Abstract
Introduction
In clinical trials comparing telavancin (TLV) with vancomycin for treatment of hospital-acquired pneumonia, TLV demonstrated lower clinical cure rates than vancomycin in patients who had mixed gram-positive and -negative infections and were concomitantly treated with either aztreonam (ATM) or piperacillin/tazobactam (PTZ). Here, we investigated therapeutic interactions between TLV and ATM or PTZ in an in vitro pharmacokinetic/pharmacodynamic (PK/PD) model under simulated reduced renal function conditions.
Methods
In vitro one-compartment PK/PD models were run over 96 h simulating TLV 10 mg/kg every 48 h, ATM 500 mg every 8 h and PTZ continuous infusion 13.5 g over 24 h alone and in combination against P. aeruginosa, E. coli and methicillin-resistant S. aureus (MRSA). The efficacy of antimicrobials was evaluated by plotting time-kill curves and calculating the reduction in log10 cfu/ml over 96 h.
Results
Against both MRSA strains, TLV was rapidly bactericidal at 4 h and maintained its activity over 96 h with no observed antagonism by either ATM or PTZ. PTZ maintained bacteriostatic and bactericidal activities against E. coli ATCC 25922 and clinical strain R1022 at 96 h, whereas both strains regrew as soon as 24 h in ATM models. Against P. aeruginosa ATCC 27853, regrowth was noted at 24 h in models simulating ATM and PTZ. The addition of TLV to ATM or PTZ had no appreciable impact on activity against the two E. coli strains and P. aeruginosa strain.
Conclusions
The combinations of TLV and either ATM or PTZ did not demonstrate any antagonistic activity. Clinical variables and patient characteristics should be further explored to determine possible reasons for discrepancies in outcomes.
Funding
Theravance Biopharma Antibiotics, Inc.
Keywords: Aztreonam, Drug interactions, Escherichia coli, Methicillin resistant, Staphylococcus aureus, Piperacillin/tazobactam, Pseudomonas aeruginosa, Telavancin
Introduction
Telavancin is a semisynthetic lipoglycopeptide with broad-spectrum activity against gram-positive bacteria with different resistance phenotypes including methicillin-resistant S. aureus (MRSA), vancomycin-intermediate S. aureus (VISA), heterogeneous VISA (hVISA) and VanB vancomycin-resistant enterococci (VRE) [1, 2]. Telavancin is approved for treatment of complicated skin and skin structure infections (cSSSIs) caused by susceptible gram-positive organisms and hospital-acquired and ventilator-associated bacterial pneumonia (HABP/VABP) caused by susceptible S. aureus in the USA [3].
Even though telavancin demonstrated a similar efficacy to vancomycin in clinical trials for treatment of both cSSSIs and nosocomial pneumonia, there are two subgroups of patients where cure rates were lower when treated with telavancin compared to vancomycin. In the Assessment of Telavancin for Treatment of Hospital-Acquired Pneumonia (ATTAIN) trials, telavancin efficacy appeared to be lower in a subset of the population with mixed gram-positive and -negative nosocomial pneumonia. In these studies, patients were randomized to either vancomycin 1 g every 12 h or telavancin 10 mg/kg every 24 h for treatment of hospital-acquired pneumonia for 7–21 days. In cases of polymicrobial infections, the addition of aztreonam or piperacillin/tazobactam was permitted. Telavancin achieved better or comparable cure rates for monomicrobial infections caused by S. aureus including MRSA. However, patients with polymicrobial pneumonia had a better cure rate when they were treated with vancomycin (79.4%) compared to telavancin (66.2%), even though the difference did not reach statistical significance [4]. While it is possible that the reduced efficacy may be due to inadequacy of treatment based on susceptibility patterns of the isolated gram-negative pathogens, the reason for these discrepancies in cure rates is unknown.
There was a concern about reduced efficacy in patients with pre-existing renal impairment in comparison to vancomycin. In the study of Assessment of Telavancin in Complicated Skin and Skin Structure Infections (ATLAS), 1867 patients were randomly assigned to vancomycin 1 g every 12 h or telavancin 10 mg/kg every 24 h for treatment of confirmed or suspected cSSSIs caused by gram-positive organisms. Clinical cure rates in patients with MRSA infections were comparable between the vancomycin and telavancin groups, achieving 86% and 91%, respectively. However, patients with creatinine clearance (CrCl) ≤50 ml/min had decreased clinical cure rates when treated with telavancin at 67.4% versus 82.7% when treated with vancomycin [5, 6].
We hypothesize that there might be antagonistic interactions between telavancin and either piperacillin/tazobactam or aztreonam, especially in patients with impaired renal functions. Therefore, the aim of this study was to perform in vitro pharmacokinetic/pharmacodynamic (PK/PD) model evaluations against MRSA and selected gram-negative pathogens, including the most frequently isolated pathogens in the ATTAIN trials, to determine whether any antagonistic relationships exist between telavancin and either aztreonam or piperacillin/tazobactam under simulated reduced renal function conditions.
Methods
Bacterial Strains
P. aeruginosa ATCC 27853, two Escherichia coli strains (ATCC 25922 and clinical isolate R1022) and two MRSA strains (ATCC 43300 and clinical isolate R5255) were evaluated in this study. These isolates were randomly selected from the isolate collection of the Anti-infective Research Laboratory at Wayne State University and consisted mostly of well-referenced ATCC strains. All gram-positive bacteria were susceptible to both vancomycin and telavancin, while gram-negative bacteria were susceptible to aztreonam and piperacillin/tazobactam.
Antimicrobial Agents
Telavancin powder was provided by its manufacturer (Theravance Biopharma Antibiotics, Inc., South San Francisco, CA). Piperacillin, tazobactam and aztreonam were purchased commercially (Sigma Chemical Co., St. Louis, MO).
Media
Cation-adjusted Mueller-Hinton broth (MHB, Difco, Detroit MI) was used for PK/PD models and susceptibility testing. Polysorbate-80 was incorporated into the broth at 0.002% for any experiment involving telavancin to minimize drug loss due to binding to plastic materials [7, 8]. Colony counts were determined using Tryptic Soy Agar (TSA, Difco, Detroit, MI) plates.
Susceptibility Testing
Minimum inhibitory concentration (MIC) values were determined by broth microdilution in duplicate at an inoculum of ~1 × 106 cfu/ml according to the CLSI guidelines [8]. Any isolate for which the MIC results were more than one dilution different was repeated. For telavancin MICs, 0.002% Polysorbate 80 (Sigma Chemical Co., St. Louis, MO) was incorporated into broth. For piperacillin/tazobactam MICs, tazobactam concentrations were fixed at 4 mg/l. All samples were incubated at 37 °C for 24 h.
In Vitro PK/PD Model
An in vitro one-compartment PK/PD model with a 250-ml capacity and input and outflow ports was used. Prior to each experiment, bacterial lawns from an overnight growth on TSA were harvested, re-suspended in MHB and injected into each model prefilled with media to obtain a starting inoculum of ~107 cfu/ml. For models with telavancin, aztreonam and antimicrobial combinations, antimicrobials were administered as boluses over a 96-h time period to simulate human pharmacokinetics. Fresh media were continuously supplied and removed from the compartment along with the drug via a peristaltic pump (Masterflex, Cole-Parmer Instrument Co., Chicago, IL) at an appropriate rate to simulate the average human half-lives (t 1/2) of the antimicrobials. Antimicrobial exposures were based on free drug pharmacokinetics pertinent to each antimicrobial agent. For models with piperacillin/tazobactam, a bolus dose was administered to achieve a steady-state concentration at T 0, and fresh media containing a constant concentration of piperacillin/tazobactam were pumped in at the appropriate rate to simulate continuous infusion of piperacillin/tazobactam as used clinically in renal failure (CrCl 20–40 ml/min) [9]. Antimicrobial simulations, including free peak concentrations, steady state concentrations and half-lives of each agent simulated in the study, were selected based on the mean population pharmacokinetic values of antimicrobial regimens clinically used in moderate to severe renal impairment with CrCl <40 ml/min [10–14]. Antimicrobial regimens evaluated included (1) telavancin 10 mg/kg every 48 h (free peak concentration 8.23 mg/l; average t 1/2 16.9 h; protein binding 90%) [12, 14]; (2) aztreonam 500 mg every 8 h (free peak concentration 55.86 mg/l; average t 1/2 4.8 h; protein binding 43%) [13]; (3) piperacillin/tazobactam continuous infusion of 13.5 g over 24 h (free steady-state concentration 37.2 mg/l; average t 1/2 of piperacillin component 2.1 h; protein biding 16%, simulated as a combination) [10, 11]; (4) telavancin 10 mg/kg every 48 h plus aztreonam 500 mg every 8 h; (5) telavancin 10 mg/kg every 48 h plus piperacillin/tazobactam by continuous infusion; (6) drug-free growth control. Models were performed in duplicate to ensure reproducibility of the study findings. Supplemental telavancin was added at an appropriate rate to combination models to compensate for the higher flow rates required to simulate clearance of aztreonam and piperacillin/tazobactam [15].
Pharmacokinetic Analysis
Pharmacokinetic samples were obtained from each model at 0, 1, 2, 4, 8, 24, 32, 48, 72 and 96 h to confirm the achievement of target antibiotic concentrations. All samples were then stored at −80 °C until ready for analysis. Telavancin and piperacillin/tazobactam concentrations were measured by bioassay using Kocuria rhizophila (formerly Micrococcus luteus) ATCC 9341 [16, 17]. For bioassay in combination models, pharmacokinetic models using a single agent were separately run to obtain the individual antibiotic concentrations because of the susceptibility of Kocuria rhizophila to study antibiotics. Briefly, blank 0.25-inch test disks were spotted with 10 μl of the standard concentrations or samples on antibiotic medium agar #11 plates, which were inoculated with a 0.5 McFarland suspension of the test organism [18]. For aztreonam, antibiotic medium number 5 agar plates pre-swabbed with Escherichia coli ATCC 25922 were used [19]. Then, the sizes of inhibition zones were measured using a laser reader (Scan® 1200, Interscience, France) after 24 h of incubation at 37 °C. Samples and standard concentrations were tested in duplicate. Pharmacokinetic parameters of the antibiotics were determined by the trapezoidal method by use of PK Analyst software (version 1.10, MicroMath Scientific Software, Salt Lake City, UT).
Pharmacodynamic Analysis
Samples were collected at 0, 4, 8, 24, 32, 48, 72 and 96 h from each model, serially diluted in normal saline and plated on TSA plates using an automatic spiral plater (WASP, DW Scientific, West Yorkshire, England). Plates were incubated overnight at 37 °C for 24 h before a colony count was performed. These methods allow reliable detection of bacterial growth with a lower limit of 2 log10 cfu/ml. The efficacy of antimicrobial agents was evaluated by plotting time-kill curves based on the number of remaining organisms and calculating the total reduction in log10 cfu/ml over the 96-h time period. Bactericidal and bacteriostatic activity was defined as a ≥3-log10 cfu/ml and <3-log10 cfu/ml reduction in colony count from the initial inoculum, respectively [20]. Antagonistic activity was defined as an increase of ≥2-log10 cfu/ml bacterial growth in comparison to the most active single agent from the combination [21]. Enhancement was defined as an increase in bacterial kill of ≥2-log10 cfu/ml for the combination compared to that of the most active single agent of that combination [22]. This article does not contain any new studies with human or animal subjects performed by any of the authors.
Changes in Susceptibility
Development of resistance was evaluated by broth microdilution for any isolates observed at 96 h. If significant resistance development (defined as a ≥twofold increase in MIC from baseline) was detected at 96 h, samples from earlier time points were tested to detect for the earliest time point in MIC elevation.
Statistical Analysis
Changes in cfu/ml at 96 h were compared by one-way analysis of variance (ANOVA) with Tukey’s post hoc test. A p value ≤0.05 was considered significant. All statistical analyses were performed using SPSS statistical software (release 22.0; SPSS, Inc., Chicago, IL).
Results
Susceptibility Testing
The E. coli strains ATCC 25922 and R1022 both possessed an aztreonam MIC of 0.125 mg/l, and the piperacillin/tazobactam MICs were 1/4 and 0.5/4 mg/l, respectively. The P. aeruginosa strain ATCC 27853 had an aztreonam MIC of 2 mg/l and piperacillin/tazobactam MIC of 4/4 mg/l. Both MRSA ATCC 43300 and clinical MRSA isolate R5255 were susceptible to telavancin, with telavancin MICs of 0.0625 mg/l. As expected, the gram-negative organisms were resistant to telavancin, and the MRSAs were resistant to aztreonam (MIC >64 mg/l) and piperacillin/tazobactam (MIC >16/4 mg/l).
In Vitro PK/PD Models
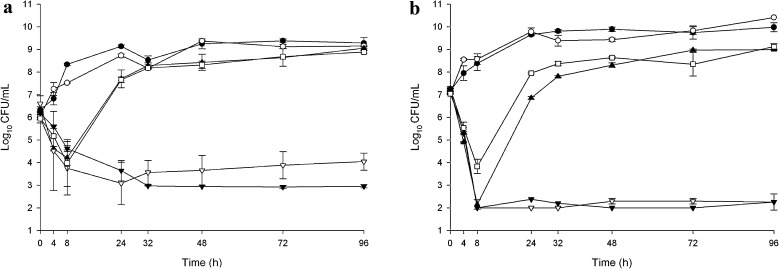
Pharmacodynamic responses to simulated antibiotic regimens against five tested strains are depicted in Figs. 1, 2 and 3. Piperacillin/tazobactam was bacteriostatic at 96 h against E. coli ATCC 25922 and bactericidal against clinical E. coli strain R1022 at 96 h. However, regrowth was observed in both strains as soon as 24 h when exposed to aztreonam despite their low MICs. The regrowth was not associated with susceptibility change. Telavancin had no effect against either E. coli strain, and the addition of telavancin to aztreonam or piperacillin/tazobactam did not result in antagonistic activity against either E. coli strain at any time point over 96 h. For E. coli R1022, changes in bacterial colony count per ml (cfu/ml) from baseline were statistically different at 4, 8, 24 and 32 h between aztreonam and aztreonam plus telavancin, with enhanced bactericidal activity in the combination (p < 0.05). Although the changes in cfu/ml from baseline at 24 h between the piperacillin/tazobactam and piperacillin/tazobactam plus telavancin were statistically significant (p < 0.05), there were no appreciable differences at 32, 48, 72 or 96 h between these two drug regimens, and at no time point did the differences meet the definition of antagonism.
Fig. 1.
In vitro activity of telavancin alone and in combination of aztreonam or piperacillin/tazobactam in E. coli ATCC 25922 (a) and clinical strain R1022 (b). Black circle growth control, white circle telavancin, white square aztreonam, white inverted triangle piperacillin/tazobactam, black triangle telavancin + aztreonam, black inverted triangle telavancin + piperacillin/tazobactam
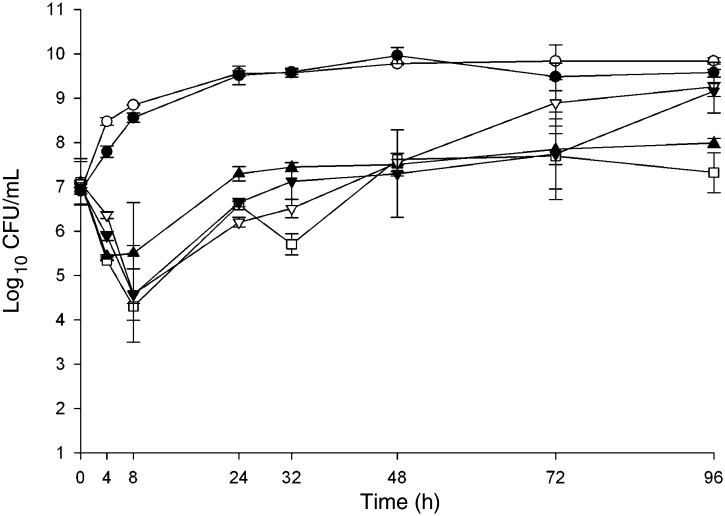
Fig. 2.
In vitro activity of telavancin alone and in combination of aztreonam or piperacillin/tazobactam in a P. aeruginosa ATCC 27853. Black circle growth control, white circle telavancin, white square aztreonam, white inverted triangle piperacillin/tazobactam, black triangle telavancin + aztreonam, black inverted triangle telavancin + piperacillin/tazobactam
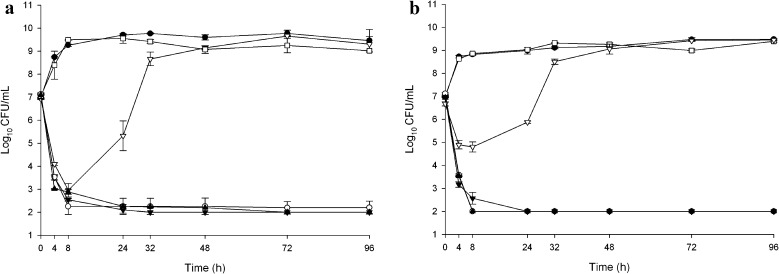
Fig. 3.
In vitro activity of telavancin alone and in combination of aztreonam or piperacillin/tazobactam in MRSA ATCC 43300 (a) and a clinical strain R5255 (b). Black circle growth control, white circle telavancin, white square aztreonam, white inverted triangle piperacillin/tazobactam, black triangle telavancin + aztreonam, black inverted triangle telavancin + piperacillin/tazobactam
P. aeruginosa ATCC 27853 demonstrated regrowth at 24 h in all models simulating aztreonam, piperacillin/tazobactam and antimicrobial combinations. No susceptibility change was detected in models simulating aztreonam, whereas piperacillin/tazobactam MIC increased from 4/4 mg/l at baseline to >64/4 mg/l at 96 h. As expected, telavancin demonstrated no significant activity against P. aeruginosa ATCC 27853. Against P. aeruginosa, aztreonam and piperacillin/tazobactam were not adversely impacted at any time point over 96 h by the addition of telavancin. Changes in colony count per ml from the baseline were not significantly different at 96 h between models simulating a single active agent and models simulating antimicrobial combinations.
Telavancin achieved bactericidal activity at 4 h against both MRSA strains evaluated in the study and maintained its activity over 96 h. Neither piperacillin/tazobactam nor aztreonam antagonized the activity of telavancin against either MRSA isolate at any time point over 96 h. Changes in bacterial cfu/ml from baseline at 96 h are summarized in Table 1.
Table 1.
Activity of telavancin alone and combined with aztreonam or piperacillin/tazobactam at 96 h
| Strains | Antibiotic | Change from baseline at 96 h |
|---|---|---|
|
E. coli ATCC 25922 |
Growth control | 2.96 ± 0.37 |
| Telavancin | 3.09 ± 0.13 | |
| Aztreonam | 2.94 ± 0.14 | |
| Piperacillin/tazobactam | −2.56 ± 0.36 | |
| Telavancin + aztreonam | 3.09 ± 0.06 (inhibited by 0.16 cfu/ml) | |
| Telavancin + piperacillin/tazobactam | −3.23 ± 0.12 (enhanced by 0.67 cfu/ml) | |
| E. coli R1022 | Growth control | 2.82 ± 0.11 |
| Telavancin | 3.37 ± 0.01 | |
| Aztreonam | 2.09 ± 0.11 | |
| Piperacillin/tazobactam | −5.03 ± 0.37 | |
| Telavancin + aztreonam | 1.72 ± 0.09 (enhanced by 0.38 cfu/ml) | |
| Telavancin + piperacillin/tazobactam | −4.88 ± 0.35 (inhibited by 0.15 cfu/ml) | |
| P. aeruginosa ATCC 27853 | Growth control | 2.67 ± 0.23 |
| Telavancin | 2.93 ± 0.14 | |
| Aztreonam | 0.23 ± 0.49 | |
| Piperacillin/tazobactam | 2.17 ± 0.7 | |
| Telavancin + aztreonam | 0.88 ± 0.63 (inhibited by 0.65 cfu/ml) | |
| Telavancin + piperacillin/tazobactam | 2.17 ± 0.59 (inhibited by 0.01 cfu/ml) | |
|
MRSA ATCC 43300 |
Growth control | 2.49 ± 0.48 |
| Telavancin | −4.92 ± 0.30 | |
| Aztreonam | 1.97 ± 0.13 | |
| Piperacillin/tazobactam | 2.38 ± 0.37 | |
| Telavancin + aztreonam | −5.09 ± 0.01 (enhanced by 0.17 cfu/ml) | |
| Telavancin + piperacillin/tazobactam | −5.13 ± 0.04 (enhanced by 0.21 cfu/ml) | |
| MRSA R5255 | Growth control | 2.5 ± 0.03 |
| Telavancin | −5.12 ± 0.04 | |
| Aztreonam | 2.37 ± 0.03 | |
| Piperacillin/tazobactam | 2.78 ± 0.08 | |
| Telavancin + aztreonam | −4.94 ± 0.01 (inhibited by 0.18 cfu/ml) | |
| Telavancin + piperacillin/tazobactam | −4.97 ± 0.04 (inhibited by 0.15 cfu/ml) |
Achieved PK parameters for aztreonam were fC max of 55.89 ± 7.87 mg/l (target 55.86 mg/l) and t 1/2 of 4.75 ± 0.63 h (target 4.8 h). Achieved PK parameters for telavancin were fC max of 8.5 ± 0.32 mg/l (target 8.23 mg/l) and t 1/2 of 16.59 ± 0.28 h (target 16.9 h). Piperacillin/tazobactam models achieved piperacillin steady-state concentrations of 31.36 ± 2.48 mg/l (target 31.2 mg/l).
Discussion
We evaluated the potential for antagonistic activities between telavancin and either aztreonam or piperacillin/tazobactam in the treatment of either MRSA or gram-negative bacilli such as E. coli and P. aeruginosa under simulated reduced renal function. Here, there were no antagonistic interactions observed between study antimicrobials against any isolates tested.
In this in vitro study, P. aeruginosa ATCC 27853 exhibited a sharp regrowth pattern at 24 h in PK/PD models simulating either aztreonam or piperacillin/tazobactam regimens. The regrowth of P. aeruginosa ATCC 27853 in piperacillin/tazobactam models appears to be associated with development of resistance to the agent during the 96-h experiment based on the increase of piperacillin/tazobactam MIC from 4/4 to 64/4 mg/l, a phenomenon that has been previously demonstrated [23]. On the other hand, regrowth of P. aeruginosa ATCC 27853 in PK/PD models evaluating aztreonam could not be attributed to emergence of resistance, as no changes in MIC were observed over 96 h. A series of time-kill assays was performed against P. aeruginosa ATCC 27853 in an effort to verify the study findings observed in PK/PD models, which demonstrated the same regrowth pattern at 24 h in the presence of aztreonam up to four times the MIC (data not shown).
The bacterial regrowth observed poses a challenge in evaluating antagonistic activity between telavancin and either gram-negative antibiotic agent, if any exists. Therefore, in addition to P. aeruginosa, we evaluated two E. coli strains, which were presumed to be more susceptible to these agents based on MIC values.
The same pattern of significant regrowth was observed in PK/PD models with both E. coli strains when they were exposed to aztreonam without any significant changes in MIC. Both E. coli ATCC 25922 and clinical strain R1022 exhibited regrowth at 24 h in the presence of aztreonam at twice the MIC in time-kill assays (−0.6 cfu/ml ± 0.07 and +0.74 cfu/ml ± 0.04 change at 24 h from baseline, respectively). Reasons for the bacterial regrowth in in vitro PK/PD models in the presence of sufficient aztreonam concentration are unknown. Bioassay sampling of the antibiotics throughout the 96-h experiments indicated that both aztreonam and piperacillin/tazobactam maintained viable activity throughout the experiment (aztreonam 24.5 ± 3.75 mg/l at 96 h).
Despite the challenge of bacterial regrowth in gram-negative models, it was evident that there were no antagonistic interactions between study antimicrobial agents against E. coli, P. aeruginosa and MRSA strains evaluated in the study. These findings may have clinical implications, as piperacillin/tazobactam and aztreonam could be combined with telavancin for broad-spectrum antimicrobial coverage. However, this leaves our initial clinical question unanswered as to why patients treated with telavancin did poorly compared to vancomycin when they had polymicrobial infections, especially in the presence of renal impairment. A possible explanation might be inadequate coverage for gram-negative organisms as suggested in the original article [4]. It is also possible that our study might not have captured all gram-negative bacilli representative of strains from the clinical trials as only a limited number of clinical isolates were tested. The in vitro nature of the experiment does not preclude a possibility of therapeutic antagonism among test antibiotics in vivo. In vitro models do not simulate the physiological conditions in humans with infections such as the pathogen-host relationship, which may also explain poor outcomes in patients who had polymicrobial infections with telavancin combinations. Similarly, our study assessed the pharmacodynamic interactions among antibiotics in the presence of infections with either gram-positive or -negative bacteria, which limited our evaluation on interactions among bacteria and their potential impact on therapeutic interactions among test antibiotics.
In addition, only one clearance was simulated in this study, limiting our ability to evaluate the impact of varying renal function on bactericidal activity of telavancin, although it is unlikely that we would find any significant difference in models simulating normal renal function. Of note, simulated impaired renal function had no negative influence on the bactericidal activity of telavancin in vitro when determined by serum inhibitory titers against a susceptible S. aureus strain in a study performed by Barriere and colleagues. [24].
Another plausible explanation for poor outcomes with telavancin combinations compared to vancomycin combinations in clinical trials is the presence of synergistic activity between vancomycin and either piperacillin/tazobactam or aztreonam, which was not addressed in the current in vitro study.
Although our study documented the lack of interactions between telavancin and aztreonam or piperacillin/tazobactam for the isolates tested, there were several limitations as stated above. These included the in vitro nature of this investigation, the limited number of strains tested, the inability to test polymicrobial interactions and the absence of a vancomycin ± aztreonam or piperacillin/tazobactam comparator arm.
Conclusion
Based on our study data, it would appear that the mechanisms for reduced efficacy of telavancin against co-infection with gram-positive and -negative bacteria do not lie in any intrinsic pharmacodynamic antagonism between telavancin and either piperacillin/tazobactam or aztreonam. Discrepancies in cure rates in clinical trials may be more attributable to clinical variables and patient characteristics enrolled in the studies, which may need to be explored further in the future research.
Acknowledgments
This work was supported by Theravance Biopharma Antibiotics, Inc., South San Francisco, CA, USA. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole and have given final approval to the version to be published.
Disclosures
Michael J. Rybak received funding support, consulted for or was on the speaker bureau for Allergan, Bayer, Cempra, Merck, The Medicine Company, Sunovian and Theravance and is partially supported by the National Institutes of Health (R21-109266-01). Juwon Yim, Jordan R. Smith, Katie E. Barber and Jessica A. Hallesy have no disclosures.
Compliance with Ethics Guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors. The study findings were presented at ICAAC/ICC 2015 in San Diego, CA, USA.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/22E4F0603737CC9F.
References
- 1.Leonard SN, Szeto YG, Zolotarev M, Grigoryan IV. Comparative in vitro activity of telavancin, vancomycin and linezolid against heterogeneously vancomycin-intermediate Staphylococcus aureus (hVISA) Int J Antimicrob Agents. 2011;37(6):558–561. doi: 10.1016/j.ijantimicag.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Zhanel GG, Calic D, Schweizer F, et al. New lipoglycopeptides: a comparative review of dalbavancin, oritavancin and telavancin. Drugs. 2010;70(7):859–886. doi: 10.2165/11534440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Theravance Biopharma Antibiotics Inc. VIBATIV (Telavancin) For injection, US Prescribing Information. 2016. https://www.vibativ.com/pdf/PrescribingInformation.pdf. Accessed 13 July 2016.
- 4.Rubinstein E, Lalani T, Corey GR, et al. Telavancin versus vancomycin for hospital-acquired pneumonia due to gram-positive pathogens. Clin Infect Dis. 2011;52(1):31–40. doi: 10.1093/cid/ciq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stryjewski ME, Graham DR, Wilson SE, et al. Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by gram-positive organisms. Clin Infect Dis. 2008;46(11):1683–1693. doi: 10.1086/587896. [DOI] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration. Anti-infective drugs advisory committee meeting 2012. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM332968.pdf. Accessed 13 July 2016.
- 7.Farrell DJ, Mendes RE, Rhomberg PR, Jones RN. Revised reference broth microdilution method for testing telavancin: effect on MIC results and correlation with other testing methodologies. Antimicrob Agents Chemother. 2014;58(9):5547–5551. doi: 10.1128/AAC.03172-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 24th Informational Supplement M100-S24. Wayne, PA2014.
- 9.Cutro SR, Holzman R, Dubrovskaya Y, et al. Extended-Infusion versus standard-infusion piperacillin-tazobactam for sepsis syndromes at a tertiary medical center. Antimicrob Agents Chemother. 2014;58(8):4470–4475. doi: 10.1128/AAC.02759-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgess DS, Waldrep T. Pharmacokinetics and pharmacodynamics of piperacillin/tazobactam when administered by continuous infusion and intermittent dosing. Clin Ther. 2002;24(7):1090–1104. doi: 10.1016/S0149-2918(02)80021-2. [DOI] [PubMed] [Google Scholar]
- 11.Dowell JA, Korth-Bradley J, Milisci M, et al. Evaluating possible pharmacokinetic interactions between tobramycin, piperacillin, and a combination of piperacillin and tazobactam in patients with various degrees of renal impairment. J Clin Pharmacol. 2001;41(9):979–986. doi: 10.1177/00912700122010960. [DOI] [PubMed] [Google Scholar]
- 12.Lodise TP, Butterfield JM, Hegde SS, Samara E, Barriere SL. Telavancin pharmacokinetics and pharmacodynamics in patients with complicated skin and skin structure infections and various degrees of renal function. Antimicrob Agents Chemother. 2012;56(4):2062–2066. doi: 10.1128/AAC.00383-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mihindu JC, Scheld WM, Bolton ND, et al. Pharmacokinetics of aztreonam in patients with various degrees of renal dysfunction. Antimicrob Agents Chemother. 1983;24(2):252–261. doi: 10.1128/AAC.24.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samara E, Shaw JP, Barriere SL, Wong SL, Worboys P. Population pharmacokinetics of telavancin in healthy subjects and patients with infections. Antimicrob Agents Chemother. 2012;56(4):2067–2073. doi: 10.1128/AAC.05915-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blaser J. In-vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. J Antimicrob Chemother. 1985;15(Suppl A):125–130. doi: 10.1093/jac/15.suppl_A.125. [DOI] [PubMed] [Google Scholar]
- 16.Hori R, Araki H, Yonezawa M, Minami S, Watanabe Y. Therapeutic effects of parenteral beta-lactam antibiotics on experimental otitis media caused by penicillin-resistant Streptococcus pneumoniae in guinea-pigs. J Antimicrob Chemother. 2000;45(3):311–314. doi: 10.1093/jac/45.3.311. [DOI] [PubMed] [Google Scholar]
- 17.Leonard SN, Vidaillac C, Rybak MJ. Activity of telavancin against Staphylococcus aureus strains with various vancomycin susceptibilities in an in vitro pharmacokinetic/pharmacodynamic model with simulated endocardial vegetations. Antimicrob Agents Chemother. 2009;53(7):2928–2933. doi: 10.1128/AAC.01544-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barcia-Macay M, Mouaden F, Mingeot-Leclercq MP, Tulkens PM, Van Bambeke F. Cellular pharmacokinetics of telavancin, a novel lipoglycopeptide antibiotic, and analysis of lysosomal changes in cultured eukaryotic cells (J774 mouse macrophages and rat embryonic fibroblasts) J Antimicrob Chemother. 2008;61(6):1288–1294. doi: 10.1093/jac/dkn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eng RH, Cherubin C, Smith SM, Buccini F. Inoculum effect of beta-lactam antibiotics on Enterobacteriaceae. Antimicrob Agents Chemother. 1985;28(5):601–606. doi: 10.1128/AAC.28.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cha R, Brown WJ, Rybak MJ. Bactericidal activities of daptomycin, quinupristin-dalfopristin, and linezolid against vancomycin-resistant Staphylococcus aureus in an in vitro pharmacodynamic model with simulated endocardial vegetations. Antimicrob Agents Chemother. 2003;47(12):3960–3963. doi: 10.1128/AAC.47.12.3960-3963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaPlante KL, Woodmansee S. Activities of daptomycin and vancomycin alone and in combination with rifampin and gentamicin against biofilm-forming methicillin-resistant Staphylococcus aureus isolates in an experimental model of endocarditis. Antimicrob Agents Chemother. 2009;53(9):3880–3886. doi: 10.1128/AAC.00134-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall Snyder A, Werth BJ, Barber KE, Sakoulas G, Rybak MJ. Evaluation of the novel combination of daptomycin plus ceftriaxone against vancomycin-resistant enterococci in an in vitro pharmacokinetic/pharmacodynamic simulated endocardial vegetation model. J Antimicrob Chemother. 2014;69(8):2148–2154. doi: 10.1093/jac/dku113. [DOI] [PubMed] [Google Scholar]
- 23.Burgess DS, Nathisuwan S. Cefepime, piperacillin/tazobactam, gentamicin, ciprofloxacin, and levofloxacin alone and in combination against Pseudomonas aeruginosa. Diagn Microbiol Infect Dis. 2002;44(1):35–41. doi: 10.1016/S0732-8893(02)00420-0. [DOI] [PubMed] [Google Scholar]
- 24.Barriere SL, Farrell DJ, Rhomberg PR, Jones RN. Serum inhibitory and bactericidal activity of telavancin in non-infected subjects with severe renal impairment or end-stage renal disease. Diagn Microbiol Infect Dis. 2014;80(4):327–329. doi: 10.1016/j.diagmicrobio.2014.09.002. [DOI] [PubMed] [Google Scholar]