Abstract
Cryptococcosis is a globally distributed invasive fungal infection that is caused by species within the genus Cryptococcus which presents substantial therapeutic challenges. Although natural human-to-human transmission has never been observed, recent work has identified multiple virulence mechanisms that enable cryptococci to infect, disseminate within and ultimately kill their human host. In this Review, we describe these recent discoveries that illustrate the intricacy of host-pathogen interactions and reveal new details about the host immune responses that either help to protect against disease or increase host susceptibility. In addition, we discuss how this improved understanding of both the host and the pathogen informs potential new avenues for therapeutic development.
Cryptococcosis was identified in 1894, when the pathologist Otto Busse and physician Abraham Buschke jointly identified Cryptococcus spp. as the cause of a chronic granuloma of the tibial bone in a 31-year-old woman. However, human cryptococcosis only became recognized as a major health threat with the onset of the AIDS pandemic in the 1980s, during which these fungal infections became a common AIDS-defining illness in patients with greatly reduced T cell function (BOX 1). Although cryptococcosis is predominantly a disease of immunocompromised patients, a recent outbreak of cryptococcosis in otherwise healthy individuals in North America and Canada (now known as the Pacific Northwest outbreak) has focused attention on the capacity of some lineages of the fungus to act as primary pathogens (see below).
Box 1. Clinical cryptococcosis.
Epidemiology
Because cryptococci are capable of extended latency in host cells43 and most humans encounter the organism in early childhood8, it has been assumed that most clinical cases represent reactivation of a longstanding, asymptomatic infection (triggered, for instance, by falling CD4+ T cell counts in HIV-infected individuals). The proportion of clinical disease representing reactivated latent disease versus primary infection is unknown in HIV-positive individuals, but a study in patients with cryptococcosis following solid-organ transplantation found that only 52% of infections are due to reactivation95, suggesting that the classical view of cryptococcosis as a reactivating infection may not be accurate.
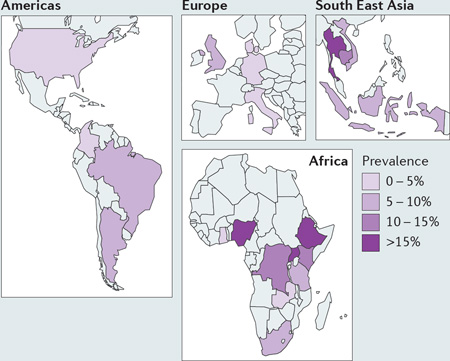
Emerging data are also highlighting the heterogeneity of cryptococcal disease worldwide, as illustrated by the prevalence of serum cryptococcal antigen (CrAg) in HIV-positive cohorts in different countries (see the figure, which displays the highest recorded prevalence per country). In addition, it is now clear that there is also considerable global heterogeneity in the fungal population structure. For example, Cryptococcus neoformans var. grubii (serotype A) is the predominant global cause of HIV-associated cryptococcal meningoencephalitis, but in China this organism frequently infects apparently immunocompetent hosts96. Similarly, particular lineages of C. neoformans vary both in virulence97–99 and in their ability to infect immunocompromised or immunocompetent individuals100. In the near future, intensive whole genome sequencing efforts for both cryptococcal isolates and affected patients may be able to explain the relative contribution of host and pathogen genotypes underlying these global patterns of disease.
Susceptibility
In contrast to other systemic fungal infections (such as candidiasis), relatively little is known about genetic risk factors for cryptococcosis. However, recent allelic association studies have shown that apparently immunocompetent individuals with cryptococcosis are significantly more likely to have defects in mannose-binding lectin101 or to be homozygous for the 232I allele of Fc γ receptor 2B (Fc γR2B)102, although these polymorphisms are common and thus, on their own, are clearly not sufficient to render an individual fully susceptible to cryptococcosis. Therefore, subtle defects in the innate immune response to fungi may underlie at least some cases of C. neoformans infection in otherwise healthy individuals. Similarly, in HIV-positive patients, allelic variation in a different FcγR, FcγR3A, also correlates with susceptibility103. In this case, individuals with a higher affinity receptor variant are at greater risk of infection, perhaps indicating that efficient uptake of the pathogen may actually aid dissemination and drive more severe disease. This is particularly striking because the same is true from the pathogen perspective: cryptococcal strains that are more avidly phagocytosed drive more aggressive disease and carry a higher risk of death in patients104. Thus, excessive phagocytosis as a result of either host or pathogen variation seems to drive cryptococcal dissemination, strongly supporting the Trojan Horse model of pathogen spread (see the main text).
Diagnosis
Diagnosis of cryptococcosis relies on detection either of the organism itself or its shed capsular glucuronoxylomannan (GXM) polysaccharide in serum or cerebrospinal fluid. This has been hugely facilitated by the introduction of the point-of-care lateral flow cryptococcal antigen assay, which is cheaper and more sensitive than earlier serological tests105. This test can detect very early dissemination and has facilitated cohort studies across the world, revealing a 2–21% prevalence of cryptococcal antigens in HIV-infected patients. As an increasing proportion of cases of cryptococcal meningoencephalitis are now presenting as unmasking of latent infection following therapy (that is, the appearance of clinical symptoms following immune reconstitution by antiretroviral treatment), wider implementation of a ‘screen-and-treat’ approach is cost effective as a public health intervention and has been demonstrated to reduce mortality in African HIV cohorts in the first year on antiretroviral therapy106.
Since its identification, cryptococcosis has been attributed to a single fungal species, Cryptococcus neoformans. However, improved molecular methods led to one variety of the pathogen, Cryptococcus neoformans var. gattii, being classified as a distinct species, Cryptococcus gattii, in 2002 (REF. 1). More recently, whole-genome sequencing-based analyses have high-lighted the complex evolutionary history of this group (BOX 2) and led to a proposal to further split C. neoformans into two species (C. neoformans and Cryptococcus deneoformans) and C. gattii into a total of five species (C. gattii, Cryptococcus bacillisporus, Cryptococcus deuterogattii, Cryptococcus tetragattii and Cryptococcus decagattii)2. As detailed biological comparisons between the various species of Cryptococcus described above have not been yet undertaken, we have adopted the simpler distinction into the two species C. gattii and C. neoformans throughout this article.
Box 2. The evolutionary history of cryptococci.
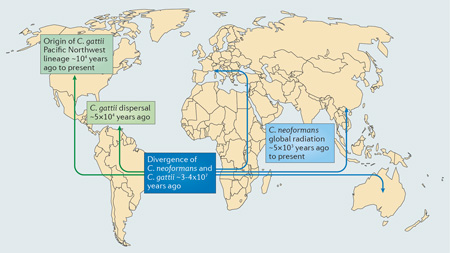
The two Cryptococcus species, Cryptococcus gattii and Cryptococcus neoformans, probably diverged from a common environmental saprophyte ancestor around 30–40 million years ago107, 108 (see the figure). For C. neoformans, extensive genetic data now indicate a common origin in sub-Saharan Africa5, 109,110. The observation that most non-African C. neoformans populations are nearclonal supports a model in which recombining African populations of cryptococci occasionally dispersed to other parts of the globe. Coalescence analyses indicate that almost all of these events have occurred within the past 5,000 years, suggesting the potential involvement of human or avian migrations in this process5.
Probing the origin and diversity of C. gattii has proven more challenging. There is a growing consensus that the evolutionary origins of this species lie within Australia and South America, as most dispersed lineages of C. gattii are near clonal (such as the lineage responsible for the Pacific Northwest outbreak) but always cluster with Australian and South American isolates during phylogenetic analyses, with an estimated origin within the past 50,000 years111–113. A recurrent theme therefore seems to be that local populations of C. gattii in endemic areas (such as Brazil) undergo continual recombination, which occasionally results in a new recombinant lineage that disperses and expands rapidly by means of clonal growth (either asexual cell division or same-sex mating)111, 112, 114.
Both species of Cryptococcus have a bipolar mating system in which cells are either mating type a (MATa) or mating type-α (MATα) (reviewed in REF 115). Classical mating involves genetic exchange between a MATa and MATα strain, followed by normal Mendelian segregation of alleles. However, both species of cryptococci are also capable of same-sex mating in which two strains of the same mating type can exchange genetic material114, 116. In addition, diploid and aneuploid strains are not uncommon117, 118, and inter- and intra-species hybrids can be found both in the environment and in patients119, 120. Thus, the global population structure of these pathogens reflects a complex mix of diversity generating recombination and aneuploidy, coupled with highly clonal amplification steps during dispersion events.
In this Review we describe the interactions between Cryptococcus spp. and its host, highlighting how this fungus subverts the host immune system to cause disease and how this interaction influences disease severity. We also discuss potential therapeutic avenues revealed by the improved understanding of these host–pathogen interactions.
Cryptococcus transmission and disease onset
In the environment, cryptococci reside in diverse ecological niches (BOX 3). Both C. neoformans and C. gattii are abundant in decaying material within hollows of various tree species, although C. gattii has been suggested to favour trees with waxier cuticles (such as the Douglas fir Pseudotsuga menziesii)3, 4. Furthermore, C. neoformans is globally distributed, whereas C. gattii has classically been viewed as a tropical or subtropical fungus. However, increased surveillance has now identified environmental reservoirs for C. gattii in the Northern USA, Canada and Northern Europe, indicating that this species may also have a wider ecological range than previously recognized.
Box 3. The evolution of virulence in cryptococci.
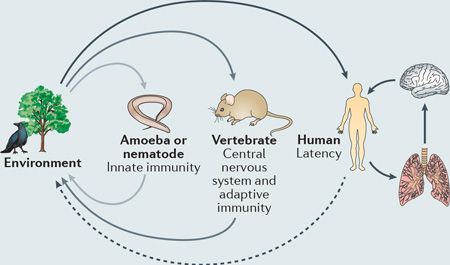
Opportunistic pathogens represent an evolutionary enigma: why has natural selection driven the acquisition of often highly specific virulence factors when most of the population remain as exclusively environmental organisms for their entire existence? This conundrum is particularly pertinent for cryptococci, which are abundant in the environment but are remarkably well suited to survive in a human host.
A compelling hypothesis to resolve this conundrum is that of accidental pathogenesis 121. This hypothesis proposes that cryptococcal pathogenesis does not result from direct selection for virulence within a mammalian host, but rather by the evolution of traits (which happen to be advantageous in mammals) in response to other selective pressures in both environmental and animal niches. So, for example, the complex polysaccharide capsule, laccase activity and the ability to synthesize melanin, all of which are Cryptococcus spp. virulence factors, are likely to offer protection against environmental pressures such as desiccation or exposure to ultraviolet light122, or aid in the colonization of plant hosts123. Similarly, cryptococci can replicate not only within vertebrate phagocytes, but also in free-living phagocytic amoebae124 (see the figure). Despite the enormous evolutionary distance between vertebrates and amoebae, many of the mechanisms used by phagocytic white blood cells to kill pathogens (for example, the generation of reactive oxygen species or the secretion of antimicrobial peptides) are identical to those used by amoebae to digest ingested prey. Thus, over millions of years, cryptococci have been selected to evolve strategies that facilitate fungal growth and persistence within amoebae that coincidentally also enable their survival within phagocytes. Such strategies include not only stress-tolerance approaches, such as resistance to reactive oxygen species125, but also elaborate mechanisms to regulate expulsion from host cells46, 47.
In addition, Cryptococcus spp. have a remarkable ability to perturb adaptive immunity, preventing complete fungal clearance and resulting in latent infections19, 126. Perhaps the ability to remain latent without perturbing their host is the strongest evidence for host adaptation by cryptococci. Because only higher vertebrates have adaptive immune systems, Cryptococcus spp. probably evolved these properties under the selective pressures of reptilian, avian or mammalian hosts within the environment, which also explains the diverse range of animals that are susceptible cryptococcosis.
Taken together, these observations suggest that interactions with both soil microorganisms (such as amoebae and nematodes) and vertebrates probably have a crucial role in the virulence potential of Cryptococcus spp. (reviewed in REF. 127). Intriguingly, laboratory studies have shown that selection pressure by amoebae can rapidly select for resistant, pseudohyphal forms of cryptococci23. These forms are attenuated in mammalian hosts and consequently frequently revert to yeast upon entry into a vertebrate host. Thus, rapid microevolutionary events may have an important role in driving cryptococcal pathogenesis in different hosts.
The paradigm of accidental pathogenesis extends beyond cryptococci to other fungal128 and even bacterial pathogens129, such as Aspergillus spp., Blastomyces spp. and Legionella spp., and it highlights two important issues. First, as pathogens adapt to changing environments due to global warming, we may see additional instances of accidental pathogenesis through the selection of new traits that promote both environmental survival and pathogenesis in humans. Second, we should be alert to the fact that changes in human behaviour and habitat use (for example, increased tourist access to remote rainforest or desert areas) may expose us to new potential pathogens that have been predisposed to infection via selection through environmental predators.
C. neoformans is particularly abundant in avian excreta4, 5, and its association with feral pigeons could be a major source of infection in densely populated urban areas. In addition, both C. neoformans and C. gattii can survive and replicate in free-living amoebae and soil nematodes, and it is possible that these alternative hosts may have an important role in determining the distribution and virulence of different cryptococcal lineages around the world (BOX 3).
With the exception of very rare iatrogenic6 or zoonotic7 transmission events, naturally acquired cases of cryptococcosis are thought to start with inhalation of fungal cells from the environment. Within the lung, Cryptococcus spp. can cause pneumonia in immunosuppressed patients, but in immunocompetent hosts the fungal cells are either cleared by the immune system or establish an asymptomatic latent infection. On subsequent immunosuppression, this latent infection can then disseminate to other tissues, most notably the central nervous system (CNS). Once established in the CNS, cryptococcosis causes an overwhelming infection of the meninges and brain tissue that is frequently accompanied by raised intracranial pressure; without rapid and effective treatment, CNS infection is invariably fatal. Despite intensive investigations, it remains unclear whether reactivation and dissemination of long-term latent pulmonary infection is a more important cause of cryptococcosis in patients than de novo acquisition from the environment, but experiments in animal models indicate that both routes can cause lethal disease.
Exposure to C. neoformans is common in humans, as most individuals produce antibodies against this fungal species by school age8. During active growth, cryptococcal cells are too large to penetrate deep into the human lung and thus the initial inoculum is thought to comprise either desiccated cells or spores. The relative contribution of these two cell types to the burden of disease remains unclear, largely owing to technical challenges associated with generating and purifying spores. However, recent studies have demonstrated that lethal brain infections can develop from spore inocula, that spores are readily phagocytosed by host immune cells and, interestingly, that rising humidity markedly increases spore viability9–11. Thus, as with other fungal pathogens such as Coccidioides immitis, environmental conditions may be an important factor in regulating human cryptococcal exposure.
Cryptococcal pathogenesis
Traditional virulence factors produced by Cryptococcus spp. (such as the capsule and melanin) and changes in fungal growth due to the host temperature (37 °C) have been previously reviewed in great detail (see for example REFS 12, 13). Instead, in this section of the Review, we focus on recently emerging concepts in cryptococcal pathogenesis.
Fungal morphology
Whether derived from spores or yeast cells, upon inhalation into a mammalian host all cryptococci transition to or maintain a yeast form. When grown under laboratory conditions, Cryptococcus cells are round and 5–7 µm in diameter. However, their cell size, structure and characteristics can vary dramatically within the host.
The best-characterized atypical morphology of Cryptococcus cells is the titan cell14 (FIG. 1). Titan cells are greater than 12 µm in diameter (excluding the capsule), polyploid, have highly crosslinked capsules and a thickened cell wall15, 16. Recent studies have shown that titan cells contain elevated levels of chitin. This polysaccharide is recognized and cleaved by host chitinases, which induces a detrimental adaptive immune response (see below)17. Intriguingly, the polyploidy observed in titan cells enhances genetic adaptation to the stressful host environment, resulting in increased within-host survival18.
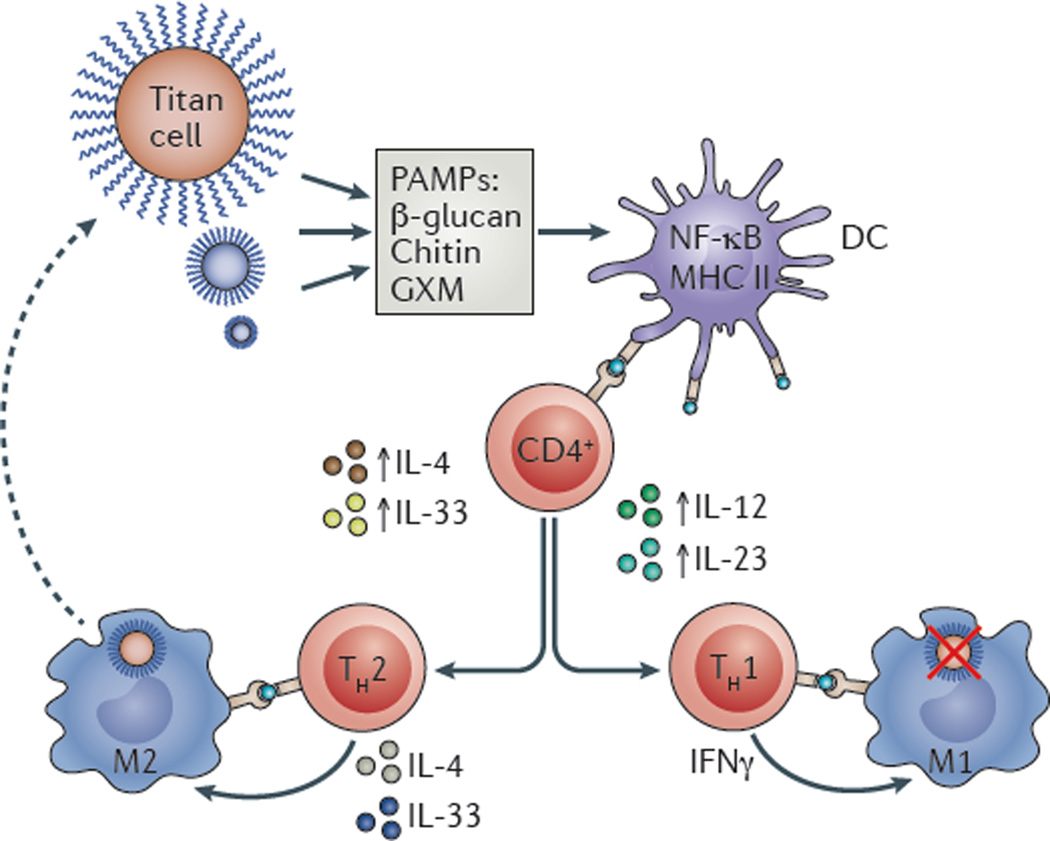
Figure 1. Inflammatory signalling in response to cryptococcal infection.
Cryptococci, which can assume a titan cell morphology, inevitably shed microbial molecules that contain pathogen-associated molecular patterns (PAMPs). Such fungal molecules are typically components of the cell wall or capsule such as β-glucan, chitin or glucuronoxylomannan (GXM), which are detected by immune sentinel cells, most notably dendritic cells (DCs). DC activation then summons T cells, inducing CD4+ T cells to secrete cytokines that activate a T helper cell 1 (TH1) response (including the secretion of interleukin-12 (IL-12) and IL-23). TH1 cells produce pro-inflammatory cytokines (such as interferon-γ (IFNγ)), which ultimately control fungal infection. However, some fungal PAMPs can influence DC activation, including modulating the levels of major histocompatibility complex class II (MHC II) or of nuclear factor-κB (NF-κB) signalling. This activates a TH2 response (mediated by the production of cytokines such as IL-4 and IL-33); this anti-inflammatory environment affects macrophage activation (M1 classic activation and M2 alternative activation) and the ability of macrophages to mediate fungal clearance.
In addition to the large titan cells, unusually small cryptococcal cells have also been observed19, 20 (FIG. 1). These socalled drop or micro cells are only 2–4 µm in size, despite having a thickened cell wall, and seem to be adapted for growth in macrophages. At present, little is known about this cell type, although they seem to be relatively metabolically inactive and therefore may have an important role during the latent stage of disease.
In the environment or under laboratory conditions, cryptococci can also grow as hyphae (during sexual reproduction) or pseudohyphae, but unlike other pathogenic fungi these morphologies are not seen in human infections21. Recent studies overexpressing the transcription factor Znf2, a master regulator that triggers the transition from yeast to hyphal growth, showed that the hyphal form elicits a robust protective immune response and is readily cleared by the host22, 23, perhaps explaining why filamentous morphologies are not seen in mammalian infections. Interestingly, however, hyphal cryptococci are protected from predation by free-living amoebae24, and thus mammalian and amoebal hosts presumably exert opposing selective pressures on this aspect of cryptococcal morphology (with mammalian hosts favouring the existence of the yeast forms and amoebae favouring hyphal forms).
Fungal ageing
Even within a clonal infection, not all cryptococcal cells are equal. For example, the age of individual cryptococcal cells has emerged as a factor that affects survival in the host and subsequent pathogenesis25. Older cells present in the initial infection, referred to as founder cells, are better able to resist phagocytosis and killing by phagocytes and are resistant to antifungal drugs. This increased resistance to phagocyte killing and antifungals is potentially due to changes in cell wall structure26 and results in the accumulation of founder cells in the brain at a higher frequency than young cells27.
Population-wide signals
In bacterial infections, quorum sensing is a well-known mechanism that regulates virulence according to population density. Interestingly, emerging data suggest that quorum sensing may also have an important role during cryptococcal pathogenesis. For example, a quorum sensing effect, mediated by an oligopeptide with 11 amino acids, was identified using mutations in the global repressor Tup1. Notably, although Tup1 is present in several species, the quorum sensing effect mediated by this oligopeptide seems to only occur in C. neoformans28. However, more recently a different signalling molecule, pantothenic acid, was shown to mediate quorum sensing both between different cryptococcal strains and between cryptococci and other, relatively distantly related fungal species29. The adhesin Cfl1 has also been shown to modulate colony morphology in a paracrine manner30. Activation of the hyphal regulator Znf2 induces expression of this adhesin, some of which is shed into the environment and triggers neighbouring cells to activate Znf2, leading to a positive feedback loop. Thus, cryptococci may communicate locally using a range of chemical messengers31.
Perhaps most unique is the observation that light-sensing pathways may also be important for virulence in Cryptococcus spp., as deletion of either BWC1 or BWC2, which encode two transcription factors that control fungal responses to light, reduces virulence in a mouse model of infection32. In the dark, Bwc1 and Bwc2 bind to DNA and repress genes involved in filamentation. However, upon light activation, they release this inhibition, leading to filamentation and upregulation of ultraviolet-resistance pathways. Thus, it is possible that an additional function of these two proteins is to detect darkness and prevent inappropriate filamentation within the host, which would induce a potent immune response and pathogen clearance.
Host immunity and pathogen subversion
One of the most remarkable discoveries of recent years has been the extent to which cryptococci can manipulate the host immune response to dampen inflammation, avoid killing by phagocytic cells and ultimately disseminate into the CNS.
Inflammatory perturbation
In general, environmental fungi trigger a potent pro-inflammatory response on entry into the human host. By contrast, cryptococci seem to be immunologically inert, driving much lower levels of pro-inflammatory cytokine release in vitro than other human fungal pathogens such as Candida albicans33. This immunological masking relies on various pathogen traits (FIG. 1).
First, the complex carbohydrates glucuronoxylomannan (GXM) and galactoxylomannan (GalXM), which make up most of the cryptococcal capsule, are extensively shed during infection and directly dampen inflammation by suppressing the pro- inflammatory nuclear factor-κB (NF-κB) pathway and driving down levels of pro-inflammatory cytokines such as tumour necrosis factor (TNF)34. In addition, emerging data indicate that cryptococcal chitin and its derivatives can also alter host inflammatory responses during infection17. Second, Cryptococcus spp. block dendritic cell maturation by reducing both major histocompatibility complex class II (MHC class II)-dependent antigen presentation and inhibiting the production of the pro-inflammatory cytokines interleukin-12 (IL-12) and IL-23 (REF. 35). Last, via a series of as-yet poorly characterized steps, cryptococci can partially repolarize the immune response, at least in mice, from a strong T helper 1 response (TH1 response) towards a weaker TH1 or often a TH2 response, which is less effective at fungal clearance17, 36–38.
Collectively, these mechanisms generate an environment that is dominated by anti-inflammatory markers such as IL-4 and IL-33 (REFS 39–41), which, as a consequence, reduce cryptococcal killing by the immune system38, 42. Therefore, modulating natural immune responses to cryptococcal infection towards a more pro-inflammatory profile offers one potential avenue for treatment. However, such approaches need to be carefully managed to avoid the potentially fatal immune ‘over reactions’ that can accompany overt inflammation, which can be just as life-threatening as the original infection (BOX 4).
Box 4. Host immunity: too little or too much?
Poor pro-inflammatory responses to cryptococci, such as those in patients with advanced HIV infection, lead to life-threatening meningoencephalitis. Consequently, immune profiling of the peripheral T cell responses and cerebrospinal fluid cytokines from patients has shown that those mounting a pro-inflammatory immune response are more likely to clear the pathogen and survive infection66. Moreover, augmenting pro-inflammatory immune responses using adjunctive interferon-γ (IFNγ) improves fungal clearance130. Conversely, individuals producing antibodies that block the activity of cytokines and interfere with appropriate pro-inflammatory responses are known to be at enhanced risk of infection131.
Although a potent immune response to Cryptococcus spp. is clearly essential for fungal clearance, too strong a response can also be harmful. For instance, a low level of anti-inflammatory activity driven by both T helper 2 (TH2) and regulatory T cells132, 133 prevents complete immune paralysis. This is also the case for the classical antifungal cytokine interleukin-17 (IL-17), which is essential for resistance to cryptococcosis134, but the effects of which must also be regulated by IL-23 to prevent damage to the host owing to excessive inflammation135. Thus, a ‘successful’ immune response to cryptococcal infection seems to be a complex blend of TH1, TH2 and TH17 responses, which must be counter-regulated to prevent either runaway fungal growth or damaging levels of inflammation.
This crucial role for ‘restraining’ pro-inflammatory signalling is particularly highlighted by the problem of immune reconstitution inflammatory syndrome (IRIS). This life-threatening inflammatory reaction occurs in some HIV-infected patients during antiretroviral therapy (ART) and is caused by an ‘overreaction’ of the newly reconstituted immune system to residual pathogen antigen. Consequently, the timing of clinical intervention is crucial; early introduction of ART is important to restore cell-mediated immunity; but if ART is introduced too early (during the initial 2 weeks following induction of antifungal treatment) at a time of high fungal load, the risk of death is increased136. Development of IRIS is particularly likely in patients who mount a poor initial pro-inflammatory response to cryptococcal infection, resulting in high residual antigen burden137. Coupled with an exaggerated baseline chemokine response in the central nervous system (CNS), this results in aberrant CNS immune responses following ART initiation, resulting in IRIS.
Excessive inflammation can also occur following withdrawal of immune suppression in solid organ transplant recipients, as well as in apparently immunocompetent patients. In such situations, steroids are often administered alongside antifungals. It remains unclear, however, whether steroids are beneficial in other contexts: a multi-centre clinical trial to address this issue (investigating the effect of adjunctive dexamethasone in patients with HIV-associated cryptococcal meningoencephalitis) has been terminated early and results are awaited138.
Avoidance and escape from phagocytes
Following entry into the lung, the first immune cell typically encountered by cryptococci is a phagocyte such as an alveolar macrophage or dendritic cell. However, cryptococci are predisposed to avoid killing by these cells, owing to their long evolutionary history of exposure to environ-mental amoebae (BOX 3). Several cryptococcal virulence factors, such as capsule synthesis, melanization and urease secretion combine to protect the fungus from the harsh environment within phagocytic cells by neutralizing reactive oxygen species and pH, allowing it to survive and proliferate within such cells43 (FIG. 2).
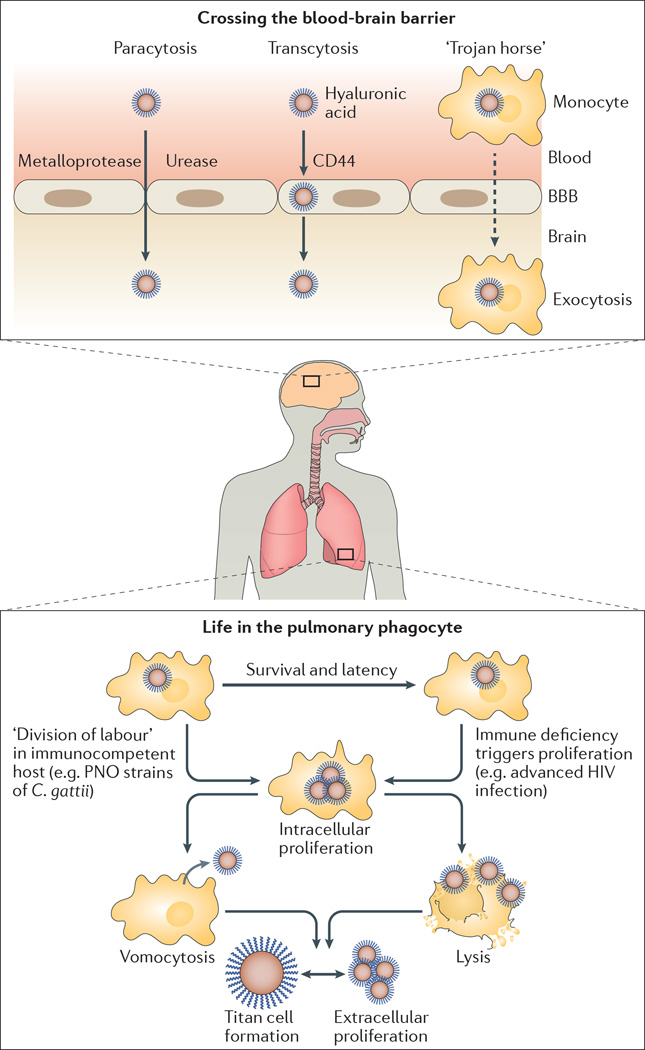
Figure 2. Infection establishment and dissemination with in the human host.
Cryptococcal cells typically enter the human host through the lung (bottom). There, they are recognized by patrolling phagocytes but can avoid uptake either by growing into very large titan cells, or by relying on the antiphagocytic properties of the fungal capsule. If uptake occurs, however, cryptococci can survive and persist in phagocytes. For most strains, a failure in host immune function is then required to allow intracellular proliferation. However, the unusual Pacific Northwest outbreak (PNO) strains of Cryptococcus gattii can proliferate in immunocompetent host cells by exploiting a poorly characterized ‘division of labour’ mechanism: in response to reactive oxygen species generated by the phagocyte, some cryptococcal cells acquire an unusual morphology characterized by extensive tubularization of their mitochondria, which increases survival of neighbouring cells (via a mechanism that remains unclear). Cryptococcus spp. proliferation within phagocytes ultimately leads either to host cell lysis or to a nonlytic escape mechanism termed vomocytosis. Upon replication in the lung, cryptococci are able to disseminate to other tissues, including the central nervous system (CNS). Entry into the CNS can occur in three ways: by squeezing between host endothelial cells (paracytosis), which involves the fungal protease Mpr1 and the enzyme urease (which probably weakens the endothelial vessel wall to facilitate entry); by moving directly through endothelial cells (transcytosis), in a process that is mediated by hyaluronic acid in the fungal capsule and the host receptor CD44; or by ‘hitching a ride’ within migrating phagocytes, through what is known as the ‘Trojan horse’ hypothesis. BBB, blood–brain barrier.
More recently, it has also become clear that cryptococci exhibit a remarkable strategy to escape from phagocytes. This process, which has been labelled vomocytosis or extrusion, involves inducing the fusion of the phagosomal membrane with the plasma membrane, resulting in the expulsion of the fungi from the phagocyte44–48. In addition, either this process or a closely related one can drive the direct ‘lateral transfer’ of cryptococci between host cells44, 45. However, the underlying mechanisms of both of these remarkable processes remain unknown.
Although cryptococci employ several mechanisms to resist phagocytosis (such as the production of titan cells15, 49 and the assembly of a thick polysaccharide capsule), fungal uptake by phagocytes can still occur. However, if uptake does occur, cryptococci perturb both phagosome maturation50 and modify the phagosome membrane to allow nutrient exchange and ultimately escape from the host cell51, 52. Notably, these effects depend on fungal virulence factors such as laccase and phospholipase B1. These enzymes have been classically thought of as having direct structural roles in melanin synthesis and membrane lipid modification, respectively, but the observation that they also mediate escape from phagocytosis suggests that they may also have more subtle roles in modifying host signalling events36, 53, 54.
Dissemination and entry into the CNS
A key feature of cryptococcal pathogenesis involves the exit of Cryptococcus spp. from the lungs into peripheral blood circulation and entry into the CNS compartment. The CNS is both an immune-privileged site and a highly sterile environment, and thus Cryptococcus spp. must have evolved potent methods to traverse the blood–brain barrier and subsist in the CNS.
There are three proposed mechanisms to penetrate this impervious barrier. First, the Cryptococcus cells could force their way between the tight junctions of the endothelial cells in a process known as paracytosis, by using proteases such as Mpr1 to promote transendothelial migration55 (FIG. 2). Impressively, when MPR1 was introduced into Saccharomyces cerevisiae, a fungus not normally able to penetrate the blood–brain barrier, S. cerevisiae gained the ability to cross endothelial cells in an in vitro transwell assay, although the target of Mpr1 remains unknown. Additional studies using powerful intravital imaging techniques demonstrated that cryptococci cross the blood–brain barrier by inducing an embolic event in the microvasculature that lines the brain56. In essence, the initial ‘capture’ of the fungus within the brain is passive, with the relatively large cells becoming trapped at points where the blood vessel narrows. However, following the initial passive arrest, cryptococcal migration into the brain tissue is an active process, as it occurs only with live fungal cells and depends on the secretion of the cryptococcal enzyme urease57. To date, the part played by urease in this process remains enigmatic; however, because urease produces ammonia, which is toxic to mammalian cells, it is possible that urease acts to locally weaken the endothelial vessel wall, facilitating fungal entry.
The second mechanism of blood–brain barrier penetration is transcytosis58 (FIG. 2). Hyaluronic acid situated on the surface of the cryptococcal cell binds to CD44 on the luminal endothelium, attaching the fungus to the host cell59. This binding then induces protein kinase C-dependent actin remodelling in the host cell, leading it to engulf the attached Cryptococcus cell60. Interestingly, recent work has revealed that the high levels of inositol present in the brain act as a trigger for this process, increasing hyaluronic acid expression by the fungus61.
Finally, Cryptococcus spp. is postulated to cross the blood–brain barrier by ‘hitchhiking’ within host phagocytes, in what is known as the ‘Trojan Horse’ hypothesis (FIG. 2). This hypothesis is supported by the observation that depletion of alveolar macrophages in mice significantly reduces cryptococcal dissemination to the CNS62, whereas infecting monocytes in vitro and transferring the cells into naive hosts substantially increases cryptococcal accumulation in the brain compared to transfer-ring Cryptococcus spp. directly; both studies support the notion that phagocytes act as fungal carriers that breach the blood–brain barrier63. Although paracytosis, transcytosis and Trojan Horse models are all fundamentally different, it is reasonable to conclude that elements of each of these models are readily observed and likely occur in concert during natural infection.
Not much is known about the physiology of Cryptococcus spp. after it has traversed the blood–brain barrier. However, a recent study of the transcriptome of cryptococcal yeasts isolated from patient samples of cerebrospinal fluid (CSF) offers some clues64. Most notably, Cryptococcus spp. are remarkably metabolically active in the CSF in vivo , showing strong upregulation of stress response genes and genes encoding enzymes that are involved in core metabolic processes; this is somewhat surprising, given that the CSF is a relatively nutrient- depleted medium. By contrast, Cryptococcus spp. growing in ex vivo CSF do not seem to be metabolically active, suggesting that the permanent cycling of CSF in vivo leads to a significantly higher nutrient content in the CSF than suggested by the analysis of ex vivo samples.
The modified fungal metabolism observed in the CSF is likely to have significant implications for pathogenesis. For instance, capsule synthesis is energetically highly demanding, and there is a positive correlation between capsular size and severity of clinical disease65. Therefore, these data suggest that yeasts in a more active metabolic state may drive more aggressive CNS infections. Furthermore, fungal cells in different metabolic states are likely to give rise to different immune responses, which may also affect disease severity. In agreement with this possibility, the presence of a pro-inflammatory response in the CSF consisting of an interplay of robust TH1 cytokines (IFNγ and IL-6), TH2 cytokines (IL-4 and IL-10) and TH17 cytokines (IL-17) has recently been shown to be highly predictive of more rapid clearance of infection and consequently improved survival in patients with HIV-associated cryptococcal meningitis66 (BOX 4).
Division of labour
The extent to which cryptococci can exploit phagocytic cells as a host has been strongly highlighted by the unusual cluster of cryptococcal disease now known as the Pacific Northwest outbreak67. Although cryptococcosis is typically a disease of immunocompromized hosts, almost all of the human and animal cases within the Pacific Northwest outbreak were immunocompetent hosts who became infected with near clonal strains of C. gattii from the VGII lineage. Both the epidemiology and aetiology of these infections differ from classical cryptococcosis (typically caused by C. neoformans in HIV-positive individuals)33, 68, which has led to vigorous efforts to establish the underlying mechanism driving virulence in the C. gattii VGII lineage.
The ability of the C. gattii VGII lineage to establish disease in individuals with a fully functional immune system seems to stem from a capacity to replicate extremely rapidly in host phagocytes (FIG. 2), presumably overwhelming the host before adaptive immunity can be triggered69. Recent data have revealed that this rapid proliferation is, in turn, driven by a remarkable mechanism of division of labour. In response to reactive oxygen species generated by the phagocyte, intracellular cryptococcal cells adopt different fates; some stop growing and acquire an unusual morphology that is characterized by extensive tubularization of their mitochondria, whereas neighbouring cells do not undergo this transition. Notably, via a mechanism that remains unclear, the cells that undergo the morphological switch then protect neighbouring cryptococci from the antimicrobial activity of the host phagocyte, enabling them to replicate rapidly and thereby maximizing the proliferative capacity of the population as a whole70. These data highlight the Cryptococcus–phagocyte interaction as a key aspect of infection that may offer powerful opportunities for therapeutic intervention in both C. neoformans and C. gattii infections.
Anti-cryptococcal therapeutics
Despite its global distribution, treatment of crypto coccosis remains a major challenge, relying on a limited arsenal of decades-old therapeutic agents. Furthermore, therapeutic outcomes are generally poor and even with amphotericinbased therapy (to target Cryptococcus spp.) and widespread access to anti-retroviral therapy (to target HIV, as most patients are immunocompromised patients with HIV), acute (that is, within 3 months) mortality following cryptococcal meningo encephalitis remains at 35–40%, both in resource-rich and resource-poor settings71, 72.
Currently used drugs
Only three classes of antifungal agents are currently used to treat cryptococcosis: polyenes (such as amphotericin B), azoles (such as fluconazole) and the pyrimidine analogue flucytosine (5-FC) (FIG. 3).
Figure 3. Current and future therapies for cryptococcosis.
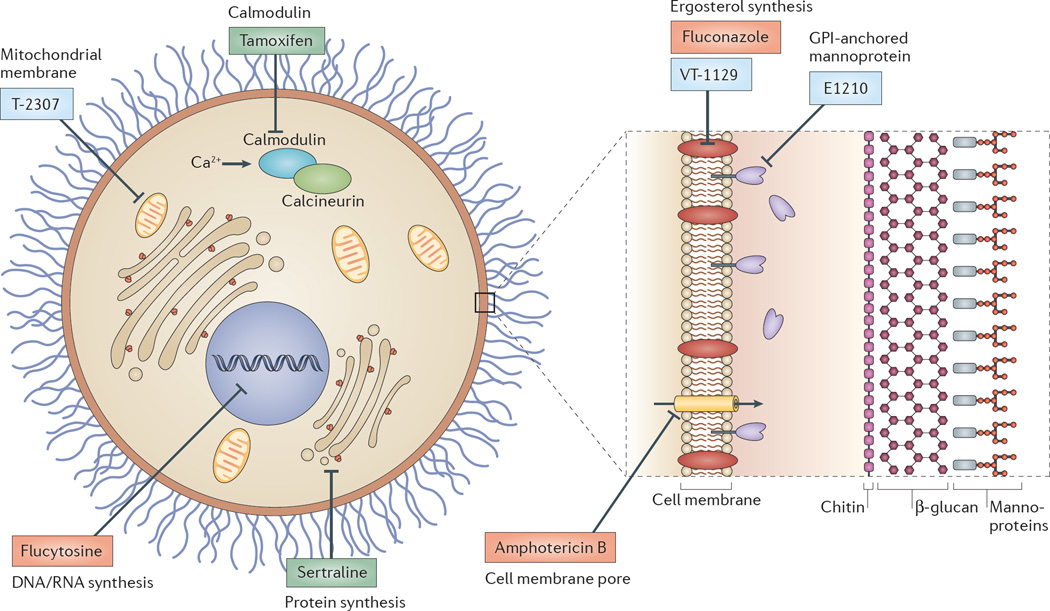
Schematic representation of a cryptococcal cell, showing key current and potential therapeutic targets and examples of antifungal drugs acting at each site. Drugs in current clinical use are shown in red, new drugs in blue and repurposed drugs in green. The three classes of antifungal agents currently used to treat cryptococcosis are polyenes (such as amphotericin B), azoles (such as fluconazole) and the pyrimidine analogue flucytosine (5-FC). Amphotericin B deoxycholate (AmBd) acts by binding to ergosterol in the cryptococcal cell wall, generating pores in the cell membrane, and by inducing cell death by oxidative damage. 5-FC is deaminated by the fungal enzyme cytosine deaminase into 5-fluorouracil (5-FU), which then inhibits thymidylate synthetase and blocks DNA synthesis, or is converted into 5-fluorouridine triphosphate, which is incorporated into RNA and disrupts protein synthesis. Fluconazole inhibits the fungal cytochrome P450 enzyme 14α-demethylase, which is required for conversion of lanosterol to ergosterol, an essential component of the fungal cell membrane. E1210 inhibits the synthesis of the cell wall component glycosylphosphatidylinositol (GPI)-anchored mannoproteins. VT-1129 blocks the activity of CYP51, an essential enzyme in the pathway to produce ergosterol. The arlyamidine T-2307 targets the fungal mitochondrial membrane. Tamoxifen (an oestrogen antagonist that is used in the treatment of breast cancer) targets calmodulin, and the antidepressant sertraline seems to target fungal protein synthesis through an unknown mechanism.
The cornerstone of treatment of cryptococcal meningoencephalitis is amphotericin B deoxycholate (AmBd), developed in the 1950s, which exerts its fungicidal effect both by binding to ergosterol in the cryptococcal cell wall (generating pores in the cell membrane) and by inducing cell death via oxidative damage73–75. AmBd is sometimes combined with 5-FC. The mechanism of action of 5-FC is deamination by the fungal enzyme cytosine deaminase into 5-fluorouracil (5-FU), which then acts through two pathways: 5-FU can be converted by cellular pyrimi-dine-processing enzymes into 5-fluorodeoxy uridine monophosphate, which inhibits thymidylate synthetase and blocks DNA synthesis; or it can be converted into 5-fluorouridine triphosphate, which is incorporated into RNA, thereby disrupting protein synthesis and leading to growth arrest. AmBd and 5-FC act synergistically to produce the fastest rates of fungal clearance from CSF76, and combination therapy results in a significant improvement in 10-week survival of patients compared with treatment with AmBd alone77. This combination remains the recommended ‘gold standard’ induction treatment in international treatment guidelines78 but presents substantial challenges in resource-poor settings, as AmBd must be administered intravenously and has notable toxicities. In addition, AmBd and 5-FC are not widely available in countries where cryptococcosis is most prevalent79.
To circumvent the problems associated with AmBd and 5-FC combination therapies, the combination of fluconazole with 5-FC (which can both be administered orally) and shorter (that is, 1-week long) AmBd-based induction treatment is being compared to the standard 2-week induction regimens in a multi-site Phase III trial in Africa80. Fluconazole is being tested because it has good oral bioavailability and excellent CSF penetration; these properties also make it a good candidate for main tenance therapy after initial treatment. Fluconazole inhibits the fungal cytochrome P450 enzyme 14α-demethylase, which is required for conversion of lanosterol to ergosterol, an essential component of the fungal cell membrane. However, fluconazole is a fungistatic (rather than fungicidal) and so is less effective at pathogen clearance and not recommended for initial therapy.
Drug resistance
Resistance to antimicrobials is a growing issue in infectious disease, and cryptococcosis is no exception. Although environmental resistance is rare, acquired resistance has been observed with all three classes of antifungals in use against Cryptococcus spp.
Polyene resistance is uncommon but has been reported in C. neoformans, with mutations in sterol synthesis and therefore alteration of the target site noted in isolates with extensive exposure to AmBd81. For 5-FC, single mutations at varying points along the 5-FU intracellular pathways lead to in vitro and clinical resistance. Therefore, monotherapy with 5-FC is not appropriate due to rapid selection of resistant Cryptococcus spp. leading to treatment failure; the drug is thus always combined with either AmBd or fluconazole. Fluconazole, like 5-FC, is fungistatic, making it liable to evolution of secondary resistance during prolonged treatment82. A key mechanism of resistance against fluconazole is the selection of intrinsically resistant cryptococcal subpopulations83 that carry specific chromosomal disomies84 and thus overexpress ERG11 (which encodes the fluconazole target enzyme lanosterol-14α-demethylase85) or have enhanced drug efflux by the ATP-binding cassette (ABC) transporter- encoding gene C. neoformans anti-fungal resistance 1 (CNAFR1)86.
New drugs
Given the ongoing high global incidence and mortality from cryptococcal meningoencephalitis, the dearth of drugs, together with toxicity and the potential for development of resistance, there is an urgent need for new drugs. Recent activity in this area has begun to highlight potential routes either for the discovery of new antifungals or for the repurposing of existing molecules showing anticryptococcal activity (FIG. 3).
An ideal antifungal drug should specifically target the fungus and not the host to avoid host cell toxicity; this is challenging given that fungal cellular processes are more closely related to mammals than those that are targeted by common antimicrobials, such as the ones used to target bacterial pathogens. Furthermore, an ideal antifungal drug should target either a virulence factor or a fungal component that is essential for fungal viability. Such a drug should be fungicidal when used alone or when combined with the widely available fluconazole, it should have good oral bioavailability (allowing it to be readily administered even in resource-poor settings) and should be able to enter cryptococcal niches within the host (such as phagocytes and the CNS).
One obvious target of such a drug is the cryptococcal cell wall. Unfortunately, the latest class of antifungals that are active against the cell wall, β-1,3D–glucan synthase inhibitors (known as echinocandins), have no substantial anti-cryptococcal activity. However, synthesis of another cell wall component, glycosylphosphatidyl inositol (GPI)-anchored mannoproteins, is inhibited by the orally active experimental molecule E1210, which has in vitro activity against Cryptococcus spp. and other medically relevant fungi (such as Candida and Scedosporium spp.) and is currently in preclinical development87.
Further along the development pipeline is VT-1129, an ergosterol synthesis inhibitor (available for oral administration) which shows good CNS penetration and is fungicidal in mouse models of Cryptococcus spp. infection. VT-1129 blocks the activity of CYP51, an essential enzyme in the pathway that produces ergosterol, and is currently entering human clinical trials (for further information see the Viamet website). Also in Phase I trials is the arlyamidine T-2307, which targets the fungal mitochondrial membrane88. T-2307 is a fungicidal injectable compound that shows comparable efficacy to AmBd in mouse models of infection.
Given the lack of market forces driving pharmaceutical development for a neglected disease such as cryptococcal meningoencephalitis, an alternative, cheaper and more expedient strategy in drug development is the repurposing of drugs that were originally developed for other conditions. Recently developed high-throughput screening techniques have advanced the repurposing effort. One such powerful tool is chemical-genetic profiling, whereby large collections of cryptococcal-knockout mutants, for which the function of a particular pathway is compromised, are screened against a library of small molecules89, and the growth behaviour of the screened strain (that is, increased or decreased susceptibility) is then recorded. A recent application of this technique with 1,448 knockout mutants of C. neoformans demonstrated distinct differences in drug susceptibility between this species and the model organism S. cerevisiae which, until now, has been the standard choice for such screens89. As proof-of-principle, this approach has identified a number of molecules that synergize strongly with fluconazole to inhibit ergosterol synthesis in C. neoformans and which are now being further investigated for potential clinical applicability. Moreover, this method has the additional advantage of providing information on the mechanism of action of lead compounds and can therefore identify both potential new drugs and potential new drug targets.
A more classical approach is to screen for compounds that trigger fungal cell lysis (detected by the release of adenylate kinase, a cytosolic enzyme, into the medium) or that alter ATP content90 (a particularly effective approach for identifying compounds that are antifungal under starvation conditions). This strategy has identified a collection of off-patent drugs91 with anticryptococcal activity that are additive or synergistic with fluconazole. These include drugs as diverse as amiodarone (a cardiac anti-arrhythmic drug), phenothiazines (widely used antipsychotics) and tamoxifen (an oestrogen antagonist used to treat breast cancer). Illustrating the utility of these approaches, tamoxifen in combination with fluconazole decreased the C. neoformans burden in the brain by ∼1 log10 CFU per gram of brain tissue, in a mouse model of infection92. Finally, another candidate that has emerged from repurposing screens is the antidepressant sertraline which is fungicidal, has high CNS penetration and seems to target fungal protein synthesis through an unknown mechanism93. Sertraline is currently being evaluated in combination with AmBd and fluconazole in a Phase II/III clinical trial94.
Outlook
The past 5 years have seen a remarkable revolution in our understanding of cryptococcosis. A deeper understanding of the natural ecology and an appreciation of the genetic and phenotypic diversity of this group of pathogens is transforming our understanding of cryptococcal pathogenesis. Meanwhile, huge progress has been made in understanding the host immune response to infection and how this process is hijacked by cryptococci to drive latency, dissemination and proliferation. However, despite these advances, cryptococcosis remains a major worldwide killer, causing hundreds of thousands of deaths per year, and the anticryptococcal drug arsenal remains limited. To address this, there is renewed focus on translational research to discover and develop new therapeutic agents and to evaluate new therapeutic strategies in a clinical setting. While progress is being made in this respect, more is urgently required, and advances in understanding the pathogenesis of Cryptococcus spp. offer new opportunities for developing therapeutics beyond the traditional approaches of killing the fungal cell or preventing its replication. In particular, the rapidly expanding understanding of the Cryptococcus–host interface opens up new avenues for potential therapy development; for instance, in modifying host pro-inflammatory responses, augmenting phagocytic clearance of the fungus, disrupting population signalling or preventing migration to the CNS. Together, such approaches offer the hope of significantly reducing the huge global burden of infection and making fatal cryptococcosis a disease of the past.
Acknowledgments
The authors gratefully acknowledge the help of S. Kannambath in preparing Figure 3 and apologize to those colleagues in the field whose work could not be included in this Review owing to space constraints. R.C.M. is supported by funding from the European Research Council, Medical Research Council, Lister Institute and Royal Society. D.L.W received support from the US National Institutes of Health (NIH) T32 training grant AI007313, a University of Minnesota Doctoral Dissertation Fellowship and a Dennis W Watson Fellowship. K.N. is supported by funding from the NIH. T.B. is supported by funding from the Wellcome Trust and the Medical Research Council (UK). N.R.H.S. is supported by a Wellcome Trust Strategic Award in Medical Mycology and Fungal Immunology to the University of Aberdeen.
Glossary
- Pacific Northwest outbreak
An unusual cluster of cryptococcal disease in otherwise healthy (rather than immunocompromised) individuals. First identified on Vancouver Island, British Columbia, in 1999 (and hence originally called the Vancouver Island outbreak), both the causative organism and cases of human and animal disease have now expanded into mainland Canada and the northwestern USA, prompting a renaming of the outbreak.
- Iatrogenic
Caused by medical treatment. For instance, infections due to contaminated surgical instruments.
- Zoonotic
A disease transmitted from animals to people.
- Polyploid
Having multiple (that is, more than two) sets of homologous chromosomes.
- Founder
The initial, small group of individuals that seeds a new population. For instance, the inoculum that starts an infection, or the first individuals to arrive on a new island habitat.
- Quorum sensing
The regulation of gene expression or behaviour in response to changes in the local population size.
- Paracrine
A signal that acts close to where it is produced, for instance on neighbouring cells.
- Filamentation
The growth of an organism by elongation without division.
- Major histocompatibility complex class II
(MHC class II). Molecules that are expressed on the surface of professional antigen-presenting cells (such as macrophages and dendritic cells) and present extracellular antigens to the immune system to coordinate an immune response.
- T helper 1 response
A response by one subtype of CD4+ helper T (TH) cell that is generally provoked by intracellular pathogens. TH2 responses, by contrast, are typically involved in the elimination of parasitic worms, harmful allergic responses and dampening of TH1-mediated inflammation. In the context of cryptococcal infection, TH1 responses are widely thought to be protective, and TH2 responses to be detrimental.
- Coalescence analyses
An evolutionary analysis method in which genetic drift is ‘.played backwards’. to calculate common ancestry of individuals within a population and thereby estimate lineage branch points within an evolutionary phylogenetic tree.
- Bipolar mating
A system to control sexual reproduction that relies on a single genetic locus at which individual organisms can carry one of two alleles, effectively generating a species with two sexes.
- Diploid
Having two homologous sets of chromosomes, one from each parent.
- Aneuploid
Having an ‘.unbalanced’. set of chromosomes; for instance, having only a single copy of one chromosome in an otherwise diploid genome.
- Melanization
The production of the dark, insoluble pigment melanin, which provides protection from high energy radiation and reactive oxygen molecules.
- Blood–brain barrier
A specialized endothelial barrier that prevents the entry of cells or large molecules into the central nervous system.
- Paracytosis
Transitioning between tissues by moving between, rather than through, adjacent cells.
- Transcytosis
Transitioning between tissues by moving directly through cells, rather than between adjacent cells.
- Hyaluronic acid
An abundant, high-molecular-weight polysaccharide that forms part of the extracellular matrix, particularly in neural tissue.
- Cerebrospinal fluid
(CSF). A clear fluid produced in the brain that bathes the central nervous tissue and is slowly turned over.
- Regulatory T cells
A type of T cell that functions to regulate the immune system, typically by suppressing the function of pro-inflammatory effector T cells.
- Fungicidal
An antimicrobial agent that kills fungi, rather than simply preventing growth.
- Fungistatic
An antimicrobial agent that prevents fungal growth, but does not kill the organism.
Footnotes
Competing interests statement
The authors declare no competing interests.
FURTHER INFORMATION
Viamet website: http://www.viamet.com/products/vt-1129
References
- 1.Kwon-Chung JK, Boekhout T, Fell JW, Diaz M. Proposal to conserve the name Cryptococcus gattii against C. hondurianus and C. basillisporus (Basidiomycota, Hymenomycetes, Tremellomycetidae) Taxon. 2002;51:804–806. [Google Scholar]
- 2.Hagen F, et al. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet. Biol. 2015;78:16–48. doi: 10.1016/j.fgb.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Springer DJ, et al. Cryptococcus gattii VGIII isolates causing infections in HIV/AIDS patients in Southern California: identification of the local environmental source as arboreal. PLoS Pathog. 2014;10:e1004285. doi: 10.1371/journal.ppat.1004285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chowdhary A, Rhandhawa HS, Prakash A, Meis JF. Environmental prevalence of Cryptococcus neoformans and Cryptococcus gattii in India: an update. Crit. Rev. Microbiol. 2012;38:1–16. doi: 10.3109/1040841X.2011.606426. [DOI] [PubMed] [Google Scholar]
- 5.Litvintseva AP, et al. Evidence that the human pathogenic fungus Cryptococcus neoformans var. grubii may have evolved in Africa. PLoS ONE. 2011;6:e19688. doi: 10.1371/journal.pone.0019688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baddley JW, et al. Transmission of Cryptococcus neoformans by organ transplantation. Clin. Infect. Dis. 2011;52:e94–e98. doi: 10.1093/cid/ciq216. [DOI] [PubMed] [Google Scholar]
- 7.Lagrou K, et al. Zoonotic transmission of Cryptococcus neoformans from a magpie to an immunocompetent patient. J. Intern. Med. 2005;257:385–388. doi: 10.1111/j.1365-2796.2005.01466.x. [DOI] [PubMed] [Google Scholar]
- 8.Goldman DL, et al. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics. 2001;107:E66. doi: 10.1542/peds.107.5.e66. [DOI] [PubMed] [Google Scholar]
- 9.Giles SS, Dagenais TR, Botts MR, Keller NP, Hull CM. Elucidating the pathogenesis of spores from the human fungal pathogen Cryptococcus neoformans. Infect. Immun. 2009;77:3491–3500. doi: 10.1128/IAI.00334-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Springer DJ, Saini D, Byrnes EJ, Heitman J, Frothingham R. Development of an aerosol model of Cryptococcus reveals humidity as an important factor affecting the viability of Cryptococcus during aerosolization. PLoS ONE. 2013;8:e69804. doi: 10.1371/journal.pone.0069804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Velagapudi R, Hsueh YP, Geunes-Boyer S, Wright JR, Heitman J. Spores as infectious propagules of Cryptococcus neoformans. Infect. Immun. 2009;77:4345–4355. doi: 10.1128/IAI.00542-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaragoza O, et al. The capsule of the fungal pathogen Cryptococcus neoformans. Adv. Appl. Microbiol. 2009;68:133–216. doi: 10.1016/S0065-2164(09)01204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonald T, Wiesner DL, Nielsen K. Cryptococcus. Curr. Biol. 2012;22:R554–R555. doi: 10.1016/j.cub.2012.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaragoza O, Nielsen K. Titan cells in Cryptococcus neoformans: cells with a giant impact. Curr. Opin. Microbiol. 2013;16:409–413. doi: 10.1016/j.mib.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okagaki LH, et al. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog. 2010;6:e1000953. doi: 10.1371/journal.ppat.1000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaragoza O, et al. Fungal cell gigantism during mammalian infection. PLoS Pathog. 2010;6:e1000945. doi: 10.1371/journal.ppat.1000945. References 15 and 16 simultaneously reported the identification of titan cells, which are likely to play a key role in cryptococcal pathogenesis
- 17.Wiesner DL, et al. Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal Infection. PLoS Pathog. 2015;11:e1004701. doi: 10.1371/journal.ppat.1004701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerstein AC, et al. Polyploid titan cells produce haploid and aneuploid progeny to promote stress adaptation. mBio. 2015;6:e01340–e01415. doi: 10.1128/mBio.01340-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alanio A, Vernel-Pauillac F, Sturny-Leclère A, Dromer F. Cryptococcus neoformans host adaptation: toward biological evidence of dormancy. mBio. 2015;6:e02580–e02614. doi: 10.1128/mBio.02580-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldmesser M, Kress Y, Casadevall A. Dynamic changes in the morphology of Cryptococcus neoformans during murine pulmonary infection. Microbiology. 2001;147:2355–2365. doi: 10.1099/00221287-147-8-2355. [DOI] [PubMed] [Google Scholar]
- 21.Neilson JB, Fromtling RA, Bulmer GS. Pseudohyphal forms of Cryptococcus neoformans: decreased survival in vivo. Mycopathologia. 1981;73:57–59. doi: 10.1007/BF00443015. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Zhai B, Lin X. The link between morphotype transition and virulence in Cryptococcus neoformans. PLoS Pathog. 2012;8:e1002765. doi: 10.1371/journal.ppat.1002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magditch DA, Liu TB, Xue C, Idnurm A. DNA mutations mediate microevolution between host-adapted forms of the pathogenic fungus Cryptococcus neoformans. PLoS Pathog. 2012;8:e1002936. doi: 10.1371/journal.ppat.1002936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin J, Idnurm A, Lin X. Morphology and its underlying genetic regulation impact the interaction between Cryptococcus neoformans and its hosts. Med. Mycol. 2015;53:493–504. doi: 10.1093/mmy/myv012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouklas T, Fries BC. Aging as an emergent factor that contributes to phenotypic variation in Cryptococcus neoformans. Fungal Genet. Biol. 2014;78:59–64. doi: 10.1016/j.fgb.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouklas T, et al. Old Cryptococcus neoformans cells contribute to virulence in chronic cryptococcosis. mBio. 2013;4:e00455–e00413. doi: 10.1128/mBio.00455-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain N, et al. Isolation characterization of senescent C. neoformans and its implications for phenotypic switching and the pathogenesis of chronic cryptococcosis. Eukaryot. Cell. 2009;8:858–866. doi: 10.1128/EC.00017-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee H, Chang YC, Nardone G, Kwon-Chung KJ. TUP1 disruption in Cryptococcus neoformans uncovers a peptide-mediated density-dependent growth phenomenon that mimics quorum sensing. Mol. Microbiol. 2007;64:591–601. doi: 10.1111/j.1365-2958.2007.05666.x. [DOI] [PubMed] [Google Scholar]
- 29.Albuquerque P, et al. Quorum sensing-mediated, cell density-dependent regulation of growth and virulence in Cryptococcus neoformans. mBio. 2014;5:e00986–e00913. doi: 10.1128/mBio.00986-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, Tian X, Gyawali R, Lin X. Fungal adhesion protein guides community behaviors and autoinduction in a paracrine manner. Proc. Natl Acad. Sci. USA. 2013;110:11571–11576. doi: 10.1073/pnas.1308173110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albuquerque PC, et al. Cryptococcus neoformans glucuronoxylomannan fractions of different molecular masses are functionally distinct. Future Microbiol. 2014;9:147–161. doi: 10.2217/fmb.13.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Idnurm A, Heitman J. Light controls growth and development via a conserved pathway in the fungal kingdom. PLoS Biol. 2005;3:e95. doi: 10.1371/journal.pbio.0030095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schoffelen T, et al. Cryptococcus gattii induces a cytokine pattern that is distinct from other cryptococcal species. PLoS ONE. 2013;8:e55579. doi: 10.1371/journal.pone.0055579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piccioni M, et al. A purified capsular polysaccharide markedly inhibits inflammatory response during endotoxic shock. Infect. Immun. 2013;81:90–98. doi: 10.1128/IAI.00553-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Angkasekwinai P, et al. Cryptococcus gattii infection dampens Th1 and Th17 responses by attenuating dendritic cell function and pulmonary chemokine expression in the immunocompetent hosts. Infect. Immun. 2014;82:3880–3890. doi: 10.1128/IAI.01773-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu Y, et al. Immune modulation mediated by cryptococcal laccase promotes pulmonary growth and brain dissemination of virulent Cryptococcus neoformans in mice. PLoS ONE. 2012;7:e47853. doi: 10.1371/journal.pone.0047853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis MJ, et al. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. mBio. 2013;4:e00264–e00213. doi: 10.1128/mBio.00264-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voelz K, Lammas DA, May RC. Cytokine signaling regulates the outcome of intracellular macrophage parasitism by Cryptococcus neoformans. Infect. Immun. 2009;77:3450–3457. doi: 10.1128/IAI.00297-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller U, et al. Abrogation of IL-4 receptor-α-dependent alternatively activated macrophages is sufficient to confer resistance against pulmonary cryptococcosis despite an ongoing Th2 response. Int. Immunol. 2013;25:459–470. doi: 10.1093/intimm/dxt003. [DOI] [PubMed] [Google Scholar]
- 40.Hardison SE, et al. Protective immunity against pulmonary cryptococcosis is associated with STAT1-mediated classical macrophage activation. J. Immunol. 2012;189:4060–4068. doi: 10.4049/jimmunol.1103455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flaczyk A, et al. IL-33 signaling regulates innate and adaptive immunity to Cryptococcus neoformans. J. Immunol. 2013;191:2503–2513. doi: 10.4049/jimmunol.1300426. [DOI] [PubMed] [Google Scholar]
- 42.Chen GH, et al. Inheritance of immune polarization patterns is linked to resistance versus susceptibility to Cryptococcus neoformans in a mouse model. Infect. Immun. 2008;76:2379–2391. doi: 10.1128/IAI.01143-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coelho C, Bocca AL, Casadevall A. The intracellular life of Cryptococcus neoformans. Annu. Rev. Pathol. 2014;9:219–238. doi: 10.1146/annurev-pathol-012513-104653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alvarez M, Casadevall A. Cell-to-cell spread and massive vacuole formation after Cryptococcus neoformans infection of murine macrophages. BMC Immunol. 2007;8:16. doi: 10.1186/1471-2172-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma H, Croudace JE, Lammas DA, May RC. Direct cell-to-cell spread of a pathogenic yeast. BMC Immunol. 2007;8:15. doi: 10.1186/1471-2172-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma H, Croudace JE, Lammas DA, May RC. Expulsion of live pathogenic yeast by macrophages. Curr. Biol. 2006;16:2156–2160. doi: 10.1016/j.cub.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 47.Alvarez M, Casadevall A. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr. Biol. 2006;16:2161–2165. doi: 10.1016/j.cub.2006.09.061. [DOI] [PubMed] [Google Scholar]
- 48.Nicola AM, Robertson EJ, Albuquerque P, Derengowski Lda S, Casadevall A. Nonlytic exocytosis of Cryptococcus neoformans from macrophages occurs in vivo and is influenced by phagosomal pH. mBio. 2011;2:e00167–e00111. doi: 10.1128/mBio.00167-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okagaki LH, Nielsen K. Titan cells confer protection from phagocytosis in Cryptococcus neoformans infections. Eukaryot. Cell. 2012;11:820–826. doi: 10.1128/EC.00121-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith LM, Dixon EF, May RC. The fungal pathogen Cryptococcus neoformans manipulates macrophage phagosome maturation. Cell. Microbiol. 2014 doi: 10.1111/cmi.12394. [DOI] [PubMed] [Google Scholar]
- 51.Davis MJ, et al. Cryptococcus neoformans-induced macrophage lysosome damage crucially contributes to fungal virulence. J. Immunol. 2015;194:2219–2231. doi: 10.4049/jimmunol.1402376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnston SA, May RC. The human fungal pathogen Cryptococcus neoformans escapes macrophages by a phagosome emptying mechanism that is inhibited by Arp2/3 complex-mediated actin polymerisation. PLoS Pathog. 2010;6:e1001041. doi: 10.1371/journal.ppat.1001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Erb-Downward JR, Noggle RM, Williamson PR, Huffnagle GB. The role of laccase in prostaglandin production by Cryptococcus neoformans. Mol. Microbiol. 2008;68:1428–1437. doi: 10.1111/j.1365-2958.2008.06245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Evans RJ, et al. Cryptococcal phospholipase B1 is required for intracellular proliferation and control of titan cell morphology during macrophage infection. Infect. Immun. 2015;83:1296–1304. doi: 10.1128/IAI.03104-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vu K, et al. Invasion of the central nervous system by Cryptococcus neoformans requires a secreted fungal metalloprotease. mBio. 2014;5:e01101–e01114. doi: 10.1128/mBio.01101-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi M, et al. Real-time imaging of trapping and urease-dependent transmigration of Cryptococcus neoformans in mouse brain. J. Clin. Invest. 2010;120:1683–1693. doi: 10.1172/JCI41963. The first observation of cryptococcal invasion into the brain in vivo
- 57.Olszewski MA, et al. Urease expression by Cryptococcus neoformans promotes microvascular sequestration, thereby enhancing central nervous system invasion. Am. J. Pathol. 2004;164:1761–1771. doi: 10.1016/S0002-9440(10)63734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang YC, et al. Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood-brain barrier. Infect. Immun. 2004;72:4985–4995. doi: 10.1128/IAI.72.9.4985-4995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jong A, et al. Involvement of human CD44 during Cryptococcus neoformans infection of brain microvascular endothelial cells. Cell. Microbiol. 2008;10:1313–1326. doi: 10.1111/j.1462-5822.2008.01128.x. [DOI] [PubMed] [Google Scholar]
- 60.Jong A, et al. Invasion of Cryptococcus neoformans into human brain microvascular endothelial cells requires protein kinase C-α activation. Cell. Microbiol. 2008;10:1854–1865. doi: 10.1111/j.1462-5822.2008.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu TB, et al. Brain inositol is a novel stimulator for promoting Cryptococcus penetration of the blood-brain barrier. PLoS Pathog. 2013;9:e1003247. doi: 10.1371/journal.ppat.1003247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kechichian TB, Shea J, Del Poeta M. Depletion of alveolar macrophages decreases the dissemination of a glucosylceramide-deficient mutant of Cryptococcus neoformans in immunodeficient mice. Infect. Immun. 2007;75:4792–4798. doi: 10.1128/IAI.00587-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Charlier C, et al. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect. Immun. 2009;77:120–127. doi: 10.1128/IAI.01065-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Y, et al. The Cryptococcus neoformans transcriptome at the site of human meningitis. mBio. 2014;5:e01087–e01013. doi: 10.1128/mBio.01087-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Robertson EJ, et al. Cryptococcus neoformans ex vivo capsule size is associated with intracranial pressure and host immune response in HIV-associated cryptococcal meningitis. J. Infect. Dis. 2014;209:74–82. doi: 10.1093/infdis/jit435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jarvis JN, et al. Cerebrospinal fluid cytokine profiles predict risk of early mortality and immune reconstitution inflammatory syndrome in HIV-associated cryptococcal meningitis. PLoS Pathog. 2015;11:e1004754. doi: 10.1371/journal.ppat.1004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Datta K, et al. Spread of Cryptococcus gattii into Pacific Northwest region of the United States. Emerg. Infect. Dis. 2009;15:1185–1191. doi: 10.3201/eid1508.081384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harris JR, et al. Cryptococcus gattii in the United States: clinical aspects of infection with an emerging pathogen. Clin. Infect. Dis. 2011;53:1188–1195. doi: 10.1093/cid/cir723. [DOI] [PubMed] [Google Scholar]
- 69.Ma H, et al. The fatal fungal outbreak on Vancouver Island is characterized by enhanced intracellular parasitism driven by mitochondrial regulation. Proc. Natl Acad. Sci. USA. 2009;106:12980–12985. doi: 10.1073/pnas.0902963106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Voelz K, et al. ‘Division of labour’ in response to host oxidative burst drives a fatal Cryptococcus gattii outbreak. Nat. Commun. 2014;5:5194. doi: 10.1038/ncomms6194. Description of a new virulence mechanism that underpins the hypervirulent Pacific Northwest outbreak
- 71.Brizendine KD, Baddley JW, Pappas PG. Predictors of mortality and differences in clinical features among patients with cryptococcosis according to immune status. PLoS ONE. 2013;8:e60431. doi: 10.1371/journal.pone.0060431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Siddiqi OK, et al. Molecular diagnosis of central nervous system opportunistic infections in HIV-infected Zambian adults. Clin. Infect. Dis. 2014;58:1771–1777. doi: 10.1093/cid/ciu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anderson TM, et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol. 2014;10:400–406. doi: 10.1038/nchembio.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Belenky P, Camacho D, Collins JJ. Fungicidal drugs induce a common oxidative-damage cellular death pathway. Cell Rep. 2013;3:350–358. doi: 10.1016/j.celrep.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gray KC, et al. Amphotericin primarily kills yeast by simply binding ergosterol. Proc. Natl Acad. Sci. USA. 2012;109:2234–2239. doi: 10.1073/pnas.1117280109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brouwer AE, et al. Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. Lancet. 2004;363:1764–1767. doi: 10.1016/S0140-6736(04)16301-0. [DOI] [PubMed] [Google Scholar]
- 77.Day JN, et al. Combination antifungal therapy for cryptococcal meningitis. N. Engl. J. Med. 2013;368:1291–1302. doi: 10.1056/NEJMoa1110404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perfect JR, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin. Infect. Dis. 2010;50:291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Loyse A, et al. Cryptococcal meningitis: improving access to essential antifungal medicines in resource-poor countries. Lancet Infect. Dis. 2013;13:629–637. doi: 10.1016/S1473-3099(13)70078-1. [DOI] [PubMed] [Google Scholar]
- 80.ISRCTN Registry. A phase III, randomised, controlled trial for the treatment of HIV-associated cryptococcal meningitis: oral fluconazole plus flucytosine or one week amphotericin B-based therapy vs two weeks amphotericin B-based therapy. 2015 ISRCTN registry [online], http://dx.doi.org/10.1186/ISRCTN45035509.
- 81.Kelly SL, et al. Resistance to amphotericin B associated with defective sterol Δ8⊗7 isomerase in a Cryptococcus neoformans strain from an AIDS patient. FEMS Microbiol. Lett. 1994;122:39–42. doi: 10.1111/j.1574-6968.1994.tb07140.x. [DOI] [PubMed] [Google Scholar]
- 82.Bicanic T, Harrison T, Niepieklo A, Dyakopu N, Meintjes G. Symptomatic relapse of HIV-associated cryptococcal meningitis after initial fluconazole monotherapy: the role of fluconazole resistance and immune reconstitution. Clin. Infect. Dis. 2006;43:1069–1073. doi: 10.1086/507895. [DOI] [PubMed] [Google Scholar]
- 83.Sionov E, Chang YC, Garraffo HM, Kwon-Chung KJ. Heteroresistance to fluconazole in Cryptococcus neoformans is intrinsic and associated with virulence. Antimicrob. Agents Chemother. 2009;53:2804–2815. doi: 10.1128/AAC.00295-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sionov E, Lee H, Chang YC, Kwon-Chung KJ. Cryptococcus neoformans overcomes stress of azole drugs by formation of disomy in specific multiple chromosomes. PLoS Pathog. 2010;6:e1000848. doi: 10.1371/journal.ppat.1000848. An elegant example of the mechanism driving antifungal resistance in cryptococci
- 85.Sionov E, Chang YC, Kwon-Chung KJ. Azole heteroresistance in Cryptococcus neoformans: emergence of resistant clones with chromosomal disomy in the mouse brain during fluconazole treatment. Antimicrob. Agents Chemother. 2013;57:5127–5130. doi: 10.1128/AAC.00694-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Posteraro B, et al. Identification and characterization of a Cryptococcus neoformans ATP binding cassette (ABC) transporter-encoding gene, CnAFR1, involved in the resistance to fluconazole. Mol. Microbiol. 2003;47:357–371. doi: 10.1046/j.1365-2958.2003.03281.x. [DOI] [PubMed] [Google Scholar]
- 87.Miyazaki M, et al. In vitro activity of E1210, a novel antifungal, against clinically important yeasts and molds. Antimicrob. Agents Chemother. 2011;55:4652–4658. doi: 10.1128/AAC.00291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shibata T, et al. T-2307 causes collapse of mitochondrial membrane potential in yeast. Antimicrob. Agents Chemother. 2012;56:5892–5897. doi: 10.1128/AAC.05954-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brown JC, et al. Unraveling the biology of a fungal meningitis pathogen using chemical genetics. Cell. 2014;159:1168–1187. doi: 10.1016/j.cell.2014.10.044. A powerful approach for identifying new antifungals and identifying their mode of action
- 90.Dehdashti SJ, et al. A high-throughput screening assay for assessing the viability of Cryptococcus neoformans under nutrient starvation conditions. Anal. Bioanal Chem. 2013;405:6823–6829. doi: 10.1007/s00216-013-7134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Butts A, et al. A repurposing approach identifies off-patent drugs with fungicidal cryptococcal activity, a common structural chemotype, and pharmacological properties relevant to the treatment of cryptococcosis. Eukaryot. Cell. 2013;12:278–287. doi: 10.1128/EC.00314-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Butts A, et al. Estrogen receptor antagonists are anti-cryptococcal agents that directly bind EF hand proteins and synergize with fluconazole in vivo. mBio. 2014;5:e00765–e00713. doi: 10.1128/mBio.00765-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhai B, Wu C, Wang L, Sachs MS, Lin X. The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrob. Agents Chemother. 2012;56:3758–3766. doi: 10.1128/AAC.00212-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.US National Library of Medicine. ClinicalTrials.gov [online], http://clinicaltrials.gov/ct2/show/NCT01802385.
- 95.Saha DC, et al. Serologic evidence for reactivation of cryptococcosis in solid-organ transplant recipients. Clin. Vaccine Immunol. 2007;14:1550–1554. doi: 10.1128/CVI.00242-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fang W, Fa Z, Liao W. Epidemiology of Cryptococcus and cryptococcosis in China. Fungal Genet. Biol. 2014;78:7–15. doi: 10.1016/j.fgb.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 97.Beale MA, et al. Genotypic diversity is associated with clinical outcome and phenotype in cryptococcal meningitis across Southern Africa. PLoS Negl. Trop. Dis. 2015;9:e0003847. doi: 10.1371/journal.pntd.0003847. A recent wide-ranging analysis that, for the first time, demonstrates an important role for cryptococcal genotype in human pathogenesis
- 98.Litvintseva AP, Mitchell TG. Most environmental isolates of Cryptococcus neoformans vargrubii (serotype A) are not lethal for mice. Infect. Immun. 2009;77:3188–3195. doi: 10.1128/IAI.00296-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wiesner DL, et al. Cryptococcal genotype influences immunologic response and human clinical outcome after meningitis. mBio. 2012;3:e00196–e00112. doi: 10.1128/mBio.00196-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khayhan K, et al. Geographically structured populations of Cryptococcus neoformans variety grubii in Asia correlate with HIV status and show a clonal population structure. PLoS ONE. 2013;8:e72222. doi: 10.1371/journal.pone.0072222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ou XT, et al. Genotypes coding for mannose-binding lectin deficiency correlated with cryptococcal meningitis in HIV-uninfected Chinese patients. J. Infect. Dis. 2011;203:1686–1691. doi: 10.1093/infdis/jir152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hu XP, et al. Association of Fcγ receptor IIB polymorphism with cryptococcal meningitis in HIV-uninfected Chinese patients. PLoS ONE. 2012;7:e42439. doi: 10.1371/journal.pone.0042439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rohatgi S, et al. Fcγ receptor 3A polymorphism and risk for HIV-associated cryptococcal disease. mBio. 2013;4:e00573–e00513. doi: 10.1128/mBio.00573-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sabiiti W, et al. Efficient phagocytosis and laccase activity affect the outcome of HIV-associated cryptococcosis. J. Clin. Invest. 2014;124:2000–2008. doi: 10.1172/JCI72950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jarvis JN, et al. Evaluation of a novel point-of-care cryptococcal antigen test on serum, plasma, and urine from patients with HIV-associated cryptococcal meningitis. Clin. Infect. Dis. 2011;53:1019–1023. doi: 10.1093/cid/cir613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mfinanga S, et al. Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label, randomised controlled trial. Lancet. 2015;385:60164–60167. doi: 10.1016/S0140-6736(15)60164-7. [DOI] [PubMed] [Google Scholar]
- 107.Findley K, et al. Phylogeny and phenotypic characterization of pathogenic Cryptococcus species and closely related saprobic taxa in the Tremellales. Eukaryot. Cell. 2009;8:353–361. doi: 10.1128/EC.00373-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xu J, Vilgalys R, Mitchell TG. Multiple gene genealogies reveal recent dispersion and hybridization in the human pathogenic fungus Cryptococcus neoformans. Mol. Ecol. 2000;9:1471–1481. doi: 10.1046/j.1365-294x.2000.01021.x. Compelling genetic evidence of an African origin for C. neoformans
- 109.Litvintseva AP, Mitchell TG. Population genetic analyses reveal the African origin and strain variation of Cryptococcus neoformans var grubii. PLoS Pathog. 2012;8:e1002495. doi: 10.1371/journal.ppat.1002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Litvintseva AP, Lin X, Templeton I, Heitman J, Mitchell TG. Many globally isolated AD hybrid strains of Cryptococcus neoformans originated in Africa. PLoS Pathog. 2007;3:e114. doi: 10.1371/journal.ppat.0030114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Billmyre RB, et al. Highly recombinant VGII Cryptococcus gattii population develops clonal outbreak clusters through both sexual macroevolution and asexual microevolution. mBio. 2014;5:e01494–e01414. doi: 10.1128/mBio.01494-14. Detailed analysis of C. gattii population structure, providing important insights into the evolution and dispersal of lineages within this species
- 112.Hagen F, et al. Ancient dispersal of the human fungal pathogen Cryptococcus gattii from the Amazon Rainforest. PLoS ONE. 2013;8:e71148. doi: 10.1371/journal.pone.0071148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Engelthaler DM, et al. Cryptococcus gattii in North American Pacific Northwest: whole-population genome analysis provides insights into species evolution and dispersal. mBio. 2014;5:e01464–e01414. doi: 10.1128/mBio.01464-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fraser JA, et al. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature. 2005;437:1360–1364. doi: 10.1038/nature04220. [DOI] [PubMed] [Google Scholar]
- 115.Idnurm A, et al. Deciphering the model pathogenic fungus Cryptococcus neoformans. Nat. Rev. Microbiol. 2005;3:753–764. doi: 10.1038/nrmicro1245. Although now 10 years old, this is still an excellent all-round introduction to the pathogen
- 116.Lin X, Hull CM, Heitman J. Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature. 2005;434:1017–1021. doi: 10.1038/nature03448. [DOI] [PubMed] [Google Scholar]
- 117.Lin X, et al. Diploids in the Cryptococcus neoformans serotype A population homozygous for the α mating type originate via unisexual mating. PLoS Pathog. 2009;5:e1000283. doi: 10.1371/journal.ppat.1000283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ni M, et al. Unisexual and heterosexual meiotic reproduction generate aneuploidy and phenotypic diversity de novo in the yeast Cryptococcus neoformans. PLoS Biol. 2013;11:e1001653. doi: 10.1371/journal.pbio.1001653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lin X, et al. αADα hybrids of Cryptococcus neoformans: evidence of same-sex mating in nature and hybrid fitness. PLoS Genet. 2007;3:1975–1990. doi: 10.1371/journal.pgen.0030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bovers M, et al. Unique hybrids between the fungal pathogens Cryptococcus neoformans and Cryptococcus gattii. FEMS Yeast Res. 2006;6:599–607. doi: 10.1111/j.1567-1364.2006.00082.x. [DOI] [PubMed] [Google Scholar]
- 121.Casadevall A. Evolution of intracellular pathogens. Annu. Rev. Microbiol. 2008;62:19–33. doi: 10.1146/annurev.micro.61.080706.093305. [DOI] [PubMed] [Google Scholar]
- 122.Wang Y, Casadevall A. Decreased susceptibility of melanized Cryptococcus neoformans to UV light. Appl. Environ. Microbiol. 1994;60:3864–3866. doi: 10.1128/aem.60.10.3864-3866.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Warpeha KM, Park YD, Williamson PR. Susceptibility of intact germinating Arabidopsis thaliana to human fungal pathogens Cryptococcus neoformans and C. gattii. Appl. Environ. Microbiol. 2013;79:2979–2988. doi: 10.1128/AEM.03697-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Steenbergen JN, Shuman HA, Casadevall A. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc. Natl Acad. Sci. USA. 2001;98:15245–15250. doi: 10.1073/pnas.261418798. The first report of cryptococcal parasitism of amoebae, and the associated proposal of an ‘accidental pathogen’ model for the evolution of cryptococcal virulence
- 125.Zaragoza O, et al. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell. Microbiol. 2008;10:2043–2057. doi: 10.1111/j.1462-5822.2008.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Garcia-Hermoso D, Janbon G, Dromer F. Epidemiological evidence for dormant Cryptococcus neoformans infection. J. Clin. Microbiol. 1999;37:3204–3209. doi: 10.1128/jcm.37.10.3204-3209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kronstad JW, et al. Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nat. Rev. Microbiol. 2011;9:193–203. doi: 10.1038/nrmicro2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Steenbergen JN, Nosanchuk JD, Malliaris SD, Casadevall A. Interaction of Blastomyces dermatitidis, Sporothrix schenckii, and Histoplasma capsulatum with Acanthamoeba castellanii. Infect. Immun. 2004;72:3478–3488. doi: 10.1128/IAI.72.6.3478-3488.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bliska JB, Casadevall A. Intracellular pathogenic bacteria and fungi—a case of convergent evolution? Nat. Rev. Microbiol. 2009;7:165–171. doi: 10.1038/nrmicro2049. [DOI] [PubMed] [Google Scholar]
- 130.Jarvis JN, et al. Adjunctive interferon-γ immunotherapy for the treatment of HIV-associated cryptococcal meningitis: a randomized controlled trial. AIDS. 2012;26:1105–1113. doi: 10.1097/QAD.0b013e3283536a93. An important clinical trial, both in improving patient outcomes and in demonstrating the direct effect of IFNy on cryptococcal immunity
- 131.Saijo T, et al. Anti-granulocyte-macrophage colony-stimulating factor autoantibodies are a risk factor for central nervous system infection by Cryptococcus gattii in otherwise immunocompetent patients. mBio. 2014;5:e00912–e00914. doi: 10.1128/mBio.00912-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grahnert A, et al. IL-4 receptor-a-dependent control of Cryptococcus neoformans in the early phase of pulmonary infection. PLoS ONE. 2014;9:e87341. doi: 10.1371/journal.pone.0087341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Schulze B, et al. CD4+ FoxP3+ regulatory T cells suppress fatal T helper 2 cell immunity during pulmonary fungal infection. Eur. J. Immunol. 2014;44:3596–3604. doi: 10.1002/eji.201444963. [DOI] [PubMed] [Google Scholar]
- 134.Murdock BJ, Huffnagle GB, Olszewski MA, Osterholzer JJ. Interleukin-17A enhances host defense against cryptococcal lung infection through effects mediated by leukocyte recruitment, activation, and γHowever, the unusual Pacific interferon production. Infect. Immun. 2014;82:937–948. doi: 10.1128/IAI.01477-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Szymczak WA, Sellers RS, Pirofski LA. IL-23 dampens the allergic response to Cryptococcus neoformans through IL-17-independent and -dependent mechanisms. Am. J. Pathol. 2012;180:1547–1559. doi: 10.1016/j.ajpath.2011.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Boulware DR, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N. Engl. J. Med. 2014;370:2487–2498. doi: 10.1056/NEJMoa1312884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chang CC, et al. Cryptococcosis-IRIS is associated with lower cryptococcus-specific IFN-y responses before antiretroviral therapy but not higher T-cell responses during therapy. J. Infect. Dis. 2013;208:898–906. doi: 10.1093/infdis/jit271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.ISRCTN Registry. Adjunctive dexamethasone in HIV-infected adults with cryptococcal meningitis. ISRCTN registry [online] 2015 http://dx.doi.org/10.1186/ISRCTN59144167.