Abstract
Objective To identify two-year trajectories of health-related quality of life (HRQOL) among children with newly diagnosed epilepsy, and evaluate key predictors of HRQOL trajectories. Methods This study is part of a prospective study of adherence and HRQOL outcomes in children with epilepsy. Caregivers completed an HRQOL questionnaire at one month post diagnosis and every three months thereafter for two years. Chart review and additional questionnaires were used to collect medical variables and seizure outcomes. Results Participants included 120 children with epilepsy and their caregiver. Unique trajectories for overall HRQOL and PedsQL™ subscales were identified and were predominantly stable. A total side effects score emerged as a consistent predictor of all HRQOL domains. Other variables (i.e., socioeconomic status, seizures, internalizing and externalizing problems) uniquely predicted HRQOL domains. Conclusions Medical and psychosocial interventions should be implemented soon after treatment initiation to target modifiable factors (e.g., side effects, anxiety symptoms), which could improve HRQOL.
Keywords: epilepsy, health-related quality of life, longitudinal research, pediatric, trajectories
Health-related quality of life (HRQOL), which encompasses the impact of an illness and associated treatment on an individual’s physical, emotional, social, and role functioning (Varni, Burwinkle, Seid, & Skarr, 2003), is compromised for many children with chronic medical conditions (Ingerski et al., 2010). Children with epilepsy, a neurological disorder affecting 1% of youth (Russ, Larson, & Halfon, 2012), are no exception, as they face many challenges including physical restrictions due to seizures (Dubow & Kelly, 2003), antiepileptic drug (AED) side effects (Morita, Glauser, & Modi, 2012), and comorbid conditions, such as attention-deficit/hyperactivity disorder and learning problems (Ott et al., 2003; Russ et al., 2012). While the primary goal of epilepsy treatment is no seizures, no side effects, and best quality of life (Glauser, 2002), scores on generic HRQOL measures remain compromised up to two years after diagnosis (Modi, Ingerski, Rausch, & Glauser, 2011; Wu, Follansbee-Junger, Rausch, & Modi, 2014). This suggests that patients continue to experience impaired physical, emotional, and role (e.g., academic performance, peer relationships) functioning despite receiving treatment with AEDs and attaining seizure control. There is a critical need to study this construct over the course of treatment, as adjustment to the illness and episodic medical events (e.g., seizures, medication changes) could negatively impact HRQOL.
HRQOL has gained increasing popularity as a relevant patient-reported outcome in several pediatric populations. While most studies have either examined the correlation between HRQOL and various demographic and disease characteristics (Gumidyala & Greenley, 2014; Ziaian et al., 2006) or the impact of an intervention on HRQOL (Rank et al., 2014; Wrotniak, Schall, Brault, Balmer, & Stallings, 2014), few have evaluated HRQOL changes over time. Among the observational research studies, most have evaluated mean differences over time (Murray et al., 2015; Neul, Minard, Currier, & Goldstein, 2013; Oberg et al., 2013; Sawicki et al., 2011), whereas few have examined long-term trajectories of HRQOL. To date, HRQOL research across pediatric conditions is equivocal with some studies showing stability over time and others demonstrating a pattern of improvement (Clarke, Eiser, & Skinner, 2008; Rodday, Terrin, & Parsons, 2013). Additionally, lower socioeconomic status has emerged as a relatively consistent predictor of HRQOL deficits (Devine et al., 2011; Tanzi, 2011), as well as changes in physical health status, such as presence of disease symptoms (Sawicki et al., 2011). While these findings can provide insight into how HRQOL may look over time, it is essential to examine HRQOL, as symptom presentation and treatment regimens are unique to each pediatric population, including epilepsy.
Potential risk factors for poor HRQOL have been examined among children with epilepsy and include various epilepsy-related (e.g., duration of epilepsy, seizure characteristics, AED regimen) and family (e.g., socioeconomic status, parental anxiety) factors (Ferro, 2014). Past cross-sectional research demonstrates that poor HRQOL in children with epilepsy is related to several disease factors, including seizure frequency and severity (Camfield, Breau, & Camfield, 2001; Cramer et al., 1999; Devinsky et al., 1999; Liu & Han, 2015; Williams et al., 2003), AED side effects (Benavente-Aguilar, Morales-Blanquez, Rubio, & Rey, 2004; Gilliam et al., 2004), medication adherence (Wu et al., 2014), and greater levels of parental anxiety (Williams et al., 2003). In longitudinal studies, side effects (Modi et al., 2011; Wu et al., 2014) and number of AEDs (Ferro et al., 2013) emerged as stronger predictors of HRQOL than seizure control. However, there is no known study that has examined these predictors together, using well-established and validated tools, in children with epilepsy.
Only one study has examined overall HRQOL trajectories in children with epilepsy (Ferro et al., 2013), which used a disease-specific instrument, the Quality of Life in Childhood Epilepsy scale. This study identified five HRQOL trajectories: low-increasing (4%), moderate-decreasing (12%), moderate-increasing (22%), high-increasing (32%), and high-stable (30%). These data highlight variability in HRQOL trajectories over two years and aid in the identification of at-risk subgroups that would benefit from intervention. Unfortunately, the disease-specific measure that was used is lengthy and unsuitable for clinical practice. Furthermore, specific subscales (e.g., Physical Restrictions, Social Interactions, and Depression) were not examined. Important next steps include classifying HRQOL trajectories for both overall and subscale scores using a clinically feasible scale, as well as identifying a comprehensive set of predictors for these trajectories.
Understanding the HRQOL trajectories for specific domains could identify patients who are experiencing problematic functioning, as well as indicate critical time points when HRQOL is compromised. Identifying modifiable and nonmodifiable predictors of trajectory group status over the course of epilepsy treatment could aid clinicians in understanding which patients are at risk for poor HRQOL and in providing timely interventions. Thus, the first objective of our study was to identify two-year HRQOL trajectories (overall and subscales) following epilepsy diagnosis and AED treatment initiation using a generic well-validated instrument, the PedsQL™. The second objective was to evaluate previously established (Ferro, 2014) demographic, medical, and psychosocial predictors of overall and subscale HRQOL trajectories. It was hypothesized that higher socioeconomic status, fewer number of AEDs and side effects, better seizure control (i.e., being in the low seizure probability trajectory), better medication adherence (i.e., being in the higher adherence trajectory groups), and better caregiver (i.e., fewer fears and concerns) and patient psychological (i.e., internalizing and externalizing symptoms) functioning would predict more favorable two-year HRQOL trajectories.
Method
Participants
Participants were recruited from the New Onset Seizure Disorder Clinic, which consists of a multidisciplinary care team (e.g., epileptologists, nurse practitioners, social workers, psychologists, pharmacists), on the day of diagnosis as part of a larger two-year longitudinal study examining adherence and outcomes in children with epilepsy. Inclusion criteria were: (1) 2–12 years old, (2) received an epilepsy diagnosis on the day of study recruitment, (3) prescribed only one AED, (4) no significant medical or developmental disorders that required daily medications, and (5) caregiver ability to read and speak English. There were 130 eligible families (children with epilepsy and a primary caregiver) that were approached for study participation. Of those initially eligible, five families declined participation owing to lack of interest or time. Of the 125 families that provided consent, one was withdrawn after study personnel learned the patient was actually ineligible owing to a pervasive developmental disorder, and four families never completed baseline measures. This resulted in 120 participants in this study cohort (92% of those initially eligible). Seventy-two percent of those initially eligible completed the final assessment (25-month follow-up). Participants who completed the study had a higher socioeconomic status (M = 55.8, SD = 20.0) compared with participants who did not complete the study [M = 44.9, SD = 19.1, t(118) = −2.735, p = .007]. There were no other demographic or medical group differences.
At baseline, the study cohort was 7.2 ± 2.9 years of age, 63% male, and 98% non-Hispanic. The majority (75%) was white, 17.5% were black, 5% were biracial, and 2.5% identified as other. The most frequent diagnosis was localization-related epilepsy (60% overall; 46.7% idiopathic, 8.3% cryptogenic, 5% symptomatic), and the remainder were diagnosed with generalized (25% overall; 19.2% idiopathic, 5% cryptogenic, less than 1% symptomatic) or unclassified (overall 15%; all idiopathic) epilepsy. Syndromes were present in 19.2% of the children (13.3% childhood/juvenile absence epilepsy, 5.8% benign rolandic epilepsy). Initial AED therapy was either carbamazepine (60%) or valproic acid (40%). Caregivers were predominately mothers (84.2%) who were married (63.3%). The mean family Duncan score (Stevens & Featherman, 1981) was 52.7 ± 20.3, which is associated with occupations such as property managers, physician’s assistants, mail carriers, sheriffs/law enforcement, and fire prevention occupations.
Measures
Health-Related Quality of Life
The caregiver-proxy report version of the PedsQL™ 4.0 (Varni, Seid, & Kurtin, 2001) is a generic measure of HRQOL and serves as the primary outcome measure of the current study. Scaled scores, ranging from 0 to 100 (higher scores reflect better HRQOL), are obtained for overall HRQOL and the following domains: physical, emotional, social, and school. The PedsQL™ has excellent psychometric properties and is a well-established measure (Palermo et al., 2008). Cronbach’s alphas for the current sample across all time points were: physical (α = .83–.92), emotional (α = .81–.87), social (α = .78–.90), and school (α = .76–.82).
Demographic and Medical Characteristics
A background information form was completed by the primary caregiver to obtain basic demographics. Socioeconomic status was measured using the Duncan score, which is an occupation-based measure (Stevens & Featherman, 1981). Scores were calculated for each family and range from 15 to 99, with higher scores reflecting higher socioeconomic status.
A medical chart review was conducted by trained coders using a standardized abstraction form to obtain seizure characteristics (i.e., type, etiology, seizure occurrence) and AED regimen.
The Pediatric Epilepsy Side Effects Questionnaire (PESQ; Morita et al., 2012) is a 19-item measure of AED side effects collected one month post diagnosis. Scores range from 0 to 100, with higher scores representing more side effects. The PESQ has strong internal consistency and test–retest reliability (Morita et al., 2012). Cronbach’s alphas for the current sample were: total (α = .96), cognitive (6 items; α = .94), motor (4 items; α = .94), behavioral (3 items; α = .92), general neurological (4 items; α = .75), and weight (2 items; α = .88).
Adherence trajectories (k = 4) have been previously identified for patients in this longitudinal study using latent class growth modeling (LCGM) of daily adherence data collected via electronic monitors (MEMS TrackCap®): severe early nonadherence, variable nonadherence, moderate nonadherence, and high adherence (Modi, Wu, Rausch, Peugh, & Glauser, 2014). These trajectories were established using mean daily adherence data for three-month periods over the course of two years. Similarly, previously established seizure trajectories (k = 2) identified the likelihood of patients having high or low seizure thresholds over the two-year study based on seizure presence or absence in three-month periods over the course of two years (Modi et al., 2014). These trajectories were used as a proxy for seizure occurrence.
Psychological Functioning
The parent-proxy version of the Behavior Assessment System for Children-2nd Edition (BASC-2; Reynolds & Kamphaus, 2004) is a reliable and valid measure of behavioral and emotional difficulties. Individual raw scores were compared with normative data for children of the same age, which resulted in standardized T-scores. The internalizing (e.g., anxiety: α = .86–.90; depressive symptoms: α = .84–.89) and externalizing (e.g., aggression: α = .76–.90; oppositional/conduct behaviors: α = .81–.87; hyperactivity: α = .48–.90) subscale scores were used for the current study.
The Parent Report of Psychosocial Care (Austin, Dunn, Huster, & Rose, 1998) addresses the concerns, needs for care, and satisfaction with care perceived by parents of children with new-onset seizures. The five-item Concerns and Fears subscale (α = .85), which assesses parental fears about the impact of the child’s seizures on functioning and outcomes, was used in the current study. Higher scores indicate a greater level of worry about the child’s seizures. Reliability and construct validity have been demonstrated (Austin et al., 1998).
Procedure
This study was approved by the hospital’s institutional review board. After consent/assent was obtained by trained research assistants, caregivers completed a demographics form and received an electronic monitor (MEMS TrackCap®) to measure adherence to the child’s prescribed AED. Disease-related variables were obtained through medical chart reviews. Subsequent study visits coincided with routine clinic appointments, which occurred one month following diagnosis and every three months thereafter for two years. During these follow-up visits, electronic monitors were downloaded and caregivers completed a battery of questionnaires. For purposes of the current study, the following caregiver-reported constructs (collected at one month following diagnosis) were examined as predictors of HRQOL: side effects, child externalizing and internalizing behaviors, and caregiver fears and concerns.
Statistical Analyses
PedsQL™ scale scores reported at a given clinic visit were used for all analyses. LCGM analyses were implemented in SAS (version 9.3; SAS Institute, Cary, NC) with the TRAJ procedure. LCGM is used when unobserved subgroups are suspected within a longitudinal data set. These unobserved groups are extracted based on response variable patterns over time within the data, and participants are assigned to one and only one of the subgroups using probabilistic estimation techniques. We used this approach to extract subgroups from longitudinal data from each of the PedsQL™ scales. All models used censored normal distributions for the outcome of interest. The number of groups was selected based on the Bayesian information criterion (BIC) statistic, model estimation convergence, and sufficient subgroups proportions for each outcome. In particular, quadratic models for change were analyzed starting with one subgroup, two subgroups, etc., up to seven subgroups within a given PedsQL™ scale. From the available models, each with a different number of groups, a model with any subgroup proportions < .09 was not considered further. The remaining models were compared with respect to BIC to determine the optimal number of subgroups. After obtaining the optimal number of groups based on quadratic trajectories for a specific PedsQL™ scale, we deleted any nonsignificant quadratic terms and re-fit our model. For the Physical subscale, this approach allowed us to retain the group with a quadratic trajectory component. However, for the School subscale, when attempting to re-fit a model with only one subgroup with a quadratic trajectory, our results for the subgroup trajectories were qualitatively different; thus, we chose to retain all our quadratic components for this scale to retain the qualitative meaning of these subgroup trajectories. Missing data were handled via maximum likelihood estimation, which is implemented with the TRAJ procedure.
After determining the appropriate number of groups and trajectory group shapes within the LCGM for a given PedsQL™ subscale, trajectory group status was then predicted with ordinal logistic regression, allowing for more than two subgroups when necessary (sometimes referred to as a “proportional odds” model; see Agresti, 2002 for more details) using the following set of predictors: adherence trajectory group status, seizure trajectory group status, side effects, socioeconomic status, number of AEDs, internalizing problems, externalizing problems, and caregiver worries. Semi-partial r2 values were calculated using R2max values from two models: (a) a model with all predictors, and (b) a model with the predictor of interest omitted (Cohen, Cohen, West, & Aiken, 2003). We used R2max values, as opposed to standard R2 measures because R2max corrects for the fact that standard R2 measures generally have an upper limit less than one for discrete variables. Thus, R2max allows for more straightforward interpretation of our (semi-)partial r2 (Nagelkerke, 1991). To balance Type I and Type II errors in this study (which contains multiple covariates and outcomes), statistical significance for these predictors was defined as p < 0.05.
Results
Determining HRQOL Trajectories
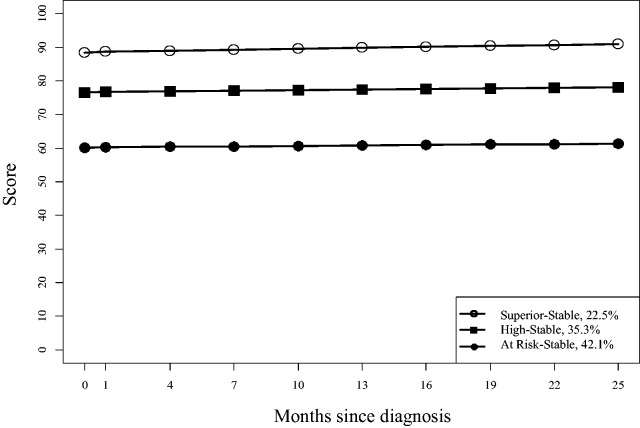
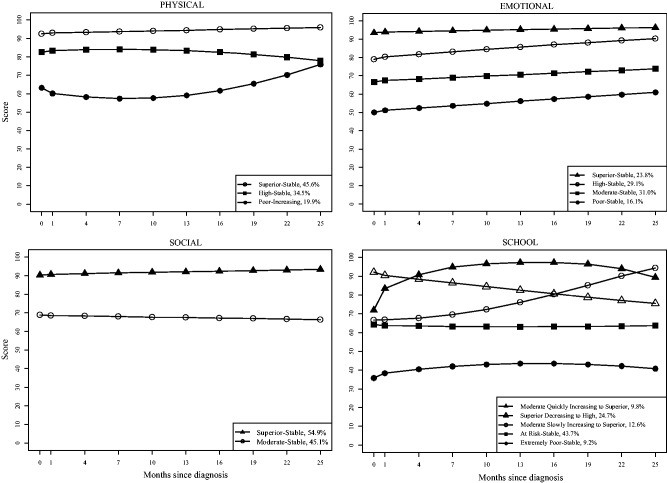
LCGM analyses (Table I) resulted in final subgroup growth model trajectories for overall HRQOL (k = 3), physical (k = 3), emotional (k = 4), social (k = 2), and school (k = 5). With the exception of the Physical and School subscales, all trajectories were stable and did not significantly change over time. The trajectories are depicted in Figures 1 and 2.
Table I.
Final Trajectory Models
| Group (%) | Parameter | Estimate (95% CI) | t | p |
|---|---|---|---|---|
| PedsQL™ physical | ||||
| Superior-stable (45.6) | Intercept | 98.3 (94.6 to 102.0) | 52.84 | <.001 |
| Time | 0.83 (0.09 to 1.57) | 2.22 | .03 | |
| High-stable (34.5) | Intercept | 86.5 (81.6 to 91.4) | 34.58 | <.001 |
| Time | −0.63 (−1.47 to 0.21) | −1.48 | .14 | |
| Poor-increasing (19.9) | Intercept | 62.9 (56.0 to 69.8) | 18.01 | <.001 |
| Time | −3.58 (−7.15 to −0.01) | −1.97 | .049 | |
| Time2 | 0.559 (0.165 to 0.953) | 2.78 | .006 | |
| PedsQL™ emotional | ||||
| Superior-stable (23.8) | Intercept | 99.5 (93.6 to 105.4) | 33.01 | <.001 |
| Time | 0.74 (−0.32 to 1.80) | 1.37 | .17 | |
| High-stable (29.1) | Intercept | 79.7 (74.2 to 85.2) | 28.59 | <.001 |
| Time | 1.60 (0.72 to 2.48) | 3.59 | <.001 | |
| Moderate-stable (31.0) | Intercept | 66.7 (61.4 to 72.0) | 25.02 | <.001 |
| Time | 0.83 (−0.01 to 1.67) | 1.94 | .053 | |
| Poor-stable (16.1) | Intercept | 50.0 (43.7 to 56.3) | 15.88 | <.001 |
| Time | 1.22 (−0.07 to 2.51) | 1.85 | .065 | |
| PedsQL™ social | ||||
| Superior-stable (54.9) | Intercept | 97.1 (93.0 to 101.2) | 46.95 | <.001 |
| Time | 0.66 (−0.14 to 1.46) | 1.61 | .11 | |
| Moderate-stable (45.1) | Intercept | 69.4 (65.3 to 73.5) | 33.05 | <.001 |
| Time | −0.29 (−1.09 to 0.51) | −0.71 | .48 | |
| PedsQL™ school | ||||
| Moderate quickly increasing to superior (9.8) | Intercept | 72.1 (58.4 to 85.8) | 10.35 | <.001 |
| Time | 13.98 (6.73 to 21.23) | 3.78 | <.001 | |
| Time2 | −1.300 (−2.027 to −0.573) | −3.50 | <.001 | |
| Moderate slowly increasing to superior (12.6) | Intercept | 66.8 (55.5 to 78.1) | 11.63 | <.001 |
| Time | −0.45 (−6.51 to 5.61) | −0.15 | .88 | |
| Time2 | 0.476 (−0.232 to 1.184) | 1.32 | .19 | |
| Superior decreasing to high (24.7) | Intercept | 97.1 (84.6 to 109.6) | 15.25 | <.001 |
| Time | −3.05 (−7.28 to 1.18) | −1.41 | .16 | |
| Time2 | 0.078 (−0.349 to 0.505) | 0.36 | .72 | |
| At risk-stable (43.7) | Intercept | 64.2 (58.4 to 70.0) | 21.73 | <.001 |
| Time | −0.41 (−2.92 to 2.10) | −0.32 | .75 | |
| Time2 | 0.040 (−0.227 to 0.307) | 0.29 | .77 | |
| Extremely poor-stable (9.2) | Intercept | 35.8 (26.5 to 45.1) | 7.54 | <.001 |
| Time | 2.77 (−1.86 to 7.40) | 1.17 | .24 | |
| Time2 | −0.248 (−0.754 to 0.258) | −0.96 | .34 | |
| PedsQL™ overall | ||||
| Superior-stable (22.5) | Intercept | 60.1 (57.1 to 63.1) | 39.40 | <.001 |
| Time | 0.14 (−0.47 to 0.75) | 0.45 | .66 | |
| High-stable (35.3) | Intercept | 76.6 (73.8 to 79.4) | 54.48 | <.001 |
| Time | 0.17 (−0.32 to 0.66) | 0.68 | .50 | |
| At risk-stable (42.1) | Intercept | 89.3 (86.9 to 91.7) | 74.2 | <.001 |
| Time | 0.36 (−0.07 to 0.79) | 1.67 | .095 | |
Figure 1.
Overall PedsQL™ trajectories.
Figure 2.
PedsQL™ subscale trajectories.
Trajectories were defined based on two components, which were the intercept (mean) and slope of HRQOL over time. Trajectories were labeled using established normative data for the PedsQL™ (Varni et al., 2003), as well as minimal clinically important difference (MCID) scores. These values are statistically derived and suggest a score threshold that represents a meaningful clinical improvement (Beaton, Boers, & Wells, 2002). For the first component (intercept/mean), a trajectory was labeled as “High” if the group mean was similar to that of the normative (healthy) sample. The trajectory was labeled as “Superior” if the mean was greater than one MCID above “High.” Conversely, the trajectory was labeled “Moderate,” “At Risk,” “Poor,” or “Extremely Poor” if it was one, two, three, or greater than four MCIDs below the mean of the normative sample, respectively. For the second component (slope), the trajectory could either be labeled as “Stable” (indicating no significant change), or “Increasing/Decreasing” (indicating significant improvement or decline over time).
Predictors of HRQOL Trajectories
The results for the predictors of interest simultaneously predicting group status for each of the PedsQL™ LCGMs are shown in Table II. Adherence group status, caregiver worry, and number of AEDs were not statistically significant predictors of any of the five HRQOL scale LCGMs. Better physical LCGM subgroup status was predicted by fewer AED side effects, low probability seizure group, and fewer internalizing problems. Better emotional subgroup status was predicted by fewer side effects and internalizing problems. Better social subgroup status was predicted by higher socioeconomic status, fewer side effects, and fewer externalizing problems. Better school subgroup status was predicted by low probability seizure group and fewer externalizing problems. Better overall subgroup status was predicted by higher socioeconomic status, fewer side effects, and fewer internalizing problems.
Table II.
Results for PedsQL™ Scales Requiring Ordinal Logistic Regression Model
| Predictors | Overall |
Physical |
Emotional |
Social |
School |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| χ2 | p | OR(95% CI) | χ2 | p | OR(95% CI) | χ2 | p | OR(95% CI) | χ2 | p | OR(95% CI) | χ2 | p | OR(95% CI) | |
| Socioeconomic status | 5.05 | .03 | 1.04 (1.00,1.07) | 0.93 | .33 | – | 2.16 | .14 | – | 7.11 | <.01 | 1.06 (1.02,1.11) | 0.49 | .48 | – |
| Number of AEDs | 3.55 | .059 | – | 1.15 | .28 | – | 1.61 | .20 | – | 3.84 | .050 | – | 060 | .44 | – |
| Side effects | 13.65 | <.001 | 0.88 (0.83,0.94) | 11.97 | <.001 | 0.92 (0.88,0.95) | 5.72 | .02 | 0.93(0.90,0.97) | 4.88 | .03 | 0.89 (0.80,0.99) | 13.66 | <.001 | 0.90 (0.86,0.95) |
| Seizures | 2.16 | .14 | – | 4.22 | .04 | 4.03 (1.07,15.26) | 2.22 | .14 | – | 0.003 | .95 | – | 0.43 | .51 | – |
| Adherence | 3.18 | .22 | – | 7.23 | .065 | – | 0.82 | .84 | – | 3.50 | .32 | – | 3.02 | .39 | – |
| Internalizing problems | 5.91 | .02 | 0.92 (0.86,0.98) | 6.45 | .01 | 0.92 (0.87,0.98) | 10.42 | <.01 | 0.91(0.86,0.96) | 1.84 | .17 | – | 1.12 | .29 | – |
| Externalizing problems | 0.04 | .84 | – | 0.49 | .48 | – | 0.11 | .74 | – | 5.23 | .02 | 0.90 (0.83,0.99) | 6.78 | <.01 | 0.94 (0.89,0.98) |
| Caregiver worry | 1.17 | .28 | – | 1.14 | .29 | – | 0.20 | .65 | – | 1.71 | .19 | – | 0.39 | .53 | – |
Note. Bolded p values are less than .05; partial r2 values for the Overall scale predictors were .04 for socioeconomic status, .12 for side effects, and .05 for internalizing problems; partial r2 values for the Physical scale predictors were .11 for side effects, .08 for seizure group, and .06 for internalizing problems; partial r2 values for the Emotional scale predictors were .08 for side effects and .09 for internalizing problems; partial r2 values for the Social scale predictors were .08 for socioeconomic status, .05 for side effects, and .05 for externalizing problems; partial r2 values for the School scale predictors were .15 for seizure group and .05 for externalizing problems.
Discussion
This study identified two-year trajectories of overall and subscale scores of the PedsQL™ among children with newly diagnosed epilepsy. All of the trajectories were statistically stable (with the exception of physical and school functioning), suggesting that emotional, social, and overall HRQOL did not change significantly during the two years following AED treatment initiation. Notably, 42% of the population had at-risk overall PedsQL™ scores, indicating significant impairments in functioning for two years. This is surprising, given that a majority of patients likely have improved seizure control and decreased side effects over time. In addition, these findings point to the impact of undesirable AED side effects on HRQOL, as this was a consistent predictor across subscales. Several other nonmodifiable (e.g., socioeconomic status) and modifiable (e.g., seizure control, emotional and behavior problems) factors predicted the HRQOL course. These findings have important implications for interventions aimed at improving HRQOL across pediatric populations, which are discussed later. Although critical periods for compromised HRQOL were not identified, it may be helpful for healthcare providers to proactively address impaired HRQOL soon after diagnosis, given the stability of HRQOL over two years.
While 80% of the participants reported superior- or high-stable physical functioning, 20% of the children experienced poor physical functioning initially, which gradually improved over two years. Membership in the poor-increasing group was predicted by more seizures, side effects, and emotional problems. Children with uncontrolled seizures may be placed under strict limitations for safety reasons, which could interfere with physical activities (e.g. running, chores), particularly in the period immediately following diagnosis. In fact, there are data to suggest that families of children with epilepsy shift their activities, which results in significantly more time spent at home (Painter, Rausch, & Modi, 2014). To assist with the adjustment to epilepsy diagnosis and treatment, providers should normalize fear associated with physical activity, provide education about safety strategies (e.g., supervised baths or shower), and encourage children with epilepsy to continue typical activities. Further, effective management of side effects is essential, as some (e.g., fatigue, drowsiness) may interfere with children with epilepsy’s ability to engage in physical activities (Ortinski & Meador, 2004). Assessment of side effects with validated tools (Morita et al., 2012) can determine whether side effects are tolerable or intolerable. Depending on the outcome, a change in AED or adjunctive therapies may be helpful for managing side effects affecting physical functioning.
With regard to emotional HRQOL, approximately 50% of the participants fell into the fair-stable or poor-stable trajectories, indicating that many children with epilepsy experience poorer emotional functioning compared with children without a chronic medical condition. Poor emotional functioning was predicted by side effects and anxious and depressive symptoms, which is consistent with past research (Reilly et al., 2015; Sano et al., 2014). Some AEDs (e.g., levetiracetam) can lead to emotion dysregulation. Additionally, it is not surprising that anxiety and depression are significant predictors owing to some overlapping items on this subscale. A model of interdisciplinary care, including medical providers, psychologists, social workers, and pharmacists, has been developed for youth with epilepsy (Guilfoyle, Follansbee-Junger, & Modi, 2013). This highlights that evidence-based treatment, including cognitive-behavioral therapy (CBT), can be incorporated into routine medical care, or a referral for outpatient services can be initiated. In fact, recent studies have demonstrated the positive impact of CBT for anxiety in children with epilepsy (Blocher, Fujikawa, Sung, Jackson, & Jones, 2013; Jones, 2014). Additionally, vitamin B6 is as a potential ameliorator of emotional side effects and is a relatively safe intervention option (Alsaadi, El Hammasi, & Shahrour, 2015; Chez, Murescan, & Kerschner, 2005; Major, Greenberg, Khan, & Thiele, 2008; Miller, 2002).
Over half of the participants experienced consistently superior social functioning during the two years following epilepsy diagnosis and treatment initiation, while the remainder had fair-stable functioning. Group status was predicted by socioeconomic status, side effects, and behavior problems. It is possible that children with epilepsy who are from lower socioeconomic status backgrounds may lack opportunities to participate in extracurricular activities that promote positive peer engagement. Children with epilepsy who experience side effects may not feel well enough to engage in social or extracurricular activities. Additionally, some AEDs can cause behavioral disturbances, which may impact their social relationships. Specifically, children who display aggressive or hyperactive behavior have more difficulties making and keeping friends (Ladd, 2006). Social skills training or school-based bullying interventions can be implemented to improve peer interactions for children with epilepsy and co-occurring peer relational issues.
Children with epilepsy had varying degrees of school-related functioning during the two year period following diagnosis and treatment initiation, which is likely related to a multitude of influencing internal and external factors (e.g., learning disabilities, attention deficit/hyperactivity disorder) and intervention efforts (e.g., implementation of school accommodation plans). Approximately 25% of the sample maintained high or superior functioning, while another 25% experienced improved school HRQOL across time. Of more concern is the fact that half of the sample experienced significant and persistent school difficulties, which were predicted by greater number of AED side effects and behavior problems. Typical AED side effects include fatigue and memory difficulties, which can negatively impact academic performance. Additionally, side effects can contribute to school absences owing to increased clinic visits or not feeling well. Neuropsychological evaluation can be useful for determining the impact of epilepsy and treatment on school performance, as well as providing educators with recommendations to maximize the student’s success. Additionally, behavior plans can be implemented to reduce these difficulties in the school setting. Social workers and other healthcare providers can assist families with accessing school resources, such as an individualized education plan (IEP) or accommodations through Section 504 of the United States Rehabilitation Act of 1973. It is likely that both testing data and the way in which school accommodations are implemented play a critical role in the course of school HRQOL. Anecdotally, our patients often report that proper implementation of 504 plans and IEPs has a tremendous positive impact on their children, especially in their academic work.
Three stable trajectories of overall HRQOL were identified and were predicted by socioeconomic status, side effects, and anxious/depressive symptoms. Forty-two percent of the patients experienced poor overall HRQOL after diagnosis and the following two years, while the remainder had HRQOL similar to or greater than the normative average. Compared with the disease-specific work done by Ferro and colleagues (2013), our data suggest that HRQOL impairments may be more widespread and persistent over time than previously thought. For the subset of patients who experience persistently poor generic HRQOL following diagnosis and treatment initiation, there may be interventions to help with these predictors. While specific interventions for side effects and emotional problems have been described above, social work can also provide resources and support for families from low socioeconomic status backgrounds.
Findings from the current study should be considered in the context of limitations. First, HRQOL was assessed using a caregiver-proxy report only owing to the young age of the sample. Although past research with the PedsQL™ documents excellent concordance between caregiver and child report (Haneef et al., 2010), there may be unique predictors of the child’s perspective of their own HRQOL. Second, HRQOL was assessed after AED initiation, which does not capture changes from pre-medication functioning. Third, the relationship between internalizing symptoms and emotional functioning may be a result of shared variance, given overlap in symptoms assessed. Fourth, there was attrition across the course of the two-year study period. This may limit generalizability of results, particularly as families from lower socioeconomic status backgrounds were more likely to drop out of the study prematurely. Fifth, some predictors were measured on an ongoing basis (i.e., seizures, adherence), while others were only assessed at baseline. Having only a single assessment of some constructs may have precluded us from detecting significant results. Finally, reliability of the chart review data could not be assessed.
Overall, this study demonstrates global HRQOL deficits that are stable over time in children with epilepsy beginning at treatment initiation. This is one of the first studies in epilepsy and the larger pediatric literature to use sophisticated trajectory analyses to identify specific patient groups based on their HRQOL scores across multiple time points. The ability to identify patients who experience persistent deficits or declines in HRQOL over time has important implications for future intervention research. Further, use of a comprehensive set of predictors that is derived from reliable and valid measurement tools allowed us to identify the critical modifiable factors that can be addressed through psychosocial and medical interventions. These include side effects, internalizing and externalizing problems, and parental fears/concerns. An important next step for future research in this area is to evaluate whether interventions to improve predictors (e.g., side effects, mood difficulties, behavior problems) result in better HRQOL over time. Additionally, future studies should evaluate the utility of examining disease-specific assessment, which may be more sensitive in detecting HRQOL change over time. Given that existing epilepsy-specific HRQOL measures are lengthy, development and validation of streamlined epilepsy-specific questionnaires (e.g., PedsQL-Epilepsy Module, development is under way) that may be more suitable for clinical practice is warranted.
Funding
This work was supported by the National Institutes of Health grant (K23HD057333 to A.C.M.) and a training grant from the National Institutes of Health (T32HD068223 to K.A.L. and R.R.R.) The authors would like to thank the families from the New Onset Seizure Clinic who participated in this two-year longitudinal study. They would also like to thank Julie Field and the research staff who were involved in data collection.
Conflicts of interest: None declared.
References
- Agresti A. (2002). Categorical data analysis (2nd ed.). Hoboken: Wiley. [Google Scholar]
- Alsaadi T., El Hammasi K., Shahrour T. M. (2015). Does pyridoxine control behavioral symptoms in adult patients treated with levetiracetam? Case series from UAE. Epilepsy and Behavior Case Reports , 4, 94–95. doi: 10.1016/j.ebcr.2015.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin J. K., Dunn D., Huster G., Rose D. (1998). Development of scales to measure psychosocial care needs of children with seizures and their parents. 1. Journal of Neuroscience Nursing , 30, 155–160. doi:10.1097/01376517-1998060000-00002 [DOI] [PubMed] [Google Scholar]
- Beaton D. E., Boers M., Wells G. A. (2002). Many faces of the minimal clinically important difference (MCID): A literature review and directions for future research. Current Opinion in Rheumatology , 14, 109–114. doi: 10.1097/00002281-200203000-00006 [DOI] [PubMed] [Google Scholar]
- Benavente-Aguilar I., Morales-Blanquez C., Rubio E. A., Rey J. M. (2004). Quality of life of adolescents suffering from epilepsy living in the community. Journal of Paediatrics and Child Health , 40, 110–113. doi: 10.1111/j.1365-2214.2004.435_1_1.x [DOI] [PubMed] [Google Scholar]
- Blocher J. B., Fujikawa M., Sung C., Jackson D. C., Jones J. E. (2013). Computer-assisted cognitive behavioral therapy for children with epilepsy and anxiety: A pilot study. Epilepsy & Behavior , 27, 70–76. doi:10.1016/j.yebeh.2012.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camfield C., Breau L., Camfield P. (2001). Impact of pediatric epilepsy on the family: A new scale for clinical and research use. Epilepsia , 42, 104–112. doi: 10.1046/j.1528-1157.2001.081420.x [DOI] [PubMed] [Google Scholar]
- Chez M. G., Murescan M., Kerschner S. (2005). Retrospective review of the effect of vitamin b6 (pyridoxine) as add-on therapy for behavioral problems associated with levetiracetam (keppra) therapy. Epilepsia, 46, 146. doi:10.1111/j.1528-1167.2005.460801_13.x [Google Scholar]
- Clarke S., Eiser C., Skinner R. (2008). Health-related quality of life in survivors of BMT for paediatric malignancy: A systematic review of the literature. Bone Marrow Transplantation , 42, 73–82. [DOI] [PubMed] [Google Scholar]
- Cohen J., Cohen P., West S., Aiken L. (2003). Applied multiple regression/correlation analysis for the behavioral sciences (3rd ed.). Mahwah, NJ: L. Erlbaum Associates. [Google Scholar]
- Cramer J. A., Westbrook L. E., Devinsky O., Perrine K., Glassman M. B., Camfield C. (1999). Development of the quality of life in epilepsy inventory for adolescents: The QOLIE-AD-48. Epilepsia , 40, 1114–1121. doi: 10.1111/j.1528-1157.1999.tb00828.x [DOI] [PubMed] [Google Scholar]
- Devine K. A., Reed-Knight B., Loiselle K. A., Simons L. E., Mee L. L., Blount R. L. (2011). Predictors of long-term health-related quality of life in adolescent solid organ transplant recipients. Journal of Pediatric Psychology , 36, 891–901. doi: 10.1093/jpepsy/jsr007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O., Westbrook L., Cramer J., Glassman M., Perrine K., Camfield C. (1999). Risk factors for poor health-related quality of life in adolescents with epilepsy. Epilepsia , 40, 1715–1720. doi: 10.1111/j.1528-1157. 1999.tb01588.x [DOI] [PubMed] [Google Scholar]
- Dubow J. S., Kelly J. P. (2003). Epilepsy in sports and recreation. Sports Medicine , 33, 499–516. doi: 10.2165/00007256-200333070-00003 [DOI] [PubMed] [Google Scholar]
- Ferro M. A. (2014). Risk factors for health-related quality of life in children with epilepsy: A meta-analysis. Epilepsia , 55, 1722–1731. doi:10.1111/epi.12772 [DOI] [PubMed] [Google Scholar]
- Ferro M. A., Camfield C. S., Levin S. D., Smith M. L., Wiebe S., Zou G., Speechley K. N. (2013). Trajectories of health-related quality of life in children with epilepsy: A cohort study. Epilepsia , 54, 1889–1897. doi:10.1111/epi.12388 [DOI] [PubMed] [Google Scholar]
- Gilliam F. G., Fessler A. J., Baker G., Vahle V., Carter J., Attarian H. (2004). Systematic screening allows reduction of adverse antiepileptic drug effects: A randomized trial. Neurology , 62, 23–27. doi: 10.1212/wnl.62.1.23 [DOI] [PubMed] [Google Scholar]
- Glauser T. A. (2002). Advancing the medical management of epilepsy: Disease modification and pharmacogenetics. Journal of Child Neurology , 17(Suppl 1), S85–S93. doi: 10.1177/08830738020170011301 [DOI] [PubMed] [Google Scholar]
- Guilfoyle S. M., Follansbee-Junger K., Modi A. C. (2013). Development and preliminary implementation of a psychosocial service into standard medical care for pediatric epilepsy. Clinical Practice in Pediatric Psychology , 1, 276–288. doi:10.1037/cpp0000031 [Google Scholar]
- Gumidyala A. P., Greenley R. N. (2014). Correlates of health-related quality of life in pediatric inflammatory bowel disease: A cumulative risk model approach. Journal of Pediatric Psychology , 39, 55–64. doi: 10.1093/jpepsy/jst073 [DOI] [PubMed] [Google Scholar]
- Haneef Z., Grant M. L., Valencia I., Hobdell E. F., Kothare S. V., Legido A., Khurana D. (2010). Correlation between child and parental perceptions of health-related quality of life in epilepsy using the PedsQL.v4.0 measurement model. Epileptic Disorders , 12, 275–282. doi:10.1684/epd.2010.0344 [DOI] [PubMed] [Google Scholar]
- Ingerski L. M., Modi A. C., Hood K. K., Pai A. L., Zeller M., Piazza-Waggoner C., Driscoll K. A., Rothenberg M. E., Franciosi J., Hommel K. A. (2010). Health-related quality of life across pediatric chronic conditions. Journal of Pediatrics , 156, 639–644. doi: 10.1016/j.jpeds.2009.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. E. (2014). Treating anxiety disorders in children and adolescents with epilepsy: What do we know? Epilepsy and Behavior , 39, 137–142. doi:10.1016/j.yebeh. 2014.06.021 [DOI] [PubMed] [Google Scholar]
- Ladd G. W. (2006). Peer rejection, aggressive or withdrawn behavior, and psychological maladjustment from ages 5 to 12: An examination of four predictive models. Child Development , 77, 822–846. doi:10.1111/j.1467-8624.2006.00905.x [DOI] [PubMed] [Google Scholar]
- Liu X., Han Q. (2015). Risk factors on health-related quality of life in children with epilepsy. Clinical Pediatrics, 54, 1334–1338. doi: 10.1177/0009922815580405 [DOI] [PubMed] [Google Scholar]
- Major P., Greenberg E., Khan A., Thiele E. A. (2008). Pyridoxine supplementation for the treatment of levetiracetam-induced behavior side effects in children: Preliminary results. Epilepsy and Behavior , 13, 557–559. doi:10.1016/j.yebeh.2008.07.004 [DOI] [PubMed] [Google Scholar]
- Miller G. (2002). Pyridoxine ameliorates adverse behavioral effects of levetiracetam in children. Epilepsia , 43(Suppl 7), 62 Retrieved from http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1528-1167 [Google Scholar]
- Modi A. C., Ingerski L. M., Rausch J. R., Glauser T. A. (2011). Treatment factors affecting longitudinal quality of life in bew onset pediatric epilepsy. Journal of Pediatric Psychology , 36, 466–475. doi:10.1093/jpepsy/jsq114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi A. C., Wu Y. P., Rausch J. R., Peugh J. L., Glauser T. A. (2014). Antiepileptic drug nonadherence predicts pediatric epilepsy seizure outcomes. Neurology , 83, 2085–2090. doi:10.1212/wnl.0000000000001023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita D. A., Glauser T. A., Modi A. C. (2012). Development and validation of the Pediatric Epilepsy Side Effects Questionnaire. Neurology , 79, 1252–1258. doi:10.1212/WNL.0b013e3182635b87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C. B., Holmbeck G. N., Ros A. M., Flores D. M., Mir S. A., Varni J. W. (2015). A longitudinal examination of health-related quality of life in children and adolescents with spina bifida. Journal of Pediatric Psychology , 40, 419–430. doi: 10.1093/jpepsy/jsu098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelkerke N. A. (1991). A note on a general definition of the coefficient of determination. Biometrika , 78, 691–692. doi:10.1093/biomet/78.3.691 [Google Scholar]
- Neul S. K., Minard C. G., Currier H., Goldstein S. L. (2013). Health-related quality of life functioning over a 2-year period in children with end-stage renal disease. Pediatric Nephrology , 28, 285–293. doi: 10.1007/s00467-012-2313-7 [DOI] [PubMed] [Google Scholar]
- Oberg J. A., Bender J. G., Morris E., Harrison L., Basch C. E., Garvin J. H., Sands S. A., Cairo M. (2013). Pediatric allo-SCT for malignant and non-malignant diseases: Impact on health-related quality of life outcomes. Bone Marrow Transplantation , 48, 787–793. doi: 10.1038/bmt. 2012.217 [DOI] [PubMed] [Google Scholar]
- Ortinski P., Meador K. J. (2004). Cognitive side effects of antiepileptic drugs. Epilepsy & Behavior , 5(Suppl 1), S60–S65. doi:10.1016/j.yebeh.2003.11.008 [DOI] [PubMed] [Google Scholar]
- Ott D., Siddarth P., Gurbani S., Koh S., Tournay A., Shields W. D., Caplan R. (2003). Behavioral disorders in pediatric epilepsy: Unmet psychiatric need. Epilepsia , 44, 591–597. doi:10.1046/j.1528-1157.2003.25002.x [DOI] [PubMed] [Google Scholar]
- Painter E., Rausch J. R., Modi A. C. (2014). Changes in daily activity patterns of caregivers of children with newly diagnosed epilepsy: A case-controlled design. Epilepsy & Behavior , 31, 1–6. doi:10.1016/j.yebeh.2013.11.001 [DOI] [PubMed] [Google Scholar]
- Palermo T. M., Long A. C., Lewandowski A. S., Drotar D., Quittner A. L., Walker L. S. (2008). Evidence-based assessment of health-related quality of life and functional impairment in pediatric psychology. Journal of Pediatric Psychology , 33, 983–996. doi:10.1093/jpepsy/jsn038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank M., Wilks D. C., Foley L., Jiang Y., Langhof H., Siegrist M., Halle M. (2014). Health-related quality of life and physical activity in children and adolescents 2 years after an inpatient weight-loss program. Journal of Pediatrics , 165, 732–737. doi:10.1016/j.jpeds.2014.05.045 [DOI] [PubMed] [Google Scholar]
- Reilly C., Atkinson P., Das K. B., Chin R. F., Aylett S. E., Burch V., Gillberg C., Scott R. C., Neville B. G. (2015). Factors associated with quality of life in active childhood epilepsy: A population-based study. European Journal of Paediatric Neurology, 19, 308–313. doi:10.1016/j.ejpn.2014.12.022 [DOI] [PubMed] [Google Scholar]
- Reynolds C. R., Kamphaus R. W. (2004). Behavior assessment system for children (2nd ed.). Circle Pines, MN: American Guidance Service, Inc. [Google Scholar]
- Rodday A. M., Terrin N., Parsons S. K. (2013). Measuring global health-related quality of life in children undergoing hematopoietic stem cell transplant: A longitudinal study. Health and Quality of Life Outcomes , 11, 26 doi:10.1186/1477-7525-11-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ S. A., Larson K., Halfon N. (2012). A national profile of childhood epilepsy and seizure disorder. Pediatrics , 129, 256–264. doi:10.1542/peds.2010-1371 [DOI] [PubMed] [Google Scholar]
- Sano F., Kanemura H., Tando T., Goto Y., Hosaka H., Sugita K., Aihara M. (2014). Depressive symptoms contribute to quality of life in children with epilepsy. European Journal of Paediatric Neurology , 18, 774–779. doi:10.1016/j.ejpn.201.08.002 [DOI] [PubMed] [Google Scholar]
- Sawicki G. S., Rasouliyan L., McMullen A. H., Wagener J. S., McColley S. A., Pasta D. J., Quittner A. L. (2011). Longitudinal assessment of health-related quality of life in an observational cohort of patients with cystic fibrosis. Pediatric Pulmonology , 46, 36–44. doi:10.1002/ppul.21325 [DOI] [PubMed] [Google Scholar]
- Stevens G., Featherman D. L. (1981). A revised socioeconomic index of occupational status. Social Science Research , 10, 364–395. doi:10.1016/0049-089x (81) 90011-9 [Google Scholar]
- Tanzi E. M. (2011). Health-related quality of life of hematopoietic stem cell transplant childhood survivors: State of the science. Journal of Pediatric Oncology Nursing , 28, 191–202. doi:10.1177/1043454211408100 [DOI] [PubMed] [Google Scholar]
- Varni J. W., Burwinkle T. M., Seid M., Skarr D. (2003). The PedsQL 4.0 as a pediatric population health measure: Feasibility, reliability, and validity. Ambulatory Pediatrics , 3, 329–341. doi:10.1367/1539-4409(2003)003 <0329:tpaapp > 2.0.co;2 [DOI] [PubMed] [Google Scholar]
- Varni J. W., Seid M., Kurtin P. S. (2001). PedsQL 4.0: Reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Medical Care , 39, 800–812. doi:10.1097/00005650-200108000-00006 [DOI] [PubMed] [Google Scholar]
- Williams J., Steel C., Sharp G. B., DelosReyes E., Phillips T., Bates S., Lange B., Griebel M. L. (2003). Parental anxiety and quality of life in children with epilepsy. Epilepsy and Behavior , 4, 483–486. doi:10.1016/s1525-5050 (03)00159-8 [DOI] [PubMed] [Google Scholar]
- Wrotniak B. H., Schall J. I., Brault M. E., Balmer D. F., Stallings V. A. (2014). Health-related quality of life in children with sickle cell disease using the Child Health Questionnaire. Journal of Pediatric Health Care , 28, 14–22. doi:10.1016/j.pedhc.2012.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. P., Follansbee-Junger K., Rausch J., Modi A. (2014). Parent and family stress factors predict health-related quality in pediatric patients with new-onset epilepsy. Epilepsia , 55, 866–877. doi:10.1111/epi.12586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziaian T., Sawyer M. G., Reynolds K. E., Carbone J. A., Clark J. J., Baghurst P. A., Couper J. J., Kennedy D., Martin A. J., Staugas R. E., French D. J. (2006). Treatment burden and health-related quality of life of children with diabetes, cystic fibrosis and asthma. Journal of Paediatrics and Child Health , 42, 596–600. doi:10.1111/j.1440-1754.2006.00943.x [DOI] [PubMed] [Google Scholar]