Abstract
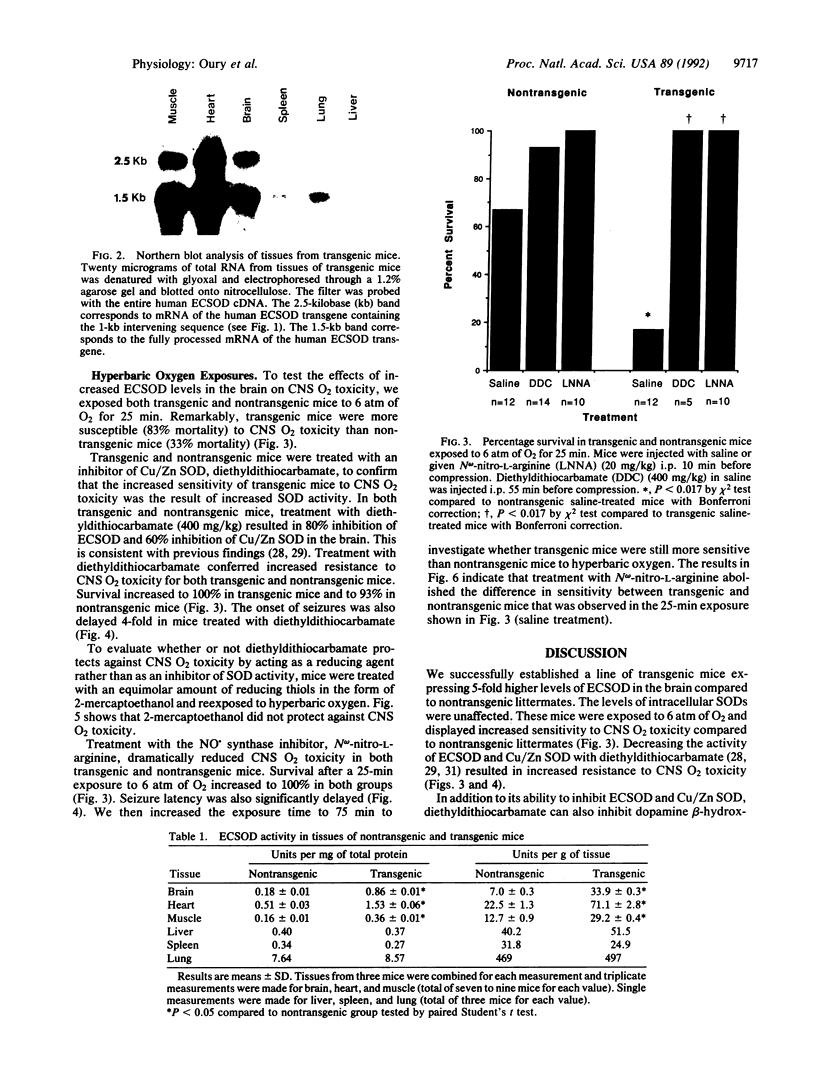
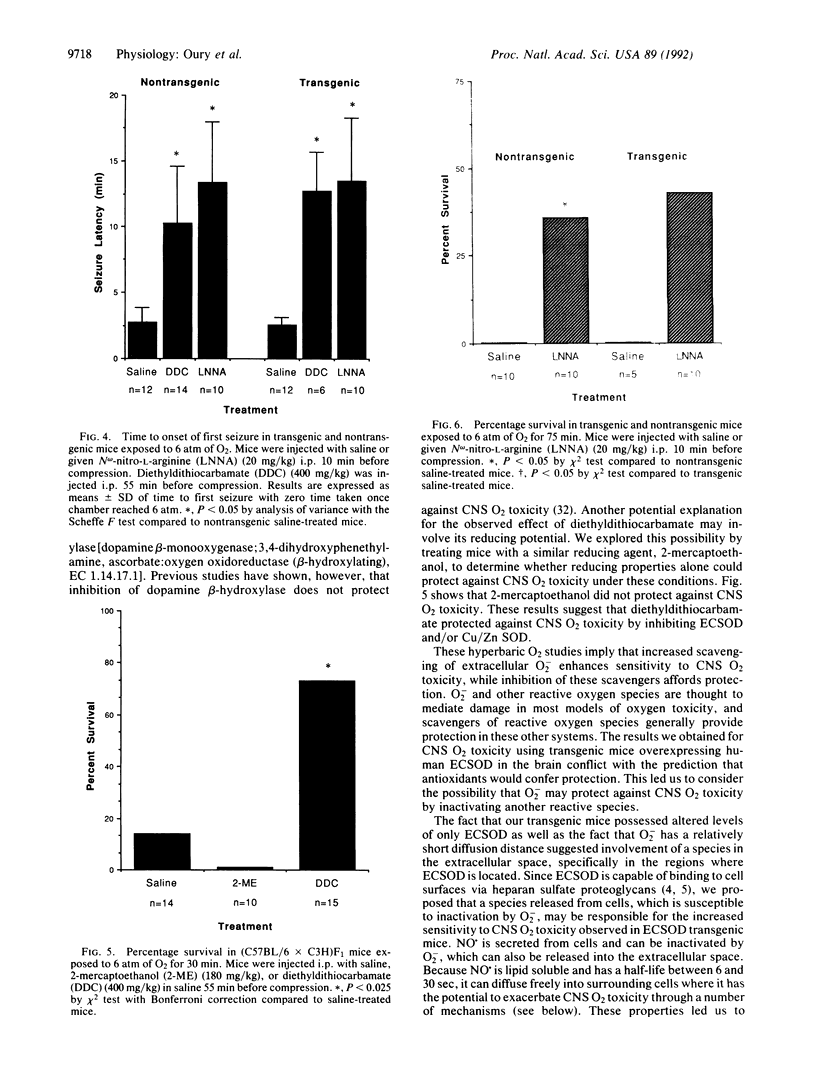
Although reactive O2 species appear to participate in central nervous system (CNS) O2 toxicity, the exact roles of different reactive O2 species are undetermined. To study the contribution of extracellular superoxide anion (O2-) to CNS O2 toxicity we constructed transgenic mice overexpressing human extracellular superoxide dismutase (ECSOD; superoxide:superoxide oxidoreductase, EC 1.15.1.1) in the brain. Remarkably, when exposed to 6 atm (1 atm = 101.3 kPA) of hyperbaric oxygen for 25 min, transgenic mice demonstrated higher mortality (83%) than nontransgenic litter-mates (33%; P < 0.017). Pretreatment with diethyldithiocarbamate, which inhibits both ECSOD and Cu/Zn superoxide dismutase (Cu/Zn SOD) activity, increased resistance to CNS O2 toxicity, in terms of both survival (100% in transgenics and 93% in nontransgenics) and resistance to seizures (4-fold increase in seizure latency in both transgenic and nontransgenic mice; P < 0.05). Thus, O2- apparently protects against CNS O2 toxicity. We hypothesized that O2- decreased toxicity by inactivating nitric oxide (NO.). To test this, we inhibited NO. synthase (EC 1.14.23) with N omega-nitro-L-arginine to determine whether NO. contributes to enhanced CNS O2 toxicity in transgenic mice. N omega-nitro-L-arginine protected both transgenic and nontransgenic mice against CNS O2 toxicity (100% survival and a 4-fold delay in time to first seizure; P < 0.05), as well as abolishing the difference in sensitivity to CNS O2 toxicity between transgenic and nontransgenic mice. These results implicate NO. as an important mediator in CNS O2 toxicity and suggest that ECSOD increases CNS O2 toxicity by inhibiting O2(-)-mediated inactivation of NO.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beubler E., Lembeck F., Stolze A. Wechselwirkungen zwischen hyperbarem Sauerstoff und Arzneimittel. Wien Klin Wochenschr. 1977 Apr 15;89(8):260–265. [PubMed] [Google Scholar]
- Bult H., Fret H. R., Van den Bossche R. M., Herman A. G. Platelet inhibition by endothelium-derived relaxing factor from the rabbit perfused aorta. Br J Pharmacol. 1988 Dec;95(4):1308–1314. doi: 10.1111/j.1476-5381.1988.tb11769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton C. A., Colton J. S. Blockade of hyperbaric oxygen induced seizures by excitatory amino acid antagonists. Can J Physiol Pharmacol. 1985 May;63(5):519–521. doi: 10.1139/y85-090. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., McCord J. M., Fridovich I. Preparation and assay of superoxide dismutases. Methods Enzymol. 1978;53:382–393. doi: 10.1016/s0076-6879(78)53044-9. [DOI] [PubMed] [Google Scholar]
- Dawson V. L., Dawson T. M., London E. D., Bredt D. S., Snyder S. H. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988 Apr 15;140(8):2829–2838. [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Murine cytotoxic activated macrophages inhibit aconitase in tumor cells. Inhibition involves the iron-sulfur prosthetic group and is reversible. J Clin Invest. 1986 Sep;78(3):790–797. doi: 10.1172/JCI112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faiman M. D., Mehl R. G., Oehme F. W. Protection with disulfiram from central and pulmonary oxygen toxicity. Biochem Pharmacol. 1971 Nov;20(11):3059–3067. doi: 10.1016/0006-2952(71)90110-9. [DOI] [PubMed] [Google Scholar]
- Forman H. J., York J. L., Fisher A. B. Mechanism for the potentiation of oxygen toxicity by disulfiram. J Pharmacol Exp Ther. 1980 Mar;212(3):452–455. [PubMed] [Google Scholar]
- Frank L., Wood D. L., Roberts R. J. Effect of diethyldithiocarbamate on oxygen toxicity and lung enzyme activity in immature and adult rats. Biochem Pharmacol. 1978 Jan 15;27(2):251–254. doi: 10.1016/0006-2952(78)90311-8. [DOI] [PubMed] [Google Scholar]
- Granger D. L., Lehninger A. L. Sites of inhibition of mitochondrial electron transport in macrophage-injured neoplastic cells. J Cell Biol. 1982 Nov;95(2 Pt 1):527–535. doi: 10.1083/jcb.95.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Taintor R. R., Cook J. L., Hibbs J. B., Jr Injury of neoplastic cells by murine macrophages leads to inhibition of mitochondrial respiration. J Clin Invest. 1980 Feb;65(2):357–370. doi: 10.1172/JCI109679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. The antioxidants of human extracellular fluids. Arch Biochem Biophys. 1990 Jul;280(1):1–8. doi: 10.1016/0003-9861(90)90510-6. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Superoxide, iron, vascular endothelium and reperfusion injury. Free Radic Res Commun. 1989;5(6):315–318. doi: 10.3109/10715768909073413. [DOI] [PubMed] [Google Scholar]
- Heikkila R. E., Cabbat F. S., Cohen G. In vivo inhibition of superoxide dismutase in mice by diethyldithiocarbamate. J Biol Chem. 1976 Apr 10;251(7):2182–2185. [PubMed] [Google Scholar]
- Hendrickson D. J., Fisher J. H., Jones C., Ho Y. S. Regional localization of human extracellular superoxide dismutase gene to 4pter-q21. Genomics. 1990 Dec;8(4):736–738. doi: 10.1016/0888-7543(90)90264-u. [DOI] [PubMed] [Google Scholar]
- Hjalmarsson K., Marklund S. L., Engström A., Edlund T. Isolation and sequence of complementary DNA encoding human extracellular superoxide dismutase. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6340–6344. doi: 10.1073/pnas.84.18.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg N., Darley-Usmar V. M., Wilson M. T., Moncada S. Production of hydroxyl radicals from the simultaneous generation of superoxide and nitric oxide. Biochem J. 1992 Jan 15;281(Pt 2):419–424. doi: 10.1042/bj2810419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson K., Marklund S. L. Extracellular superoxide dismutase in the vascular system of mammals. Biochem J. 1988 Oct 1;255(1):223–228. [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L. Extracellular superoxide dismutase in human tissues and human cell lines. J Clin Invest. 1984 Oct;74(4):1398–1403. doi: 10.1172/JCI111550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. L., Holme E., Hellner L. Superoxide dismutase in extracellular fluids. Clin Chim Acta. 1982 Nov 24;126(1):41–51. doi: 10.1016/0009-8981(82)90360-6. [DOI] [PubMed] [Google Scholar]
- Marklund S. L. Human copper-containing superoxide dismutase of high molecular weight. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7634–7638. doi: 10.1073/pnas.79.24.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheis G., Sherman M. P., Buckberg G. D., Haybron D. M., Young H. H., Ignarro L. J. Role of L-arginine-nitric oxide pathway in myocardial reoxygenation injury. Am J Physiol. 1992 Feb;262(2 Pt 2):H616–H620. doi: 10.1152/ajpheart.1992.262.2.H616. [DOI] [PubMed] [Google Scholar]
- Mulligan M. S., Hevel J. M., Marletta M. A., Ward P. A. Tissue injury caused by deposition of immune complexes is L-arginine dependent. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6338–6342. doi: 10.1073/pnas.88.14.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazono K., Watanabe N., Matsuno K., Sasaki J., Sato T., Inoue M. Does superoxide underlie the pathogenesis of hypertension? Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10045–10048. doi: 10.1073/pnas.88.22.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Oxygen-derived free radicals, endothelium, and responsiveness of vascular smooth muscle. Am J Physiol. 1986 May;250(5 Pt 2):H815–H821. doi: 10.1152/ajpheart.1986.250.5.H815. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M., Vanhoutte P. M. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986 May;250(5 Pt 2):H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- Schuman E. M., Madison D. V. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 1991 Dec 6;254(5037):1503–1506. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]
- Stadler J., Billiar T. R., Curran R. D., Stuehr D. J., Ochoa J. B., Simmons R. L. Effect of exogenous and endogenous nitric oxide on mitochondrial respiration of rat hepatocytes. Am J Physiol. 1991 May;260(5 Pt 1):C910–C916. doi: 10.1152/ajpcell.1991.260.5.C910. [DOI] [PubMed] [Google Scholar]
- Tibell L., Hjalmarsson K., Edlund T., Skogman G., Engström A., Marklund S. L. Expression of human extracellular superoxide dismutase in Chinese hamster ovary cells and characterization of the product. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6634–6638. doi: 10.1073/pnas.84.19.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton M., Granger D. L., Durack D. T. Mitochondrial iron loss from leukemia cells injured by macrophages. A possible mechanism for electron transport chain defects. J Immunol. 1988 Aug 15;141(4):1311–1317. [PubMed] [Google Scholar]
- Zhang J., Piantadosi C. A. Prevention of H2O2 generation by monoamine oxidase protects against CNS O2 toxicity. J Appl Physiol (1985) 1991 Sep;71(3):1057–1061. doi: 10.1152/jappl.1991.71.3.1057. [DOI] [PubMed] [Google Scholar]
- de Nucci G., Gryglewski R. J., Warner T. D., Vane J. R. Receptor-mediated release of endothelium-derived relaxing factor and prostacyclin from bovine aortic endothelial cells is coupled. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2334–2338. doi: 10.1073/pnas.85.7.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]