Abstract
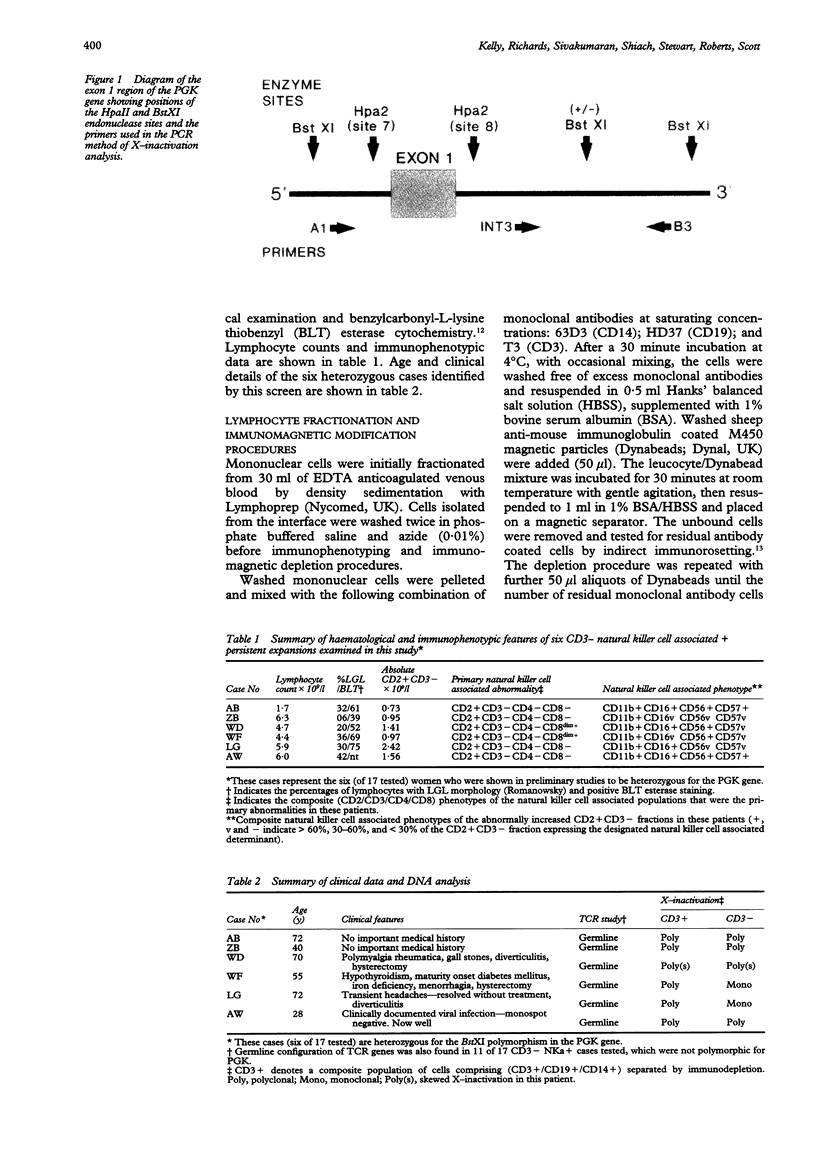
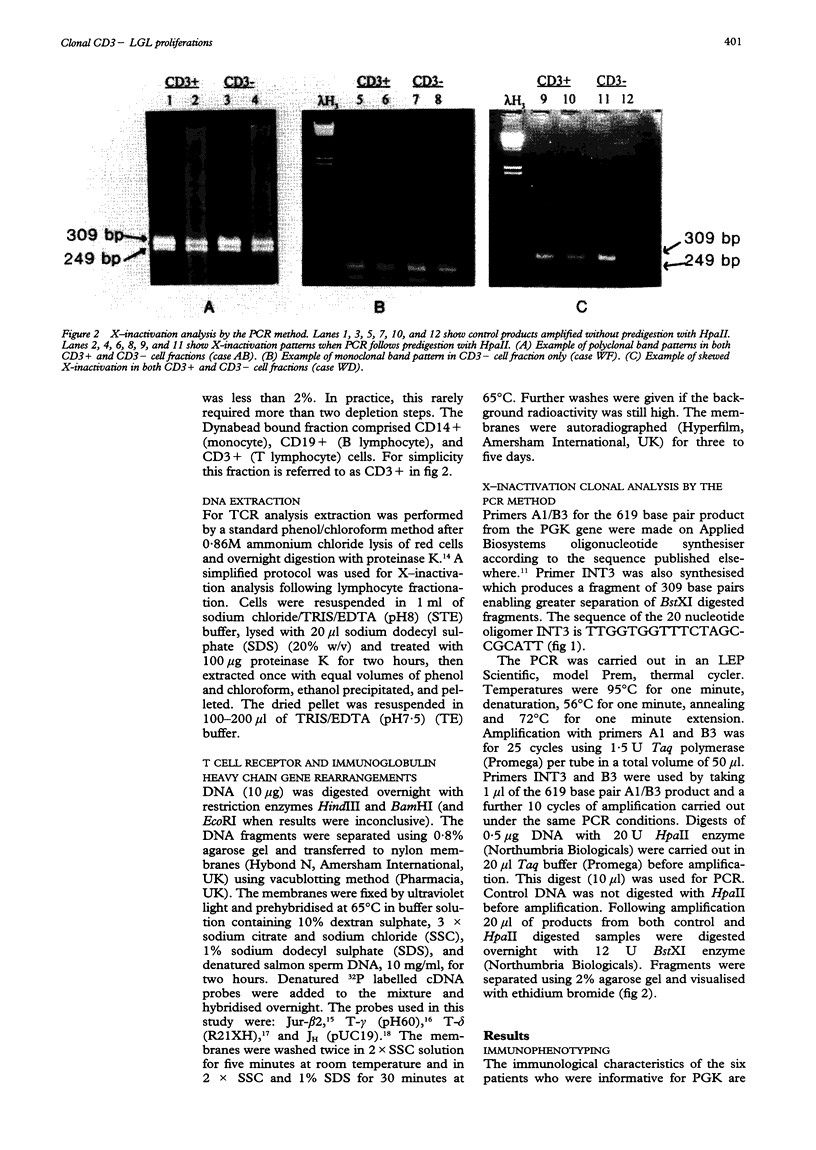
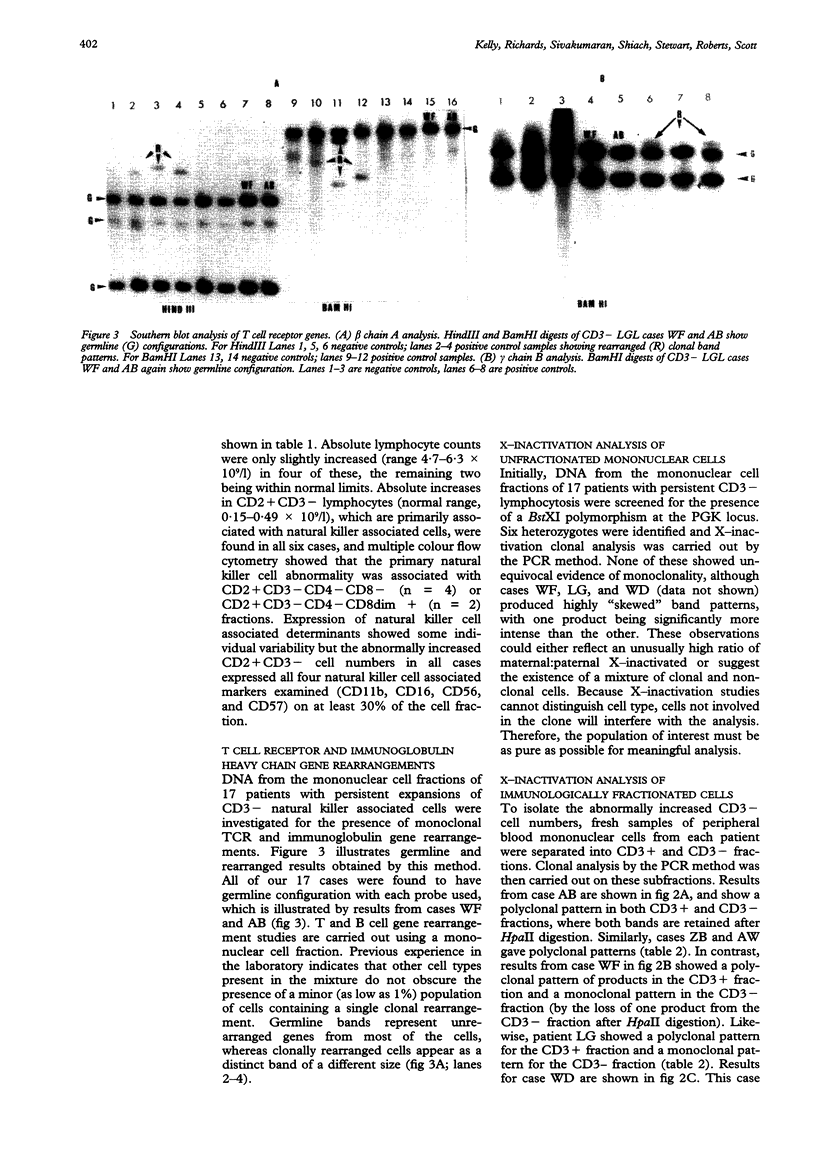
AIMS--To examine persistent CD3-large granular lymphocytosis (LGL) cases for clonality, both by lineage specific (T cell receptor) and lineage independent (X-inactivation) molecular methods; and to find out whether X-inactivation studies are more appropriate than gene rearrangement studies for this subset of LGL disorders. METHODS--Patients were selected who had LGL of more than six months' duration and identified as CD3- by immunophenotyping. T cell receptor studies and, where possible, X-inactivation studies of the phosphoglycerate kinase (PGK) gene were carried out. Analysis of subpopulations was carried out on cases heterozygous for PGK by the use of a polymerase chain reaction (PCR) method for X-inactivation. RESULTS--Of 17 CD3- LGL cases studied, all were found to be germline for beta, gamma, and delta T cell receptor studies, and immunoglobulin heavy chain genes. However, six of these were analysed by X-inactivation of the PGK gene and two cases gave clonal band patterns but only within the CD3- subpopulation. CONCLUSIONS--Clonal analysis by the lineage independent method of X-inactivation allows clonal expansion undetected by T and B cell specific markers to be identified. It is therefore a more appropriate method for the analysis of CD3- LGL. This has implications for diagnosis in CD3- LGL disorders.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boehm T., Buluwela L., Williams D., White L., Rabbitts T. H. A cluster of chromosome 11p13 translocations found via distinct D-D and D-D-J rearrangements of the human T cell receptor delta chain gene. EMBO J. 1988 Jul;7(7):2011–2017. doi: 10.1002/j.1460-2075.1988.tb03040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Gale R. E., Wheadon H., Goldstone A. H., Burnett A. K., Linch D. C. Frequency of clonal remission in acute myeloid leukaemia. Lancet. 1993 Jan 16;341(8838):138–142. doi: 10.1016/0140-6736(93)90004-z. [DOI] [PubMed] [Google Scholar]
- Kawa-Ha K., Ishihara S., Ninomiya T., Yumura-Yagi K., Hara J., Murayama F., Tawa A., Hirai K. CD3-negative lymphoproliferative disease of granular lymphocytes containing Epstein-Barr viral DNA. J Clin Invest. 1989 Jul;84(1):51–55. doi: 10.1172/JCI114168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefranc M. P., Rabbitts T. H. Two tandemly organized human genes encoding the T-cell gamma constant-region sequences show multiple rearrangement in different T-cell types. Nature. 1985 Aug 1;316(6027):464–466. doi: 10.1038/316464a0. [DOI] [PubMed] [Google Scholar]
- Loughran T. P., Jr, Starkebaum G., Aprile J. A. Rearrangement and expression of T-cell receptor genes in large granular lymphocyte leukemia. Blood. 1988 Mar;71(3):822–824. [PubMed] [Google Scholar]
- Loughran T. P., Jr, Starkebaum G., Kidd P., Neiman P. Clonal proliferation of large granular lymphocytes in rheumatoid arthritis. Arthritis Rheum. 1988 Jan;31(1):31–36. doi: 10.1002/art.1780310105. [DOI] [PubMed] [Google Scholar]
- MacKarill I. D., Scott C. S., Stark A. N., Limbert H. J., Master P. S., Jones R. A., Earnshaw A. Preparation of chromic chloride solutions for indirect rosetting: a rapid method. Med Lab Sci. 1987 Oct;44(4):401–404. [PubMed] [Google Scholar]
- Nash R., McSweeney P., Zambello R., Semenzato G., Loughran T. P., Jr Clonal studies of CD3- lymphoproliferative disease of granular lymphocytes. Blood. 1993 May 1;81(9):2363–2368. [PubMed] [Google Scholar]
- Newland A. C., Catovsky D., Linch D., Cawley J. C., Beverley P., San Miguel J. F., Gordon-Smith E. C., Blecher T. E., Shahriari S., Varadi S. Chronic T cell lymphocytosis: a review of 21 cases. Br J Haematol. 1984 Nov;58(3):433–446. doi: 10.1111/j.1365-2141.1984.tb03990.x. [DOI] [PubMed] [Google Scholar]
- Ohno T., Kanoh T., Arita Y., Fujii H., Kuribayashi K., Masuda T., Horiguchi Y., Taniwaki M., Nosaka T., Hatanaka M. Fulminant clonal expansion of large granular lymphocytes. Characterization of their morphology, phenotype, genotype, and function. Cancer. 1988 Nov 1;62(9):1918–1927. doi: 10.1002/1097-0142(19881101)62:9<1918::aid-cncr2820620909>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Rabbitts T. H., Stinson A., Forster A., Foroni L., Luzzatto L., Catovsky D., Hammarström L., Smith C. I., Jones D., Karpas A. Heterogeneity of T-cell beta-chain gene rearrangements in human leukaemias and lymphomas. EMBO J. 1985 Sep;4(9):2217–2224. doi: 10.1002/j.1460-2075.1985.tb03917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansoni P., Girasole G., Manara G. C., Snelli G., Passeri G., Allavena P., Rossi V., De Panfilis G., Passeri M. Lymphocytes of a patient with lymphoproliferative disease of large granular lymphocytes express high natural killer, ADCC, and LAK activity. Clin Immunol Immunopathol. 1990 Jul;56(1):9–21. doi: 10.1016/0090-1229(90)90164-l. [DOI] [PubMed] [Google Scholar]
- Scott C. S., Richards S. J. Classification of large granular lymphocyte (LGL) and NK-associated (NKa) disorders. Blood Rev. 1992 Dec;6(4):220–233. doi: 10.1016/0268-960x(92)90018-l. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Stegagno M., Wright K. A., Krane S. M., Amento E. P., Colvin R. B., Duquesnoy R. J., Kurnick J. T. Clonal dominance among T-lymphocyte infiltrates in arthritis. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1179–1183. doi: 10.1073/pnas.85.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniwaki M., Tagawa S., Nishigaki H., Horiike S., Misawa S., Shimazaki C., Maekawa T., Fujii H., Kitani T., Abe T. Chromosomal abnormalities define clonal proliferation in CD3- large granular lymphocyte leukemia. Am J Hematol. 1990 Jan;33(1):32–38. doi: 10.1002/ajh.2830330107. [DOI] [PubMed] [Google Scholar]
- Viard J. P., Bach J. F. Clonality in autoimmune diseases. Semin Hematol. 1991 Jan;28(1):57–65. [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Preisinger A. C., Willard H. F., Michelson A. M., Riggs A. D., Orkin S. H. Clonal analysis using recombinant DNA probes from the X-chromosome. Cancer Res. 1987 Sep 15;47(18):4806–4813. [PubMed] [Google Scholar]
- Wagner L., Base W., Wiesholzer M., Sexl V., Bognar H., Worman C. P. Detection of BLT substrate-specific proteases in individual human peripheral blood leucocytes and bone marrow cells. Application of the method to the classification of leukaemia. J Immunol Methods. 1991 Sep 13;142(2):147–155. doi: 10.1016/0022-1759(91)90101-k. [DOI] [PubMed] [Google Scholar]
- Yoshikai Y., Anatoniou D., Clark S. P., Yanagi Y., Sangster R., Van den Elsen P., Terhorst C., Mak T. W. Sequence and expression of transcripts of the human T-cell receptor beta-chain genes. Nature. 1984 Dec 6;312(5994):521–524. doi: 10.1038/312521a0. [DOI] [PubMed] [Google Scholar]
- van Kamp H., Jansen R., Willemze R., Fibbe W. E., Landegent J. E. Studies on clonality by PCR analysis of the PGK-1 gene. Nucleic Acids Res. 1991 May 25;19(10):2794–2794. doi: 10.1093/nar/19.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]





