;16:423. doi: 10.1186/s13075-014-0423-2. http://dx.doi.org/10.1186/s13075-014-0423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okamura K, Yonemoto Y, Arisaka Y, et al. The assessment of biologic treatment in patients with rheumatoid arthritis using FDG-PET/CT. Rheumatology (Oxford) 2012;51:1484–1491. doi: 10.1093/rheumatology/kes064. http://dx.doi.org/10.1093/rheumatology/kes064. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi N, Chubachi A, Kume M, et al. A clinical analysis of 52 adult patients with hemophagocytic syndrome: the prognostic significance of the underlying diseases. Int J Hematol. 2001;74:209–213. doi: 10.1007/BF02982007. http://dx.doi.org/10.1007/BF02982007. [DOI] [PubMed] [Google Scholar]
- 28.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. http://dx.doi.org/10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 29.Karantanis D, Subramaniam RM, Peller PJ, et al. The value of [(18)F]fluorodeoxyglucose positron emission tomography/computed tomography in extranodal natural killer/T-cell lymphoma. Clin Lymphoma Myeloma. 2008;8:94–99. doi: 10.3816/CLM.2008.n.010. http://dx.doi.org/10.3816/CLM.2008.n.010. [DOI] [PubMed] [Google Scholar]