Abstract
Background. In recent years, with the popularity of CHM, its hepatotoxicity has also been increasingly noticed. However, there are still veils on causative herbs and clinical characteristics. Aim. To systematically review data on CHM induced liver injury with particular focus on causative herbs and clinical characteristics. Methods. Using terms related to CHM and liver injury, PubMed and three Chinese electronic databases were searched, which was limited to the past 5 years. Publications meeting our eligibility criteria were included and further analyzed. Results. In total, 4 single herbs, 21 patent drugs, and 4 decoctions were reported to be of hepatotoxicity, with He-Shou-Wu being the most common one (65/114). Dang-Gui and other 5 herbs were the most common ingredients of patent drugs and decoctions. All patients were assessed using the RUCAM scale, with 26 being highly probable and 28 being probable. For these 54 cases, the latent period was 30 (47) days, and 81.48% were labeled as hepatocellular injuries. Most patients (96.3%) recovered, apart from the fact that one died and one is receiving liver transplantation. Conclusions. CHM should be used carefully for hepatotoxicity. Liver injury from CHM is similar to that from conventional medicines in clinical characteristics. Details about causative herbs should be illustrated, and more RUCAM should be used in future.
1. Introduction
Traditional Chinese medicine (TCM), originated in ancient China, has been widely used to treat diseases for thousands of years, using Chinese herbal medicine (CHM), acupuncture, moxibustion, and other body practices. In recent decades, TCM has been increasingly popular around the world [1–4]. As the main part of TCM, CHM is usually combined in formulas and taken orally as decoction, powders, and other forms, following TCM theories. Although CHM plays an important role in health care, more and more liver injury cases from CHM are reported. While the exact number is unavailable, nearly 20% of drug-induced liver injuries (DILI) were due to CHM in China [5, 6]. Therefore, it is of great importance to study CHM induced liver injury.
To learn CHM induced liver injury, some questions are inevitable, namely, which herbs can lead to liver injury specifically, when it will cause liver injury, and what the clinical characteristics are. Though some reviews [7–10] have been published to give detailed information, they did not take Chinese electronic database into account, in which large amounts of data about use and adverse events of CHM are found. While these reviews did put an emphasis on causality assessment by the Roussel Uclaf Causality Assessment Method (RUCAM) scale [11] and positive reexposure tests, they paid little attention to strict definition of hepatotoxicity. A low threshold of liver enzyme values may allow cases with nonspecific increases in.
To help clinicians and TCM practitioners know more about and avoid CHM induced liver injury, we systematically reviewed publications, giving a list of Chinese herbal medicines with possible hepatotoxicity and summarizing associated clinical characteristics. Since more attention has been paid to DILI in recent years and the diagnosis has also developed both in China and the world, our review focused on literature of the past 5 years.
2. Methods
2.1. Literature Search
Our review was planned and performed in conformity with Cochrane Handbook for Systematic Reviews of Interventions [12] and Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement [13], which was also published on the PROSPERO register, with a registration number being CRD42016036053. A literature search in PubMed database and three Chinese electronic databases, including China National Knowledge Infrastructure (CNKI), Wan Fang database, and VIP database, was independently carried out by two investigators, using the terms “herb∗,” “Chinese medicine,” “traditional medicine,” and “complementary and alternative medicine” and “liver injury,” “hepatotoxicity,” “liver disease,” and “hepatitis.” The maximal number of articles was obtained using terms in all possible combinations. The search was limited to English and Chinese language articles and restricted between 2011 and March 1, 2016.
2.2. Eligibility Criteria
Articles included have to meet the following criteria. (1) Studies on human subjects are included. (2) Liver injury is specifically induced by Chinese herbal medicines, which include single herbs, patent drugs, and decoctions made up of herbal ingredients. Herbs included should be usually used by TCM practitioners or officially listed in the Chinese Pharmacopoeia [14]. (3) Liver injury is defined as elevations of ALT above 5 times the upper limit of normal (ULN) and/or ALP above 2 times ULN. If ALT > 5ULN and ALP ≤ ULN or if both ALT and ALP are elevated, R ≥ 5, the liver injury is hepatocellular. If ALP > 2ULN and ALT ≤ ULN or if both ALT and ALP are elevated, R ≤ 2, the liver injury is cholestatic. If ALT > 5N and ALP > N and 2 < R < 5, the liver injury is mixed. (4) Causality assessment is done using the RUCAM scale [11], with a score no less than 3 points. If the pattern of liver injury is hepatocellular, a subtype of RUCAM for hepatocellular injury is used, and if it is cholestatic or mixed, a subtype for the cholestatic or mixed injury is performed.
2.3. Study Selection and Data Extraction
Included articles were independently reviewed by two authors, based on title/abstract firstly and full-text secondly. During the process of full-text selection, disagreements were resolved by discussion, and if an agreement could not be reached, a third author would make a decision. The following data were recorded: causative herbs, demographic information, regional distribution, primary diseases, usage and dosage, latent period, laboratory results, pattern of liver injury, causality assessment, reexposure results, and clinical outcomes.
2.4. Statistical Analysis
A descriptive analysis was used. Enumeration data was described with frequency distribution, while measurement data was described with centralized tendency. Normally distributed data was described as Mean ± Standard Deviation, while data obeying abnormal distribution was presented as Median (Interquartile Range). All statistical analyses were performed using SPSS software (version 20.0). Cases with incomplete clinical information were also included in this review, but only those with well-defined values for each parameter were included in the statistical analysis.
3. Results
3.1. Literature Selection and Characteristics
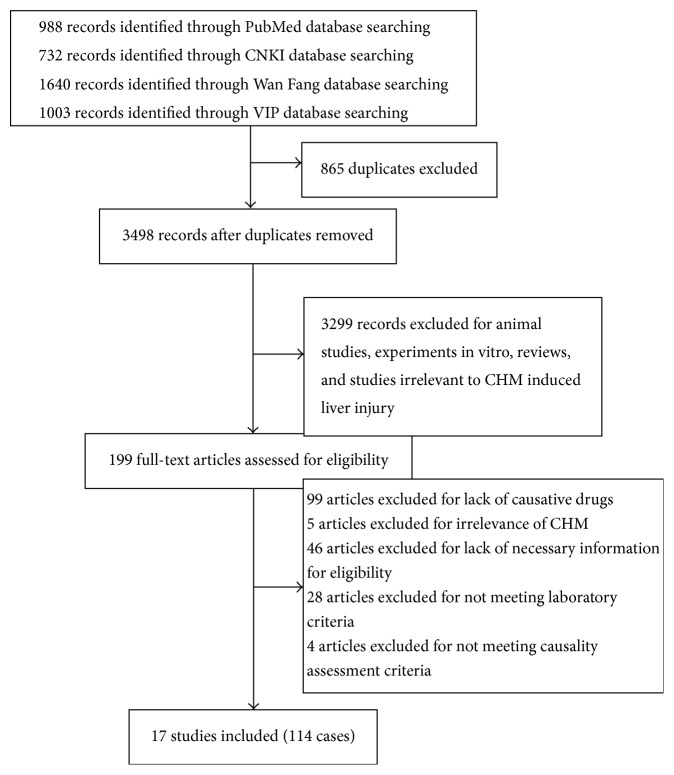
The initial search produced 4363 articles (Figure 1), of which 865 were excluded for duplicates and 3299 were eliminated as animal studies, experiments in vitro, reviews, and studies irrelevant to CHM. After further evaluation, 17 articles [15–31] fulfilled the eligibility criteria, of which 14 were case reports, 2 were case series, and 1 was cross-sectional study. 114 cases were included in total, of which 83 cases were from China, 26 were from Korea, 4 were from the United States, and 1 was from Japan. Detailed information of CHM, like locality and specific taxa, was not mentioned in all 17 articles, and only 4 of 17 described correct scientific names of herbs [18, 19, 21, 22]. Among six articles reporting liver injury attributed to Chinese patent drugs [15–17, 21, 23, 29], there was one [29] without detailed herbal ingredients, six without herbal contents, three [15, 23, 29] without recommended dosage and usage, and three [16, 23, 29] without brand names or manufacturers. Only 1 of 4 articles reporting decoctions mentioned detailed herbal dosage [30].
Figure 1.
Flow chart of literature selection.
3.2. Identification of CHM with Reported Hepatotoxicity
In total, 4 kinds of single herbs, 21 patent drugs, and 4 decoctions made up of multiple herbs were reported to have caused liver injury, including He-Shou-Wu [Reynoutria multiflora (Thunb.) Moldenke], Cang-Er-Zi [Xanthium strumarium subsp. sibiricum (Patrin ex Widder) Greuter], Huang-Yao-Zi (Dioscorea bulbifera L.), Lei-Gong-Teng (Tripterygium wilfordii Hook. f.), Yang Xue Sheng Fa Jiao Nang, Bai Dian Feng Jiao Nang, Xiao Yin Pian, Qu Bai Ba Bu Pian, Bu Shen Sheng Fa Tang, Ze Qi Chong Ji, Xian Ling Gu Bao Jiao Nang, Gu Kang Jiao Nang, Zhuang Gu Jiao Nang, Ling Zhi Yi Shou Jiao Nang, Ling Zhi Jiao Nang, Hui Chun Ru Yi Jiao Nang, Ru Bi San, ShuXiong Jiao Nang, Zeng Sheng Ping, Long Bi Shu, Zhi Xue Jiao Nang, Move Free, Ban Tu Wan, Kamishoyosan, Qi Bao Mei Ran Wan, herbal extracts containing Hu-Ji-Sheng [Viscum coloratum (Kom.) Nakai] and Ye-Ge (Pueraria montana var. lobata (Willd.) Sanjappa & Pradeep), herbal tea containing Kelp, and two decoctions consisting of CHM. Detailed information is listed in Table 1. Of the total 114 cases, liver injury caused by He-Shou-Wu accounted for 65, which was the most common one in our review. With regard to primary diseases of included cases, dermatosis, grey hair, and alopecia took up 11/30, which was the largest proportion. Other applications involved were osteoarthrosis (5/30), health promotion (3/30), diabetes mellitus (2/30), and mammary gland disorders (2/30). In addition, a further analysis about ingredients of patent drugs and decoctions was performed. Totally, 72 kinds of herbs were involved in 4 decoctions and 8 patent drugs with detailed ingredients, reported in articles or the Chinese Pharmacopoeia, of which Dang-Gui [Angelica sinensis (Oliv.) Diels], He-Shou-Wu, Di-Huang [Rehmannia glutinosa (Gaertn.) DC.], Chuan-Xiong (Ligusticum striatum DC.), Hong-Hua (Chelonopsis pseudobracteata var. rubra C. Y. Wu & H. W. Li), Bai-Xian-Pi (Dictamnus albus L.), and Bu-Gu-Zhi [Psoralea cordata (Thunb.) Salter] were the most common ones. Details are shown in Figure 2.
Table 1.
List of CHM with reported hepatotoxicity.
| CHM | Part used/ingredients | Potential toxicity mechanism | Application | Recommended dosage | Cases | References |
|---|---|---|---|---|---|---|
| He-Shou-Wu | Radix | Probably associated with anthraquinone derivatives, lipid peroxidation, or an immune response [32, 33] | Alopecia and grey hair | 3~6 g/d (unprocessed herb), 6~12 g/d (processed herb) | Preparation unmentioned, 25 | Jung et al., 2011 [18] |
| 4 unprocessed, 14 processed | Dong et al., 2014 [19] | |||||
| 1 unprocessed | Zhang et al., 2014 [27] | |||||
| Preparation unmentioned, 2 | Yuan and Chen, 2014 [25] | |||||
| Preparation unmentioned, 1 | Yang et al., 2014 [28] | |||||
| Preparation unmentioned, 18 | Ren and Xu, 2015 [29] | |||||
|
| ||||||
| Cang-Er-Zi | Fruit | Probably kaurene glycosides induced liver injury via oxidative stress as lipid peroxidation in liver [34] | To eliminate wind and dampness | 3~10 g/d | 1 | Wang et al., 2013 [24] |
|
| ||||||
| Huang-Yao-Zi | Rhizoma | Probably oxidative stress injury caused by diosbulbin [35] | Thyroid nodule | 4.5~9 g/d | 1 | Jiang and Yang, 2014 [26] |
|
| ||||||
| Lei-Gong-Teng | Radix | Probably associated with triptolide, lipid peroxidation, and an immune response [36, 37] | Osteoarthrosis | NA | 4 | Ren and Xu, 2015 [29] |
|
| ||||||
| Yang Xue Sheng Fa Jiao Nang | Shu-Di-Huang, Dang-Gui, Qiang-Huo (Notopterygium incisum Ting ex H. T. Chang), Mu-Gua (Chaenomeles sinensis (Thouin) Koehne), Chuan-Xiong, Bai-Shao (Paeonia lactiflora Pall.), Tu-Si-Zi (Cuscuta chinensis Lam.), Tian-Ma (Gastrodia elata Blume), Zhi-Shou-Wu | Alopecia | 4 granules, twice a day | 4 | Ren and Xu, 2015 [29] | |
|
| ||||||
| Bai Dian Feng Jiao Nang | Bu-Gu-Zhi 33.33 g, Huang-Qi (Astragalus propinquus Schischkin) 33.33 g, Hong-Hua 33.33 g, Chuan-Xiong 33.33 g, Dang-Gui 33.33 g, Xiang-Fu (Cyperus rotundus L.) 33.33 g, Tao-Ren (Prunus persica (L.) Batsch) 33.33 g, Dan-Shen (Salvia miltiorrhiza Bunge) 33.33 g, Wu-Shao-She (Zaocys) 33.33 g, Zi-Cao (Lithospermum erythrorhizon Siebold & Zucc.) 33.33 g, Bai-Xian-Pi 33.33 g, Shan-Yao (Dioscorea oppositifolia L.) 33.33 g, Gan-Jiang 33.33 g, Long-Dan (Gentiana scabra Bunge) 33.33 g, Yan-Ji-Li (Tribulus terrestris L.) 433.33 g |
Leucoderma | 3~4 granules, twice a day | 4 | Ren and Xu, 2015 [29] | |
|
| ||||||
| Xiao Yin Pian | Di-Huang, Dan-Pi (Paeonia officinalis L.), Chi-Shao, Dang-Gui, Ku-Shen (Sophora flavescens Aiton), Jin-Yin-Hua (Lonicera japonica Thunb.), Xuan-Shen (Scrophularia ningpoensis Hemsl.), Niu-Bang-Zi (Arctium lappa L.), Chan-Tui (Cicadae periostracum), Bai-Xian-Pi, Fang-Feng (Saposhnikovia divaricata (Trucz.) Schischk.), Da-Qing-Ye (Wrightia laevis Hook. f.), Hong-Hua | Psoriasis | 5~7 tablets, three times a day | 3 | Ren and Xu, 2015 [29] | |
|
| ||||||
| Qu Bai Ba Bu Pian | — | Leucoderma | NA | 2 | Ren and Xu, 2015 [29] | |
|
| ||||||
| Bu Shen Sheng Fa Tang | — | Alopecia | NA | 1 | Ren and Xu, 2015 [29] | |
|
| ||||||
| Ze Qi Chong Ji | — | Psoriasis | NA | 1 | Ren and Xu, 2015 [29] | |
|
| ||||||
| Xian Ling Gu Bao Jiao Nang | — | Osteoarthrosis | NA | 3 | Ren and Xu, 2015 [29] | |
|
| ||||||
| Gu Kang Jiao Nang | — | Osteoarthrosis | NA | 2 | Ren and Xu, 2015 [29] | |
|
| ||||||
| Zhuang Gu Jiao Nang | — | Osteoarthrosis | NA | 1 | Ren and Xu, 2015 [29] | |
|
| ||||||
| Ling Zhi Yi Shou Jiao Nang | — | Health promotion | NA | 2 | Ren and Xu, 2015 [29] | |
|
| ||||||
| Ling Zhi Jiao Nang | — | Health promotion | NA | 1 | Ren and Xu, 2015 [29] | |
|
| ||||||
| Hui Chun Ru Yi Jiao Nang | — | Symptoms like dizziness, a poor memory, fatigue, tinnitus, and soreness in the lower back and knees | NA | 1 | Ren and Xu, 2015 [29] | |
|
| ||||||
| Ru Bi San | — | Hyperplasia of mammary gland | NA | 1 | Ren and Xu, 2015 [29] | |
|
| ||||||
| ShuXiong Jiao Nang | San-Qi (Panax pseudoginseng Wall. var. notoginseng (Burkill) Hoo et Tseng) 166.7 g, Hong-Hua 166.7 g, Chuan-Xiong 333.3 g | Hyperplasia of mammary gland | 3 granules, three times a day | 1 | Ren and Xu, 2015 [29] | |
|
| ||||||
| Zeng Sheng Ping | — | Tumor | NA | 1 | Ren and Xu, 2015 [29] | |
|
| ||||||
| Long Bi Shu | Bu-Gu-Zhi, Yi-Mu-Cao (Leonurus artemisia (Lour.) S. Y. Hu), Jin-Qian-Cao (Lysimachia christinae Hance), Hai-Jin-Sha (Lygodium japonicum (Thunb.) Sw.), Amber, Shan-Ci-Gu (Iphigenia indica Kunth) | Hyperplasia of prostate gland | 3 granules, twice a day | 4 | Ren and Xu, 2015 [29] | |
|
| ||||||
| Zhi Xue Jiao Nang | — | Hemorrhoids | NA | 2 | Ren and Xu, 2015 [29] | |
|
| ||||||
| Move Free | Containing glucosamine, chondroitin, methylsulfonylmethane, black catechu, maltodextrin, Huang-Qin (Scutellaria baicalensis Georgi) | Arthritis | NA | 1 | Yang et al., 2012 [21] | |
| 1 | Dhanasekaran et al., 2013 [17] | |||||
|
| ||||||
| Ban Tu Wan | Di-Huang, Shu-Di-Huang, Zhi-Shou-Wu, Dang-Gui, Dan-Shen, Bai-Shao, Wu-Wei-Zi (Schisandra chinensis (Turcz.) Baill.), Qiang-Huo, Mu-Gua | Alopecia | 5 g, three times a day | 1 | Cortez et al., 2012 [16] | |
|
| ||||||
| Kamishoyosan | Chai-Hu (Bupleurum chinense DC.), Dan-Pi, Bai-Zhu (Atractylodes macrocephala Koidz.), Ri-Ben-Dang-Gui (Angelica acutiloba (Sieb. et Zucc.) Kitagawa), Fu-Ling (Poria cocos (Schw.) Wolf.), Zhi-Zi (Gardenia jasminoides J. Ellis), Bai-Shao, Sheng-Jiang (Zingiber officinale Roscoe), Gan-Cao (Glycyrrhiza uralensis Fisch.), Bo-He (Mentha haplocalyx Briq.) | Postmenopausal syndrome | NA | 1 | Inoue et al., 2011 [23] | |
|
| ||||||
| Qi Bao Mei Ran Wan | Zhi-Shou-Wu, Dang-Gui, Bu-Gu-Zhi, Gou-Qi (Lycium chinense Mill.), Tu-Si-Zi, Fu-Ling, Niu-Xi (Achyranthes bidentata Blume) | Grey hair | NA | 1 | Li et al., 2015 [15] | |
|
| ||||||
| Herbal extracts containing Hu-Ji-Sheng and Ye-Ge | Health promotion | NA | 1 | Kim et al., 2015 [22] | ||
|
| ||||||
| Herbal tea containing Kelp | Type 2 diabetes mellitus | NA | 1 | Viswanathan and Patel, 2013 [20] | ||
|
| ||||||
| Decoction: Lian-Qiao (Forsythia suspensa (Thunb.) Vahl) 10 g, Pu-Gong-Ying (Taraxacum mongolicum Hand.-Mazz.) 10 g, Zi-Hua-Di-Ding (Viola philippica Cav.) 10 g, Ye-Ju-Hua (Chrysanthemum indicum L.) 10 g, Bai-Zhi (Angelica dahurica (Fisch. ex Hoffm.) Benth. et Hook. f. ex Franch. et Sav.) 10 g, Huang-Qin 10 g, Xuan-Shen 10 g, Gan-Cao 10 g, Sheng-Shi-Gao (Gypsum fibrosum) 15 g, Dan-Pi 10 g, Bai-Xian-Pi 10 g, Bai-Mao-Gen (Imperata cylindrica (L.) Raeusch.) 10 g, Yan-Ji-Li 10 g, Tao-Ren 10 g, Hong-Hua 10 g, Dong-Gua-Pi (Benincasa hispida (Thunb.) Cogn.) 10 g, Di-Gu-Pi (Lycium chinense Mill.) 10 g, Di-Fu-Zi (Kochia scoparia (L.) Schrad.) 10 g, Sheng-Bai-Zhu 10 g, Chuan-Xiong 10 g, Fu-Ling-Pi 10 g, Fa-Ban-Xia (Pinellia ternata (Thunb.) Makino) 10 g, Chen-Pi (Citrus reticulata Blanco) 10 g, Tu-Fu-Ling (Smilax glabra Roxb.) 10 g | Eczema | NA | 1 | Mao et al., 2013 [30] | ||
|
| ||||||
| Pi Fu Bing Xue Du Wan and decoction: Chai-Hu, Dang-Gui, Bai-Zhu, Fu-Ling, Bo-He, Wu-Mei (Prunus mume (Siebold) Siebold & Zucc.), Fang-Feng, Yin-Chai-Hu (Stellaria dichotoma L. var. lanceolata Bge.), Wu-Wei-Zi, Pi-Pa-Ye (Eriobotrya japonica (Thunb.) Lindl.), Chuan-Shan-Jia (Manis squama), Ze-Xie (Alisma plantago-aquatica Linn.), He-Shou-Wu, Lu-Lu-Tong (Liquidambar formosana Hance), Xu-Duan (Dipsacus inermis Wall.), Nv-Zhen-Zi (Ligustrum lucidum Ait.), Han-Lian-Cao (Eclipta prostrata (L.) L.), Gui-Zhi (Cinnamomum cassia Presl) | Psoriasis | NA | 1 | Wei, 2011 [31] | ||
NA = not available.
Figure 2.
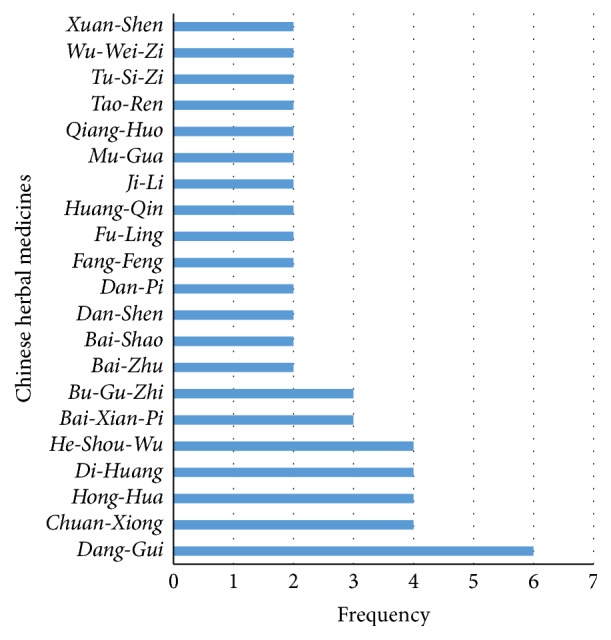
Frequency distribution of herbal ingredients of patent drugs and decoctions was performed, and herbs used more than once were given in the figure.
3.3. Causality Assessment
Causality assessment is necessary for the diagnosis of CHM induced liver injury. While expert consensus opinion and the RUCAM scale are considered as preferred algorithms to establish causality in suspected herb-induced liver injury (HILI), the former one is not widely available since it is cumbersome, costly, and time consuming. RUCAM scale is much more widely used by clinicians and researchers, which is structured, quantitative, and validated for liver injury. Of the total 17 articles included, 7 directly used the RUCAM scale to assess causality, while the other 10 articles provided associated information, based on which RUCAM can be performed. Four articles [9, 25, 28, 31] explicitly excluded HEV by serology tests, and four [20, 22, 26, 30] demonstrated exclusion of viral hepatitis without specific mention of HEV. Fifty-six cases of one article [29], scored no less than 3 points, were not supplied with detailed information and cannot be graded by RUCAM. Other cases were grouped into different likelihood levels, with 26 being highly probable (score > 8), 28 being probable (6–8), and 4 being possible (3–5). In a further analysis, 54 cases with more than 6 points of RUCAM were identified, in which He-Shou-Wu, Huang Yao Zi, Move Free, Kamishoyosan, Qi Bao Mei Ran Wan, herbal extracts containing Hu-Ji-Sheng and Ye-Ge, herbal tea containing Kelp, and a decoction consisting of CHM were causative. Since these herbs were more likely to cause liver injury, more attention should be paid. Furthermore, one case attributed to He-Shou-Wu was reported with a positive reexposure result, which accorded with the criteria of reexposure [11], while another case caused by kamishoyosan did not supply detailed ALT level of the first time, which, consequently, could not be diagnosed as reexposure.
3.4. Clinical Characteristics
Patients' features and clinical characteristics of liver injury from CHM were summarized. Of the 58 cases with detailed information, all 54 with probable or highly probable causality grading were included. Thus, we focused on these 54 cases and showed their clinical characteristics. While 35 were male and 19 were female, there was an average age of 47.13 ± 12.24 years, ranging from 17 to 78. The latent period was 30 (47) days, which is consistent with DILI [38]. Nine of eleven cases with well-defined information of actual and recommended dosage were involved in excessive intake of CHM, indicating that excessive use may be related to the incidence of liver injury. Concerning laboratory parameters, the average serum levels of ALT and AST were 1246 (824.25) IU/L and 931.52 ± 598.36 IU/L, respectively, and the average serum level of ALP was 225.08 ± 170.66 IU/L. The high laboratory values may be due to our restriction criteria for hepatotoxicity, which helps eliminate unspecific liver enzymes' increases and substantiate causality at higher probability. Cases were further identified by the pattern of liver injury, consisting of forty-four (81.48%) of hepatocellular injuries, eight (14.81%) of mixed injuries, and two (3.7%) of cholestatic injuries. Most of patients recovered from CHM induced liver injury, with a percentage of 96.3%, apart from the fact that one died and one is receiving liver transplantation. Additional details are available in Table 2.
Table 2.
Clinical information of included cases with highly probable or probable causality grading.
| CHM | Sex/age (y) | Usage and dosage | Exposure | ALT (IU/L)/AST (IU/L)/ TBIL (mg/dl)/ALP (IU/L) |
Pattern | RUCAM grade (scores) | Outcome | Reexposure | Countries |
|---|---|---|---|---|---|---|---|---|---|
| Sheng-He-Shou-Wu | M/45 | 1 kg soaked in 2.5 kg wine, 25 mL/day | 7 d | 2870/2599/2.26/109 | Hepatocellular | Highly probable (13) | Recovery | NA | China |
| Sheng-He-Shou-Wu | M/63 | 1 kg soaked in 22 kg wine, 250 mL/day | 15 d | 601/1515/2.87/181 | Hepatocellular | Highly probable (11) | Recovery | NA | China |
| He-Shou-Wu | M/29 | 100 g consumed as tea, 100 mL/day | 29 d | 1792/899/5.51/138 | Hepatocellular | Highly probable (11) | Recovery | NA | China |
| He-Shou-Wu | M/40 | 1 kg soaked in 10 kg wine, 300 mL/day | 28 d | 878/853/7.47/112 | Hepatocellular | Highly probable (10) | Recovery | NA | China |
| He-Shou-Wu | M/40 | 1 kg soaked in 20 kg alcohol, 50 mL/day | 43 d | 4095/2473/1.7/220 | Hepatocellular | Highly probable (10) | Recovery | NA | China |
| He-Shou-Wu | M/55 | 1 kg soaked in 15 kg alcohol, 300 mL/day | 4 d | 1056/769/10.29/176 | Hepatocellular | Highly probable (10) | Recovery | NA | China |
| He-Shou-Wu | M/61 | NA | 1 d | 818/NA/1.77/109 | Hepatocellular | Highly probable (10) | Recovery | Positive | Korea |
| He-Shou-Wu | M/61 | NA | 60 d | 885/NA/21.2/224 | Mixed | Highly probable (10) | Recovery | NA | Korea |
| Sheng-He-Shou-Wu | M/40 | 1 kg soaked in 10 kg wine, 200 mL/day | 1 d | 3120/1615/16.78/151 | Hepatocellular | Highly probable (10) | Recovery | NA | China |
| Move Free | F/78 | a recommended dose of 1 tablet twice a day | 21 d | 1626/1053/7.2/354 | Hepatocellular | Highly probable (10)† | Recovery | NA | USA |
| He-Shou-Wu | F/45 | Not available | 7 d | 1196/507/0.88/816 | Hepatocellular | Highly probable (9) | Recovery | NA | China |
| He-Shou-Wu | M/31 | 10 g powder consumed directly, 20 g/day | 14 d | 2026/858/2.19/180 | Hepatocellular | Highly probable (9) | Recovery | NA | China |
| He-Shou-Wu | M/56 | 1 slice consumed as tea, 100 mL/day | 120 d | 2313/1202/5.81/305 | Hepatocellular | Highly probable (9) | Recovery | NA | China |
| He-Shou-Wu | F/37 | 1 tablespoon consumed as decoction, 250 mL/day | 52 d | 922/319/9.06/116 | Hepatocellular | Highly probable (9) | Recovery | NA | China |
| He-Shou-Wu | F/46 | 1 tablespoon consumed as decoction, 250 mL/day | 50 d | 1127/297/1.94/203 | Hepatocellular | Highly probable (9) | Recovery | NA | China |
| He-Shou-Wu | F/18 | 1 tablespoon consumed as decoction, 100 mL/day | 67 d | 1074/348/0.8/89 | Hepatocellular | Highly probable (9) | Recovery | NA | China |
| He-Shou-Wu | M/40 | 3 tablespoons consumed as decoction, 100 mL/day | 23 d | 1987/872/11/182 | Hepatocellular | Highly probable (9) | Recovery | NA | China |
| He-Shou-Wu | M/34 | NA | 30 d | 1452/NA/25.3/111 | Hepatocellular | Highly probable (9) | Recovery | NA | Korea |
| He-Shou-Wu | M/58 | NA | 35 d | 1898/NA/13.4/134 | Hepatocellular | Highly probable (9) | Recovery | NA | Korea |
| He-Shou-Wu | M/45 | NA | 30 d | 1400/NA/2.04/125 | Hepatocellular | Highly probable (9) | Recovery | NA | Korea |
| He-Shou-Wu | M/49 | NA | 90 d | 1235/NA/32.9/465 | Mixed | Highly probable (9) | Recovery | NA | Korea |
| He-Shou-Wu | M/46 | NA | 2 d | 1287/NA/19.7/146 | Hepatocellular | Highly probable (9) | Recovery | NA | Korea |
| He-Shou-Wu | M/62 | NA | 90 d | 1174/NA/9.2/175 | Hepatocellular | Highly probable (9) | Recovery | NA | Korea |
| He-Shou-Wu | F/63 | NA | 30 d | 943/NA/4.2/137 | Hepatocellular | Highly probable (9) | Recovery | NA | Korea |
| He-Shou-Wu | M/44 | NA | 7 d | 1077/NA/15/197 | Hepatocellular | Highly probable (9) | Recovery | NA | Korea |
| Herbal extracts containing Hu-Ji-Sheng and Ye-Ge | M/55 | NA | 30 d | 1528/1108/6.3/160 | Hepatocellular | Highly probable (9) | Recovery | NA | Korea |
| He-Shou-Wu | M/38 | 2 slices consumed as tea, 500 mL/day | 1 d | 1815/1351/5.3/108 | Hepatocellular | Probable (8) | Recovery | NA | China |
| He-Shou-Wu | M/48 | 100 g mixed with 150 g bee honey, 8 g/day | 10 d | 1891/798/7.9/159 | Hepatocellular | Probable (8) | Recovery | NA | China |
| He-Shou-Wu | F/61 | 10–20 g/d | 60 d | 56/61/28.07/601 | Cholestatic | Probable (8)† | Recovery | NA | China |
| He-Shou-Wu | M/57 | NA | 30 d | 853/NA/28.1/173 | Mixed | Probable (8) | Death | NA | Korea |
| He-Shou-Wu | F/47 | NA | 60 d | 1947/NA/30.4/218 | Hepatocellular | Probable (8) | Recovery | NA | Korea |
| He-Shou-Wu | M/59 | NA | 30 d | 1245/NA/1.6/155 | Hepatocellular | Probable (8) | Recovery | NA | Korea |
| He-Shou-Wu | F/46 | NA | 30 d | 1804/NA/6.2/81 | Hepatocellular | Probable (8) | Recovery | NA | Korea |
| He-Shou-Wu | M/45 | NA | 20 d | 271/NA/2.9/164 | Mixed | Probable (8) | Recovery | NA | Korea |
| He-Shou-Wu | M/65 | NA | 10 d | 1107/NA/21.9/197 | Hepatocellular | Probable (8) | Recovery | NA | Korea |
| He-Shou-Wu | F/42 | NA | 10 d | 500/NA/1.6/181 | Mixed | Probable (8) | Recovery | NA | Korea |
| He-Shou-Wu | M/42 | NA | 60 d | 1706/NA/26.3/147 | Hepatocellular | Probable (8) | Recovery | NA | Korea |
| He-Shou-Wu | F/48 | NA | 3 d | 1142/NA/15.9/145 | Hepatocellular | Probable (8) | Recovery | NA | Korea |
| Sheng-He-Shou-Wu | M/50 | 1 kg soaked in 5 kg wine, 500 mL/day | 20 d | 1992/1155/6.73/185 | Hepatocellular | Probable (8) | Recovery | NA | China |
| Qi Bao Mei Ran Wan | M/26 | Taken at the recommended dosages | 30 d | 1674/617/3.2/normal | Hepatocellular | Probable (8)† | Recovery | NA | China |
| He-Shou-Wu | F/41 | 15 tablets per day | 21 d | 104/85/21.7/947 | Cholestatic | Probable (7)† | Recovery | NA | China |
| He-Shou-Wu | M/37 | 1 tablespoon consumed as decoction, 1500 mL/day | 7 d | 1613/835/2.26/177 | Hepatocellular | Probable (7) | Recovery | NA | China |
| He-Shou-Wu | F/54 | NA | 4 d | 1752/NA/8.4/286 | Hepatocellular | Probable (7) | Recovery | NA | Korea |
| He-Shou-Wu | M/24 | NA | 60 d | 1652/NA/31.9/140 | Hepatocellular | Probable (7) | Liver transplantation | NA | Korea |
| He-Shou-Wu | M/42 | NA | 120 d | 1677/NA/15.8/93 | Hepatocellular | Probable (7) | Recovery | NA | Korea |
| He-Shou-Wu | M/41 | NA | 30 d | 520/NA/9.9/143 | Mixed | Probable (7) | Recovery | NA | Korea |
| Huang-Yao-Zi | F/66 | 30 g decocted in water for oral dose per day | 21 d | 1042/1006/16.44/normal | Hepatocellular | Probable (7)† | Recovery | NA | China |
| He-Shou-Wu YanShouPian | M/17 | 15 tablets per day | 45 d | 1501/545/18.63/155 | Hepatocellular | Probable (6)† | Recovery | NA | China |
| He-Shou-Wu | F/54 | NA | 180 d | 1519/NA/11.7/187 | Hepatocellular | Probable (6) | Recovery | NA | Korea |
| He-Shou-Wu/1 | M/53 | NA | 180 d | 1227/NA/33.2/370 | Mixed | Probable (6) | Recovery | NA | Korea |
| Herbal tea containing Kelp | F/40 | 3 cups per day | 60 d | 435/219/9.2/435 | Mixed | Probable (6)† | Recovery | NA | USA |
| Kamishoyosan | F/48 | NA | 60 d | 972/900/12.8/420 | Hepatocellular | Probable (6)† | Recovery | NA | Japan |
| Move Free | F/62 | 4 tablets/d for two and a half weeks tapered down to 2 tablets/d for four days | 21 d | 1247/893/6.9/297 | Hepatocellular | Probable (6)† | Recovery | NA | USA |
| Decoction [30] | F/51 | Brewed with water, two times a day | 23 d | 758/1262/9.92/normal | Hepatocellular | Probable (6)† | Recovery | NA | China |
NA = not available, ALT = alanine aminotransferase, AST = aspartate aminotransferase, TB = total bilirubin, and ALP = alkaline phosphatase. †The article did not provide an outcome of RUCAM scale but detailed information based on which a score was given.
4. Discussion
DILI is one of the most common causes of hepatitis in the world. According to some studies, DILI accounted for about 11% of acute liver failure cases [39, 40]. As a result of popularity, liver injury induced by CHM is on the increase around the world. To help TCM practitioners avoid CHM induced liver injury and supply clinicians with more associated data, we systematically reviewed publications, focusing on developments in the recent 5 years.
Characteristics of included cases were summarized, while problems in the original papers were brought up. Detailed information about CHM, like locality, botanical classification, brand names, detailed contents, and usage and dosage, was unavailable in most publications, which is of great importance to improve the reliability of studies. CHM with reported hepatotoxicity were identified, of which He-Shou-Wu attracted our attention. He-Shou-Wu is one of the most popular CHM, officially listed in the Chinese Pharmacopoeia. According to Ben Cao Gang Mu (Compendium of Materia Medica), an ancient book recording therapeutic effects of CHM, He-Shou-Wu is usually used to treat alopecia and white hair by nourishing the liver and kidneys. In recent years, with the increasing use of it, numbers of hepatotoxicity cases have been reported [18, 19, 41, 42]. Although efforts have been made, the toxicity mechanism is still not fully elucidated. Probably, the hepatotoxicity is related to some bioactive compounds, anthraquinone derivatives [32, 43]. Based on TCM theory, processing is believed to be able to reduce the toxicity of herbs, including He-Shou-Wu, but both processed and unprocessed He-Shou-Wu were reported to cause liver injury in publications. The phenomenon does not necessarily mean that preparation is of no use to hepatotoxicity. Since the use of the processed one is much more common than the unprocessed one and the hepatotoxicity can be affected by many other factors, the relationship between processing and hepatotoxicity cannot be concluded for now. Our review also indicated that hepatotoxicity was much more common with dermatosis and osteoarthrosis, which reminds dermatologists or orthopedists of avoiding those recorded herbs. Since people increasingly focus on health care, CHM used for health promotion also should be taken carefully.
It is worth noting that formulae consisting of multiple herbs, including patent drugs and decoctions, prevail in CHM with potential hepatotoxicity. In the view of TCM, liver injury from these drugs and decoctions is often related to some ingredients with hepatotoxicity. After a review of related literature, in our results, He-Shou-Wu, Bai-Xian-Pi, and Bu-Gu-Zhi have been reported to be of hepatotoxicity [10, 44], while Dang-Gui, Di-Huang, Chuan-Xiong, and Hong-Hua have not been, among which Dang-Gui, Di-Huang, and Hong-Hua have been reported to be with hepatoprotective effect [45–47]. These three herbs are often used in TCM prescriptions, especially used for skin and gynecological diseases, which may account for their high frequency. TCM practitioners always use a prescription to treat diseases, which blends together a number of herbs with specific functions. Sometimes, prescription compatibility may also play an important role in attenuating adverse events of CHM [48]. It is definitely necessary for clinicians to supply detailed ingredients and contents in articles as far as possible. However, during literature selection, lots of articles were excluded for lack of detailed information of multiple ingredients, which is the same as situation on the online websites, LiverTox [49] and HepaTox [50].
Since syndrome differentiation and individualized treatment are main features of TCM, excessive use of CHM is necessary and universal sometimes. It is also essential to further analyze its relationship with hepatotoxicity on the basis of more data. In our results, the values of liver tests were relatively high, which may be related to our strict inclusion criteria. The majority of cases were grouped into levels of highly probable or probable, indicating a high quality of causality assessment. What is more, many articles included did not provide a score of RUCAM, while the tool is widely accepted by researchers and clinicians [51–53]. The RUCAM scale is not without problems, especially for CHM induced liver injury. Previous information of CHM is not always available, and contamination by heavy metals and adulteration also provide challenges [54], which should be clarified before causality assessment. But it at least provides us with a framework, within which clinicians can organize the history taking and laboratory tests.
Based on our work, some suggestions were offered. First, CHM identified with reported hepatotoxicity should be used carefully. Secondly, since there is uncertain accuracy in determining the relationship between CHM and liver injury in most reports, we would like to adopt a strict definition of hepatotoxicity and the RUCAM scale, which contributes to excluding cases of other causes and offers causality evidence. Thirdly, when clinicians and researchers plan to report cases or carry out associated studies, it is advisable that details of causative herbs or patent drugs, like locality, botanical classification, brand names, detailed herbal ingredients and contents, and usage and dosage, should be provided at full length. What is more, it is necessary to build up a new website that provides up-to-date, comprehensive, and unbiased information about CHM induced liver injury and standardizes submission of associated information. Finally, since CHM is general designation of various kinds of herbs, we consider it improper to compare CHM with a single medicine.
In contrast with previous reviews, we adopted a high threshold of laboratory tests to avoid nonspecific liver injuries and included electronic data in China, which explained the difference from results of other reviews. Also, due to much more attention attracted in recent years, the increasing number of articles [7], and the development of diagnosis [55], our review focused on publications of the past 5 years. Of course, our review has its limitations. Above all, gray literature was not included, since cases unpublished were unavailable. A large number of publications searched failed to provide essential information associated with our inclusion criteria, which leads to missing data and may result in a risk of bias. Moreover, detailed information of herbs in the primary articles, like plant family, subfamily, species, subspecies, and locality, was unmentioned, which contributes to excluding adulteration and contamination. Finally, while the strict definition of hepatotoxicity helps us exclude unspecific liver injury, it may cause some toxic herbs to be missed. In fact, numbers of articles, especially articles in Chinese, were excluded for a different standard of diagnosis with ours.
In conclusion, CHM, especially He-Shou-Wu and those for dermatosis and osteoarthrosis, should be used carefully, and routine liver tests may be needed. Although cases have been increasingly reported, details about causative herbs need to be particularly illustrated. Liver injury from CHM is similar to that from conventional medicines in patent period, injury pattern, and prognosis. Further studies are needed on toxicity mechanisms and biomarkers, and more RUCAM should be used in future cases.
Supplementary Material
Detailed information about all included cases was supplied in the supplementary table, including causative herbs, demographic information, regional distribution, usage and dosage, latent period, laboratory results, pattern of liver injury, causality assessment, reexposure results, and clinical outcomes.
Acknowledgments
The authors' work was supported by the National Science and Technology Major Project of the 11th five-year plan in China (no. 2008ZX10005-006).
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Dossett M. L., Davis R. B., Lembo A. J., Yeh G. Y. Complementary and alternative medicine use by US adults with gastrointestinal conditions: results from the 2012 National Health Interview Survey. The American Journal of Gastroenterology. 2014;109(11):1705–1711. doi: 10.1038/ajg.2014.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu T.-G., Xiong S.-Q., Yan Y., Zhu H., Yi C. Use of Chinese herb medicine in cancer patients: a survey in southwestern China. Evidence-Based Complementary and Alternative Medicine. 2012;2012:5. doi: 10.1155/2012/769042.769042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin H.-K., Jeong S.-J., Huang D. S., Kang B.-K., Lee M. S. Usage patterns and adverse experiences in traditional Korean medicine: results of a survey in South Korea. BMC Complementary and Alternative Medicine. 2013;13, article 340 doi: 10.1186/1472-6882-13-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shumer G., Warber S., Motohara S., et al. Complementary and alternative medicine use by visitors to rural Japanese family medicine clinics: results from the international complementary and alternative medicine survey. BMC Complementary and Alternative Medicine. 2014;14(1, article 360) doi: 10.1186/1472-6882-14-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Group of Hepatobiliary Disease of Digestive Disease Branch of Chinese Medical Association. A multicenter survey on hospital inpatients with drug-induced acute liver injury in China. Zhong Hua Xiao Hua Za Zhi. 2007;27(7):437–440. [Google Scholar]
- 6.Zhou Y., Yang L., Liao Z. L., He X. Y., Zhou Y. Y., Guo H. Epidemiology of drug-induced liver injury in China: a systematic analysis of the Chinese literature including 21 789 patients. European Journal of Gastroenterology and Hepatology. 2013;25(7):825–829. doi: 10.1097/meg.0b013e32835f6889. [DOI] [PubMed] [Google Scholar]
- 7.Teschke R., Wolff A., Frenzel C., Schulze J., Eickhoff A. Herbal hepatotoxicity: a tabular compilation of reported cases. Liver International. 2012;32(10):1543–1556. doi: 10.1111/j.1478-3231.2012.02864.x. [DOI] [PubMed] [Google Scholar]
- 8.Teschke R., Wolff A., Frenzel C., Schulze J. Review article: herbal hepatotoxicity—an update on traditional Chinese medicine preparations. Alimentary Pharmacology and Therapeutics. 2014;40(1):32–50. doi: 10.1111/apt.12798. [DOI] [PubMed] [Google Scholar]
- 9.Teschke R., Zhang L., Long H., et al. Traditional Chinese Medicine and herbal hepatotoxicity: a tabular compilation of reported cases. Annals of Hepatology. 2015;14(1):7–19. [PubMed] [Google Scholar]
- 10.Lee W.-J., Kim H.-W., Lee H.-Y., Son C.-G. Systematic review on herb-induced liver injury in Korea. Food and Chemical Toxicology. 2015;84:47–54. doi: 10.1016/j.fct.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Danan G., Teschke R. RUCAM in drug and herb induced liver injury: the update. International Journal of Molecular Sciences. 2016;17(1, article 14) doi: 10.3390/ijms17010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins J. P., Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. 2011. http://handbook.cochrane.org/ [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D. G., The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. International Journal of Surgery. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Pharmacopoeia Commission of the Ministry of Health. Pharmacopoeia of The People's Republic of China, 1st Div. Beijing, China: China Chemical Industry Press; 2015 (Chinese) [Google Scholar]
- 15.Li X., Qu C., He Q., et al. Acute hepatitis induced by a Chinese herbal product Qibao Meiran Wan: a case study. International Journal of Clinical and Experimental Medicine. 2015;8(7):11624–11627. [PMC free article] [PubMed] [Google Scholar]
- 16.Cortez E., Boulger C., Bernard A. Ban Tu Wan hepatotoxicity. BMJ Case Reports. 2012;2012:4. doi: 10.1136/bcr-2012-006438.006438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhanasekaran R., Owens V., Sanchez W. Chinese skullcap in move free arthritis supplement causes drug induced liver injury and pulmonary infiltrates. Case Reports in Hepatology. 2013;2013:4. doi: 10.1155/2013/965092.965092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung K. A., Min H. J., Yoo S. S., et al. Drug-induced liver injury: twenty five cases of acute hepatitis following ingestion of Polygonum multiflorum thunb. Gut and Liver. 2011;5(4):493–499. doi: 10.5009/gnl.2011.5.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong H., Slain D., Cheng J., Ma W., Liang W. Eighteen cases of liver injury following ingestion of Polygonum multiflorum . Complementary Therapies in Medicine. 2014;22(1):70–74. doi: 10.1016/j.ctim.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Viswanathan L., Patel A. Hepatotoxicity associated with herbal tea containing kelp. ACG Case Reports Journal. 2013;1(1):55–57. doi: 10.14309/crj.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang L., Aronsohn A., Hart J., Jensen D. Herbal hepatoxicity from Chinese skullcap: a case report. World Journal of Hepatology. 2012;4(7):231–233. doi: 10.4254/wjh.v4.i7.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim H. J., Kim H., Ahn J. H., Suk H. J. Liver injury induced by herbal extracts containing mistletoe and kudzu. Journal of Alternative and Complementary Medicine. 2015;21(3):180–185. doi: 10.1089/acm.2014.0228. [DOI] [PubMed] [Google Scholar]
- 23.Inoue H., Yamazaki S., Shimizu M., et al. Liver injury induced by the Japanese herbal drug Kamishoyosan. Gastroenterology and Hepatology. 2011;7(10):692–695. [PMC free article] [PubMed] [Google Scholar]
- 24.Wang M., Qu J. T., Dai B., Mei C. L. Fructus Xanthii poisoning: a case report and literature review. Zhong Guo Quan Ke Yi Xue. 2013;16(2):218–220. [Google Scholar]
- 25.Yuan C., Chen L. Two cases of recurrent jaundice induced by oral use of He-Shou-Wu. Lin Chuang He Li Yong Yao. 2014;10(7, article 78) [Google Scholar]
- 26.Jiang A. H., Yang L. M. One case of liver injury induced by Huang Yao Zi. Zhong Guo Bao Jian Ying Yang. 2014;(5, article 3187) [Google Scholar]
- 27.Zhang W. L., Ma J. W., Dong J. Case analysis of liver injury induced by Sheng-He-Shou-Wu. Kong Jun Yi Xue Za Zhi. 2014;2(30, article 121) [Google Scholar]
- 28.Yang M., Rao H. Y., Wei L. Drug-induced liver injury: take care of herbal and dietary supplements. Jian Kang Guan Li. 2014;12:57–60. [Google Scholar]
- 29.Ren X. F., Xu J. M. A clinical study on cases with traditional chinese medicine induced liver injury. An Hui Yi Yao. 2015;19(10):1997–2000. [Google Scholar]
- 30.Mao L., Li S. Q., Liang Z. Q. Drug-induced hepatitis due to oral traditional chinese medicine decoction. Zhong Guo Yi Yao. 2013;1(8):121–122. [Google Scholar]
- 31.Wei G. H. Liver injury caused by traditional chinese medicine for psoriasis. Jian Kang Bi Du ZaZhi. 2011;2, article 163 [Google Scholar]
- 32.Ma J., Zheng L., He Y.-S., Li H.-J. Hepatotoxic assessment of Polygoni Multiflori Radix extract and toxicokinetic study of stilbene glucoside and anthraquinones in rats. Journal of Ethnopharmacology. 2015;162:61–68. doi: 10.1016/j.jep.2014.12.045. [DOI] [PubMed] [Google Scholar]
- 33.Lv G. P., Meng L. Z., Han D. Q., Li H. Y., Zhao J., Li S. P. Effect of sample preparation on components and liver toxicity of Polygonum multiflorum . Journal of Pharmaceutical and Biomedical Analysis. 2015;109:105–111. doi: 10.1016/j.jpba.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y., Han T., Xue L.-M., et al. Hepatotoxicity of kaurene glycosides from Xanthium strumarium L. fruits in mice. Pharmazie. 2011;66(6):445–449. doi: 10.1691/ph.2011.0844. [DOI] [PubMed] [Google Scholar]
- 35.Wang J., Ji L., Liu H., Wang Z. Study of the hepatotoxicity induced by Dioscorea bulbifera L. rhizome in mice. BioScience Trends. 2010;4(2):79–85. [PubMed] [Google Scholar]
- 36.Li X.-X., Du F.-Y., Liu H.-X., Ji J.-B., Xing J. Investigation of the active components in Tripterygium wilfordii leading to its acute hepatotoxicty and nephrotoxicity. Journal of Ethnopharmacology. 2015;162:238–243. doi: 10.1016/j.jep.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Li J., Shen F., Guan C., et al. Activation of Nrf2 protects against triptolide-induced hepatotoxicity. PLoS ONE. 2014;9(7) doi: 10.1371/journal.pone.0100685.e100685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chalasani N. P., Hayashi P. H., Bonkovsky H. L., Navarro V. J., Lee W. M., Fontana R. J. ACG clinical guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. The American Journal of Gastroenterology. 2014;109(7):950–966. doi: 10.1038/ajg.2014.131. [DOI] [PubMed] [Google Scholar]
- 39.Lee W. M., Squires R. H., Jr., Nyberg S. L., Doo E., Hoofnagle J. H. Acute liver failure: summary of a workshop. Hepatology. 2008;47(4):1401–1415. doi: 10.1002/hep.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reuben A., Koch D. G., Lee W. M. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52(6):2065–2076. doi: 10.1002/hep.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bae S. H., Kim D. H., Bae Y. S., et al. Toxic hepatitis associated with Polygoni multiflori . The Korean Journal of Hepatology. 2010;16(2):182–186. doi: 10.3350/kjhep.2010.16.2.182. [DOI] [PubMed] [Google Scholar]
- 42.Ou P., Chen Y., Li B., et al. Causes, clinical features and outcomes of drug-induced liver injury in hospitalized patients in a Chinese tertiary care hospital. SpringerPlus. 2015;4, article 802 doi: 10.1186/s40064-015-1600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lv G. P., Meng L. Z., Han D. Q., Li H. Y., Zhao J., Li S. P. Effect of sample preparation on components and liver toxicity of Polygonum multiflorum . Journal of Pharmaceutical and Biomedical Analysis. 2015;109:105–111. doi: 10.1016/j.jpba.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 44.Cheung W. I., Tse M. L., Ngan T., et al. Liver injury associated with the use of Fructus Psoraleae (Bol-gol-zhee or Bu-gu-zhi) and its related proprietary medicine. Clinical Toxicology. 2009;47(7):683–685. doi: 10.1080/15563650903059136. [DOI] [PubMed] [Google Scholar]
- 45.Wang K., Song Z., Wang H., Li Q., Cui Z., Zhang Y. Angelica sinensis polysaccharide attenuates concanavalin A-induced liver injury in mice. International Immunopharmacology. 2016;31:140–148. doi: 10.1016/j.intimp.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y.-M., Zhu L.-L., Li R., et al. Xijiao Dihuang Decoction (犀角地黄汤) and Rehmannia glutinosa Libosch. protect mice against lipopolysaccharide and tumor necrosis factor alpha-induced acute liver failure. Chinese Journal of Integrative Medicine. 2015 doi: 10.1007/s11655-015-2141-2. [DOI] [PubMed] [Google Scholar]
- 47.Wu S., Yue Y., Tian H., et al. Carthamus red from Carthamus tinctorius L. exerts antioxidant and hepatoprotective effect against CCl4-induced liver damage in rats via the Nrf2 pathway. Journal of Ethnopharmacology. 2013;148(2):570–578. doi: 10.1016/j.jep.2013.04.054. [DOI] [PubMed] [Google Scholar]
- 48.Zhang B., Zhang Q., Liu M., et al. Increased involvement of Panax notoginseng in the mechanism of decreased hepatotoxicity induced by Tripterygium wilfordii in rats. Journal of Ethnopharmacology. 2016;185:243–254. doi: 10.1016/j.jep.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 49.Livertox. Chinese and Other Asian Herbal Medicines. 2016, http://livertox.nih.gov/ [PubMed]
- 50.Hepatox. http://www.hepatox.org/chPatentDrug.
- 51.Rochon J., Protiva P., Seeff L. B., et al. Reliability of the Roussel Uclaf Causality Assessment Method for assessing causality in drug-induced liver injury. Hepatology. 2008;48(4):1175–1183. doi: 10.1002/hep.22442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teschke R., Frenzel C., Schulze J., Eickhoff A. Herbal hepatotoxicity: challenges and pitfalls of causality assessment methods. World Journal of Gastroenterology. 2013;19(19):2864–2882. doi: 10.3748/wjg.v19.i19.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teschke R., Wolff A., Frenzel C., Schwarzenboeck A., Schulze J., Eickhoff A. Drug and herb induced liver injury: council for International Organizations of Medical Sciences scale for causality assessment. World Journal of Hepatology. 2014;6(1):17–32. doi: 10.4254/wjh.v6.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Teschke R., Eickhoff A., Schulze J. Drug- and herb-induced liver injury in clinical and translational hepatology: causality assessment methods, quo vadis? Journal of Clinical and Translational Hepatology. 2013;1(1):59–74. doi: 10.14218/JCTH.2013.D002X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tran T., Lee W. M. DILI: new insights into diagnosis and management. Current Hepatitis Reports. 2013;12(1):53–58. doi: 10.1007/s11901-012-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed information about all included cases was supplied in the supplementary table, including causative herbs, demographic information, regional distribution, usage and dosage, latent period, laboratory results, pattern of liver injury, causality assessment, reexposure results, and clinical outcomes.