Abstract
The oxygenated short aldehyde methylglyoxal (MG) is produced in plants as a by-product of a number of metabolic reactions, including elimination of phosphate groups from glycolysis intermediates dihydroxyacetone phosphate and glyceraldehyde 3-phosphate. MG is mostly detoxified by the combined actions of the enzymes glyoxalase I and glyoxalase II that together with glutathione make up the glyoxalase system. Under normal growth conditions, basal levels of MG remain low in plants; however, when plants are exposed to abiotic stress, MG can accumulate to much higher levels. Stress-induced MG functions as a toxic molecule, inhibiting different developmental processes, including seed germination, photosynthesis and root growth, whereas MG, at low levels, acts as an important signaling molecule, involved in regulating diverse events, such as cell proliferation and survival, control of the redox status of cells, and many other aspects of general metabolism and cellular homeostases. MG can modulate plant stress responses by regulating stomatal opening and closure, the production of reactive oxygen species, cytosolic calcium ion concentrations, the activation of inward rectifying potassium channels and the expression of many stress-responsive genes. MG appears to play important roles in signal transduction by transmitting and amplifying cellular signals and functions that promote adaptation of plants growing under adverse environmental conditions. Thus, MG is now considered as a potential biochemical marker for plant abiotic stress tolerance, and is receiving considerable attention by the scientific community. In this review, we will summarize recent findings regarding MG metabolism in plants under abiotic stress, and evaluate the concept of MG signaling. In addition, we will demonstrate the importance of giving consideration to MG metabolism and the glyoxalase system, when investigating plant adaptation and responses to various environmental stresses.
Keywords: abiotic stress, glyoxalases, methylglyoxal, reactive oxygen species, signaling crosstalk, stress tolerance mechanism
Introduction
Most plants live in environments where they are constantly exposed to one or combinations of various abiotic stressors, such as extreme temperatures, salinity, drought, and excessive light, which can severely limit plant growth and development. For many important crop plants, exposure to stress(es) ultimately results in a considerable reduction in potential yields (Atkinson and Urwin, 2012). The interaction between abiotic stressors and plants is complex, eliciting multiple morphological, physiological, biochemical and molecular changes that can ultimately result in varying degrees of stress adaptation, enabling some plants to grow and develop under environmentally induced stress. Because of the number of metabolic pathways involved, and the compexity of their regulation, it is often difficult for researchers to identify the major regulatory components involved in the abiotic stress responses of plants (Sharma et al., 2013). Plants subjected to stress often produce toxic aldehydes (Hoque et al., 2012a,b,c; Hoque M.A. et al., 2012; Mano, 2012), of which methylglyoxal (CH3COCHO; MG) is the most ubiquitous. The reactive alpha-ketoaldehyde MG is cytotoxic to plant cells at high cellular concentrations, but it may act as an important signaling molecule at low concentrations (Yadav et al., 2005a,b; Singla-Pareek et al., 2006; Hossain et al., 2009; Kaur et al., 2015a,b). MG is produced in plant cells as a result of glycolysis, and its celluar concentrations are maintained at very low levels in the absence of any environmental stress (Kaur et al., 2015b). However, in response to abiotic stressors celluar concentrations of MG rapidly increase (Yadav et al., 2005a,b). Accumulation of MG can disrupt the normal functioning of cells, leading to alterations in metabolic behavior and, in some instances, the death of plants (Hossain et al., 2011). The glyoxalase pathway has evolved to enable plants, and other organisms, to withstand the detrimental effects of MG overproduction, by limiting the accumulation of MG in the cells under stress (Singla-Pareek et al., 2006, 2008; Alvarez Viveros et al., 2013). MG and the glyoxalases are now considered as potential markers for evaluating plant abiotic stress tolerance (Hossain et al., 2009; Kaur et al., 2014a,b,c; Nahar et al., 2015a). Although significant progress has been made in investigating MG metabolism and toxicity in plants, the role of MG as a signaling molecule in stress responses and the acquisition of stress tolerance in plants still remain unclear. In this review, we will summarize recent findings regarding MG metabolism and the glyoxalase system in plants under abiotic stress, evaluate the concept of MG signaling, and discuss the importance of MG metabolism in modulating plant abiotic stress responses and tolerance.
MG Synthesis in Plants
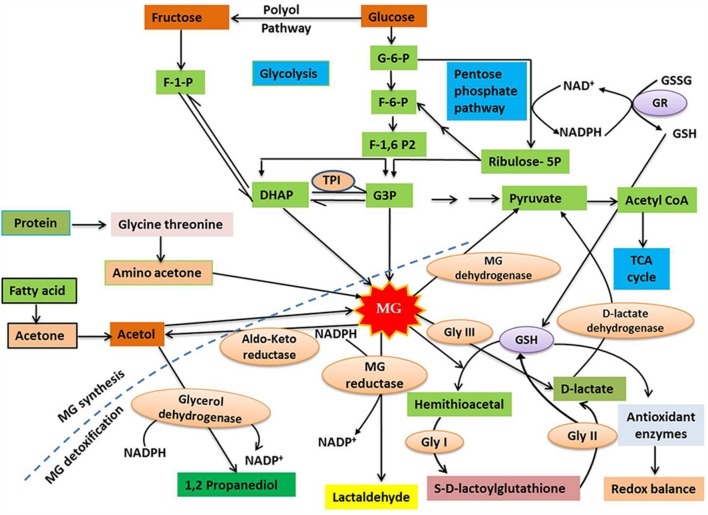
In plant cells, the cytosol, chloroplasts and mitochondria are all considered to be potential sites of MG production. However, the specific rate and sites of MG production vary depending upon the cell or tissue type, the plant organ (e.g. leaves or roots), and the physiological state of the whole plant (Kaur et al., 2015a,b). Spontaneous production of MG occurs as a consequence of glycolysis, in metabolically active plant cells, from the reaction of the triose sugar phosphates glyceraldehyde-3-phosphate (G3P) and dihydroxyacetone phosphate (DHAP), both of which are photosynthetic intermediates (Yadav et al., 2005a; Takagi et al., 2014; Kaur et al., 2015a,b). This reaction is considered to be the principal route for MG formation under normal physiological conditions (Figure 1). Triose phosphates are unstable metabolites and show a high tendency to release an α-carbonyl proton, producing an enediolate phosphate intermediate that has a relatively low energy barrier for the elimination of phosphate groups (Richard, 1984). Thus, MG is formed by the deprotonation followed by the spontaneous β-elimination of the phosphate groups of triose phosphates (Richard, 1993). The enzymatic formation of MG occurs through the triose phosphate isomerase (TPI) that hydrolyzes G3P and DHAP, and removes phosphate to yield MG (Phillips and Thornalley, 1993). MG may also be formed by Amadori rearrangement during production of a Schiff base, which involves the reaction of the aldehyde groups of sugars with free amino acids or the amino acids of proteins (Vistoli et al., 2013). Other possible sources for MG formation include the auto-oxidation of surgars, as well as the metabolism of acetone and aminoacetone (Kalapos, 1999), although there is little evidence that these routes occur in plants.
FIGURE 1.
A diagrammatic representation of methylglyoxal (MG) synthesis and detoxification in plants (modified from Ko et al., 2005; Hossain et al., 2011). MG is primarily produced as a by-product of carbohydrate metabolism, with small amount produced during protein and lipid metabolism. Cytotoxic MG is efficiently degraded to form D-lactate by the action of the enzymes Gly I and Gly II, with the help of GSH. In addition, GSH-independent Gly III is able to convert MG to D-lactate. D-lactate dehydrogenase finally converts D-lactate into pyruvate, which enters TCA cycle via acetyl CoA. The broken line separates the synthesis and detoxification pathways of MG. For further discussion, see the text. Abbreviations are defined in the text.
MG Detoxification in Plants via Glyoxalase and Other Metabolic Pathways
Methylglyoxal detoxification involves the conversion of MG to less toxic molecules, thus limiting its detrimental effects. The major route for MG detoxification in plants is the glyoxalase pathway, whose prescence was demonstrated in plants over 20 years ago (Norton et al., 1990; Maiti et al., 1997). In plant cells, the glyoxalase pathway is present in the cytosol and organelles, with high levels of glyoxalase enzyme activity found in chloroplasts and mitochondria (Yadav et al., 2008; Rabbani and Thornalley, 2012). There are two main enzymes associated with the glyoxalase pathway; glyoxalase I (Gly I; lactoylglutathione lyase; EC 4.4.1.5) and glyoxalase II (Gly II; hydroxyacylglutathione hydrolase; EC 3.1.2.6). These enzymes function in tandem to transform MG, and other 2-oxoaldehydes, to 2-hydroxyacids with the release of glutathione (GSH) (Thornalley, 1990). The detoxification of MG involves two irreversible reactions catalyzed by glyoxalases. The first step involves the reaction of MG with GSH, resulting in the formation of hemithioacetal that is then converted to S-D-lactoylglutathione (SLG) in a reaction catalyzed by Gly I. In the second step, which is catalyzed by Gly II, GSH is regenerated and D-lactate is formed by the hydrolysis of SLG (Figure 1). D-lactate, which is also considered a toxic compound if overaccumulated, is converted into pyruvate by D-lactate dehydrogenase (Engqvist et al., 2009; Wienstroer et al., 2012). Pyruvate, the major catabolic product of MG, can enter the tricarboxylic acid (TCA) cycle via acetyl CoA (Figure 1). The availability of cellular GSH is an important factor for MG detoxification via the glyoxalase system as the lack of GSH restricts hemithioacetal formation, resulting in MG accumulation (Hossain et al., 2011). Recently, a novel glyoxalase enzyme, named glyoxalase III (Gly III), was detected in plants, providing a shorter route for MG detoxification (Ghosh et al., 2016). Gly III contains a DJ-1/PfpI domain, and the presence of this domain has been used to confirm the existence of Gly III-like proteins in various plant species. Conventional glyoxalases (Gly I and Gly II) detoxify MG by converting it to D-lactate, with the help of GSH, but Gly III is able to irreversibly convert MG to D-lactate in a single step, without the need for GSH (Figure 1).
In addition to the glyoxalase system, several other pathways contribute to the detoxification of MG in plants. Other enzymes, including NADPH-dependent reductases, such as the aldo-keto reductases and aldehyde/aldose reductases, involved in detoxifying reactive carbonyls (Yamauchi et al., 2010), can reduce MG to the corresponding alcohol (Simpson et al., 2009; Narawongsanont et al., 2012). Another pathway is the irreversible oxidation of reactive aldehydes, including MG, to their corresponding carboxylic acids, which is catalyzed by aldehyde dehydrogenases (Kirch et al., 2005). However, the glyoxalase system is the most efficient MG detoxification system in plants under normal physiological conditions (Ghosh et al., 2016), and this pathway is very important for plants under stress (Singla-Pareek et al., 2006; Alvarez Viveros et al., 2013).
MG Levels in Plants Under Stressful Conditions
Under normal metabolic conditions, plants usually maintain a lower level (30-75 μM) of MG (Yadav et al., 2005a; Hossain et al., 2009); however, an abrupt increase was observed in respone to abiotic stresses (Yadav et al., 2005a; Hossain et al., 2009; Mostofa et al., 2015a,b). Salt stress-induced inceases in MG levels were found in various plant species, including pumpkin (Cucurbita maxima L.) by 77%, tobacco (Nicotiana tabacum L., cv. BY-2) by 67% and potato (Solanum tuberosum L. cv. Taedong Valley) by 50%, compared with the respective controls (Hossain et al., 2009; Banu et al., 2010; Upadhyaya et al., 2011; Ghosh et al., 2014). Increased MG levels were also found in mung bean (Vigna radiata L.), Lepidium sativum and rice plants in response to drought (90–107%), and excessive Cd (60–260%) and Cu (106–156%) stresses, respectively, when compared with control counterparts (Nahar et al., 2015b; Mostofa et al., 2015b,c). These findings indicate that the increase in MG levels is a common response of plants to a variety of abiotic stressors, and that stress-induced MG could act as a generic signal molecule for plants under adverse environmental conditions.
MG Toxicity in Plant Cells During Plant Growth and Development
In plant cells, MG accumulation has been shown to correlate with increased levels of intracellular oxidative stress, due to the enhanced reactive oxygen species (ROS) production (Maeta et al., 2005; Kalapos, 2008). MG accumulation may indirectly result in increased ROS production by decreasing available GSH levels and by impairing the function of antioxidant enzymes in plants under oxidative stress. In addition, MG can function as a Hill oxidant and catalyze the photoreduction of O2 to superoxide (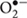 ) in photosystem I (PSI) (Saito et al., 2011). The production of
) in photosystem I (PSI) (Saito et al., 2011). The production of 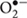 is deleterious as it can cause oxidative damage to cellular components.
is deleterious as it can cause oxidative damage to cellular components.
Methylglyoxal is an α,β-dicarbonyl compound that can act both as a genotoxic and a glycation agent (Rabbani and Thornalley, 2014). MG has two functional groups; a ketone group and an aldehyde group, the latter being more reactive than the former (Leoncini, 1979). The dicarbonyl group within MG can readily react with the amine groups of proteins and nucleic acids, including DNA and RNA. The accumulation of MG is often called dicarbonyl stress, which has been implicated as a cause of tissue damage and aging (Rabbani and Thornalley, 2014). MG reacts with the amino acids lysine, cysteine and arginine producing glycated proteins, often referred to as advanced glycation end products (AGEs) (Ahmed and Thornalley, 2007), which can cause inactivation of proteins and oxidative damage to key cellular components (Thornalley, 2006). AGEs and dicarbonyl compounds, including MG, often accumulate in plant leaves upon exposure to high light or elevated CO2 concentrations (Qiu et al., 2008; Bechtold et al., 2009). Thus, it appears that the increase in sugar accumulation and changes in the metabolic flux of sugars, which occur at high CO2 concentrations, promote the production of MG and other reactive carbonyls, resulting in the accumulation of AGEs. In summary, excessive MG accumulation in plant cells under stress can inhibit cell proliferations, and cause the inactivation and/or degradation of proteins, inactivation of antioxidant defenses, leading to disruption of many cellular functions (Hoque et al., 2010; Hoque M.A. et al., 2012).
Indeed, MG showed toxicity to photosynthesis in the chloroplasts of spinach (Spinacia oleracea L.) (Mano et al., 2009), and the accumulation of MG in the pdtpi mutant, which lacks the plastid isoform of TPI, exhibited greatly reduced growth and increased chlorosis (Chen and Thelen, 2010). Yadav et al. (2005a) reported that accumulation of MG, as a result of salt stress, directly and adversely influenced plant developmental processes, such as seed germination and seedling growth, in tobacco plants. Similarly, Engqvist et al. (2009) found that Arabidopsis plants grown on MS medium supplemented with MG (0.1 or 1 mM MG) exhibited a significant reduction in shoot and root growth. Later, the same group reported a dose-dependent decrease in root and shoot growth of Arabidopsis, tomato and tobacco plants grown on MS medium containing 1 mM MG (Wienstroer et al., 2012). Furthermore, Hoque et al. (2012b) examined the inhibitory effects of MG on growth and development in Arabidopsis and suggested that 1 mM MG is toxic enough to significantly inhibit seed germination and root elongation in seedlings. However, concentrations lower than 0.1 mM MG had no influence on seed germination, but did reduced the rate of root elongation. In addition, concentrations of 1 mM MG or higher resulted in seedling chlorosis within 4 days of treatment. Recently, Kaur et al. (2015a) also reported that MG exposure caused a significant growth reduction in rice seedlings (Oryza sativa cv. IR4), with acute effects on root elongation in a concentration-dependent manner. The above findings highlight the growth inhibitory effects of MG on plants, and indicate that the MG levels causing toxic effects to plants vary depending on plant species, exposure time and perhaps age of plants.
MG As A Signaling Molecule in Plants Under Stress
MG-Induced ROS Regulation
In plants, ROS are primarily formed at low levels as metabolic by-products of photosynthesis and respiration in organelles through enzymatic reactions that take place in plant cell walls and the apoplastic space in response to pathogens (Ahmad et al., 2010; Sharma et al., 2012). In plants, the rates of ROS production dramatically increase under abiotic or biotic stress (Sharma et al., 2012), leading to the onset of oxidative stress. The enzymes involved directly in ROS production include plasmamembrane NAD(P)H oxidases, cell-wall peroxidases (Grant et al., 2000; Torres et al., 2002), apoplastic amine oxidases, oxalate oxidases and heme-containing peroxidases (Sewelam et al., 2016). Recently, Kaur et al. (2014a) reported that when MG levels in plant cells increased due to stress, ROS generation increased directly due to the presence of MG or indirectly due to the formation of AGEs. It has been reported that application of exogenous MG to tobacco plants, at concentrations between 0.5 and 10 mM, reduced the activities of antioxidant enzymes, such as glutathione S-transferase (GST) and ascorbate peroxidase (APX), leading to oxidative stress (Hoque et al., 2010; Hoque M.A. et al., 2012). In addition, Saito et al. (2011) also demonstrated that MG induced 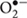 production in chloroplasts during photosynthesis. MG, at concentrations to 1 mM, has also been shown to cause a reversible induction of
production in chloroplasts during photosynthesis. MG, at concentrations to 1 mM, has also been shown to cause a reversible induction of 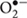 production in leaves of both wild-type Arabidopsis and NAD(P)H oxidase knock-out atrbohD atrbohF mutant plants, suggesting that salicylhydroxamic acid (SHAM)-sensitive peroxidases could be involved in this oxidative burst.
production in leaves of both wild-type Arabidopsis and NAD(P)H oxidase knock-out atrbohD atrbohF mutant plants, suggesting that salicylhydroxamic acid (SHAM)-sensitive peroxidases could be involved in this oxidative burst.
Regulation of Stomatal Conductance Involving Cytosolic Ca2+ Oscillation and Inward Kin Channel Activation
Plants appear to have well-developed systems to sense and react to diverse environmental stimuli (Jia and Zhang, 2008). Stomata, which control CO2 uptake and minimize transpirational water loss, are capable of responding to various environmental stimuli, e.g., light levels, CO2 levels, temperature and humidity, and are often used as a model system to investigate cell-to-cell signaling in plants (Schroeder et al., 2001; Kim et al., 2010). Stomatal closure is an adaptive mechanism in plants, enabling them to survive in adverse environments (Osakabe et al., 2014). Stomatal closure is associated with increased concentrations of cytosolic calcium, [Ca2+]cyt, with oscillations in [Ca2+]cyt occuring in guard cells in response to a diverse range of environmental stimuli (Pei et al., 2000; Mori et al., 2006; Young et al., 2006).
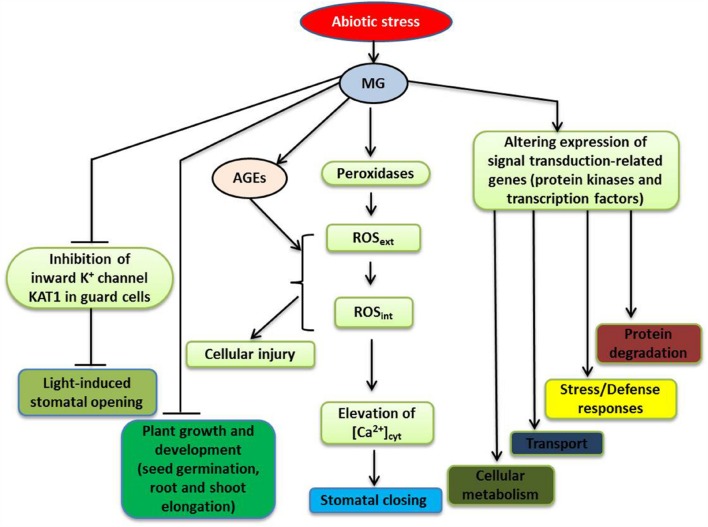
To investigate the mode of action of MG in stomatal guard cell signal transduction, Hoque et al. (2012a,c) investigated stomatal movement in Arabidopsis treated with different concentrations of MG. They found that at concentrations of MG up to 1 mM, MG behaved like a signal molecule as it induced stomatal closure, in a dose-dependant and reversible manner, without reducing the viability of guard cells. The induction of stomatal closure by MG involved an extracellular peroxidase-mediated oxidative burst and [Ca2+]cyt oscillations (Figure 2). However, this MG-controlled induction did not require endogenous abscisic acid (ABA) nor endogenous methyl jasmonate (MeJA), and was not affected by deficiency in NAD(P)H oxidases. Thus, the studies of Hoque et al. (2012a,c) provided evidence that MG can induce stomatal closure, which is an important adaptive response of plants to environmentally induced stress.
FIGURE 2.
A schematic model depicting the signaling roles of MG in plant during abiotic stresses (modified from Hoque et al., 2015; Kaur et al., 2015a). Stress-induced MG participates in signal transduction by altering the expression of a number of genes, such as those encoding protein kinases and transcription factors (TFs), triggering various responses, including changes in general metabolism and ion/metabolite transport, stress and defense responses, as well as protein degradation. For further discussion, see the text. Abbreviations are defined in the text.
Regulation of stomatal opening can greatly influence plant productivity and stress management (Dietrich et al., 2001), with inhibition of light-induced stomatal opening likely being occurred in plants under stress. The uptake of K+ into the guard cells accompanies light-induced stomatal opening, and inward-rectifying potassium (Kin) channels play important roles in regulating K+ uptake (Schroeder et al., 2001). The K+ transporter of Arabidopsis thaliana KAT1 gene is expressed in stomatal guard cells, and plants with a dominant negative mutation in this gene have reduction of Kin channel currents, which results in a reduced ability in regulating K+ ion flow and suppression of light-induced stomatal opening (Kwak et al., 2001). It has also been demonstrated that MG, in a concentration-dependent manner, can interfere with light-induced stomatal opening in Arabidopsis, and that this interference involves inhibition of Kin channel currents in guard cells, partially due to suppression of KAT1 channel activity (Hoque et al., 2012a,c) (Figure 2). According to Sato et al. (2009, 2010), protein kinase C (PKC) and stress-activated protein kinase SnRK2.6 (Snf1-related protein kinase 2.6) phosphorylate the C-terminal regions of KAT1, which modulates the activity of KAT1 channel. It is possible that MG can restrain the Kin channel activity by modifying C-terminal regions of KAT1, as well as other components, which inhibits stomatal opening.
Expression of Stress-Responsive Genes in Co-ordination with ABA
Abiotic stresses, including drought, salinity and extreme temperatures, can induce the expression of many defense-related genes in plants. Stress-induced genes are important for plant survival as they encode proteins with both direct and indirect protective functions, and proteins that play important roles in signal transduction and gene regulation, both of which are important for coordinated stress responses (Ma et al., 2012; Thao and Tran, 2012; Yoshida et al., 2015). The plant hormone ABA is an important signal molecule for plant growth and development, as well as various physiological processes, including abiotic stress responses (Fujita et al., 2011, 2013; Osakabe et al., 2014). Many stress-inducible genes exhibit ABA-dependent gene expression patterns (Hadiarto and Tran, 2011; Todaka et al., 2015).
As ABA plays an important role in the integration of stress signals and downstream regulation of stress responses in plants (Hubbard et al., 2010; Weiner et al., 2010), it is possible that ABA could be involved in the responses that occur following MG accumulation. Hoque et al. (2012b) investigated the expression of the stress- and ABA-responsive genes RD29B and RAB18 in Arabidopsis wild-type and ABA-deficient (aba2-2) mutant plants in response to MG treatment. They reported that MG significantly enhanced transcriptional levels of RD29B and RAB18 in WT seedlings in a dose-dependent manner. In contrast, the transcription of neither RD29B nor RAB18 was affected by MG in aba2-2 mutant plants, indicating that ABA is involved in MG-induced up-regulation of RD29B and RAB18 genes. This finding suggests that stress-induced MG may regulate stress-responsive genes in ABA-dependent pathway for plant adaptation to stress.
MG-Responsive Signal Transduction Pathways
Plants have developed effective detection mechanisms and efficient signal transduction pathways to enable them to respond to various environmental stresses (Petrov et al., 2015). These pathways often involve multiple genes/proteins, operating in a coordinated manner, to regulate the expression patterns of the key genes, enabling plants to respond to a diverse range of external stimuli (Hadiarto and Tran, 2011; Gururani et al., 2015; Li and Tran, 2015). Kaur et al. (2015a) used microarray analysis to investigate gene expression profiles in rice exposed to exogenous MG, and study the molecular basis of MG responses. MG affected genes involved in hormone signaling, cell-to-cell communications, and chromatin remodeling. A number of genes encoding bZIP, MYB, NAC, WRKY, AP2/EREBP, and zinc finger transcription factors (TFs) were also found to be MG-responsive. In addition, various genes encoding protein kinases, including mitogen-activated protein kinases (MAP kinases), calcium/calmodulin-dependent protein kinases (CDPKs), Ser/Thr protein kinases, histidine kinases and receptor-like kinases, and OsRR2 type-A response regulator showed changes in their expression patterns. Since cellular MG levels increase in plants in response to stressful conditions, altered expression patterns of stress-inducible genes encoding TFs and protein kinases are expected to be observed following MG application (Figure 2). Using in silico analysis, Kaur et al. (2015a) identified conserved motifs as MG-responsive elements (MGREs) in the upstream regions of MG-responsive genes and provided the putative MGRE sequences (CTXXCTC and GGCGGCGX). The ability of MG to influence the stress-responsive signaling network highlights the importance of MG in plant stress responses.
Glyoxalases in Plant Abiotic Stress Responses and Adaptation to Environmental Stressors
The glyoxalase system is involved in various cellular functions, but the involvement of this system in plant stress responses and tolerance is regarded as its most significant role (Kaur et al., 2014a). The glyoxalase system regulates MG levels in plants under stress and regenerates GSH. GSH and a high GSH/GSSG ratio are required to help protect plants against oxidative stress (Yadav et al., 2005a,b; Noctor et al., 2012), and GSH is directly or indirectly required for the functioning of various antioxidant enzymes, including GST, glutathione peroxidase (GPX), and APX (Yadav et al., 2008). Several studies have shown close links between the antioxidant and glyoxalase systems in plants, suggesting a direct influence of the glyoxalase system on ROS detoxification (Yadav et al., 2005a; El-Shabrawi et al., 2010; Upadhyaya et al., 2011; Mostofa et al., 2014a, 2015a).
An increase in glyoxalase enzyme activities occurs in plants in response to many different stressors, including osmotic stress, extremes of temperature, heavy metals and exposure to stress-related hormones, including MeJA, ABA and salicylic acid (SA) (Hossain and Fujita, 2009; Hossain et al., 2009). Transcriptomic and proteomic analyses of various plant species have improved our knowledge and understanding of the roles of glyoxalases in plant stress responses and tolerance (Singla-Pareek et al., 2003, 2006; Hossain et al., 2009; Lin et al., 2010; Mustafiz et al., 2011). Plant glyoxalase genes (Gly I and Gly II) have been cloned from various plant species and characterized in detail. The expression of Gly I and Gly II genes has been shown to be up-regulated in many plant species by a diverse range of environmental cues, and plants overexpressing either Gly I or Gly II showed enhanced plant abiotic stress tolerance (Singla-Pareek et al., 2003, 2006, 2008; Lin et al., 2010; Alvarez Viveros et al., 2013; Wu et al., 2013; Kaur et al., 2014a,c). The genetic manipulation of the glyoxalase system in plants has successfully contributed to improved tolerance to multiple abiotic stresses, such as salinity, heavy metals and MG treatments (Table 1). Transgenic plants overexpressing glyoxalase pathway genes have lower MG and ROS levels when exposed to stress, because they have better GSH homeostasis and retain better antioxidant enzyme functionality. Thus, glyoxalase enzyme levels can be used as phenomic biomarkers to indicate degrees of stress tolerance, and plants with high glyoxalase enzyme levels are potentially tolerant to a wide range of abiotic stresses (Kaur et al., 2014c). In Table 1, we summarized most of the successful transgenic studies that showed that transgenic plants, including important crop plants, overexpressing individually or together Gly I and Gly II have increased stress tolerance.
Table 1.
Glyoxalase genes overexpressed in transgenic plants exhibiting enhanced abiotic stress tolerance.
| Gene | Plant species | Response phenotype | Reference |
|---|---|---|---|
| Gly I | Tobacco (Nicotiana tabacum) | Improved salt stress tolerance | Veena et al., 1999 |
| Gly I | Black gram (Vigna mungo) | Improved salt stress tolerance | Bhomkar et al., 2008 |
| Gly I | Arabidopsis thaliana | Improved salt stress tolerance | Roy et al., 2008 |
| Gly I | Rice (Oryza sativa) | Improved salt stress tolerance | Verma et al., 2005 |
| Gly I | Tobacco (Nicotiana tabacum) | Improved salt stress tolerance | Yadav et al., 2005a |
| Gly I | Tobacco (Nicotiana tabacum) | Improved zinc tolerance | Lin et al., 2010 |
| Gly I | Tobacco (Nicotiana tabacum | Improved tolerance to MG, salt stress, excessive mannitol and H2O2 | Wu et al., 2013 |
| Gly I | Tobacco (Nicotiana tabacum) | Improved tolerance to MG and salt stress | Mustafiz et al., 2014 |
| Gly II | Rice (Oryza sativa) | Improved salinity tolerance | Singla-Pareek et al., 2008 |
| Gly II | Mustard (Brassica juncea) | Improved salinity tolerance | Saxena et al., 2011 |
| Gly II | Rice (Oryza sativa) | Improved salinity tolerance | Wani and Gosal, 2011 |
| Gly II | Arabidopsis thaliana | Improved salt and anoxic stress tolerance | Devanathan et al., 2014 |
| Gly II | Tobacco (Nicotiana tabacum) | Improved salinity tolerance | Ghosh et al., 2014 |
| Gly I + Gly II | Tobacco (Nicotiana tabacum) | Improved salinity tolerance and set viable seeds under zinc-spiked soils | Singla-Pareek et al., 2003, 2006; Yadav et al., 2005b |
| Gly I + Gly II | Tomato (Solanum lycopersicum) | Improved salt stress tolerance | Alvarez Viveros et al., 2013 |
| Gly I + Gly II | Carrizo citrange (Citrus sinensis × Poncirus trifoliata) | Improved salinity tolerance | Alvarez-Gerding et al., 2015 |
Gly, glyoxalase.
In addition to the transgenic approach, alternative methods, such as treatments of seeds prior to sowing and/or plants with exogenous chemicals, e.g., plant growth regulators, osmoprotectants, signaling molecules etc., can also alter the glyoxalase system in plants, thereby improving stress tolerance (Table 2). For instance, treatment of rice seedlings with Ca has been shown to increase the activities of Gly I and Gly II, contributing to the reduction in As- and Cd- induced growth inhibition (Rahman et al., 2015a,b). Mostofa and Fujita (2013) reported that a SA pre-treatment of rice seedlings under Cu stress alleviated Cu-toxicity by increasing the capacity of both antioxidant and glyoxalase systems. Table 2 lists most of the important studies in which chemical treatments were used to influence the glyoxalase system, leading to enhanced stress tolerance.
Table 2.
Effects of exogenous chemicals on glyoxalase systems and abiotic stress tolerance.
| Plant species | Types of stresses | Exogenous chemicals | Responses of glyoxalases (Gly I and II) | Concentration of MG | Reference |
|---|---|---|---|---|---|
| Rice (Oryza sativa L.) | As, Cd | Ca | Gly I ↑ Gly II ↑ (As) | ↓ | Rahman et al., 2015a,b |
| Gly I ↑ Gly II ↑ (Cd) | |||||
| Rice (Oryza sativa L.) | Cu | SA | Gly I ↑ | ND | Mostofa and Fujita, 2013; Mostofa et al., 2014a |
| Gly II ↑ | |||||
| Rice (Oryza sativaL.) | Heat | Spd | Gly I ↑ | ↓ | Mostofa et al., 2014b |
| Gly II ↑ | |||||
| Rice (Oryza sativa L.) | NaCl, Cu | Tre | Gly I ↕ Gly II ↑ (NaCl) | ↓ | Mostofa et al., 2015a,b |
| Gly I ↑ Gly II ↑ (Cu) | |||||
| Rice (Oryza sativa L.) | Cd, NaCl | H2S | Gly I ↓ Gly II ↑ (Cd) | ↓ | Mostofa et al., 2015c,d |
| Gly I ↓ Gly II ↑ (NaCl) | |||||
| Mung bean (Vigna radiata L.) | Cd | Pro and GB | Gly I ↑ | ND | Hossain et al., 2010 |
| Gly II ↑ | |||||
| Mustard (Brassica junceaL.) | Drought | Pro and GB | Gly I ↕ Gly II ↑ | ND | Hossain et al., 2014 |
| Tea (Camellia sinensisL.) | Cold | Pro and GB | Gly I ↑ | ND | Kumar and Yadav, 2009 |
| Gly II ↑ | |||||
| Tobacco (Nicotiana tabacumL.) | NaCl | Pro and GB | Gly I ↑ | ↓ | Hoque et al., 2008 |
| Gly II ↕ | |||||
| Mung bean (Vigna radiata L.) | Heat, Drought | GSH | Gly I ↓ Gly II ↑ (Drought) | ↓ | Nahar et al., 2015b,c |
| Gly I ↑ Gly II ↑ (Heat) | |||||
| Wheat (Triticum aestivum) | Heat, NaCl | NO | Gly I ↑ Gly II ↕ (Heat) | ND | Hasanuzzaman et al., 2011a, 2012b |
| Gly I ↑ Gly II ↑ (NaCl) | |||||
| Rapeseed (Brassica napus) | Drought, NaCl, Cd | Se | Gly I ↑ Gly II ↑ (Drought) | ND | Hasanuzzaman and Fujita, 2011; Hasanuzzaman et al., 2011b, 2012a |
| Gly I ↑ Gly II ↑ (NaCl) | |||||
| Gly I ↑ Gly II ↑ (Cd) | |||||
| Ficusconcinna | Heat | BRs | Gly I ↑ Gly II ↑ | ↓ | Jin et al., 2015 |
As, Cd, Cu, Ca, SA, Spd, Tre, H2S, Pro, GB, GSH, NO, Se, and BRs correspond to arsenic, cadmium, copper, calcium, salicylic acid, spermidine, trehalose, hydrogen sulfide, proline, glycinebetaine, glutathione, nitric oxide, selenium, and brassinosteroids, respectively. Gly, glyoxalase; ↑, increased; ↕, unchanged; ↓, decreased; ND, not determined.
Conclusion and Future Perspectives
Recent studies of MG metabolism have revealed many important functions of MG related to stress responses and tolerance in plants. The excessive accumulation of MG in plants is an inevitable stress, but MG can stimulate the components of different stress-protection pathways (Figure 2; Engqvist et al., 2009; Hoque et al., 2012a,b,c; Wienstroer et al., 2012; Kaur et al., 2015a), which could be considered as an acclimation/adaptation process. The glyoxalase pathway scavenges MG and confers tolerance to multiple stresses; and thus, MG levels and glyoxalase pathway are closely associated with abiotic stress tolerance in plants. The signaling roles of MG in up-regulating stress-responsive pathways and its potential to active multiple pathways have made MG a suitable marker for abiotic stress tolerance in plants. Recent progress made by genome-wide and in silico analyses has revealed intricate regulatory networks associated with MG signaling, which control gene expression, protein modification and the metabolite composition of plants. Further omic studies investigating the roles of MG would be worthwhile to improve our understanding of multiple abiotic stress tolerance. In-depth understanding of the interactions of MG with Ca2+, ROS, NO, H2S, plant hormones, TFs, and the glyoxalase system, as well as with other MG detoxification systems in different subcellular compartments will reveal more regulatory roles for MG in plant abiotic stress responses and tolerance.
Author Contributions
TH and MH conceived the idea. TH, MH, MM, DB, MF, and L-ST wrote the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ahmad P., Jaleel C. A., Salem M. A., Nabi G., Sharma S. (2010). Roles of enzymatic and non-enzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 30 161–175. 10.3109/07388550903524243 [DOI] [PubMed] [Google Scholar]
- Ahmed N., Thornalley P. J. (2007). Advanced glycation endproducts: what is their relevance to diabetic complications? Diab. Obes. Metab. 9 233–245. 10.1111/j.1463-1326.2006.00595.x [DOI] [PubMed] [Google Scholar]
- Alvarez-Gerding X., Cortés-Bullemore R., Medina C., Romero-Romero J. L., Inostroza-Blancheteau C., Aquea F., et al. (2015). Improved salinity tolerance in Carrizo Citrange Rootstock through overexpression of glyoxalase system genes. Bio. Med. Res. Int. 2015 827951 10.1155/2015/827951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez Viveros M. F., Inostroza-Blancheteau C., Timmermann T., González M., Arce-Johnson P. (2013). Overexpression of GlyI and GlyII genes in transgenic tomato (Solanum lycopersicum Mill.) plants confers salt tolerance by decreasing oxidative stress. Mol. Biol. Rep. 40 3281–3290. 10.1007/s11033-012-2403-4 [DOI] [PubMed] [Google Scholar]
- Atkinson N. J., Urwin P. E. (2012). The interaction of plant biotic and abiotic stresses: from genes to the field. J. Exp. Bot. 63 3523–3544. 10.1093/jxb/ers100 [DOI] [PubMed] [Google Scholar]
- Banu M. N. A., Hoque M. A., Watanabe-Sugimoto M., Islam M. A., Uraji M., Matsuoka M., et al. (2010). Proline and glycinebetaine ameliorated NaCl stress via scavenging of hydrogen peroxide and methylglyoxal but not superoxide or nitric oxide in tobacco cultured cells. Biosci. Biotechnol. Biochem. 74 2043–2049. 10.1271/bbb.100334 [DOI] [PubMed] [Google Scholar]
- Bechtold U., Rabbani N., Mullineaux P. M., Thornalley P. J. (2009). Quantitative measurement of specific biomarkers for protein oxidation, nitration and glycation in Arabidopsis leaves. Plant J. 59 661–671. 10.1111/j.1365-313X.2009.03898.x [DOI] [PubMed] [Google Scholar]
- Bhomkar P., Upadhyay C. P., Saxena M., Muthusamy A., Prakash N. S., Poggin M., et al. (2008). Salt stress alleviation in transgenic Vigna mungo L. Hepper (blackgram) by overexpression of the glyoxalase I gene using a novel Cestrum yellow leaf curling virus (CmYLCV) promoter. Mol. Breed. 22 169–181. [Google Scholar]
- Chen M., Thelen J. J. (2010). The plastid isoform of triose phosphate isomerase is required for the postgerminative transition from heterotrophic to autotrophic growth in Arabidopsis. Plant Cell 22 77–90. 10.1105/tpc.109.071837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanathan S., Erban A., Perez-Torres R., Kopka J., Makaroff C. A. (2014). Arabidopsis thaliana glyoxalase 2-1 is required during abiotic stress but is not essential under normal plant growth. PLoS ONE 9:e95971 10.1371/journal.pone.0095971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich P., Sanders D., Hedrich R. (2001). The role of ion channels in light-dependent stomatal opening. J. Exp. Bot. 52 1959–1967. 10.1093/jexbot/52.363.1959 [DOI] [PubMed] [Google Scholar]
- El-Shabrawi H., Kumar B., Kaul T., Reddy M. K., Singla-Pareek S. L., Sopory S. K. (2010). Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 24 85–96. 10.1007/s00709-010-0144-6 [DOI] [PubMed] [Google Scholar]
- Engqvist M. K. M., Drincovich M. F., Flügge U. I., Maurino V. G. (2009). Two D-2-hydroxy-acid dehydrogenases in Arabidopsis thaliana with catalytic capacities to participate in the last reactions of the methylglyoxal and β-oxidation pathways. J. Biol. Chem. 284 25026–25037. 10.1074/jbc.M109.021253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y., Fujita M., Shinozaki K., Yamaguchi-Shinozaki K. (2011). ABA-mediated transcriptional regulation in response to osmotic stress in plants. J. Plant Res. 124 509–525. 10.1007/s10265-011-0412-3 [DOI] [PubMed] [Google Scholar]
- Fujita Y., Yoshida T., Yamaguchi-Shinozaki K. (2013). Pivotal role of the AREB/ABF-SnRK2 pathway in ABRE-mediated transcription in response to osmotic stress in plants. Physiol. Plant 147 15–27. 10.1111/j.1399-3054.2012.01635.x [DOI] [PubMed] [Google Scholar]
- Ghosh A., Kushwaha H. R., Hasan M. R., Pareek A., Sopory S. K., Singla-Pareek S. L. (2016). Presence of unique glyoxalase III proteins in plants indicates the existence of shorter route for methylglyoxal detoxification. Sci. Rep. 6 18358 10.1038/srep18358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Pareek A., Sopory S. K., Singla-Pareek S. L. (2014). A glutathione responsive rice glyoxalase II, OsGLYII-2 functions in salinity adaptation by maintaining better photosynthesis efficiency and anti-oxidant pool. Plant J. 80 93–105. 10.1111/tpj.12621 [DOI] [PubMed] [Google Scholar]
- Grant M., Brown I., Adams S., Knight M., Ainslie A., Mansfield J. (2000). The RMP1 plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. Plant J. 23 441–450. 10.1046/j.1365-313x.2000.00804.x [DOI] [PubMed] [Google Scholar]
- Gururani M. A., Venkatesh J., Tran L. S. (2015). Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant 8 1304–1320. 10.1016/j.molp.2015.05.005 [DOI] [PubMed] [Google Scholar]
- Hadiarto T., Tran L. S. (2011). Progress studies of drought-responsive genes in rice. Plant Cell Rep. 30 297–310. 10.1007/s00299-010-0956-z [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M., Fujita M. (2011). Selenium pretreatment up-regulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol. Trace Elem. Res. 143 1758–1776. 10.1007/s12011-011-8958-4 [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M., Hossain M. A., Fujita M. (2011a). Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 5 353–365. 10.1007/s11816-011-0189-9 [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M., Hossain M. A., Fujita M. (2011b). Selenium-induced up-regulation of the antioxidant defense and methylglyoxal detoxification system reduces salinity-induced damage in rapeseed seedlings. Biol. Trace Elem. Res. 143 1704–1721. 10.1007/s12011-011-8958-4 [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M., Hossain M. A., Fujita M. (2012a). Exogenous selenium pretreatment protects rapeseed seedlings from cadmium-induced oxidative stress by up-regulating antioxidant defense and methylglyoxal detoxification systems. Biol. Trace Elem. Res. 149 248–261. 10.1007/s12011-012-9419-4 [DOI] [PubMed] [Google Scholar]
- Hasanuzzaman M., Nahar K., Alam M. M., Fujita M. (2012b). Exogenous nitric oxide alleviates high temperature induced oxidative stress in wheat (Triticum aestivum L.) seedlings by modulating the antioxidant defense and glyoxalase system. Aust. J. Crop Sci. 6 1314–1323. [Google Scholar]
- Hoque M. A., Banu M. N. A., Nakamura Y., Shimoishi Y., Murata Y. (2008). Proline and glycinebetaine enhance antioxidant defense and methylglyoxal detoxification systems and reduce NaCl-induced damage in cultured tobacco cells. J. Plant Physiol. 165 813–824. 10.1016/j.jplph.2007.07.013 [DOI] [PubMed] [Google Scholar]
- Hoque M. A., Uraji M., Banu M. N. A., Mori I. C., Nakamura Y., Murata Y. (2010). The effects of methylglyoxal on glutathione S-transferase from Nicotiana tabacum. Biosci. Biotechnol. Biochem. 74 2124–2126. 10.1271/bbb.100393 [DOI] [PubMed] [Google Scholar]
- Hoque M. A., Uraji M., Banu M. N. A., Mori I. C., Nakamura Y., Murata Y. (2012). Methylglyoxal inhibition of cytosolic ascorbate peroxidase from Nicotiana tabacum. J. Biochem. Mol. Toxicol. 26 315–321. 10.1002/jbt.21423 [DOI] [PubMed] [Google Scholar]
- Hoque T. S., Hossain M. A., Mostofa M. G., Burritt D. J., Fujita M. (2015). “Signalling roles of methylglyoxal and the involvement of the glyoxalase system in plant abiotic stress responses and tolerance,” in Plant-Environment Interaction: Responses and Approaches to Mitigate Stress, eds Azooz M. M., Ahmad P. (Chichester: John Wiley & Sons Ltd; ), 311–326. 10.1002/9781119081005.ch17 [DOI] [Google Scholar]
- Hoque T. S., Okuma E., Uraji M., Furuichi T., Sasaki T., Hoque M. A., et al. (2012a). Inhibitory effects of methylglyoxal on light-induced stomatal opening and inward K+ channel activity in Arabidopsis. Biosci. Biotechnol. Biochem. 76 617–619. 10.1271/bbb.110885 [DOI] [PubMed] [Google Scholar]
- Hoque T. S., Uraji M., Tuya A., Nakamura Y., Murata Y. (2012b). Methylglyoxal inhibits seed germination and root elongation and up-regulates transcription of stress-responsive genes in ABA-dependent pathway in Arabidopsis. Plant Biol. 14 854–858. 10.1111/j.1438-8677.2012.00607.x [DOI] [PubMed] [Google Scholar]
- Hoque T. S., Uraji M., Ye W., Hossain M. A., Nakamura Y., Murata Y. (2012c). Methylglyoxal-induced stomatal closure accompanied by peroxidase-mediated ROS production in Arabidopsis. J. Plant Physiol. 169 979–986. 10.1016/j.jplph.2012.02.007 [DOI] [PubMed] [Google Scholar]
- Hossain M. A., Fujita M. (2009). Purification of glyoxalase I from onion bulbs and molecular cloning of its cDNA. Biosci. Biotechnol. Biochem. 73 2007–2013. 10.1271/bbb.90194 [DOI] [PubMed] [Google Scholar]
- Hossain M. A., Hasanuzzaman M., Fujita M. (2010). Up-regulation of antioxidant and glyoxalase systems by exogenous glycinebetaine and proline in mung bean confer tolerance to cadmium stress. Physiol. Mol. Biol. Plants 16 259–272. 10.1007/s12298-010-0028-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M. A., Hossain M. Z., Fujita M. (2009). Stress-induced changes of methylglyoxal level and glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Aust. J. Crop Sci. 3 53–64. [Google Scholar]
- Hossain M. A., Mostofa M. G., Burritt D. J., Fujita M. (2014). Modulation of reactive oxygen species and methylglyoxal detoxification systems by exogenous glycinebetaine and proline improves drought tolerance in mustard (Brassica juncea L.). Int. J. Plant Biol. Res. 2 1014. [Google Scholar]
- Hossain M. A., Teixeira da Silva J. A., Fujita M. (2011). “Glyoxalase system and reactive oxygen species detoxification system in plant abiotic stress response and tolerance: an intimate relationship,” in Abiotic Stress in Plants-Mechanisms and Adaptations, eds Shanker A., Venkateswarlu B. (Rijeka: INTECH-Open Access Publisher; ), 235–266. [Google Scholar]
- Hubbard K. E., Nishimura N., Hitomi K., Getzoff E. D., Schroeder J. I. (2010). Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions. Genes Dev. 24 1695–1708. 10.1101/gad.1953910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W., Zhang J. (2008). Stomatal movements and long-distance signaling in plants. Plant Signal. Behav. 3 772–777. 10.4161/psb.3.10.6294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S. H., Li X. Q., Wang G. G., Zhu X. T. (2015). Brassinosteroids alleviate high-temperature injury in Ficus concinna seedlings via maintaining higher antioxidant defence and glyoxalase systems. AoB Plants 7:plv009 10.1093/aobpla/plv009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalapos M. P. (1999). Methylglyoxal in living organisms: chemistry, biochemistry, toxicology and biological implications. Toxicol. Lett. 110 145–175. 10.1016/S0378-4274(99)00160-5 [DOI] [PubMed] [Google Scholar]
- Kalapos M. P. (2008). The tandem of free radicals and methylglyoxal. Chem. Biol. Interact. 171 251–271. 10.1016/j.cbi.2007.11.009 [DOI] [PubMed] [Google Scholar]
- Kaur C., Ghosh A., Pareek A., Sopory S. K., Singla-Pareek S. L. (2014a). Glyoxalases and stress tolerance in plants. Biochem. Soc. Trans. 42 485–490. 10.1042/BST20130242 [DOI] [PubMed] [Google Scholar]
- Kaur C., Kushwaha H. R., Mustafiz A., Pareek A., Sopory S. K., Singla-Pareek S. L. (2015a). Analysis of global gene expression profile of rice in response to methylglyoxal indicates its possible role as a stress signal molecule. Front. Plant Sci. 6:682 10.3389/fpls.2015.00682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur C., Mustafiz A., Sarkar A., Ariyadasa T. U., Singla-Pareek S. L., Sopory S. K. (2014b). Expression of abiotic stress inducible ETHE1-like protein from rice is higher in roots and is regulated by calcium. Physiol. Plant. 152 1–16. 10.1111/ppl.12147 [DOI] [PubMed] [Google Scholar]
- Kaur C., Sharma S., Singla-Pareek S. L., Sopory S. K. (2015b). “Methylglyoxal, triose phosphateisomerase and glyoxalase pathway: implications in abiotic stress and signaling in plants,” in Elucidation of Abiotic Stress Signaling in Plants, ed. Pandey G. K. (New York, NY: Springer; ), 347–366. [Google Scholar]
- Kaur C., Singla-Pareek S. L., Sopory S. K. (2014c). Glyoxalase and methylglyoxal as biomarkers for plant stress tolerance. Crit. Rev. Plant Sci. 33 429–456. 10.1007/s00709-010-0144-6 [DOI] [Google Scholar]
- Kim T. H., Bohmer M., Hu H., Nishimura N., Schroeder J. I. (2010). Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 61 561–591. 10.1146/annurev-arplant-042809-112226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirch H. H., Schlingensiepen S., Kotchoni S., Sunkar R., Bartels D. (2005). Detailed expression analysis of selected genes of the aldehyde dehydrogenase (ALDH) gene superfamily in Arabidopsis thaliana. Plant Mol. Biol. 57 315–332. 10.1007/s11103-004-7796-6 [DOI] [PubMed] [Google Scholar]
- Ko J., Kim I., Yoo S., Min B., Kim K., Park C. (2005). Conversion of methylglyoxal to acetol by Escherichia coli aldo-keto reductases. J. Bacteriol. 187 5782–5789. 10.1128/JB.187.16.5782-5789.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Yadav S. K. (2009). Proline and betaine provide protection to antioxidant and methylglyoxal detoxification systems during cold stress in Camellia sinensis (L.) O. Kuntze. Acta Physiol. Plant. 31 261–269. 10.1007/s11738-008-0227-6 [DOI] [Google Scholar]
- Kwak J. M., Murata Y., Baizabal-Aguirre V. M., Merrill J., Wang M., Kemper A., et al. (2001). Dominant negative guard cell K+ channel mutants reduce inward-rectifying K+ currents and light-induced stomatal opening in Arabidopsis. Plant Physiol. 127 473–485. 10.1104/pp.010428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoncini G. (1979). The role of alpha-oxoaldehydes in biological systems. Ital. J. Biochem. 28 285–294. [PubMed] [Google Scholar]
- Li W., Tran L. S. (2015). Are karrikins involved in plant abiotic stress responses? Trends Plant Sci. 20 535–538. 10.1016/j.tplants.2015.07.006 [DOI] [PubMed] [Google Scholar]
- Lin F., Xu J., Shi J., Li H., Li B. (2010). Molecular cloning and characterization of a novel glyoxalase I gene TaGly I in wheat (Triticum aestivum L.). Mol. Biol. Rep. 37 729–735. 10.1007/s11033-009-9578-3 [DOI] [PubMed] [Google Scholar]
- Ma Y., Qin F., Tran L. S. (2012). Contribution of genomics to gene discovery in plant abiotic stress responses. Mol. Plant 5 1176–1178. 10.1093/mp/sss085 [DOI] [PubMed] [Google Scholar]
- Maeta K., Izawa S., Inoue Y. (2005). Methylgyoxal, a metabolite derived from glycolysis, functions as a signal initiator of the high osmolarity glycerol-mitogen-activated protein kinase cascade and calcineurin/Crz1-mediated pathway in Saccharomyces cerevisiae. J. Biol. Chem. 280 253–260. 10.1074/jbc.M408061200 [DOI] [PubMed] [Google Scholar]
- Maiti M. K., Krishnasamy S., Owen H. A., Makaroff C. A. (1997). Molecular characterization of glyoxalase II from Arabidopsis thaliana. Plant Mol. Biol. 35 471–481. 10.1023/A:1005891123344 [DOI] [PubMed] [Google Scholar]
- Mano J. (2012). Reactive carbonyl species: their production from lipid peroxides, action in environmental stress, and the detoxification mechanism. Plant Physiol. Biochem. 59 90–97. 10.1016/j.plaphy.2012.03.010 [DOI] [PubMed] [Google Scholar]
- Mano J., Miyatake F., Hiraoka E., Tamoi M. (2009). Evaluation of the toxicity of stress-related aldehydes to photosynthesis in chloroplasts. Planta 230 639–648. 10.1007/s00425-009-0964-9 [DOI] [PubMed] [Google Scholar]
- Mori I. C., Murata Y., Yang Y., Munemasa S., Wang Y. F., Andreoli S., et al. (2006). CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biol. 4:e327 10.1371/journal.pbio.0040327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofa M. G., Fujita M. (2013). Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by up-regulating antioxidative and glyoxalase systems. Ecotoxicology 22 959–973. 10.1007/s10646-013-1073-x [DOI] [PubMed] [Google Scholar]
- Mostofa M. G., Hossain M. A., Fujita M. (2015a). Trehalose pretreatment induces salt tolerance in rice (Oryza sativa L.) seedlings: oxidative damage and co-induction of antioxidant defense and glyoxalase systems. Protoplasma 252 461–475. 10.1007/s00709-014-0691-3 [DOI] [PubMed] [Google Scholar]
- Mostofa M. G., Hossain M. A., Fujita M., Tran L. S. (2015b). Physiological and biochemical mechanisms associated with trehalose-induced copper-stress tolerance in rice. Sci. Rep. 5 11433 10.1038/srep11433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofa M. G., Rahman A., Ansary M. M. U., Watanabe A., Fujita M., Tran L. S. (2015c). Hydrogen sulfide modulates cadmium-induced physiological and biochemical responses to alleviate cadmium toxicity in rice. Sci. Rep. 5 14078 10.1038/srep14078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofa M. G., Saegusa D., Fujita M., Tran L. S. (2015d). Hydrogen sulfide regulates salt tolerance in rice by maintaining Na+/K+ balance, mineral homeostasis and oxidative metabolism under excessive salt stress. Front. Plant Sci. 6:1055 10.3389/fpls.2015.01055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofa M. G., Seraj Z. I., Fujita M. (2014a). Exogenous sodium nitroprusside and glutathione alleviate copper toxicity by reducing copper uptake and oxidative damage in rice (Oryza sativa L.) seedlings. Protoplasma 251 1373–1386. 10.1007/s00709-014-0639-7 [DOI] [PubMed] [Google Scholar]
- Mostofa M. G., Yoshida N., Fujita M. (2014b). Spermidine pretreatment enhances heat tolerance in rice seedlings through modulating antioxidative and glyoxalase systems. Plant Growth Regul. 73 31–44. 10.1007/s10725-013-9865-9 [DOI] [Google Scholar]
- Mustafiz A., Ghosh A., Tripathi A. K., Kaur C., Ganguly A. K., Bhavesh N. S., et al. (2014). A unique Ni2+-dependent and methylglyoxal-inducible rice glyoxalase I possesses a single active site and functions in abiotic stress response. Plant J. 78 951–963. 10.1111/tpj.12521 [DOI] [PubMed] [Google Scholar]
- Mustafiz A., Singh A. K., Pareek A., Sopory S. K., Singla-Pareek S. L. (2011). Genome-wide analysis of rice and Arabidopsis identifies two glyoxalase genes that are highly expressed in abiotic stresses. Funct. Integr. Genomics 11 293–305. 10.1007/s10142-010-0203-2 [DOI] [PubMed] [Google Scholar]
- Nahar K., Hasanuzzaman M., Alam M. M., Fujita M. (2015a). Roles of exogenous glutathione in antioxidant defense system and methylglyoxal detoxification during salt stress in mung bean. Biol. Plant. 59 745–756. 10.1007/s10535-015-0542-x [DOI] [Google Scholar]
- Nahar K., Hasanuzzaman M., Alam M. M., Fujita M. (2015b). Glutathione-induced drought stress tolerance in mung bean: coordinated roles of the antioxidant defence and methylglyoxal detoxification systems. AoB Plants 7 lv069 10.1093/aobpla/plv069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahar K., Hasanuzzaman M., Alam M. M., Fujita M. (2015c). Exogenous glutathione confers high temperature stress tolerance in mung bean (Vigna radiata L.) by modulating antioxidant defense and methylglyoxal detoxification system. Environ. Exp. Bot. 112 44–54. 10.1016/j.envexpbot.2014.12.001 [DOI] [Google Scholar]
- Narawongsanont R., Kabinpong S., Auiyawong B., Tantitadapitak C. (2012). Cloning and characterization of AKR4C14 a rice aldo-ketoreductase, from Thai Jasmine rice. Protein J. 31 35–42. 10.1007/s10930-011-9371-8 [DOI] [PubMed] [Google Scholar]
- Noctor G., Mhamdi A., Chaouch S., Han Y. I., Neukermans J., Marquez-Garcia B., et al. (2012). Glutathione in plants: an integrated overview. Plant Cell Environ. 35 454–484. 10.1111/j.1365-3040.2011.02400.x [DOI] [PubMed] [Google Scholar]
- Norton S. J., Talesa V., Yuan W. J., Principato G. B. (1990). Glyoxalase-I and Glyoxalase-II from Aloe vera: purification, characterization and comparison with animal glyoxalases. Biochem. Int. 22 411–418. [PubMed] [Google Scholar]
- Osakabe Y., Yamaguchi-Shinozaki K., Shinozaki K., Tran L. S. (2014). ABA control of plant macroelement membrane transport systems in response to water deficit and high salinity. New Phytol. 202 35–49. 10.1111/nph.12613 [DOI] [PubMed] [Google Scholar]
- Pei Z. M., Murata Y., Benning G., Thomine S., Klüsener B., Allen G. J., et al. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406 731–734. 10.1038/35021067 [DOI] [PubMed] [Google Scholar]
- Petrov V., Hille J., Mueller-Roeber B., Gechev T. S. (2015). ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 6:69 10.3389/fpls.2015.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S. A., Thornalley P. J. (1993). The formation of methylglyoxal from triose phosphates. Investigation using a specific assay for methylglyoxal. Eur. J. Biochem. 212 101–105. [DOI] [PubMed] [Google Scholar]
- Qiu Q. S., Huber J. L., Booker F. L., Jain V., Leakey A. D. B., Fiscus E. L., et al. (2008). Increasing protein carbonylation in leaves of Arabidopsis and soybean in response to elevated [CO2]. Photosynth. Res. 97 155–166. 10.1007/s11120-008-9310-5 [DOI] [PubMed] [Google Scholar]
- Rabbani N., Thornalley P. J. (2012). Methylglyoxal, glyoxalase I and dicarbonyl proteome. Amino Acids 42 1133–1142. 10.1007/s00726-010-0783-0 [DOI] [PubMed] [Google Scholar]
- Rabbani N., Thornalley P. J. (2014). Dicarbonyl proteome and genome damage in metabolic and vascular disease. Biochem. Soc. Trans. 42 425–432. 10.1042/BST20140018 [DOI] [PubMed] [Google Scholar]
- Rahman A., Mostofa M. G., Nahar K., Alam M. M., Hasanuzzaman M., Fujita M. (2015a). Calcium mitigates arsenic toxicity in rice seedlings by reducing arsenic uptake and modulating the antioxidant defense and glyoxalase systems and stress markers. Biomed Res. Int. 2015 340812 10.1155/2015/340812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A., Mostofa M. G., Nahar K., Hasanuzzaman M., Fujita M. (2015b). Exogenous calcium alleviates cadmium-induced oxidative stress in rice (Oryza sativa L.) seedlings by regulating the antioxidant defense and glyoxalase systems. Braz. J. Bot. 39 393 10.1007/s40415-015-0240-0 [DOI] [Google Scholar]
- Richard J. P. (1984). Acid-base catalysis of the elimination and isomerization-reactions of triose phosphates. J. Am. Chem. Soc. 10 4926–4936. 10.1021/ja00329a050 [DOI] [Google Scholar]
- Richard J. P. (1993). Mechanism for the formation of methylglyoxal from triosephosphates. Biochem. Soc. Trans. 21 549–553. 10.1042/bst0210549 [DOI] [PubMed] [Google Scholar]
- Roy S. D., Saxena M., Bhomkar P. S., Pooggin M., Hohn T., Bhalla-Sarin N. (2008). Generation of marker free salt tolerant transgenic plants of Arabidopsis thaliana using the gly I gene and cre gene under inducible promoter. Plant Cell Tiss. Organ Cult. 95 1–11. 10.1007/s11240-008-9402-0 [DOI] [Google Scholar]
- Saito R., Yamamoto H., Makino A., Sugimoto T., Miyake C. (2011). Methylglyoxal functions as Hill oxidant and stimulates the photoreduction of O2at photosystem I: a symptom of plant diabetes. Plant Cell Environ. 34 1454–1464. 10.1111/j.1365-3040.2011.02344.x [DOI] [PubMed] [Google Scholar]
- Sato A., Gambale F., Dreyer I., Uozumi N. (2010). Modulation of the Arabidopsis KAT1 channel by an activator of protein kinase C in Xenopus laeves oocytes. FEBS J. 277 2318–2328. 10.1111/j.1742-4658.2010.07647.x [DOI] [PubMed] [Google Scholar]
- Sato A., Sato Y., Fukao Y., Fujiwara M., Umezawa T., Shinozaki K., et al. (2009). Threonine at position 306 of the KAT1 potassium channel is essential for channel activity and is a target site for ABA-activated SnRK2/OST1/SnRK2.6 protein kinase. Biochem. J. 424 439–448. 10.1042/BJ20091221 [DOI] [PubMed] [Google Scholar]
- Saxena M., Roy S. D., Singla-Pareek S. L., Sopory S. K., Bhalla-Sarin N. (2011). Overexpression of the glyoxalase II gene leads to enhanced salinity tolerance in Brassica juncea. Open Plant Sci. J. 5 23–28. 10.2174/1874294701105010023 [DOI] [Google Scholar]
- Schroeder J. I., Allen G. J., Hugouvieux V., Kwak J. M., Waner D. (2001). Guard cell signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52 627–658. 10.1146/annurev.arplant.52.1.627 [DOI] [PubMed] [Google Scholar]
- Sewelam N., Kazan K., Schenk P. M. (2016). Global plant stress signaling: reactive oxygen species at the cross-road. Front. Plant Sci. 7:187 10.3389/fpls.2016.00187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P., Jha A. B., Dubey R. S., Pessarakli M. (2012). Reactive oxygen species, oxidative damage, and antioxidative defense mechanisms in plants under stressful conditions. J. Bot. 2012 217037 10.1155/2012/217037 [DOI] [Google Scholar]
- Sharma R., De Vleesschauwer D., Sharma A. K., Ronald P. C. (2013). Recent advances in dissecting stress-regulatory crosstalk in rice. Mol. Plant 6 250–260. 10.1093/mp/sss147 [DOI] [PubMed] [Google Scholar]
- Simpson P. J., Tantitadapitak C., Reed A. M., Mather O. C., Bunce C. M., White S. A., et al. (2009). Characterization of two novel aldo-keto reductases from Arabidopsis: expression patterns, broad substrate specificity, and an open active-site structure suggest a role in toxicant metabolism following stress. J. Mol. Biol. 392 465–480. 10.1016/j.jmb.2009.07.023 [DOI] [PubMed] [Google Scholar]
- Singla-Pareek S. L., Reddy M. K., Sopory S. K. (2003). Genetic engineering of the glyoxalase pathway in tobacco leads to enhanced salinity tolerance. Proc. Natl. Acad. Sci. U.S.A. 100 14672–14677. 10.1073/pnas.2034667100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla-Pareek S. L., Yadav S. K., Pareek A., Reddy M. K., Sopory S. K. (2006). Transgenic tobacco overexpressing glyoxalase pathway enzymes grow and set viable seeds in zinc-spiked soils. Plant Physiol. 140 613–623. 10.1104/pp.105.073734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla-Pareek S. L., Yadav S. K., Pareek A., Reddy M. K., Sopory S. K. (2008). Enhancing salt tolerance in a crop plant by overexpression of glyoxalase II. Transgenic Res. 17 171–180. 10.1007/s11248-007-9082-2 [DOI] [PubMed] [Google Scholar]
- Takagi D., Inoue H., Odawara M., Shimakawa G., Miyake C. (2014). The Calvin cycle inevitably produces sugar-derived reactive carbonyl methylglyoxal during photosynthesis: a potential cause of plant diabetes. Plant Cell Physiol. 55 333–340. 10.1093/pcp/pcu007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thao N. P., Tran L. S. (2012). Potentials toward genetic engineering of drought-tolerant soybean. Crit. Rev. Biotechnol. 32 349–362. 10.3109/07388551.2011.643463 [DOI] [PubMed] [Google Scholar]
- Thornalley P. J. (1990). The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem. J. 269 1–11. 10.1042/bj2690001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornalley P. J. (2006). “Quantitative screening of protein glycation, oxidation, and nitration adducts by LC-MS/MS: protein damage in diabetes, uremia, cirrhosis, and Alzheimer’s disease,” in Redox Proteomics: From Protein Modifications to Cellular Dysfunction and Diseases, eds Dalle-Donne I., Scaloni A., Butterfield D. A. (Hoboken, NJ: John Willey & Sons; ), 681–727. [Google Scholar]
- Todaka D., Shinozaki K., Yamaguchi-Shinozaki K. (2015). Recent advances in the dissection of drought-stress regulatory networks and strategies for development of drought-tolerant transgenic rice plants. Front. Plant Sci. 6:84 10.3389/fpls.2015.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres M. A., Dangl J. L., Jones J. D. G. (2002). Arabidopsis gp91phox homologues, AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. U.S.A. 99 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya C. P., Venkatesh J., Gururani M. A., Asnin L., Sharma K., Ajappala H. (2011). Transgenic potato overproducing L-ascorbic acid resisted an increase in methylglyoxal under salinity stress via maintaining higher reduced glutathione level and glyoxalase enzyme activity. Biotechnol. Lett. 33 2297–2307. 10.1007/s10529-011-0684-7 [DOI] [PubMed] [Google Scholar]
- Veena, Reddy V. S., Sopory S. K. (1999). Glyoxalase I from Brassica juncea: molecular Cloning, regulation and its over-expression confer tolerance in transgenic tobacco under stress. Plant J. 17 385–395. 10.1046/j.1365-313X.1999.00390.x [DOI] [PubMed] [Google Scholar]
- Verma M., Verma D., Jain R. K., Sopory S. K., Wu R. (2005). Overexpression of glyoxalase I gene confers salinity tolerance in transgenic japonica and indica rice plants. News Lett. 22 58–62. [Google Scholar]
- Vistoli G., De Maddis D., Cipak A., Zarkovic N., Carini M., Aldini G. (2013). Advanced glycation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation. Free Radic. Res. 47 3–27. 10.3109/10715762.2013.815348 [DOI] [PubMed] [Google Scholar]
- Wani S. H., Gosal S. S. (2011). Introduction of OsglyII gene into Oryza sativa for increasing salinity tolerance. Biol. Plant. 55 536–540. 10.1007/s10535-011-0120-9 [DOI] [Google Scholar]
- Weiner J. J., Peterson F. C., Volkman B. F., Cutler S. R. (2010). Structural and functional insights into core ABA signaling. Curr. Opin. Plant Biol. 13 495–502. 10.1016/j.pbi.2010.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienstroer J., Engqvist M. K. M., Kunz H. H., Flügge U. I., Maurino V. G. (2012). D-Lactate dehydrogenase as a marker gene allows positive selection of transgenic plants. FEBS Lett. 586 36–40. 10.1016/j.febslet.2011.11.020 [DOI] [PubMed] [Google Scholar]
- Wu C., Ma C., Pan Y., Gong S., Zhao C., Chen S., et al. (2013). Sugar beet M14 glyoxalase I gene can enhance plant tolerance to abiotic stresses. J. Plant Res. 126 415–425. 10.1007/s10265-012-0532-4 [DOI] [PubMed] [Google Scholar]
- Yadav S. K., Singla-Pareek S. L., Ray M., Reddy M. K., Sopory S. K. (2005a). Methylglyoxal levels in plants under salinity stress are dependent on glyoxalase I and glutathione. Biochem. Biophys. Res. Commun. 337 61–67. 10.1016/j.bbrc.2005.08.263 [DOI] [PubMed] [Google Scholar]
- Yadav S. K., Singla-Pareek S. L., Ray M., Reddy M. K., Sopory S. K. (2005b). Transgenic tobacco plants overexpressing glyoxalase enzymes resist an increase in methylglyoxal and maintain higher reduced glutathione levels under salinity stress. FEBS Lett. 579 6265–6271. 10.1016/j.febslet.2005.10.006 [DOI] [PubMed] [Google Scholar]
- Yadav S. K., Singla-Pareek S. L., Sopory S. K. (2008). An overview on the role of methylglyoxal and glyoxalases in plants. Drug Metabol. Drug Interact. 23 51–68. 10.1515/DMDI.2008.23.1-2.51 [DOI] [PubMed] [Google Scholar]
- Yamauchi Y., Hasegawa A., Taninaka A., Mizutani M., Sugimoto Y. (2010). NADPH-dependent reductases involved in the detoxification of reactive carbonyls in plants. J. Biol. Chem. 286 6999–7009. 10.1074/jbc.M110.202226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Mogami J., Yamaguchi-Shinozaki K. (2015). Omics approaches toward defining the comprehensive abscisic acid signaling network in plants. Plant Cell Physiol. 56 1043–1052. 10.1093/pcp/pcv060 [DOI] [PubMed] [Google Scholar]
- Young J. J., Mehta S., Israelsson M., Godoski J., Grill E., Schroeder J. I. (2006). CO2 signaling in guard cells: calcium sensitivity response modulation, a Ca2+-independent phase, and CO2 insensitivity of the gca2 mutant. Proc. Natl. Acad. Sci. U.S.A. 103 7506–7511. 10.1073/pnas.0602225103 [DOI] [PMC free article] [PubMed] [Google Scholar]