Abstract
The Fischer 344 (F344) rat was used by the National Toxicology Program (NTP) for over 5 decades for toxicity and carcinogenicity studies. However, in 2006, the NTP decided to switch to a different rat stock due largely to high background control incidences of Leydig cell tumors (LCTs) and mononuclear cell leukemia (MNCL), also known as large granular lymphocytic (LGL) leukemia. In the current review, we aim (1) to provide a summary of NTP bioassays with treatment-associated effects involving MNCL and LCTs in addition to male F344-specific tunica vaginalis mesothelioma (TVM); (2) to describe important pathobiological differences between these F344 rat tumor responses and similar target tissue-tumor response in humans; and (3) to present the NTP reasons for switching away from the F344 rat. We show that due to the highly variable background incidence of F344 MNCL, more reliance on historical control data than is usual for most tumor responses is warranted to evaluate potential effect of any chemical treatment in this rat strain. The high spontaneous incidence of LCTs in the testes of male F344 rats has made this tumor endpoint of little practical use in identifying potential testicular carcinogenic responses. TVM responses in F344 rats have a biological plausible relationship to LCTs unlike TVM in humans. Given their high spontaneous background incidence and species-specific biology, we contend that MNCL and LCT, along with TVM responses, in F344 rat carcinogenicity studies are inappropriate tumor types for human health risk assessment and lack relevance in predicting human carcinogenicity.
Keywords: Cancer bioassay, carcinogenesis bioassay, Leydig cell tumor, LGL leukemia, mononuclear cell leukemia, National Toxicology Program, staging leukemia, tunica vaginalis mesothelioma
Introduction
The Fischer 344 (F344) rat was originally produced by Dr Maynie Rose Curtis at Columbia University in September 1920 from the 344th brother–sister mating of rats from the Fischer commercial breeder colony (Rao & Boorman 1990). This inbred rat became a favorite strain for use in tumor transplantation studies in the 1950s (Dunning & Curtis 1957). Due to its small size, what was considered at the time to be favorable fertility, and consistent response to a number of chemical carcinogens, it was selected as the rat of choice for National Cancer Institute (NCI) cancer bioassays in 1970 (Weisburger 1983; Cameron et al. 1985; Goodman et al. 1985). Use of the F344 rat by the NCI and the National Toxicology Program (NTP) in carcinogenicity studies over 5 decades has led to the creation of the largest rat cancer bioassay database in the world. In 2006, the NTP made a decision to switch from the F344, first to the Wistar rat and subsequently to the Sprague–Dawley rat for their toxicity and carcinogenicity studies (King-Herbert & Thayer 2006; King-Herbert et al. 2010). Because NTP toxicity and carcinogenicity testing practices have tended to create a testing paradigm followed by other investigators, it is unlikely that the F344 rat will see much use in carcinogenesis bioassays in the future.
There are multiple objectives to our review. First, to provide background on the NTP and discuss its reasons for switching away from use of the F344 rat. Second, to provide a retrospective summary and evaluation of mononuclear cell leukemia (MNCL) 1 , Leydig cell tumors (LCTs) and tunica vaginalis mesothelioma (TVM) in the several hundred F344 cancer bioassays conducted by the NTP. Third, to critically evaluate the relevance of these specific F344 rat tumors to their counterparts in humans.
Based on their high spontaneous background incidence and species-/strain-specific biology, our conclusion is that these tumor responses in F344 rat carcinogenicity studies differ from and/or are due to different mechanisms from those in humans. Thus, increased frequencies of these tumors in F344 rats do not predict human carcinogenicity.
This is not intended to be a comprehensive review of all existing literature on MNCL 2 , LCTs and TVMs, and it is not our intention to challenge the final NTP conclusions from the corresponding cancer bioassays. It is important to note that NTP conclusions are made in regards to the strength of evidence that a chemical exposure is responsible for increased incidence of neoplasms in rodents. The conclusions are not intended to evaluate human relevance. The intent of this review is to examine the large database and to specifically comment on the significance of these responses with respect to human health risk.
Methods
Source material for general information on the NTP carcinogenesis bioassays plus data and commentary on MNCL, LCTs and TVMs is derived from the publically available NTP database and published NTP toxicity/carcinogenicity technical reports (http://ntp.niehs.nih.gov).
Background incidence distributions for Figures 1 and 2 were modeled using the mean incidences and range of incidences of spontaneous neoplasms in F344 rats as reported by Haseman et al. (1998) for MNCL, LCT and combined adenoma and carcinoma liver tumors. Liver tumors were chosen for comparative purposes to illustrate incidence and variability of a common tumor type. The modeled distributions were generated in an Excel spreadsheet using random numbers that were based on the mean incidence and a rough standard deviation for each tumor type. The rough standard deviation was generated by taking the reported range (Incidencemax − Incidencemin) divided by four, which is an accepted statistical practice (Triola 2010). The random number generation was repeated 27 times to produce a modeled distribution for the feeding studies and 18 times to produce a modeled distribution for the inhalation studies. These values were selected based on the number of studies used to compile the descriptive statistics taken from Haseman et al. (1998) (27 feeding studies and 18 inhalation studies).
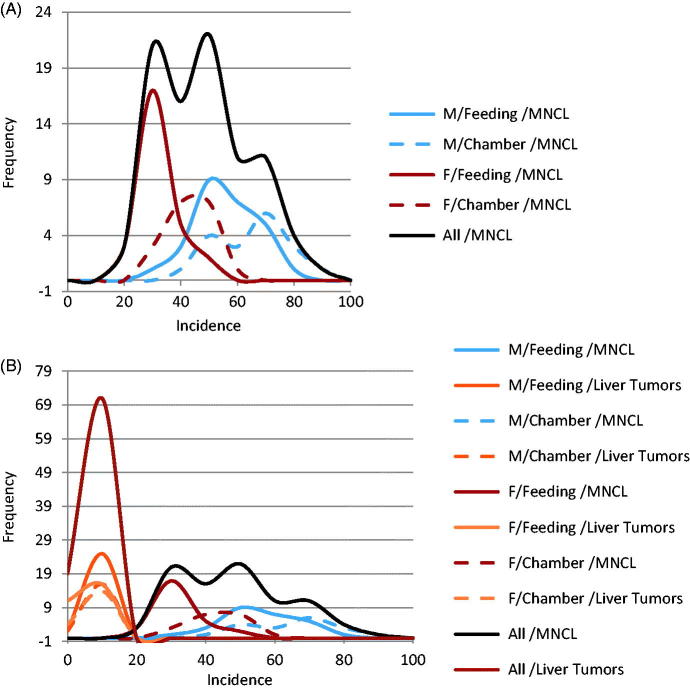
Figure 1.
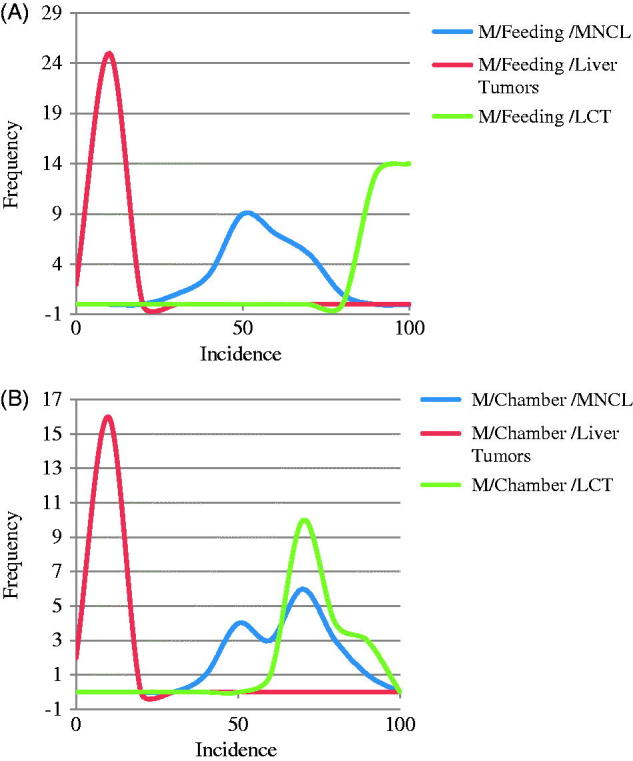
Comparison in male rats of distribution of background incidence for high incidence MNCL and LCTs versus Liver tumor incidence in (A) feeding studies and (B) Chamber studies. MNCLs have both a high incidence and wide distribution in comparison to the other tumor types which makes it difficult to determine if differences between treatment and control are treatment related or due to random chance. The difference in background incidence levels for LCTs in feeding studies (A) and chamber studies (B) maybe explains why five out of the seven NTP studies identified as having increased LCT incidence were inhalation studies. Possible biological reasons for the differences in background incidence are discussed in the text.
Figure 2.
Highly variable and unpredictable nature of the MNCL background tumor incidence. Background incidence in Haseman at al., 1998 was separated by sex and study type. As can be seen above there are relatively large differences in background incidence for MNCL between sexes as well as between exposure methods (A). Contrast this with the effect sex and exposure type has on liver tumor incidence (B). Other factors that affect background incidence of MNCL are discussed in the text.
A brief history of the NCI/NTP carcinogenesis bioassay
As mentioned previously, the F344 rat was derived in the 1920s to fill a need for a reproducible cancer model (Lindsey 1979). The F344 was favored for early tumor transplantation studies (Dunning & Curtis 1957) due to its size and low spontaneous tumor rate (with the exception of the high rate of LCTs). Dr. Dunning provided F344 breeding stock to Walter Heston at the NCI who, in turn, provided breeding stock to the NIH Division of Research Services in 1951 (Cameron et al. 1985). After that the NIH colony was used as the source of F344 rats for cancer research in the 1960s. What followed were some comparisons of the F344 rat response with the Sprague–Dawley, the ACI rat and the Osborne Mendel rat with the ultimate conclusion that the F344 rat provided a more consistent response to a spectrum of chemical carcinogen classes. This led to the selection of the inbred F344 rat as the rat choice for the NCI bioassay program, largely based on the sensitivity of the F344 rat to chemically induced liver tumors (Cameron et al. 1985).
The NCI cancer bioassay program transferred to the NTP in 1970. The initial emphasis of the bioassay hazard assessment program was on carcinogenicity but a substantial amount of refinement occurred from the origin of the early NCI bioassays to the contemporary study design. The majority of the studies from the early 1970s have been relatively well standardized, thereby allowing meaningful retrospective study of tumor responses. The default group size has been 50 males and 50 females per dose along with increased group sizes to accommodate interim evaluations and special studies. The earliest studies involved a high dose that was the estimated maximum tolerated dose (MTD) and a lower dose that was one-half the MTD plus control groups. This approach evolved to include an additional lower dose but the conceptual reliance on an MTD has remained a part of the testing paradigm. More recent modifications of the NTP rodent bioassay testing approach include more emphasis on non-cancer endpoints, incorporation of mechanistic endpoints into studies, use of molecular biology to better understand the relevance of the observed responses and a default study design incorporating in utero exposure to assess the effects of chemical exposure through the entire life cycle.
The basic NTP testing scenario is to have the toxicity and carcinogenicity studies conducted at contract research laboratories using F344 rats from the NTP colony and with study data submitted to NTP for quality assurance and pathology peer review. A draft technical report is next prepared by NTP scientists and made publicly available for comment. The draft report is peer reviewed by an external panel of scientific experts who either endorse the conclusions of the NTP or recommend modification of those conclusions.
In their cancer bioassay technical reports that provide conclusions regarding carcinogenic responses, the NTP uses five categories of evidence of carcinogenic activity to summarize the strength of evidence observed in each species and sex. There are two categories for positive results (clear evidence and some evidence); one category for uncertain findings (equivocal evidence); one category for no observable effects (no evidence); and one category for experiments that cannot be evaluated due to major flaws (inadequate study). For the judgment of clear evidence, there is a dose-related increase of malignant neoplasms, a dose-related increase in a combination of benign and malignant neoplasms or a dose-related increase in benign neoplasms where there is evidence of progression to malignancy. In a some evidence determination, the data show a treatment-related increased incidence of neoplasia where the strength of the response is less than that for clear evidence. There may not necessarily be a clear dose-response and the neoplasms may be benign, malignant, or a combination of benign and malignant. For a call of equivocal evidence, the data are interpreted as showing a marginal increase in neoplasia that may be chemically related. No evidence of carcinogenicity is used when there is no treatment-related increase in neoplasia. These categories refer to the strength of the experimental evidence and not to potency or mechanism.
During the formal external peer review of individual draft technical reports, there are frequently discussions and debate about the levels of evidence for carcinogenicity proposed by the NTP scientists. Highlights of these discussions are included in the final technical report for each rodent carcinogenicity bioassay.
NTP switch from the F344 rat
The NTP decision to no longer use the F344 rat in its toxicity and carcinogenicity bioassays was based on several factors (King-Herbert & Thayer 2006; King-Herbert et al. 2010). Admittedly, there was some concern about switching due to loss of a very robust historical database. Given 5 decades of using the F344 rat despite the high spontaneous incidence of LCTs, it is apparent that no single factor was enough to cause a switch to a different rat strain or stock. Other problems in addition to the high spontaneous LCT incidence that prompted the decision to switch included a high and variable background occurrence of MNCL in both sexes, a high incidence of early mortality due to MNCL, time-dependent decreased fecundity and relatively poor reproductive performance, sporadic seizures and idiopathic chylothorax. With the NTP wanting to standardize a rat of choice across all studies, including reproductive toxicity assessments, and the plan for in utero exposures in future bioassays, high fecundity was paramount. The high incidence of LCTs precluded adequate assessment of testicular effects. The variable background incidence of MNCL progressively increased since the 1970s and became a major cause of early mortality in carcinogenesis studies. In addition, the MNCL response showed sporadic exacerbation by treatment or unexplained decrease following splenic toxicity. The sporadic seizures and idiopathic chylothorax were perhaps less significant issues but were additional factors leading to the decision to switch. The initial switch to the Wistar rat (Crl:WI[Han]) was short-lived due to its small litter size. The current NTP rat of choice for toxicity and carcinogenicity studies is the Sprague–Dawley (Hsd:Sprague–Dawley SD). Their choice of mouse has remained the B6C3F1.
Mononuclear cell leukemia
Early history of MNCL
The initial report of what was most likely MNCL occurred during transplantation studies of mammary adenocarcinomas in F344 rats by Dunning and Curtis (1957). The leukemia cells were initially observed in blood vessels of transplanted adenocarcinomas. Upon subsequent subcutaneous passages, the leukemia cells outgrew the adenocarcinoma cells leading to progressively decreased latency between passages. After the 115th transplant generation, the leukemia growth led to death between 14 and 25 days post-transplantation with infiltration of the subcutis, lungs, liver, spleen and lymph nodes by leukemia cells and hemorrhage. Description of the leukemia cells in stained smears is consistent with morphological features of MNCL, although the characteristic eosinophilic cytoplasmic granules described in later accounts were apparently not prominent. The leukemia was readily transferred by injection of tissue fragments from the inoculation site, by injection of fragments of affected liver and by subcutaneous or intraperitoneal injection of whole blood. The clinical features of the transplanted leukemia described by Dunning are essentially the same as detailed in later reports following transplantation.
Natural history of spontaneous and transplanted MNCL
The first relatively complete descriptions of spontaneous MNCL were reported by Moloney and colleagues in inbred female Wistar–Furth rats (Moloney et al. 1969) and subsequently in female F344 rats (Moloney et al. 1970). They suggested the term MNCL based on its morphological features that differed from other known rat leukemias. They describe leukemic cells as having reddish cytoplasmic granules and disease features including splenic enlargement, leukemic infiltration of liver parenchyma and anemia with a 2- to 6-week clinical course resulting in death. Numerical and/or morphological abnormalities were seen in some metaphase spreads of the leukemic cells. In a subsequent report, Moloney and King found that splenectomy at an early age greatly reduced the incidence of MNCL in inbred Wistar–Furth and in F344 rats (sex not specified) suggesting an origin or early development occurred in the spleen (Moloney & King 1973). Due to the relative increase in use of F344 rats in the 1970s, MNCL was soon recognized as an important cause of spontaneous death in older F344 rats (Davey & Moloney 1970; Sass et al. 1975; Sacksteder 1976; Coleman et al. 1977; Goodman et al. 1979). Spontaneous MNCL is rarely seen in Sprague–Dawley rats with incidences below 1% (Frith 1988).
A variety of variables, factors and events influence the incidence of MNCL in F344 rats (Table 1). Since many of these variables occur in specific study designs, it is important to use the most appropriate historical control data in interpretation of study results. For this reason, NTP has maintained several historical control databases to account for some of the variables affecting MNCL incidences. The high and variable background incidence of MNCL has been clearly noted as critical in assessing the relevance of potential treatment-related increases to human health risk (Lington et al. 1997; Caldwell 1999; Thomas et al. 2007).
Table 1.
Variables, factors and events influencing the incidence of MNCL in F344 rats.
| Variable/factor/event | Effect on MNCL incidence | References |
|---|---|---|
| Gender | Incidence higher in male than in female controls | Haseman et al. 1985Haseman 1983Haseman et al. 1998Rao & Haseman 1993 |
| Diet – Commercial rodent chow versus NIH-07 | 72% increased incidence in males fed NIH-07 diet | Rao & Haseman 1993 |
| Diet – NIH-07 versus NTP-2000 | Slight decreased incidence in males and females in NTP 2000 diet feeding studies but not in inhalation studies | Haseman et al. 2003 |
| Diet restriction | Decreased incidence. Progression is retarded but rats live longer and eventually develop MNCL | Hursting et al. 1993Imai et al. 1990Shimokawa et al. 1993Thurman et al. 1994Turturro et al. 1994Lipman et al. 1999Stefanski et al. 1990 |
| Corn oil gavageSafflower oil gavageTricaprylin gavage | Decreased incidence in males (up to 57% lower compared to non-gavage NIH-07 diet or water gavage); females not affected | Hursting et al. 1994Rao & Haseman 1993Haseman et al. 1985Haseman & Rao 1992NTP TR 426 |
| Body weight at 14 weeks | Positive correlation with MNCL as adult | Turturro et al. 1994 |
| Body weight at 12 months | Slight increased incidence in both sexes | Haseman et al. 2003 |
| Inhalation chamber control | Increased incidence in females versus feeding study | Haseman et al. 1985, 1998, 2003 |
| Splenectomy at 1–2 months of age | Significant decreased incidence later in life | Moloney & King 1973 |
| Splenic toxicity | Significant decreased incidence in both sexes | Elwell et al. 1996. (Report on 13 cases with decreased incidence). |
| StressEpinephrine and prostaglandins | Ben-Eliyahu et al. 1999Inbar et al. 2011 | |
| Increased liver tumors | Decreased incidence | Haseman 1983Harada et al. 1990 |
| Hypophysectomy | Decreased incidence | Ward et al. 1990Stefanski et al. 1990 |
| Germfree versus conventional | MNCL not seen in germfree F344 | Sacksteder 1976 |
| Radiation | Decreased incidence | Hellman et al. 1982 |
| Time trends | Increased diagnoses over time in both sexes | Rao et al. 1990Haseman & Rao 1992 |
| Refinement of diagnostic criteria | Increased incidence in both sexes based on recognition of early stages of MNCL | Rao et al. 1990 |
| 60 Hz Magnetic field | No effect on MNCL | Morris et al. 1999Sasser et al. 1996 |
Several definitive studies of spontaneous and transplantable MNCL beginning in the early 1980s and continuing over the following 20 years serve to extend our knowledge of the clinical and pathological features of this disease. Spontaneous MNCL arises in the splenic marginal zone with subsequent infiltration of the red pulp, splenic lymphoid depletion and erythrophagocytosis by MNCL cells (Ward & Reynolds 1983; Stromberg & Vogtsberger 1983; Losco & Ward 1984). There is secondary involvement of the liver with distention of sinusoids by leukemic cells leading to hepatocellular degeneration, some evidence of nodular regeneration (Shiga & Narama 2015), and necrosis in severe cases. Many other organs are affected with bone marrow involvement occurring late in the disease. Paraneoplastic events associated with spontaneous MNCL include immune mediated hemolytic anemia, thromobocytopenia, myelofibrosis and osteosclerosis in some cases (Stromberg & Vogtsberger 1983; Stromberg et al. 1983c). Disease onset typically starts after 18 months of age leading to death by 22 months. The spontaneous latency from identification of leukemia to death from anemia and organ failure is 10–12 weeks (Stromberg et al. 1983c, 1990) although a 5-week latency was reported during the early identification of this disease (Moloney et al. 1970). MNCL is the main cause of death in F344 rats 20 months and older (Sass et al. 1975; Sacksteder 1976; Coleman et al. 1977; Goodman et al. 1979; Stromberg & Vogtsberger 1983; Kodell et al. 1995). Clinical pathology features include severe anemia, altered coagulation parameters, white blood cell counts as high as 400,000/mm3 and elevated serum enzymes reflecting liver and other organ damage (Stromberg et al. 1983b, 1983c).
The MNCL transplant model
While initial transplantation was carried out by injection of leukemic blood or pieces of affected tissue (Dunning & Curtis 1957), transplantation studies of MNCL starting in the 1980s involved obtaining leukemic cells from spleens of spontaneous cases with isolation of the MNCL cells on Ficoll-isopaque gradients and adjusting cell suspensions with phosphate buffered saline (Stromberg et al. 1985). Isolated cells were typically injected ip or iv. In contrast to the natural disease occurring in rats older than 20 months, all ages of F344 rats regardless of sex are susceptible to the transplanted MNCL cells. Progression of the transplant disease is dependent upon the number of injected cells with a dose of 2 × 107 leukemia cells resulting in a mean longevity of 67 days (Stromberg et al. 1985).
Many features of the transplantation MNCL model mimic the natural spontaneous disease (Stromberg et al. 1985) supporting the idea of using the transplant model as a short-term assay for identification of anti-leukemia chemicals (Dieter et al. 1989, 1990). Clinical feature including emaciation, icterus and anemia are similar to spontaneous MNCL. Macroscopic hallmark features of splenomegaly, hepatomegaly, lymphadenopathy and petechial hemorrhages in lymph nodes, lungs and brain are present in both natural and transplant diseases. The morphological features and clinical pathology in the MNCL transplant disease, including paraneoplastic immune mediated hemolytic anemia, thrombocytopenia, myelofibrosis and osteosclerosis, are similar to the natural disease. As there is progressive involvement of the liver and other tissues, serum enzyme markers reflecting tissue damage are elevated.
The most notable difference in the transplantation model is a shorter clinical course with a dose-dependent and serial-dependent decreased latency. In the extreme, after 23 serial passages, rats inoculated ip with 107 leukemia cells died between 12 and 16 days after transplantation (Stromberg et al. 1990). Transplanted MNCL has decreased pentose-shunt enzymes and malate dehydrogenase possibly related to the rapid growth rate of transplanted tumor cells (Dieter et al. 1985). Other features unique to the transplantation model are an increased clinical malignancy and cytotoxicity with increased serial passage, more frequent bone marrow and lymph node infiltration, and the fact that all ages of F344 rats are susceptible to transplantation (Reynolds et al. 1984; Dieter et al. 1985; Stromberg et al. 1985). This latter observation suggests that the natural disease may be an age-associated genetic event.
Staging MNCL
Starting in the mid-1980s the NTP began to define different stages in the progression of MNCL in their technical reports to better define whether treatment potentially accelerated the progression of the leukemia. Three stages of MNCL have been defined (Dunnick et al. 1989; Stefanski et al. 1990; Frith et al. 1993; Stefanski et al. 1995).
Stage 1 is primarily restricted to the spleen with minimal splenic enlargement but increased leukemia cells in the splenic red pulp. Some depletion of small lymphocytes in germinal centers, periarteriolar lymphoid sheaths and marginal zones has been documented in early stages of the disease (Losco & Ward 1984). The liver may contain a small number of leukemia cells in the sinusoids. There is no evidence of involvement of any other organs.
Stage 2 is primarily restricted to the spleen and liver. There is splenic enlargement with increased leukemia cells expanding the red pulp but lymphoid follicles and periarteriolar lymphoid sheaths are still evident. Liver involvement includes increased leukemia cells beginning to expand the sinusoids. The vasculature in other organs may also contain leukemia cells but without obvious aggregates of leukemia cells.
Stage 3 involves advanced disease in multiple organs including lung, lymph nodes, kidney, adrenal gland and brain. Splenomegaly is pronounced with effacement of normal splenic architecture and the liver is enlarged and mottled. Centrilobular hepatocellular degeneration and necrosis are often present.
Cytological, immunophenotypic and functional features of F344 MNCL cells
Cytological features of MNCL cell have been characterized from natural cases and from different transplant cell lines (Ward & Reynolds 1983; Stromberg et al. 1985). The MNCL cell ranges from 12 to 20 microns in diameter and contains a single round to reniform eccentric nucleus with dense heterochromatin and a single nucleolus. There is abundant gray-blue cytoplasm that contains red cytoplasmic granules that are slightly less than 1 micron in size and have been shown to be lysosomes by electron microscopy (Stromberg et al. 1983a). MNCL cells are variably immunopositive for OX-8, BC-84 and W3/13 and have variable NK-cell activity, features characteristic of normal rat large granular lymphocytes (LGLs) (Ward & Reynolds 1983). More complete immunophenotypic features of MNCL cells can be found in several references (Stromberg & Vogtsberger 1983; Reynolds et al. 1984; Reynolds 1985; Ward et al. 1990).
In their examination of the NK functionality of MNCL cells, Reynolds and colleagues showed that both NK and antibody-dependent cell-mediated cytotoxicity was similar to that of normal LGL cells but that the MNCL cells lacked a functional T-cell receptor beta chain (Reynolds et al. 1985). In addition to identifying phenotypic features similar to LGL cells, Stromberg and colleagues identified adherence to glass, phagocytosis and NaF-sensitive esterase granule staining, all features of MNCL cells that are characteristic of monocytes (Stromberg et al. 1983a). The functional ability to phagocytose erythrocytes and expression of csf-1 receptor also suggest a relationship to a monocyte/macrophage lineage (Kusewitt et al. 1982; Stefanski et al. 1990). However, Reynolds and colleagues distinguish MNCL cells from monocytes by their lack of peroxidase, lysozyme, non-specific esterase and non-adherence in cell culture (Ward & Reynolds 1983; Reynolds et al. 1984). Thus, while an exact cell of origin for F344 rat MNCL is unknown, immunological and functional features show a relationship to lymphocytes and monocytes with strong evidence of LGL lineage. MNCL cell lines have differing degrees of NK activity (cytotoxicity) and different cell surface antigens as indication that leukemic cells are heterogeneous and not clonal (Ward & Reynolds 1983).
F344 MNCL and human LGL leukemia
There are two major human lymphoproliferative diseases with LGL features that have morphological resemblance to F344 rat MNCL. One is a CD3+ leukemia of LGL without NK activity and the other a surface CD3- leukemia with NK activity (Steinway et al. 2014).
The CD3+ LGL leukemia is cytotoxic, and is characterized by an increase in the number of mature CD8+ CD57+ T-cell receptor αβ+ T lymphocytes (Steinway et al. 2014). Clinically, it presents as a chronic disease, associated with autoimmune conditions (e.g. rheumatoid arthritis), recurrent infections, anemia and neutropenia (Johansson et al. 2015). This is an indolent condition, with median survival of more than 10 years (Steinway et al. 2014), and therefore, based upon pathogenesis, is clearly unrelated to the F344 MNCL (Chan et al. 2008a). An aggressive form of this condition has been described in the literature (Gentile et al. 1994; Alekshun et al. 2007), with clinical resemblance to the MNCL form observed in F344 rats. However, this aggressive entity is considered so rare, that it was not included in the 2008 World Health Organization Classification of Hematologic Malignancies.
The cells which are representative of the lymphoproliferative disease with lineage from CD3− LGL lymphoid cells are typically CD16+ CD56 + (Sokol & Loughran 2006). According to the 2008 World Health Organization Classification, two types of conditions exist: chronic NK lymphoproliferative disease (LPD), defined as a provisional entity and aggressive NK cell leukemia (ANKCL). Analogous to the CD3+ LGL leukemia, chronic NK-LPD is an indolent disorder with similar clinical phenotype, although autoimmune disorders are less prevalent in this condition (Poullot et al. 2014). ANKCL is extremely rare, with only 98 cases reported worldwide (R. Irons, personal communication), mostly in Asia or Central/South America (Suzuki et al. 2004; Semenzato et al. 2012). It usually presents acutely, and is associated with B symptoms (particularly fever, but also night sweats and weight loss), jaundice, lymphadenopathy, hepatosplenomegaly, circulating leukemic cells and cytopenia (Zhang et al. 2014). It has a multi-organ profile, and is characterized by extensive bone marrow involvement and often presents with the hemophagocytic syndrome (Hasserjian & Harris 2007). ANKCL bears a very poor prognosis, and is acutely fatal with median survival time from diagnosis of approximately 58 days (Song et al. 2002). It has an Epstein–Barr virus (EBV) etiology, and unique clonal expression of selected EBV genes found in the tumor cells in almost all of the cases (Ohshima et al. 1998). The pathogenesis of ANKCL in humans likely requires three key events: (1) antigenic stimulation; (2) EBV infection; and (3) dominant cellular oncogenic transformation (Ryder et al. 2007). It is important to note that all three of these events are required for ANKCL development in humans. Its clinical and pathological features are very similar to F344 rat MNCL (Cheung et al. 2003; Ruskova et al. 2004; Suzuki et al. 2004; Ryder et al. 2007; Chan et al. 2008b). However, the F344 rat MNCL does not have a viral etiology (Kawa-Ha et al. 1989; Hart et al. 1992), has a much higher incidence rate compared to ANKCL, has a variable nature, and is strain specific. Therefore, the commonly occurring F344 rat MNCL cannot be considered a relevant predictor of human disease.
NTP studies with potential MNCL responses
From 1978 to 2006 when the NTP stopped using the F344 rat in cancer bioassays, there have been 40 F344 rat studies with a potential chemical-related increased incidence of MNCL and 26 chemicals with a chemical-related decrease in MNCL in one or both sexes. The majority of these 66 studies had chemical-related increased incidence of neoplasia at tissue sites other than the hematopoietic system. Here we will focus our review on potentially positive MNCL responses. The majority of the chemicals with decreased incidences of MNCL had an associated splenic toxicity in pre-chronic studies at similar doses to the 2-year carcinogenicity studies (Elwell et al. 1996). Among the 40 chemicals with a potential positive MNCL response, two [bisphenol A (NTP TR215) and phenol (NTP TR203)] were ultimately concluded by the NTP to not be carcinogenic based on more appropriate statistical analysis for bisphenol A and lack of a convincing dose response for phenol. Of the remaining 38 studies, nine were documented in NTP technical reports as having MNCL as the only tumor response in one or both sexes. Consequently, levels of evidence of carcinogenicity for 29 chemical studies are based on the tumor response in the hepatic, pulmonary, renal, endocrine and/or other non-hematopoietic system tissues in addition to a MNCL response.
The rat studies with decreased incidences of MNCL are listed in Table 2. Fifteen of these 26 chemicals had one or more tumor responses in rats and/or mice primarily involving liver, kidney, lung and/or endocrine tissues. The magnitude of the MNCL decrease was dramatic, with incidence values frequently reaching 0%. The relationship of the decreased incidence to splenic toxicity underscores the importance of the splenic microenvironment in the development of MNCL. It is noted that splenectomy at 2 months of age had previously been shown to dramatically reduce the incidence of MNCL later in life (Moloney & King 1973).
Table 2.
Decreased frequency of MNCL in NTP studies.
| NTP TR | Carcinogenic in rodents with decreased MNCL (as per Elwell et al. 1996) | Splenic toxicity | Sex |
|---|---|---|---|
| TR-351 | p-Chloroaniline hydrochloride | x | M&F |
| TR-222 | C.I. Disperse Yellow 3 | x | M&F |
| TR-226 | C.I. Solvent Yellow 14 | x | M&F |
| TR-225 | D & C Red 9 | x | M&F |
| TR-360 | N,N-Dimethylaniline | x | M&F |
| TR-266 | Monuron | x | M&F |
| TR-442 | p-Nitrobenzoic acid | x | M&F |
| TR-383 | 1-Amino-2,4-dibromoanthroquinone | x | M&F |
| TR-407 | C.I. Pigment Red 3 | x | M&F |
| TR-337 | Nitrofurazone | x | M&F |
| TR-448 | Isobutyl nitrite | x | M&F |
| TR-233 | 2-Biphenylamine hydrochloride | x | M&F |
| TR-205 | 4,4′-Oxydianiline | x | M&F |
| TR-271 | HC Blue 1 | x | M&F |
| TR-216 | 11-Aminodecanoic acid | M&F | |
| Not carcinogenic in rodents with decreased MNCL (as per Elwell et al. 1996) | |||
| TR-211 | C.I. Acid Orange 10 | x | M&F |
| TR-330 | 4-Hexylresorcinol | x | M&F |
| TR-411 | C.I. Pigment Red 23 | M&F | |
| TR-240 | Propyl gallate | M&F | |
| TR-322 | Phenylephrine hydrochoride | M&F | |
| Decreased MNCL (Post Elwell et al. 1996) | |||
| TR-527 | Malachite green | F | |
| TR-527 | Leukomalachite green | M&F | |
| TR-494 | Anthraquinone | M&F | |
| TR-383 | 1-Amino-2,4-dibromoanthraquinone | M&F | |
| TR-493 | Emodin | M&F | |
| TR-426 | Corn oil, safflower oil, tricaprylin | M |
Identification of the 38 chemicals with potential MNCL responses along with statistical analyses is provided as Supplemental Table S1. We have listed these studies based on the levels of evidence of carcinogenicity for the MNCL response as per the data in the NTP technical reports (Table 3). This has been challenging for a variety of bioassay outcomes. When there is an overwhelming tumor response in one or more non-hematopoietic tissues, MNCL may not have received particular mention but was simply included in the overall level of evidence determination for that bioassay. In other situations where a bioassay is classified as having clear evidence of carcinogenicity in a non-hematopoietic tissue, a suspicious MNCL response may be listed as “may have been related” to treatment – basically an equivocal call. When the MNCL response in treated groups falls within the historical control range, there has often been documented discussion and debate during the draft technical report peer review regarding how to classify the leukemia response. Since MNCL is a common cause of early mortality in F344 rat studies, a life-table or poly 3 statistical analysis has tended to dominate the ultimate classification of the MNCL response with respect to its level of evidence of carcinogenicity, especially when a treatment-related increased incidence is associated with decreased survival in a treated group. For those studies where the NTP concluded that MNCL was a carcinogenic response, we note that many of the responses occurred in only one sex. In those studies where there is dose-related and robust (p < 0.01) increased incidence of MNCL in both sexes, we believe a judgment of clear evidence of carcinogenicity is appropriate.
Table 3.
Male and female F344 rat MNCL responses in NTP studies.
| MNCL/leukemia response |
||||||
| Chemicals |
NTP TR |
Sex |
Positive |
Negative |
||
| Studies evaluated prior to use of NTP’s levels of evidence of carcinogenicity | ||||||
| Allyl isovalerate | 253 | M | X | |||
| F | X | |||||
| 2-Amino-5-nitrothiazole | 053 | M | X | |||
| F | X | |||||
| Butyl benzyl phthalate | 213 | M | a | |||
| F | X | |||||
| 3,3′-Dimethoxybenzidine-4,4′-diisocyanate | 128 | M | X | |||
| F | X | |||||
| Lasiocarpine | 039 | M | X | |||
| F | b | |||||
| 2,4,6-Trichlorophenol | 155 | M | X | |||
| F | X | |||||
| Chemicals |
NTP TR |
Sex |
Cleasr |
Some |
Equivocal |
None |
| Studies with clear evidence of carcinogenicity for MNCL in one or both genders | ||||||
| o-Nitroanisole | 416 | M | X | |||
| F | X | |||||
| Furan | 402 | M | X | |||
| F | X | |||||
| Tetrachloroethylene | 311 | M | X | |||
| F | X | |||||
| C.I. Direct Blue 15 | 397 | M | X | |||
| F | X | |||||
| Tetrafluoroethylene | 450 | M | X | |||
| F | X | |||||
| 2,2-bis(Bromomethyl)-1,3-propanediole | 452 | M | X | |||
| F | X | |||||
| Gallium arsenide | 492 | M | X | |||
| F | X | |||||
| Glycidol | 374 | M | X | |||
| F | X | |||||
| Mirex | 313 | M | X | |||
| F | X | |||||
| Studies with some evidence of carcinogenicity for MNCL in one or both genders | ||||||
| Dibromoacetic acid | 537 | M | X | |||
| F | X | |||||
| Dichlorvos | 342 | M | X | |||
| F | X | |||||
| Dimethyl morpholino-phosphoramidate | 298 | M | X | |||
| F | X | |||||
| Hydroquinone | 366 | M | X | |||
| F | X | |||||
| Iodinated glycerol | 340 | M | X | |||
| F | X | |||||
| 2-Mercaptobenzothiozole | 332 | M | X | |||
| F | X | |||||
| Riddelliine | 508 | M | Xc | |||
| F | Xc | |||||
| Studies with equivocal evidence of carcinogenicity for MNCL in one or both genders | ||||||
| Acetaminophen | 394 | M | X | |||
| F | X | |||||
| Alpha methylstyrene | 543 | M | X | |||
| F | X | |||||
| Ampicillin trihydrate | 318 | M | X | |||
| F | X | |||||
| Benzophenone | 533 | M | X | |||
| F | X | |||||
| Chlorinated paraffins | 308 | M | X | |||
| F | X | |||||
| Chlorinated water | 392 | M | X | |||
| F | X | |||||
| Chloraminated water | 392 | M | X | |||
| F | X | |||||
| C.I. Acid Red 114 | 405 | M | X | |||
| F | X | |||||
| Diallylphthalate | 284 | M | X | |||
| F | X | |||||
| 3,3′-Dimethylbenzidine dihydrochloride | 390 | M | Xd | |||
| F | Xd | |||||
| Dimethyl methylphosphonate | 323 | M | X | |||
| F | X | |||||
| Indium phosphide | 499 | M | X | |||
| F | X | |||||
| 4-Methylimidazole | 535 | M | X | |||
| F | X | |||||
| Methyl isobutyl ketone | 538 | M | X | |||
| F | X | |||||
| Pyridine | 470 | M | X | |||
| F | X | |||||
| Tris(2-chloroethyl) phosphate | 391 | M | X | |||
| F | X | |||||
a = Inadequate study; b = Combined lymphoma and granulocytic leukemia; c = Study terminated at 72 weeks; d = 14-month study duration.
Factors consistent with a weight-of-evidence approach in decisions regarding chemical safety and risk assessment include use of historical control data, a clear dose-response, having the effect in both sexes, lesion latency, the stage of the disease and the appropriate degree of statistical stringency. For example, in pairwise comparisons, the use of a statistical cut-off of p < 0.05 for rare tumors and p < 0.01 for commonly occurring tumors has been a reasonable suggestion (Haseman 1983). A major factor that has been of persistent concern in evaluating a MNCL response in NTP F344 rat bioassays is the high and variable background incidence in study controls. Exactly how the factors listed in Table 1 influence the incidence of MNCL is unknown. Due to the influence of gender and route of exposure on the incidence of MNCL, NTP has employed multiple historical control databases in evaluating MNCL responses. We considered these factors in our commentary on each of the 38 studies with an indication of a treatment-related increased incidence of MNCL. Thomas et al. provide a general review of changes over time in NTP historical control rates of MNCL in tabular form, in their 2007 paper. They document a progressive increase in MNCL in males from 7.9% in 1971 to 52.5% in 1995–1998 and in females from 2.1% in 1971 to 24.2% in 1995–1998.
Early studies evaluated before use of levels of evidence of carcinogenicity
There are six early studies conducted and evaluated by NCI or NTP prior to the introduction of levels of evidence of carcinogenicity by NTP (Table 4). These studies are simply listed as positive for leukemia. Based on a review of archived slides from five of the studies, some of the leukemia in these studies is consistent with MNCL. Slides were not available for lasiocarpine (NTP TR039), a known carcinogen. The butyl benzyl phthalate (NTP TR213) study was subsequently repeated (see NTP TR458) and no treatment-related increased incidence of MNCL was present in the repeat study. For four of these six early studies there are no historical control data for MNCL. In these early studies, the data evaluation is less complete compared to subsequent studies, and MNCL was combined with lymphoma for purposes of evaluation. We are unable to draw firm conclusions regarding the MNCL incidence in these cases.
Table 4.
NCI/NTP F344 rat studies with a positive leukemia response started and evaluated prior to introduction of NTP levels of evidence of carcinogenicity.
| Two-year study highlights | Overall MNCL incidencesa | MNCL historical control data | Authors’ commentary |
|---|---|---|---|
| Allyl Isovalerate (NTP TR 253) | |||
| This corn oil gavage study started in 1979 and there was good survival and body weight gain in rats. Marginal increases in pancreatic acinar and preputial tumors were present in treated males along with an increase in MNCL. No increased incidence of tumors was seen in female rats or male B6C3F1 mice. Treated female B6C3F1 mice had increased incidence of lymphoma. | F 4/50 (8%), 6/50 (12%), 8/50 (16%)M 1/50 (2%), 4/50 (8%), 7/50 (14%) | 13.2% (range 2–42%)49.6% (range 2–24%) | There was discussion during the peer review regarding how biologically relevant the leukemia response was and debate regarding which of several different historical controls should be used in the evaluation. It is noted that the incidences of MNCL are unusually low in all groups and within the laboratory historical control range. |
| 2-Amino-5-nitrothiazole (NTP TR 053) | |||
| There was dose-related early mortality in rats in this dosed-feed study. A mixture of hematopoietic neoplasms was present in male and female rats but only considered a positive response in males. According to the TR, there was no clear evidence of carcinogenicity in female rats or in either sex of B6C3F1 mice. | All lymphomas/leukemias combined.F 2/24 (8%), 9/24 (37%), 11/24 (46%), 1/24 (4%)M 13/50 (26%), 19/50 (38%), 28/49 (57%) | No historical control data provided in TR. | The leukemia was consistent with MNCL. There was a marginally significant trend for leukemia but no significant pairwise statistical flag for MNCL. During the 1978 peer review, it was suggested that the male response might be within normal statistical variation. It is not clear why the female response was not regarded as positive. |
| Butyl benzyl phthalate (NTP TR 213) | |||
| This is the first of two dietary studies. This study was considered inadequate for male rats due to toxicity and early mortality. Female rats had a marginally increased incidence of MNCL as the only tumor response. There was no increased in tumor incidences in B6C3F1 mice. | F 7/49 (14%), 7/49 (14%), 18/50 (36%)M Study inadequate | 19% (range 12–24%) | Peer reviewed in December 1978. Study repeated starting in 1989 (TR 458) using same or higher doses. In the repeat study there was an increase in pancreatic acinar tumors but no increase in MNCL. MNCL incidences – [F 21/50 (42%), 20/50 (40%), 21/50 (42%), 19/50 (38%); M 31/50 (62%), 28/50 (56%), 34/50 (68%), 30/50 (60%)]. We note the high background incidence of MNCL in this second study. |
| 3,3′-Dimethoxybenzidine-4,4′-diisocyanate (NTP TR 128) | |||
| Test agent was administered by gavage and then followed by dosed-feed. Exposure was for 78 weeks plus 26 weeks without treatment. There was dose-related increased mortality in both sexes. The increased incidence of leukemia/malignant lymphoma was present along with skin and Zymbal gland tumors, and endometrial stromal polyps in treated rats. The B6C3F1 mouse study was negative for carcinogenicity. | F 1/20 (5%), 8/50 (16%), 13/48 (27%)M 0/20 (0%), 19/50 (38%), 17/50 (34%) | No historical control data provided in TR | The leukemia was morphologically characteristic of MNCL. The lymphoma diagnoses were also consistent with MNCL. The low leukemia incidence and small group size of the controls is noted. The 1978 peer review comments were supportive of the leukemia/malignant lymphoma diagnoses. |
| Lasiocarpine (NTP TR 039) | |||
| In this dosed-feed study, there was significant dose-related mortality in male and female rats. Liver angiosarcomas and hepatocellular tumors were treatment-related in both sexes. The leukemia was diagnosed as granulocytic leukemia. Lymphoma/leukemia was increased in low and mid-dose female rats. There was no associated mouse study. | Lymphoma/leukemia combinedF 2/24 (8%), 9/24 (37%), 11/24 (46%), 1/23 (4%)M 3/24 (12%), 3/24 (12%), 11/24 (46%), 7/24 (29%) | No historical control data provided in TR | Slides were not available to determine if the leukemia was consistent with MNCL. During the 1977 peer review by the Data Evaluation/Risk Assessment Subgroup of the Clearinghouse on Environmental Carcinogens, it was pointed out that the incidence of leukemia in treated rats was approximately the same as in female controls in a contemporary study of hexa-chlorophene. The small experimental group size was noted. |
| 2,4,6-Trichlorophenol (NTP TR 155) | |||
| This was a dosed-feed study with good survival but lower body weight in treated rats. The leukemia in male rats was described a monocytic with mature and blast forms. Liver tumors were increased in treated B6C3F1 mice. | F 3/20 (15%), 11/50 (22%), 11/50 (22%)M 4/20 (20%), 25/50 (50%), 29/50 (58%) | No historical control data provided in TR | The monocytic leukemia was confirmed to the characteristic of MNCL. The study summary noted a large number of circulating monocytes in male and female rats. |
TR: NTP Technical Report; M: Male; F: Female; MNCL: Mononuclear cell leukemia.
a = Tumor incidences arranged starting with controls and progressing through increased doses.
Studies with clear evidence of carcinogenicity for MNCL
Study detail and commentary on nine studies in which the MNCL response in one or both sexes was considered to be clear evidence of carcinogenicity by the NTP are summarized in Table 5. For furan (NTP TR402) and tetrafluoroethylene (NTP TR450) the data are consistent with a judgment of clear evidence of carcinogenicity. In both cases there is a robust dose-response in both sexes. For the remaining seven studies, the call of clear evidence of carcinogenicity for MNCL is debatable. For tetrachloroethylene (NTP TR311), the call of clear evidence of carcinogenicity was questioned during the formal peer review due to the high rates of MNCL in controls. MNCL incidences in treated groups were within historical control ranges for glycidol (NTP TR374) and C.I. Direct Blue 15 (NTP TR397) and these two studies along with 2,2-bis(bromomethyl)-1,3-propanediol (NTP TR452) have robust multisite tumor responses representing significant pathophysiology as well as competing causes of early mortality. In considering the appropriate degree of statistical significance for common tumor responses, the marginally significant life table statistical flags for mirex (NTP TR313), o-nitroanisole (NTP TR416) and gallium arsenide (NTP TR492) do not strongly support the conclusion that there is clear evidence of carcinogenicity for MNCL. For o-nitroanisole (NTP TR416), MNCL was the only identified tumor response in the main study, and it was suggested (but not accepted by consensus vote of the peer review panel) that the clear evidence of carcinogenicity for MNCL in both sexes be reduced to some evidence for males and equivocal evidence for females. For three studies with clear evidence of MNCL carcinogenicity in one sex, there was some evidence of carcinogenicity for MNCL in females (tetrachloroethylene NTP TR311) and equivocal evidence in males (C.I. Direct Blue 15 NTP TR397 and tetrafluoroethylene NTP TR450).
Table 5.
NTP studies where at least one sex had MNCL classified as clear evidence of carcinogenicity.
| Two-year study highlights | Overall MNCL incidencesa | MNCL historical control data | Authors’ commentary |
|---|---|---|---|
| 2,2-Bis(bromomethyl)-1,3-propanediol (NTP TR 452) | |||
| This dietary administration study started in March 1989 with a high-dose male stop-study group. There were epithelial tumors in several tissues in rats and mice. There was an increase in tunica vaginalis mesotheliomas in male rats and increased incidence of MNCL. Oral cavity and esophageal tumors were present in female rats. Harderian gland and lung tumors were present in treated B6C3F1 mice. | F 15/50 (30%), 13/51 (25%), 19/53 (36%), 19/52 (37%)M 27/51 (53%), 29/53 (55%), 40/51 (78%), 34/55 (62%), 25/60 (42% – stop exposure group) | (Not provided in TR)M 48.9% ± 8.8% (range 32–62%) | The MNCL response in the top two doses of the main study was significantly different by the life table test and, therefore, MNCL was listed as part of the clear evidence of carcinogenicity in male rats. It is noted that only the mid-dose exceed the historical control range and MNCL was not increased in the stop exposure group of males. |
| C.I. Direct Blue 15 (NTP TR 397) | |||
| Drinking water study started in 1983 with 9 and 15-month interim sacrifices and with 22-month final sacrifice due to excessive mortality. Multisite positive carcinogen in both sexes including skin, Zymbal’s gland, liver, oral cavity, intestine, etc. MNCL was listed as clear evidence of carcinogenicity in females and as may have been related to chemical treatment in males. There was no study in mice. | F 7/50(14%), 13/35(37%), 27/65(42%), 15/50(30%)M 17/50(34%), 19/35(54%), 28/65(43%), 20/50(40%) | F 20% ± 8% (range 6–40%)M 37% ± 16% (range 10–72%) | Overall incidences within historical control ranges for both sexes. Life table statistical flags are significant. Competing risks from multiple fatal neoplasms. The wide historical control range in males makes that response uncertain. Contribution of MNCL to NTP conclusions of clear evidence in females and equivocal evidence in males is questionable. |
| Furan (NTP TR 402) | |||
| This corn oil gavage study started in June 1982 and included a high-dose stop-exposure. There was a high incidence of liver cholangiocarcinoma in the stop study as well as the main study. MNCL was listed as clear evidence of carcinogenicity in both sexes. There were hepatocellular and adrenal tumors in treated B6C3F1 mice. | F 8/50 (16%), 9/50 (18%), 17/50 (34%), 21/50 (42%)M 8/50 (16%), 11/50 (22%), 17/50 (34%), 25/50 (50%) | F 26.8% ± 7% (range 16–38%)M 21.3% ± 8.9% (range 4–38%) | The MNCL response exceeded the historical control range in at least one treated group in both sexes but was close to the upper historical control limit in females. There was a clear dose-response for MNCL in both sexes consistent with the NTP call of clear evidence of carcinogenicity. |
| Gallium arsenide (NTP TR 492) | |||
| There was good survival in this inhalation study that started in September 1993. There were no neoplastic effects in males. There were lung and adrenal tumors in addition to MNCL classified as clear evidence of carcinogenicity in females. There were no neoplastic effects in B6C3F1 mice. | F 22/50 (44%), 21/50 (42%), 18/50 (36%), 33/50 (66%)M 19/50 (38%), 28/50 (56%), 33/60 (66%), 28/50 (56%) | F 35.0% ± 5.9% (range 24–47%)M 58.0% ± 8.0% (range 42–70%) | The incidence of MNCL in females exceeded the historical control range only at the high dose and statistical significance was only p = 0.021 for this group by the poly 3 test. The control incidence of MNCL in males was unusually low and the incidence in the exposed males was within the historical control range. The call of clear evidence of carcinogenicity in females is questionable. |
| Glycidol (NTP TR374) | |||
| Water gavage study started in 1981. Multisite positive carcinogen in both sexes including mesothelioma, mammary gland neoplasia, glioma, oral mucosa tumors, intestine tumors, etc. Early deaths due to fatal neoplasms. MNCL in females listed as part of clear evidence of carcinogenicity. Multisite neoplasia in male and female B6C3F1 mice. | F 13/50 (26%), 14/50 (28%), 20/50 (40%)M 25/50 (50%), 33/50 (66%), 21/50 (42%) | F 20% ± 8% (range 6–40%)M 37% ± 16% (range 10–72%) | Overall incidences of MNCL within historical control ranges for both sexes. Competing risks from multiple fatal neoplasms. There was discussion of the MNCL response during the peer review. The contribution of MNCL to the NTP conclusion of clear evidence in female rats is questionable. |
| o-Nitroanisole (NTP TR 416) | |||
| This dietary study was started in September 1984 and included a stop-exposure group with interim sacrifices. While the stop study had urinary and intestinal epithelial neoplasia, the only tumor response in the main study was MNCL. The study was classified as clear evidence of carcinogenicity in both sexes of rats based on the combination of the main and stop studies. Liver tumors were present in the treated B6C3F1 mice. | F 14/50 (28%), 11/50 (22%), 14/50 (28%), 26/50 (52%)M 26/50 (52%), 25/50 (50%), 42/50 (84%), 34/50 (68%) | F 26.6% ± 8.8% (range 14–36%)M 48.1% ± 7/7% (range 32–62%) | This is the first NTP study where the combined results of the main and stop studies were used to classify the carcinogenic response. There was discussion during the peer review that the MNCL should be considered some evidence in males and equivocal evidence in females. Life table statistics are not highly convincing. Ultimately, the peer review supported the NTP call of clear evidence of carcinogenicity for MNCL. |
| Mirex (NTP TR 313) | |||
| First dietary study started in June 1977. Second dietary study at higher doses started in females 6 months later. Results combined from the two studies. Good survival. NTP reported clear evidence of carcinogenicity in males based on liver, kidney and adrenal responses and clear evidence of carcinogenicity in females based on liver and MNCL. There was no corresponding B6C3F1 mouse study. | F 14/104(13%), 8/52 (15%), 11/52 (21%), 14/52 (27%), 18/52 (35%), 27/104 (26%), 14/52 (27%)M 16/52 (31%), 17/52 (33%), 15/52 (29%), 22/52 (42%), 21/52 (40%), 10/53 (19%) | F 19% ± 7% (range 6–38%)M 29% ± 12% (range 10–60%) | MNCL response was weak in females without an early response and this was noted during the peer review. In the first study, the female MNCL was considered lethal while in the second study the female MNCL was considered incidental. Statistical flags are low and not convincing. |
| Tetrachloroethylene (NTP TR311) | |||
| Inhalation study started in 1981. Reduced survival in males exposed to highest concentration may have been due to MNCL. NTP reported clear evidence of carcinogenicity in male rats based on MNCL and renal tubular neoplasms and some evidence of carcinogenicity in female rats based on MNCL. B6C3F1 mice had treatment-related increased hepatocellular neoplasia. | F 18/50(36%), 30/50(60%), 29/50(58%)M 28/50(56%), 37/50(74%), 37/50(74%) | F 29% ± 6% (range 22–35%)(Laboratory)F 19% ± 7% (range 6–38%)(NTP)M 47% ± 15% (range 32–68%)(Laboratory)M 29% ± 12% (range 10–60%)(NTP) | The frequency distribution of MNCL stages 1, 2 and 3 was similar and not statistically significant among the controls and exposed rats. Comments during the NTP peer review regarding concerns about the high laboratory control rates of MNCL in this study were made suggesting conclusions regarding MNCL are questionable for both sexes. |
| Tetrafluoroethylene (NTP TR 450) | |||
| This inhalation study, started in June 1988, resulted in liver and kidney neoplasms in both sexes along with MNCL as clear evidence of carcinogenicity in females and equivocal evidence regarding MNCL in males. There was reduced body weight gain and increased early mortality for both sexes at the high concentration. Both sexes of treated B6C3F1 mice had liver neoplasms. | F 16/50 (32%), 31/50 (62%), 23/50 (46%), 36/50 (72%)M 34/50 (68%), 43/50 (86%), 38/50 (76%), 31/50 (62%) | F 42.0% ± 7.2% (range 30–54%)(Laboratory)40.1% ± 7.2% (range 30–54%) (NTP)56.0% ± 8.7% (range 38–66%) (Laboratory)54.4% ± 8.7% (range 34–66%) (NTP) | The control incidence of MNCL in males slightly exceeded the laboratory historical control. There was a clear increased incidence of MNCL at the low- and mid-concentrations in both sexes. The male response was considered uncertain due to the high control incidence. The female MNCL response is consistent with clear evidence of carcinogenicity. |
TR: NTP Technical Report; M: Male; F: Female; MNCL: Mononuclear cell leukemia.
a = Tumor incidences arranged starting with controls and progressing through increased doses.
In summary, with the exception of two of the nine studies with a judgment of clear evidence of carcinogenicity for MNCL, the data supporting clear evidence of carcinogenicity for MNCL from the remaining seven studies is less compelling. Clear evidence of carcinogenicity was only present in one sex for the seven studies, three studies had competing causes for reduced survival, and statistical significance is of low stringency for three studies.
Studies with some evidence of carcinogenicity for MNCL
For all the seven studies where at least one sex had a determination of some evidence of carcinogenicity for MNCL (Table 6), there was some discussion during the formal peer review about the MNCL response. The discussions during the peer review of dibromoacetic acid (NTP TR537) resulted in downgrading some evidence to equivocal evidence of carcinogenicity for MNCL in males. For riddelliine (NTP TR508) the MNCL response was initially included under clear evidence of carcinogenicity in both sexes along with liver tumors but the MNCL response is documented in the peer review comments as being more consistent with some evidence of carcinogenicity. The dimethyl morpholinophosphoramidate (NTP TR298) study represents the first time that the only evidence of a carcinogenic response in both sexes was MNCL. Consequently, this narrowly focused the comments during the formal peer review, and although the final decision was some evidence of carcinogenicity for both sexes, there was a contrary opinion expressed regarding the female MNCL responses, indicating that the review group had some uncertainty about the NTP interpretation. For females in the dibromoacetic acid (NTP TR537) study, the only tumor response was some evidence of carcinogenicity for MNCL. In this study, there was equivocal evidence of MNCL carcinogenicity in males (dibromoacetic acid NTP TR537).
Table 6.
NTP studies where at least one sex had MNCL classified as some evidence of carcinogenicity.
| Two-year study highlights | Overall MNCL incidencesa | MNCL historical control data | Authors’ commentary |
|---|---|---|---|
| Dibromoacetic acid (NTP TR 537) | |||
| This drinking water study was started in March 2000 with good survival in treated groups. There was some evidence of carcinogenicity based on tunica vaginalis mesotheliomas in males with equivocal evidence for MNCL in males. MNCL was the only tumor response in females and was called some evidence of carcinogenicity. Liver and lung tumors were present in treated B6C3F1 mice. | F 11/50 (22%), 13/50 (26%), 16/50 (32%), 22/50 (44%)M 17/50 (34%), 31/50 (32%), 24/50 (48%), 13/50 (26%) | F 23.5% ± 4.4% (range 20–30%)M 31.6% ± 3.3% (range 26–34%) | There was significant debate regarding the MNCL call of some evidence of carcinogenicity for females during the formal public peer review and that discussion continued on the following day. It was noted that the historical control range was possibly artificially tight being based on only four studies. NTP downgraded the evidence of carcinogenicity from some to equivocal in males based on the peer review discussion. |
| Dichlorvos (NTP TR 342) | |||
| There was good survival in this corn oil gavage study started in January 1981 with MNCL in males and pancreatic acinar tumors in male and female rats. Forestomach tumors were present in male and female treated B6C3F1 mice. | F 17/50 (34%), 21/50 (42%), 23/50 (46%)M 11/50 (22%), 20/50 (40%), 21/50 (42%) | F (Not provided in TR)M 9% ± 7% (range 2–18%) (Laboratory)15% ± 9% (range 2–44%) (NTP) | There was discussion during the formal public peer review pointing out the higher than usual concurrent control incidence of MNCL. The MNCL response was considered contributory to the call of some evidence of carcinogenicity. |
| Dimethyl morpholinophosphoramidate (NTP TR 298) | |||
| In this corn oil gavage study started in April 1980, there was reduced survival in high-dose rats. The only tumor response attributed to treatment was some evidence of carcinogenicity for MNCL in both male and female rats. No treatment-associated tumors were present in B6C3F1 mice. | F 9/50 (18%), 13/5 (26%), 12/49 (24%), 18/50 (36%)M 14/50 (28%), 21/50 (42%), 19/50 (38%), 25/50 (50%) | F 29.3% ± 13.0% (range 16–42%) (Laboratory)16.1% ± 8.9% (range 2–42%) (NTP)M 17.3% ± 11.7% (range 4–26%) (Laboratory)12.2% ±7.6% (range 2–26%) (NTP) | There was increased incidence of MNCL in the high-dose for both sexes and the severity of the MNCL was greater in treated than in control rats. On the other hand, the MNCL incidence in male controls exceeded the historical control while that for the female controls was lower than the mean historical control. There was some concern voiced regarding the MNCL call during the formal public peer review. |
| Hydroquinone (NTP TR 366) | |||
| There was good survival in this water gavage study started in August 1982. Renal tubular tumors in the male rats and MNCL in females were considered some evidence of carcinogenicity. Liver tumors were present in female treated B6C3F1 mice. | F 9/55 (16%), 15/55 (27%), 22/55 (40%)M 28/55 (51%), 26/55 (47%), 31/55 (56%) | F 25% ± 15% (range 8–46%) (NTP)M (Not provided in TR) | It is noted that the control incidence of MNCL is lower than the historical control incidence. Some concern regarding the MNCL call was raised during the formal public peer review period due to the high and variable control incidence of MNCL. Most of the discussion, however, was focused on the male renal tumor response. |
| Iodinated glycerol (NTP TR 340) | |||
| This water gavage study was started in April 1981 and there was reduced survival in high-dose male rats plus some evidence of carcinogenicity for MNCL and thyroid follicular carcinomas. There were no treatment-related tumors in female rats. Female B6C3F1 mice had some evidence of carcinogenicity. | F 15/50 (30%), 14/50 (28%), 14/50 (28%)M 14/50 (28%), 29/50 (58%) 24/50 (48%) | F (Not provided in TR)M 39% ± 16% (range 14–60%) | The call of some evidence of carcinogenicity was based largely on the MNCL response in the male rats since the incidences of the thyroid tumors & nasal adenomas were marginal. There was some discussion regarding the MNCL call raised during the formal public review of the study. It is noted that the MNCL incidences for males fall within the historical control range. |
| 2-Mercaptobenzothiazole (NTP TR 332) | |||
| There was reduced survival in treated male and high-dose females in this corn oil gavage study started in July 1981. The some evidence of carcinogenicity call in males was based on MNCL as well as preputial gland, pancreatic ascinar and adrenal pheochromocytomas. Females also had pheochromocytomas (some evidence). There was an equivocal liver tumor response in female B6C3F1 mice. | F 6/50 (12%), 14/50 (28%), 9/50 (8%)M 7/50 (14%), 16/50 (32%), 3/50 (6%) | F 19% ± 9% (range 4–42%) (NTP)M 14% ± 8%(range 2–28%) (NTP) | The MNCL call of some evidence of carcinogenicity was discussed during the formal public peer review and there were two opinions expressed that the levels of evidence for carcinogenicity in males should be equivocal. The MNCL response was present only at the low dose with poor survival at the high dose. |
| Riddelliine (NTP TR 508) | |||
| Riddelliine, a gavage study starting in 1996, had an unbalanced design with only a control and high-dose group for male rats. Both genders of rats are listed as clear evidence of carcinogenicity for MNCL. The male rat study was terminated at 72 weeks due to a high incidence (clear evidence) of hepatic hemangiosarcomas. Male B6C3F1 mice had hepatic hemangiosarcomas while females had lung tumors. | F 12/50(24%), 8/50 (16%), 13/50 (26%), 18/50 (36%), 18/50 (35%), 14/50 (28%)M 2/50 (4%), 9/50 (18%) | F 29.1% ± 8.4% (range 16–42%)M Not applicable. Early study termination. | The overall incidences of MNCL were within the historical control range. We have included riddelliine in our listing of some evidence based on comments made during the formal public peer review that the female MNCL response by itself should be classified as some evidence of carcinogenicity. In light of the early termination and high incidence of hepatic hemangiosarcomas in treated males, attributing any levels of evidence for MNCL carcinogenicity is questionable. |
TR: NTP Technical Report; M: Male; F: Female; MNCL: Mononuclear cell leukemia.
a = Tumor incidences arranged starting with controls and progressing through increased doses.
In summary, there was discussion during the formal peer review regarding the MNCL response for all the seven studies with some evidence of MNCL carcinogenicity. Despite some comments by peer reviewers indicating that historical control data are important, the final NTP level of evidence calls for MNCL carcinogenicity were largely driven by life table statistical analyses versus concurrent controls.
Studies with equivocal evidence of carcinogenicity for MNCL
There are 16 studies where there was equivocal evidence of carcinogenicity for MNCL in one or both sexes (Table 7). In the case of acetaminophen (NTP TR 394), there was discussion during the formal peer review regarding the MNCL but the fact that there was a negative study in F344 rats at a higher dose of acetaminophen (Hiraga & Fujii, 1985) was not considered. In 10 of the 16 bioassays where one or both sexes had a final determination of equivocal evidence of carcinogenicity for MNCL, there was discussion centered on the MNCL response during the NTP formal peer review. For four bioassays [chlorinated water (NTP TR392), chloraminated water (NTP TR392), pyridine (NTP TR470) and 4-methylimidazole (NTP TR535)], MNCL was the only tumor response identified in one or both sexes. In those cases, the discussion during the formal peer review was particularly focused on MNCL. Different factors influenced the final judgment regarding equivocal evidence of carcinogenicity for MNCL. For 12 of the 16 bioassays, the MNCL incidences fell within the relevant historical control range or were only one percentage point above the upper range value. An unusually high incidence of MNCL in control and treated groups was present in the benzophenone (NTP TR533) study and an unusually low concurrent control value was present in the tris(2-chloroethyl) phosphate (NTP TR391) study. The initial NTP opinions were downgraded from some to equivocal evidence based on a highly variable background incidence for the diallylphthalate (NTP TR284) study and based on the lack of a convincing response in the opposite sex for pyridine (NTP TR470) in addition to the fact that a concurrent dose water study in the same lab had a 38% control incidence versus the 24% incidence for pyridine. These factors demonstrate that the NTP has sometimes considered the variable MNCL response in evaluating the level of evidence of carcinogenicity and that a potential MNCL response in only one sex is less convincing than a response present in both sexes. For the 3,3′-dimethylbenzidine dihydrochloride (NTP TR390) study terminated at 14 months, equivocal evidence of carcinogenicity for MNCL is questionable due to the relatively short duration of the study.
Table 7.
Occurrence of MNCL in NTP studies with at least one sex classified as equivocal evidence of carcinogenicity.
| Two-year study highlights | Overall MNCL incidencesa | MNCL historical control data | Authors’ commentary |
|---|---|---|---|
| Acetaminophen (NTP TR 394) | |||
| Survival was similar among all groups in an acetaminophen 2-year study started in December 1982 at dietary concentrations of 0, 600, 3000 and 6000 ppm. The incidence of MNCL was increased in female acetaminophen groups. There was no evidence of carcinogenicity in male rats or in B6C3F1 male and females. | F 9/50 (18%), 17/50 (34%), 15/50 (30%), 24/50 (48%)M 27/50 (54%), 26/50 (52%), 24/50 (48%), 20/50 (40%) | F 16.5% ± 7/9% (range 6–28%) (Lab)F 20.8% ± 8.1% (range 6–40%) (NTP)M Not provided in TR | High dose MNCL response was considered equivocal evidence of carcinogenicity in females during NTP formal public peer review due to uncertainty that increased incidence was due to acetaminophen exposure. Lack of leukemic response in other rat studies including a F344 rat study with acetaminophen exposures up to 13,000 ppm is noted in NTP Technical Report 394. |
| Alpha-Methylstyrene (NTP TR 543) | |||
| This inhalation study was started in 2001. There were increased kidney tumors in males with equivocal evidence of carcinogenicity for MNCL. No increase in tumors was seen in females. B6C3F1 mice had increased liver tumors. | F 18/50 (36%), 21/50 (42%), 21/50 (42%), 22/50 (44%)M 26/50 (52%), 32/50 (64%), 29/50 (58%), 38/50 (76%) | F Not provided in TRM 47.1% ± 10.3% (range 32–66%) | No recorded comments on MNCL were documented during the formal public peer review where the focus was on kidney tumors. The male MNCL response is primarily a high-dose effect. |
| Ampicillin trihydrate (NTP TE 318) | |||
| This corn oil gavage study was started in 1980. There was good survival in both sexes and in mice. There was equivocal evidence of carcinogenicity in male rats based on pheochromocytomas and a marginal increase in MNCL. Female rats and B6C3F1 mice had no evidence of carcinogenicitiy. | F 14/50 (28%), 18/50 (36%), 13/50 (26%)M 5/5 (10%), 14/50 (28%), 13/50 (26%) | F Not provided in TRM 14% ± 8% (range 2–28%) (NTP) | There was considerable discussion regarding the MNCL during the formal publc peer review raising concern about how to classify the MNCL response. Ultimately a consensus vote supported equivocal evidence of carcinogenicity for the male rats. It is noted that the incidences of MNCL in males are within the historical control range. |
| Benzophenone (NTP TR 533) | |||
| In this dosed-feed study started in 1999, there was early mortality in high-dose males. There was an increase in renal tubular tumors in males. MNCL was considered equivocal evidence of carcinogenicity in both sexes. Mice had liver tumors (males) and histiocytic sarcoma (females). | F 19/50 (38%), 25/50 (50%), 30/50 (60%), 29/50 (58%)M 27/50 (54%), 41/50 82%), 39/50 (78%), 24/50 (48%) | F 24.6% ± 9.5% (range 12–38%)M 49.1% ± 11.9% (range 30–68%) | The extent of organ involvement with MNCL increased with dose in females and decreased with dose in males. It was noted during the formal public peer review that the incidences of MNCL in control and treated rats was unusually high. |
| Chlorinated paraffins (NTP TR 308) | |||
| This corn oil gavage was started in 1980. There was decreased body weight and survival in male and female rats. Liver, thyroid and kidney tumor responses were present in both sexes of rats and B6C3F1 mice. | F 11/50 (22%), 22/50 (44%), 16/50 (32%)M 7/50 (14%), 12/50 (24%), 14/50 (28%) | F 12% ± 6% (range 4–20%) (Lab)18% ± 9% (range 4–42%) (NTP)M 6% ± 6% (range 2–18%) (Lab)14% ± 8% (range 2–28%) (NTP) | The study was classified as clear evidence of carcinogenicity while the MNCL response was equivocal evidence of carcinogenicity with indication that it may have been related to treatment. It was recommended that the fact that the maximum tolerated dose was exceeded should be mentioned in the technical report abstract. |
| Chlorinated water (TR 392) | |||
| This drinking water study was started in 1985 with MNCL as the only tumor response attributed to treatment, seen in female rats and considered equivocal evidence of carcinogenicity. B6C3F1 mice had no evidence of carcinogenicity. | F 8/50 (16%), 7/50 (14%), 19/51 (37%), 16/50 (32%)M 25/51 (49%), 25/51 (49%), 27/50 (54%), 29/51 (57%) | F 25% ± 6.1% (range 14–36%) (NTP Feed studies)26% ± 8.5% (range 16–33%) (NTP drinking water studies)M Not provided in TR | There was considerable discussion during the formal public peer review with suggestions to downgrade the call to no evidence of carcinogenicity. However, that motion was defeated in the final voting and equivocal evidence of carcinogenicity was listed in the technical report. |
| Chloraminated water (TR 392) | |||
| This drinking water was started in 1985 and run along with the chlorinated water study (same TR 392). MNCL in female rats was the only response and was called equivocal evidence of carcinogenicity. | F 8/50 (16%), 11/50 (22%), 15/50 (30%), 16/50 (32%)M 25/51 (49%), 26/50 (52%), 29/51 (57%), 30/50 (60%) | F 25% ± 6.1% (range 14–36%) (NTP Feed studies)26% ± 8.5% (range 16–33%) (NTP drinking water studies)M Not provided in TR | The study is even less convincing than the chlorinated water study with all responses falling within the historical control range. A concern is with the high and variable incidence of MNCL in historic controls and the fact that study incidences were within the historic control range. The final call was equivocal evidence of carcinogenicity for MNCL in female rats. |
| C.I. Acid Red 114 (NTP TR 405) | |||
| This dose water study started in 1983 had early mortality due to multiple tumor sites in both sexes. MNCL in female was listed as an uncertain finding (equivocal evidence) regarding carcinogenicity. No mouse study was done. | F 12/50 (24%), 13/35 (37%), 18/65 (28%), 5/50 (10%)M 20/50 (40%), 20/35 (57%), 37/65 (57%), 12/50 (24%) | F 25% ± 6.3% (range 14–36%)M Not provided in TR | Most of the discussion during the peer review indicated a consideration that MNCL should be listed as a tumor response. Hence, it was listed as uncertain (equivocal evidence) evidence of carcinogenicity. It is noted that the incidences fall within or are just above (37% versus 36%) the historical control range. Early mortality was due to toxicity and not MNCL. |
| Diallylphthalate (NTP TR 284) | |||
| There was good survival in this rat only corn oil gavage study started in 1980. No evidence of carcinogenicity in male rats. | F 15/50 (30%), 15/43 (35%), 25/49 (51%)M 13/50 (26%), 12/50 (24%), 14/50 (28%) | F 29% ± 13% (range 16–42%) (Lab)16% ± 9% (range 2–42%) (NTP)M Not provided in TR | This study was called some evidence of carcinogenicity based on MNCL but was downgraded to equivocal evidence during the formal public peer review based on variability in the control incidence and difficulty in definitively diagnosing this lesion. |
| 3,3′-Dimethylbenzidine dihydrochloride (NTP TR 390) | |||
| This was a drinking water study started in 1983 that lasted only 14 months due to mortality. Multiple tumor sites in male and female rats were documented. There was no concurrent B6C3F1 mouse study. | F 1/60 (2%), 3/45 (7%), 6/75 (8%), 4/60 (7%)M 0/60 (0%), 1/44 (2%), 1/75 (1%), 1/60 (2%). | F No historical control data for 14-month intervalM No historical control data for 14-month interval. | The frequency of MNCL is low since it is a late occurring disease in control as well as in treated rats. However, the TR speculates that MNCL may have been related to treatment (equivocal evidence of carcinogenicity) based on its possible early occurrence in treated females. |
| Dimethyl methylphosphonate (NTP TR 323) | |||
| This corn oil gavage study was started in 1981. Significant weight loss and decreased survival were present at the high-dose with clear evidence of carcinogenicity based on renal tumors in male and no evidence of carcinogenicity in female rats. The male B6C3F1 mouse study was inadequate and the female mice had no evidence of carcinogenicity. | F 10/50 (20%), 9/50 (18%), 12/50 (24%)M 10/50(20%), 11/50 (22%), 17/50 (34%) | F Not provided in TRM 19% ± 9% (range 4–28%) (Lab)14% ± 8% (range 2–28%) (NTP) | The increased incidence of MNCL in males was not considered part of the clear evidence call as noted during the formal public peer review and would probably fall into the category of equivocal evidence of carcinogenicity but was not indicated in the technical report. The majority of the MNCL cases were at stage 3. |
| Indium Phosphide (NTP TR 499) | |||
| This inhalation study was started in 1996 producing lung and adrenal tumors in both sexes. MNCL was an uncertain finding in male and female rats. Lung and liver tumors were seen in treated B6C3F1 mice. | F 14/50 (28%), 21/50 (42%), 14/50 (28%), 24/50 (48%)M 16/50 (32%), 23/50 (46%), 29/50 (58%), 25/50 (50%) | F 29.1% ± 8.5% (range 16–42%)M 43.5% ± 9.6% (range 32–54%) | There was no discussion of MNCL during the peer review. The TR report commented that the occurrence of MNCL in both sexes suggests equivocal evidence of carcinogenicity. |
| 4-Methylimidazole (NTP TR 535) | |||
| This dosed feed study started in 2000 and there was equivocal evidence of carcinogenicity in female rats based on MNCL as the only response in rats. B6C3F1 mice had increased lung tumors. | F 9/50 (18%), 7/50 (14%), 16/50 (32%), 20/50 (40%)M 15/50 (30%), 18/50 (36%), 22/50 (44%), 20/50 (40%) | F 23.8% ± 9.1% (range 12–38%)M 46.8% ± 13.0% (range 30–68%) | MNCL was discussed during the formal public peer review and ultimately considered equivocal evidence of carcinogenicity. The female MNCL response was one tumor outside the control range. |
| Methyl isobutyl ketone (NTP TR 538) | |||
| This inhalation study started in 2000 resulted in kidney tumors in males and possible kidney tumors in females. Males had equivocal evidence of MNCL. B6C3F1 mice had increased liver tumors. | F 14/50 (28%), 21/50 (42%), 12/50 (24%), 16/50 (32%)M 25/50 (50%), 26/50 (52%), 32/50 (64%), 35/50 (70%) | F Not provided in TRM 47.1% ± 10.3% (range 32–66%) | No recorded comment on MNCL during the formal public peer review. |
| Pyridine (NTP TR 470) | |||
| This is a dosed water study started in 1991 with some evidence of carcinogenicity in males based on kidney tumors and with MNCL as the only response in females. Liver tumors were increased in male and female B6C3F1 mice. | F 12/50 (24%), 16/50 (32%), 22/50 (44%), 23/50 (46%)M 29/50 (58%), 32/50 (64%), 26/50 (52%), 27/50 (54%) | F 30.9% ± 10.0% (range 16–44%)M Not provided in TR | Female incidences of MNCL were mostly within historical control range with the high-dose just outside that range. The control incidence in a concurrent dosed water study at the same lab was 38%. |
| Tris(2-chloroethyl) phosphate (NTP TR391) | |||
| Survival in high dose males and females was reduced in this 2-year corn oil gavage study. Renal tubular neoplasms were increased in both sexes. Slight increases in MNCL and thyroid follicular neoplasms (equivocal evidence) were seen in both sexes. There was an interim sacrifice at 66 weeks. Male B6C3F1 had a marginal increase in neoplasms. | F 14/50(28%), 16/50(32%), 20/50(40%)M 5/50(10%), 14/50(28%), 13/50(26%) | F 15.3 ± 10.6%% (range 0–38%) (NTP)M 14.9 ± 10.8% (range 0–44%) (NTP) | NTP concluded clear evidence of carcinogenicity based on renal neoplasms and equivocal evidence based on MNCL using a life table statistical test. In males, there was a lower than expected control incidence of MNCL. In females, the MNCL incidence was marginal and restricted to the high dose group. |
TR: NTP Technical Report; M: Male; F: Female; MNCL: Mononuclear cell leukemia.
a = Tumor incidences arranged starting with controls and progressing through increased doses.
By definition, equivocal evidence of carcinogenicity represents an uncertain judgment. This uncertainty was reflected in the discussion and debate during the formal peer review for the majority of the 16 studies with equivocal evidence of carcinogenicity for MNCL. In these studies, there were definitive treatment-related tumor responses in non-hematopoietic tissue sites. However, for 12 of the 16 studies, the MNCL response was within or only one tumor outside the relevant historical control range. When the judgment regarding MNCL carcinogenicity is uncertain, responses that fall within the historical control should get more consideration with less dependence on the implied precision of a particular statistical test.
Conclusions
Review of the natural history and characteristics of the F344 rat MNCL reveals two important features: its species- and strain-specificity and its unusually high and variable background incidence. The high and variable background incidence is present in both sexes and is in dramatic contrast to other tumor types such as hepatocellular adenomas and carcinomas (Figures 1 and 2). There is sufficient evidence from the literature documenting that MNCL is a distinct form of LGL leukemia that is highly prevalent in both sexes of F344 rats and extremely rare in other stocks and strains of laboratory rats. Furthermore, the only potential human LGL counterpart is an extremely rare but aggressive leukemia that, unlike the F344 MNCL, has a viral etiology. Thus, the evidence indicates that MNCL, a spontaneous tumor that occurs at high incidence in aging F344 rats, is distinct from human LGL and, therefore, MNCL data should not be used in assessing potential human health hazards.
Examination of 66 NTP F344 rat studies reveals 26 studies with significant and dramatic treatment-related decreases in the incidence of MNCL. The majority of these studies had tumor responses in non-hematopoietic tissue sites and often some indication of splenic toxicity in prechronic studies. Of the 40 studies with potential chemical treatment-related increases in MNCL, two were ultimately considered to not be carcinogenic and 38 had differing levels of evidence of carcinogenicity based on NTP determination with confirmation by peer review. For many of these 38 studies, there was discussion and debate during formal peer review indicating that for most studies with clear or some evidence of carcinogenicity for MNCL, the treatment-associated increases fell within an appropriate historical control range. While in conventional toxicology practice the concurrent control is the most appropriate comparator for assessing typical tumor responses, the high background incidence levels, variability and unpredictable nature of this tumor type makes it difficult to differentiate between a true positive and a false positive (Figure 2). Due to these multiple biological factors that impact the background incidence of MNCL, we contend that analyses of F344 rat MNCL warrant more reliance on historical control data than is usual for most tumor responses. The traditional statistical approach may have an increased Type I error rate due to the biology of this unique leukemia.
Leydig cell tumors
Features of Leydig cell (LC) proliferative lesions
The two most common proliferative lesions of the testes in the F344 rats are the Leydig cell (LC) hyperplasia and LC adenoma. These tumors are age-related and appear spontaneously in this strain, and their incidence approaches 100% in 18–24-month-old F344 rats. LCTs are infrequent and less severe in the Wistar and Sprague–Dawley rats (Creasy et al. 2012), with less than 2% incidence rate reported. Nevertheless, according to Creasy et al. (2012), the incidence of this type of tumor in different strains of Wistar rats may reach 40%, depending upon the commercial source providing the rats. LCTs in rodents are almost always benign and malignancy is rarely reported (Boorman et al. 1990; Cook et al. 1999). Nolte et al (2011), who reviewed the spontaneous occurrence of LC adenomas in F344, Sprague–Dawley and Wistar rats, found only a single case of LC carcinoma in a Wistar rat.
The distinction between LC hyperplasia and adenoma is difficult, since LC adenoma is considered as a continuum of the hyperplasia, and it is not possible to distinguish between these two based on cellular characteristics alone (Steinbach et al. 2015). Therefore, when discussing historical data dealing with LC adenoma, it is important to describe the methodology used to define the adenoma.
The main morphological features of LC hyperplasia are the presence of focal, multifocal and diffuse collections of LCs between the seminiferous tubules, with no or minimal compression of the surrounding tissue. Most importantly, in the case of focal hyperplasia, the diameter of the lesion is smaller than or equal to three seminiferous tubules. The recently published INHAND criteria emphasized that an additional criterion for distinguishing between “normal” and LC hyperplasia is the degree of demarcation of the focal lesion from the surrounding tissue (Creasy et al. 2012).
Leydig cell (LC) adenoma is a proliferative lesion exceeding the diameter of three seminiferous tubules, usually with peripheral compression of adjacent seminiferous tubules. LC adenomas may contain cystic areas and less differentiated areas composed of either basophilic cells with scanty cytoplasm or elongated spindle-shaped cells.
Chemically induced proliferative LC lesions
The high incidence of LCTs in the F344 rat reflects hormone imbalance between testicular LH receptor levels and serum testosterone (Turek & Desjardins 1979). Cook et al. (1999) described five mechanisms by which chemicals can influence hormone balance and disrupt the hypothalamic–pituitary–testes (HPT) axis leading to LC hyperplasia and tumors. These include agonists of estrogens, GnRH and dopamine receptors, androgen receptor antagonists, and inhibitors of 5α-reductase. They concluded that the agents associated with LC proliferation disturb hormonal homeostasis by endocrine, paracrine or autocrine mechanisms, leading to LC growth and/or inhibition of LC death. Of interest, it appears, that genotoxic compounds that induce LCs also operate via disruption of hormonal homeostasis, however whether that is due to DNA-reactive agents initiating LCs with LH then promoting tumor development, or via an unidentified hormonal mechanism unrelated to their genotoxic properties cannot be concluded. What is important is differentiating between genotoxic and non-genotoxic compounds since genotoxic agents, which produce LC hyperplasia or LCTs, would be considered relevant to humans. The distinction is that site concordance is not essential for genotoxic compounds as this mechanism can produce alternative tumor types in humans. However, the hormonal disruption cascade by non-genotoxic compounds that leads to LCTs in rodents is unique to rats. Initiation of a similar cascade would not occur in other organ types, and this mechanism in rats is not relevant to humans. Therefore, the induction of LCTs in F344 is not relevant for prediction of LCTs in humans and is not a mechanism that would be anticipated to produce alternative tumor types in humans.
Factors influencing the spontaneous incidence of LCTs
There is a variety of diverse factors associated with the variable incidence of LCTs. Nolte et al. (2011) used the Registry of Industrial Toxicology Animal-data (RITA) database, which included 7453 male rats from different strains, in order to analyze the variables that may influence the spontaneous formation of LCTs. Some of the factors from analysis of the RITA database and from other publications are discussed below.
Strain and breeder
Commercial breeder-dependent differences in the spontaneous incidence of LC adenoma were seen in two F344 rat studies with LC adenoma incidences of 76% for one animal source and 90% for the other (Nolte et al. 2011). Similar breeder-related differences were also present in SD rats with incidence rates between 2.3 and 6.3%. In the Wistar rat, the mean incidence of LC adenoma was 39.9% for one breeder, while in other commercial sources of Wistar rats the incidences ranged between 2.8 and 12.5%. Taken together, these observations indicate that animal strain is the most relevant factor in influencing LCT incidences with commercial sources having a secondary but significant influence on LCT incidences for a given rat stock or strain.
Dependence on body weight
There is strong evidence that increased body weight and associated increases in prolactin serum levels reduce LCT incidence in the rat (Sharpe & McNeilly 1979; Nolte et al. 2011). However, Nolte et al. (2011) pointed out that there were no obvious differences in the mean terminal body weight between animals with or without LC adenomas in the RITA database.
Age dependence
The incidence of LCTs is age dependent (Nolte et al. 2011). Occurrence is rare in the first year of life but LCTs are frequently observed in the male F344 rat in the 2nd year. In any case, LCTs are generally not fatal and, therefore it was concluded that their presence does not reduce the survival.
Dependence on administration route
An analysis evaluating the correlation between the route of administration and LCT incidence for specific commercial breeders revealed a higher incidence in studies with dietary admixture compared to gavage administration (Nolte et al. 2011). Rather than a direct effect of diet, it was suggested that the effect was dependent on year of study start given that dietary studies were conducted, on average, 6–13 years earlier than the gavage studies. Of importance here was the negative correlation between year of study start and LCT incidence for both breeders used in the analyses. Similarly, there was slightly but consistently improved survival in NTP feed studies relative to NTP inhalation studies, regardless of using either the NIH-07 or NTP-2000 diets (Haseman et al. 2003). Ultimately, the increased survival may influence the increased incidence of LCT reported in the feeding studies.
Individual caging versus group caging
Analysis of the RITA database did not provide a definite conclusion regarding the potential influence of the caging parameter on tumor incidence. However, Haseman et al. (1997, 2003), who analyzed data from 22 separate 2-year feed and inhalation studies, found that differences in animal care and housing protocols may be a major factor contributing to the observed differences in LCT incidence rates. Their analysis found a relatively high incidence of pituitary gland tumors and a decreased incidence of LCTs in NTP dermal and inhalation studies (which use individual housing) compared to NTP gavage and feeding studies (which use group housing). A link between the number of animals per cage and stress levels has been proposed, with singly housed animals having a higher degree of stress (Nyska et al. 2002). The single-housed animals in the NTP dermal and inhalation studies would have higher stress levels leading to increased serum corticosteroids, which can impair testosterone synthesis by LCs (Nyska et al. 1998; Ben-Eliyahu et al. 1999; Nyska et al. 2002).
Effect of sexual activity
Weisburger et al. (2002) investigated whether breeding was a possible protective factor against LCT development. Comparing virgins versus sexually active rats, the authors concluded that functionality of the testicular apparatus does not seem to influence the occurrence of this neoplasm in F344 rats, apparently contrary to some observations made 70 years earlier (Dunning & Curtis 1946).
Human Leydig cell tumors
In general, human testicular cancers are rare, representing 1% of all human cancers, with ∼52,000 new cases of testicular cancers estimated worldwide in 2008 (Purdue et al. 2005; Le Cornet et al. 2014). Although LCTs are the most common non-germ cell testicular tumors (Cheville 1999), they comprise only 1–3% of all primary testicular tumors, the majority of human testicular tumors having a germ cell origin (Kim et al. 1985; Vukina et al. 2015). In children, testicular tumors are rare; however, the relative prevalence of LCTs is much higher than in adults, reaching 8% of primary testicular tumors (Vukina et al. 2015).
LCTs can be diagnosed at any age, but they have two age peaks: between 5 and 10 years, which is in contrast to the rarity of the tumor in rats younger than 1 year, and in adulthood, between 20 and 60 years (Fernandez et al. 2004).
Almost all the cases present as a unilateral and unifocal mass, representing a discrete solid tumor that displaces the bordering parenchyma (Kim et al. 1985). In most of the cases, the tumors are very small, measuring less than 1 cm in diameter (Maizlin et al. 2004). When the size is greater than 5 cm in diameter, malignancy should be strongly suspected (Kim et al. 1985). Sometimes, increased endocrine function of the tumor cells can result in clinical signs and symptoms, such as precocious puberty or gynecomastia. However, clinical symptoms are not observed very commonly (Ozyavuz et al. 1993; Gana et al. 1995), and are estimated to be present in only 30% of LCT patients (Woodward et al. 2002).
A strong risk factor for testicular cancers in humans is undescended testis, testicular dysgenesis syndrome and infertility (Giwercman et al. 1987; Hoei-Hansen et al. 2003; Skakkebaek et al. 2003). Indeed, malignancy occurs in 3.5–14.5% of undescended testis (Maqdasy et al. 2015), although seminoma is the most prevalent type of cancer in these cases (Trabert et al. 2013). Nevertheless, a clear association has not been found between LCTs and undescended testis, and the etiology of LCTs in humans is still largely unknown (Tsitouridis et al. 2014).
Macroscopically, LCTs are well-defined solid tumors, which occasionally demonstrate lobulated masses. They have a variable coloration, ranging from yellow to brown or gray-white, depending on the lipid content (Kim et al. 1985; Tsitouridis et al. 2014). Histopathologically, these tumors are characterized by sheets or septated lobules of minimally pleomorphic low N:C ratio cells. They have an eosinophilic cytoplasm, cytologically bland nuclei and prominent nucleoli (Kim et al. 1985; Vukina et al. 2015). Zona fasciculata-type vacuolated cells are occasionally seen inside the tumor, and can sometimes even be the predominant cells (Kim et al. 1985). Other features that have been described less commonly include spindle cell cytology, adipose metaplasia and osseous metaplasia (Ulbright et al. 2002). While Reinke crystals are considered a characteristic finding in LCTs, they are present in only the minority of the cases (Kim et al. 1985). Sometimes a thin fibrous capsule can be seen separating the tumor from the compressed normal testicular tissue (Unluer et al. 1990). Almost all of the cells of LCTs in humans will be positive for inhibin, Mart-1 and calretinin stains (Vukina et al. 2015).
Similar to rats, human LCTs are generally benign (Farkas et al. 2000). Therefore, some studies suggest conservative or testis-sparing surgery instead of orchidectomy, especially in young patients with a single testicle (Masoudi et al. 1999; Giannarini et al. 2010). While in children LCTs are always benign, in adults approximately 10% have a malignant phenotype with metastatic potential, especially in older men (median age of 62.1 years) (Gonzalez et al. 2007; Hendry et al. 2015). The distinction of malignant potential is based on specific histopathological characteristics that include: large tumor size (>5 cm), cytological atypia, increased mitotic activity, increased MIB-1 expression, necrosis, vascular invasion, infiltrative margins, extension beyond the testicular parenchyma and DNA aneuploidy (Albers et al. 2011). The most common sites of metastasis are the regional lymph nodes, followed by the liver, lungs and bone (Kim et al. 1985; Tsitouridis et al. 2014).
In general, LCTs in humans share many similarities to their counterparts in rats, having a relatively benign nature and similar morphologic and clinical characteristics. Nevertheless, in humans, the tumor is very rare, in contrast to the much higher prevalence in F344 rats. Furthermore, in humans, LCT has two age peaks, while in F344 rats there is a gradually increasing incidence with age.
NTP studies with an LCT response
Features of seven NTP studies in F344 rats that have an increased LCT response to treatment are summarized in Table 8 and statistical analyses of these LCT responses are provided in Supplemental Table S2. Of these seven studies, one had clear evidence of carcinogenicity for LCTs (Isoprene, NTP TR486) and the remaining six were judged to have equivocal evidence of carcinogenicity for LCTs. For five of the seven studies, the route of administration was inhalation, with this relatively high frequency of association possibly linked to the stress of single cage housing as discussed previously (i.e. the single caging environment ultimately increases the power of the inhalation studies to detect treatment related effects). Interestingly, in all five of these inhalation studies a kidney tumor response was reported, but a biological connection between the two responses is not apparent. In the case of kava kava extract (NTP TR571), a corn oil gavage study, the equivocal evidence of carcinogenicity represents the only tumor response in the study.
Table 8.
NTP studies that had a Leydig cell tumor response.
| Two-year study highlights | Overall incidencesa | Historical control data | Authors’ commentary |
|---|---|---|---|
| Cumene (NTP TR 452) | |||
| Inhalation exposure to cumene was started in June 2001. There was clear evidence of carcinogenicity for nose and kidney and equivocal evidence of carcinogenicity for LCT. Lung, spleen and thyroid tumor responses were present in male B6C3F1 mice. | 36/50 (72%), 38/50 (76%), 40/50 (80%), 46/50 (92%) | Mean 76.8% ± 5.9% (range 66–84%) | The judgment that the LCT response represents equivocal evidence of carcinogenicity is reasonable in that there is a clear dose response. Furthermore, the incidence of LC hyperplasia was increased in the low- and intermediate-dose groups, and the severity was increased in the high-dose group. |
| Ethylbenzene (NTP TR 466) | |||
| This inhalation study was started in March 1990 and had a reduced survival in high-dose males. There was clear evidence of carcinogenicity based on kidney neoplasms. The Leydig cell tumor response was also listed as clear evidence. Male B6C3F1 mice had an increase in lung tumors. | 36/50 (72%), 33/50 (66%), 40/50 (80%), 44/50 (88%) | Mean 68.7% ± 8.7% (range 54–83%) | An increase incidence of unilateral and bilateral a Leydig cell tumors was slightly above the historical control in the highest concentration. There was no treatment-related increase in Leydig cell hyperplasia. At best, the Leydig cell tumor response should more appropriately be consistent with equivocal evidence of carcinogenicity |
| Isoprene (NTP TR 486) | |||
| This inhalation study was started in June 1993. Clear evidence of carcinogenicity was identified for mammary and kidney tumors in addition to Leydig cell tumors. There was no accompanying mouse carcinogenicity study. | 33/50 (66%), 37/50 (74%), 44/50 (88%), 48/50 (96%) | Mean 69.4% ± 9.7% (range 46–83%) | Although the concurrent control is unusually low, the mid- and high-dose responses are robust. Leydig cell proliferation was present in a previously published 26-week study supporting the clear evidence of carcinogenicity call for the Leydig cell tumor response. |
| Kava Kava (NTP TR 571) | |||
| Kava kava extract was administered by corn oil gavage in a study that started in August 2004. There were no tumor responses in male, aside from the equivocal evidence of carcinogenicity for LCTs. Male B6C3F1 mice had an increase in liver hepatoblastomas. | 37/49 (76%), 44/50 (88%), 49/50 (98%), 46/50 (92%) | Mean 88.4% ± 8.6% (range 76–94%) | The equivocal evidence of carcinogenicity for LCTs in this study was associated with a reduced latency and a dose-related decrease in Leydig cell hyperplasia. These observations suggest early conversion of hyperplasia to adenoma. It is noted that the control incidence is the lowest recorded for corn oil gavage studies. |
| Leucomalachite Green (NTP TR 527) | |||
| The dietary study of leucomalachite green was started in November 1998. Treatment in males was associated with equivocal evidence of carcinogenicity for thyroid and Leydig cell tumors with treatment-related decreases in pituitary adenomas and MNCL. There was no accompanying mouse study. | 37/48 (77%), 42/47 (89%), 43/48 (90%), 45/47 (96%) | Mean 85.7% (range 69–90%) | The incidence of bilateral interstitial cell tumors was increased in treated rats removed from study prior to the terminal sacrifice. The call of equivocal evidence of carcinogenicity for ICT is reasonable. |
| Tetrafluoroethylene (NTP TR 450) | |||
| This inhalation study, started in June 1988, resulted in clear evidence of liver and kidney carcinogenesis and equivocal evidence of carcinogenesis for MNCL in males. There was reduced body weight and early mortality at the highest concentration. Bothe sexes of treated B6C3F1 mice had liver neoplasms. | 39/50 (78%), 40/50 (80%), 48/50 (96%), 47/50 (94%) | Mean 68.7% ± 8.7% (range 54–83%) | The Leydig cell tumor response may have been related to treatment (equivocal evidence of carcinogenicity) but it is noted that the control incidence is within the historical control range. There was no treatment-related increase in Leydig cell hyperplasia or adenoma at a 15-month interim sacrifice. |
| Tetralin (NTP TR 561) | |||
| This inhalation study started in June 2003. There was some evidence of carcinogenicity based on kidney tumors and equivocal evidence of carcinogenicity for LCTs. There was no evidence of carcinogenicity in male B6C3F1 mice. | 29/50 (58%), 39/50 (78%), 31/50 (62%), 41/50 (82%) | Mean 71.7% ± 8.5% (range 58–84%) | There was some discussion during the formal public peer review where it was pointed out that all of the LCT responses are within the historical control range. The judgment that the response is equivocal evidence of carcinogenicity for LCTs is based primarily on statistical evaluation. Since the concurrent control incidence of LCTs is the lowest observed in inhalation studies, the judgment of equivocal evidence of carcinogenicity for LCTs is questionable. |
TR: NTP Technical Report; M: Male; F: Female; LCT: Leydig cell tumor; MNCL: Mononuclear cell leukemia.
a = Tumor incidences arranged starting with controls and progressing through increased doses.
The background incidence of LCTs in the F344 rat is exceptionally high, making it practically impossible to assess testicular carcinogenic potential using this strain of rat (Figure 1(a)). The NTP historical control rate of LCTs has been constant over time ranging from 54% to as high as 98%. The fact that five of the seven NTP studies with at least an equivocal treatment-related LCT response are from inhalation studies is noteworthy because the spontaneous incidence of LCTs in inhalation study controls is lower than in other routes of test article administration, thereby allowing identification of potential treatment-related increases based on statistical evidence (Figure 1(b)). This supports that strains with high background incidences of tumor types do not have enough statistical power to detect effects, using common bioassay protocols. To obtain robust data one would need to either increase the animal numbers used or switch the strain of animal used.
It is of interest to note that in one study male F344 rats as well as male Wistar rats were exposed to pyridine (NTP TR470), and there was equivocal evidence of carcinogenicity for LCTs in the Wistar but not the F344 rat. Since the control group incidence of LCT was 85% in the F344 rat study, the lack of evidence for LCT carcinogenicity in the F344 rat study is possibly due to the reduced power of the F344 rat to detect true positives due to the high background incidence.
Taken together, it is obvious that analysis of LCT data taken from NTP studies should be judged very carefully. First, the background incidence of LCT in historical controls can reach 100% in some cases; second, there is a proposed link between cage-related stress conditions in inhalation studies and lower incidence of LCT that confounds determinations of chemical influence; and, finally, the mechanism for LCT induction in rats differs from the mechanism for induction of LCTs in humans. Thus an increased frequency of LCTs in F344 rats is not predictive of LCT induction in humans or useful for human health risk assessment. The mechanism of LCT induction in rats by non-genotoxic compounds is a testis-specific process that does not occur in humans, and, therefore, is not anticipated to be relevant for other potential tumor types in humans.
Conclusions
The high spontaneous incidence of LCTs in F344 rats has made this bioassay model of little practical use in identifying potential testicular carcinogenic responses. Inhalation studies may be a questionable exception due to a stress response in single cage housing, but even then a putative LCT response in NTP studies has ranked only as equivocal evidence of testicular carcinogenicity. In contrast to the age-related high incidence in F344 rats, human LCTs are rare and have two age peaks of occurrence. Thus, the F344 LCT is not relevant to human testicular cancer, except possibly for the rare cases of men with genetic susceptibility to develop LCTs, such as individuals harboring somatic mutations in the LHCG-R or GNAS genes or germline mutations in FH (Libé et al. 2012). We believe that use of the F344 rat strain has long been recognized as not being useful for identification of testicular carcinogens and applaud the NTP for finally switching to a different rat for toxicity and carcinogenicity studies.
Tunica vaginalis mesothelioma
Features and pathogenesis of tunica vaginalis mesotheliomas in F344 rats
An extensive published review of TVM induction in rats is available (Maronpot et al. 2009). Spontaneous TVM in the rat is an age-associated tumor with background incidences ranging from 0.2% to 5%, and with most descriptions in the literature based on observations in F344 rats. Histomorphological features of tunica vaginalis and peritoneal mesotheliomas are similar in all species, including humans (Ilgren & Wagner, 1991; Ilgren, 1993). In rats, these tumors typically start as single-layered collections of hyperplastic mesothelium overlying a thin fibrovascular stroma initially localized in the tunica vaginalis. With continued growth they can form small papillary projections of hyperplastic mesothelium as well as multilayered sheets and nests of cuboidal to polygonal cells with round nuclei and single prominent nucleoli. The mesothelioma growths can form glandular and tubular structures, can have a sarcomatous phenotype, and may form cystic structures lined by flattened mesothelium. Rat TVMs may be classified as epithelial, sarcomatous or mixed, consistent with mesothelioma classification in humans. Implant metastasis, induced by transcoelomic spread from the primary tumor in the tunica vaginalis, can be seen throughout the peritoneal cavity. Features of malignancy include pleomorphism, cytological atypia and local invasiveness but even without these features TVMs are generally regarded as malignant even if confined to the scrotal sac. Immunohistochemical features of mesotheliomas are useful in differentiating them from adenocarcinomas (Maronpot et al. 2009). Pathological features of treatment-associated TVM in the F344 rat are similar to spontaneous cases but generally have a reduced latency and greater extension into the peritoneal cavity. They are generally less invasive and less pleomorphic than mesotheliomas associated with exposure to asbestos or nanotubes (Maronpot et al. 2009).
Most of what is known about TVMs is from the uniquely sensitive F344 rat. Both spontaneous and xenobiotic-induced TVMs are closely associated with and most likely secondary to LCTs, a common spontaneous tumor in F344 rats. Hormone imbalance is a likely key event associated with both spontaneous and treatment-induced TVMs (Turek & Desjardins 1979; Tanigawa et al. 1987; Shipp et al. 2006). The high incidence of LCTs in the F344 rat also reflects hormone imbalance between testicular LH receptors levels and serum testosterone and is potentially causally linked to development of TVMs in this rat (Turek & Desjardins 1979). One hypothesis is that the hormone imbalance associated with LCTs exposes the tunica vaginalis compartment to transudates containing altered androgen levels leading to release of growth factors, mesothelial mitogenesis and development of TVM (Karpe et al. 1982; Gerris & Schoysman 1984). An alternative hypothesis implicates mechanical pressure from the LCTs, leading to tunica vaginalis mesothelial cell expression of autocrine growth factor stimulating mitogenesis (Gerwin et al. 1987; Tanigawa et al. 1987; Versnel et al. 1988). In response to shearing forces and pressure from an adjacent testis enlarged by the presence of a LCT, mesothelial cells produce autocrine growth factors that lead to mitogenesis.
Although hormone imbalance and mechanical pressure are likely key events in the genesis of TVM, xenobiotics or their metabolites can potentially reach the tunica vaginalis mesothelium and directly or indirectly lead to mesothelial mitogenesis and development of TVM. Oxidative stress secondary to reactive oxygen species is considered an important mediator of asbestos-associated mesotheliomas and could play a role in the development of TVM (Attanoos & Gibbs 1997; Schurkes et al. 2004). Cell cycle alterations in testicular mesothelium have been seen following subchronic exposure to acrylamide (Lafferty et al. 2004), a known xenobiotic associated with induction of TVM in F344 rats.
In addition to 17 NTP studies with a treatment-related increased incidence in TVM, there are reports in the literature that additional xenobiotics have caused TVM in F344 rats from different commercial colonies (Maronpot et al. 2009). This observation reflects the sensitivity of the F344 rat from different commercial sources to development of TVM. The strain specificity of the F344 rat to develop TVM following xenobiotic exposure by other than a peritoneal injection route is unique to the F344 rat and is not seen in other strains or stocks of rats, even following sustained increased LH levels (Prentice et al. 1992). Furthermore, in hazard identification studies conducted by NTP and others with parallel studies in mice, peritoneal mesothelioma responses have never been seen in mice or in female rats.
TVM in humans
Just like in rats, the tunica vaginalis in humans is of mesothelial origin, similar to the pleura, peritoneum and pericardium. It is derived from the processus vaginalis, an outpouching of the peritoneum, which descends to cover the testis in the scrotum. Unlike in the rat, the cranial end of the processus vaginalis closes, forming a closed cavity, which is not connected to the peritoneal cavity (Woodward et al. 2003; Hassan & Alexander 2005; Garriga et al. 2009; Maronpot et al. 2009). Several mesothelial lesions can arise from the paratesticular area, and include reactive mesothelial hyperplasia, mesothelial cysts, adenomatoid tumor, well-differentiated papillary mesothelioma and malignant mesothelioma (Erdogan et al. 2014).
Most of the mesotheliomas in humans develop in the pleura, comprising 68–85% of all malignant mesothelioma cases. Of the remainder, 9.1–24.1% of malignant mesotheliomas cases develop in the peritoneum (Chen & Hsu 2009). TVM is the rarest mesothelioma, representing only 0.3–5% of all mesotheliomas (Chekol & Sun 2012). Since its first description in 1957 (Barbera & Rubino 1957), approximately 250 cases have been reported (Chen & Hsu 2009; Bisceglia et al. 2010; Chekol & Sun 2012; Mensi et al. 2012; Bandyopadhyay et al. 2015), and in Italy, the standardized incidence rate for TVM is 0.2 cases per million (Bisceglia et al. 2010; Marinaccio et al. 2010). Clinically, the tumor usually presents as recurrent or enlarging hydrocele, and not as a scrotal mass. Other less common presentations include inguinal hernia, epididymitis, spermatocele, testicular torsion and testicular traumatic injury (Plas et al. 1998; Hamm et al. 1999; Kato et al. 2012; Yen et al. 2012; Segura-Gonzalez et al. 2015).
While exposure to asbestos has been linked to TVM development, it is reported in only 30–40% of the cases (Guney et al. 2007; Ikegami et al. 2008). Trauma, herniorrhaphy, ionizing radiation and long-standing hydrocele are additional reported risk factors (Peterson et al. 1984; Goel et al. 2008). Like pleural mesotheliomas (Peterson et al. 1984), TVMs have been suggested to develop following chronic inflammatory condition, and recurrent epididymitis has been proposed as a risk factor for the development of TVM (Yen et al. 2012). It should be noted that while many studies have shown the presence of SV40 in pleural mesotheliomas in humans, its presence in TVM is not well documented, and was negative in several reports (Xiao et al. 2000; Erdogan et al. 2014).
A wide age range has been reported for this tumor, ranging between 7 and 91 years, with the mean age at diagnosis being 53.5 years (Boyum & Wasserman 2008; Chekol & Sun 2012). Only 10% of the cases occur in patients younger than 25 years old (Plas et al. 1998).
The molecular pathogenesis for mesothelioma in general is still not entirely clear. A homozygous deletion of the 9q21 locus is one of the most common genetic alterations found in mesothelioma (Husain et al. 2013), and recently germline mutation in BAP1 (BRCA 1 associated protein) was suggested to predispose to malignant mesothelioma (Testa et al. 2011). Additional genomic changes include losses in 1p36, 22q12 and 14q32 and gains in 5p and 7p (Kivipensas et al. 1996; Takeda et al. 2012).
Three clinicopathologic types of malignant mesotheliomas of the male genital tract have been described: diffuse tubulopapillary mesothelioma, well-differentiated papillary mesothelioma and multicystic mesothelioma (Rajan et al. 2013). Macroscopically, the tumor appears as a firm, solid, cystic, yellow or white pearly mass. Thickening of the tunica vaginalis is commonly observed, and nodules of different sizes can cover it. The tumor can invade adjacent structures, such as the testicular parenchyma, epididymis and spermatic cord (Segura-Gonzalez et al. 2015).
Microscopically, three subtypes of malignant mesotheliomas are recognized: epithelial, mesenchymal (or sarcomatous) and mixed (Hassan & Alexander 2005). Most of the tumors are of the epithelial type, with papillary, tubulopapillary and solid patterns. The mixed type is the second in frequency, and the pure sarcomatous type is very rare (Jones et al. 1995; Plas et al. 1998). The neoplastic cells are typically cuboid, with scant eosinophilic cytoplasm (Plas et al. 1998; Chekol & Sun 2012). Mesothelial hyperplasia of the tunica may sometimes be extensive, and mimic malignant mesothelioma, especially of the epithelial type. Such hyperplasia may result from stubborn or repetitive serosal injury inflammation in hydroceles and inguinal hernia sacs (Churg 2003; Lee et al. 2014). Biomarkers have been utilized to differentiate between these two conditions, including p53, Ki-67 and GLUT-1, but no antibody has had an absolute discriminatory value (Attanoos et al. 2003; Kato et al. 2007; Taheri et al. 2008; Lagana et al. 2012; Lee et al. 2013).
Immunohistochemically, TVMs show similar reaction as pleural mesotheliomas, with positive staining to calretinin antibody, Wilms tumor (WT1), epithelial membrane antigen, thrombomodulin and cytokeratin 7. The tumor is negative to cytokeratin 20 and carcinoembryonic antigen, and variable expression of cytokeratin 5/6 is evident (Amin et al. 1995; Winstanley et al. 2006; Pacheco et al. 2009).
Metastasis is reported in less than 15% of cases, and 35.3% of the patients that undergo staging lymphadenectomy do not have any tumors (Plas et al. 1998; Abe et al. 2002; Chekol & Sun 2012). The first line of treatment is radical orchidectomy, but unfortunately, local recurrence is common due to the aggressive nature of the tumor, occurring in 53% of the cases, usually within 2 years of diagnosis (Boyum & Wasserman 2008). Median overall survival ranges from 14 to 23 months in most of the series (Segura-Gonzalez et al. 2015). The most common sites for metastasis are the retroperitoneal lymph nodes, followed by inguinal and iliac lymph nodes. When distant metastasis occurs, it commonly involves the lung, liver and rarely bones (Bandyopadhyay et al. 2015). History positive for asbestos exposure is significantly associated with shorter interval before tumor recurrence (Plas et al. 1998).
Since the tunica vaginalis in the adult human does not directly connect to the peritoneal cavity, human TVMs originate only from the scrotal sac where they are locally more invasive and metastatic compared to TVM in the F344 rat (Guney et al. 2007). Furthermore, the rarity of LCTs in humans indicates an alternative mechanism for TVM induction in humans in comparison to the likely primary mode of action in the F344 rat (secondary to LCTs for TVM induction). Consequently, the specificity of the TVM response in the male F344 rat is not likely to be relevant to other species, including humans.
NTP studies with increased incidences of TVMs
Fourteen of 17 NTP studies with a treatment-related increased incidence of TVM in F344 rats had clear evidence of carcinogenicity or were considered positive for four studies completed before NTP began categorizing studies using levels of evidence of carcinogenicity (Table 9). Statistical analyses are provided in Supplemental Table S3. Thirteen studies were multi-site carcinogens with two or more additional tumor responses in tissues other than the peritoneal mesothelium while in three studies (methyleugenol (NTP TR491), o-nitrotoluene (NTP TR504) and o-toluidine hydrochloride (NTP TR153), TVM was the only tumor response. Pentachlorophenol (NTP TR 483) had some evidence of carcinogenicity for TVM with a questionable nasal tumor response. The positive TVM responses were typically clear and robust with the exception of two studies [1-bromopropane (NTP TR564) and nitrofurazone (NTP TR337)] with equivocal evidence of carcinogenicity where the TVM response was only one tumor above the upper incidence of the control range. TVMs were all considered malignant, started in the tunica vaginalis mesothelium, and usually spread to multiple sites within the peritoneal cavity. Among the 17 studies with a TVM response, there were three studies that incorporated stop exposures at 3 (o-nitrotoluene) or 12 (methyleugenol and pentachlorophenol) months, usually at a higher dose, and had increased incidences of TVM. For pentachlorophenol, the TVM response was present in the stop exposure group but not in the main study groups.
Table 9.
NTP studies that had a tunica vaginalis mesothelioma (TVM) response.
| Two-year study highlights | Overall incidencesa | Historical control data | Authors’ commentary |
|---|---|---|---|
| Acrylamide (NTP TR575) | |||
| This drinking water study was started in April 2004 and yielded multiple tumor sites (pancreas, heart, thyroid) along with TVM as clear evidence of carcinogenicity. B6C3F1 male mice had Harderian gland, lung and stomach tumors. | 2/48 (4%), 2/48 (4%), 1/48 (1%), 5/48 (10%), 8/48 (17%) | 4.8% (range 3.3–6.4%) | Although there was no debate about the TVM response during the formal public peer review, it is noted that a TVM response was seen in two other F344 rat studies (Johnson et al. 1986; Friedman et al. 1995) but not in a Wistar rat study (Maronpot et al. 2015). The F344 rat TVM response is apparently rat strain-specific. |
| 2,2-Bis(bromomethyl)-1,3-propanediol (NTP TR452) | |||
| This dietary study started in April 1986 had epithelial tumors in multiple tissue sites along with TVM. Tumor responses were clear evidence of carcinogenicity. B6C3F1 males had Harderian gland, skin and lung tumors. | 0/51 (0%), 3/53 (6%), 8/51 (16%), 9/55 (16%), 26/60 (43%) | 3% ± 2.4% (range 0–8%) | There was a clear dose related increased incidence of TVM to support the clear evidence of carcinogenicity in this study. |
| Bromochloroacetic acid (NTP TR549) | |||
| This drinking water study was started in July 2000. Large intestine tumors along with TVM are listed as clear evidence of carcinogenicity. Islet cell and liver tumors were considered equivocal evidence of carcinogenicity. B6C3F1 males had a liver tumor response. | 1/50 (2%), 5/50 (10%), 10/50 (20%), 6/50 (12%) | 3% ± 2.8% (range 0–6%) | The increased incidence of TVM in treated rats supports the clear evidence of carcinogenicity call for TVM. |
| 1-Bromopropane (NTP TR564) | |||
| There was clear evidence of carcinogenicity for intestine and skin in this inhalation study started in July 2002. Islet cell tumors and TVM were considered to be equivocal evidence of carcinogenicity. There was no tumor response in B6C3F1 male mice. | 0/50 (0%), 2/50 (4%), 2/50 (4%), 4/50 (8%) | 1.4% ± 2.2% (range 0–6%) | The equivocal evidence call for TVMs is reasonable given that this tumor is rare. The high- dose incidence just barely exceeded the historical control range. |
| Cytembena (NTP TR207) | |||
| Cytembena was given by intraperitoneal injection in this early study that was peer review in June 1980. TVMs were present in multiple organs indicating a positive carcinogenic response. The B6C3F1 mouse study was negative for carcinogenicity. | 3/50 (6%), 26/50 (52%), 26/50 (52%) | No data provided in the TR. | The increased incidence of TVM was associated with chronic peritonitis. Female rats also had peritonitis but did not have mesotheliomas. |
| 1,2-Dibromoethane (NTP TR210) | |||
| This inhalation study peer reviewed in June 1980 had high early mortality in high-dose males. The study was positive for carcinogenicity based on nasal tumors, TVMs, and hemangiosarcomas.Male B6C3F1 mice had increased lung tumors. | 0/50 (0%), 5/50 (10%), 1/50 (2%) | No data provided in the TR. | Although historical control data were apparently not available, the increased incidence of TVM in the low-dose males represented a significant increase supporting TVM as a positive carcinogenic response. |
| 2,3-Dibromo-1-propanol (NTP TR400) | |||
| This dermal study was started in 1980 and was terminated at 55 weeks due to reduced survival. There were epithelial tumors at multiple tissue sites along with an increase in TVM as clear evidence of carcinogenicity. | 0/50 (0%), 1/50 (2%), 4/50 (8%) | There were no historical control data for this 55-week duration study. | The dose-related increase in TVM, although not dramatic, should be considered in terms of the study duration and is supportive the clear evidence of carcinogenicity. |
| 3,3′-Dimethoxybenzidine dihydrochloride (NTP TR372) | |||
| This drinking water study started in 1982. TVM were considered along with epithelial tumors in multiple sites as clear evidence of carcinogenicity. There was no corresponding mouse study. | 2/60 (3%), 1/45 (2%), 7/75 (9%), 6/60 (10%) | 3% (Lab); 3% ± 3% (NTP) | The dose-related increased incidence of TVM supports the clear evidence of carcinogenicity determination. |
| 3,3′-Dimethylbenzidine dihydrochloride (NTP TR390) | |||
| This drinking water study started in 1982 had increased early mortality in all treated rats. There was clear evidence of carcinogenicity for epithelial tumors at multiple tissue sites in addition to TVM. There was no corresponding mouse study. | 0/60(0%), 0/45 (0%), 3/75 (4%), 4/60 (7%) | 3% (Lab); 2.9% ± 2.6% (NTP) | The modest but dose-related increased incidence of TVM supports the call of clear evidence of carcinogenicity. |
| Ethyl telluric (NTP TR152) | |||
| This dietary study reported in 1979 and peer reviewed at NCI had an increase in TVM as the only positive tumor response. B6C3F1 mice had Harderian gland tumors. | 0/20 (0%), 2/49 (4%), 8/50 (16%) | 2.9% (Lab) | Although the increased incidences in the treated rats were not statistically significant, the 16% incidence of TVM in the high-dose group clearly exceeded the laboratory historical control range indicating this study was positive for TVM carcinogenicity. |
| Glycidol (NTP TR374) | |||
| Glycidol was given by water gavage in 1979 and is a multisite carcinogen showing clear evidence of carcinogenicity for epithelial tumors and TVM. There was increased mortality in treated groups. B6C3F1 males had an increase of multiple epithelial tumors. | 3/50(6%), 34/50 (68%), 39/50 (78%) | 1% ± 2% for water gavage; 3% ± 3% for untreated rats. | Despite an unusually high control incidence, the dramatic increased incidence of TVM is supportive of clear evidence of carcinogenicity. |
| Methyleugenol (NTP TR491) | |||
| This gavage study used methylcellulose as the vehicle and was started in 1994. There was increased early mortality in treated groups and a 52-week stop-study. Liver tumors and neuroendocrine stomach tumors as well as an increased incidence of TVM supported clear evidence of carcinogenicity with doses up to 150 mg/kg. TVMs were also increased in the stop study (300 mg/kg). B6C3F1 mice had increased incidences of liver and neuroendocrine tumors. | 1/50 (2%), 3/50 (6%), 5/50 (10%), 12/50 (24%)Stop study:1/50 (2%), 5/50 (10%) | 1.7% ±2.2% (range 0–6%) | In addition to a treatment-related increased incidence of TVM, a higher dose for 52 weeks followed by only vehicle also had clear evidence of TVM carcinogenicity. |
| Nitrofurazone (NTP TR337) | |||
| This dietary study started in 1981 had increased mortality in the high dose. There was equivocal evidence of carcinogenicity for skin and preputial tumors as well as TVM. There were no treatment-related tumor responses in B6C3F1 males. | 1/50 (2%), 7/50 (14%), 2/50 (4%) | 3% ± 3% (Lab)3% ± 3% (NTP) | It is not clear why the 14% incidence of TVM was considered only as equivocal evidence of a carcinogenic response. The reduced incidence in the high-dose group reflects reduced survival. |
| o-Nitrotoluene (NTP TR504) | |||
| This dietary study was started in 1996 and included a 13-week stop study. There was increased early mortality in treated rats. There was clear evidence of carcinogenicity for tumors of skin, mammary gland, lung and for TVM at doses up to 2000 ppm. Male B6C3F1 mice did not have any treatment-related increase in tumors. | 2/60 (3%), 20/60 (33%), 29/60 (48%), 44/60 (73%)Stop study:44/60 (73%) @ 2000 ppm54/60 (90%) @ 5000 ppm | 3.7% ± 2.9%(range 0–10%) | The high incidences of TVM exclusively in male rats, including significant increases with only a 3-month exposure provide strong support for clear evidence of carcinogenicity. |
| Pentachlorophenol (NTP TR483) | |||
| This dietary study was started in 1992 with does up to 600 ppm and included a 52-week stop study at 1000 ppm. Some evidence of carcinogenicity for TVM was present only in the stop-study along with a questionable nasal tumor response. There was no corresponding mouse study. | Stop study: 1/50 (2%), 9/50 (18%) | 3% ± 2.3% (range 0–8%) | There was considerable discussion and debate during the formal public peer review regarding the TVM response and concerns that the 1000 ppm dose in the stop-study exceeded the maximum tolerated dose and is the reason for the some evidence of carcinogenicity call. |
| o-Toluidine hydrochloride (NTP TR153) | |||
| This was a dermal study reported in 1979 and peer reviewed at NCI. There was early mortality in treated rats. The TVM response in the mid- and high-dose was considered positive for carcinogenicity along with subcutaneous fibromas and sarcomas in multiple organs. There was no increase in tumors in B6C3F1 mice. | 0/20 (0%), 17/50 (34%), 9/49 (18%) | Not provided in the TR | The increased incidence of TVM supports the conclusion of positive carcinogenicity for o-toluidine hydrochloride. |
| Trimethalolpropane triacrylate (NTP TR576) | |||
| This dermal study started in 2005 and there was some early mortality in high-dose males. TVM was the only positive tumor response and was considered equivocal evidence of carcinogenicity. There was no increased tumor response in male B6C3F1 mice. | 0/50 (0%), 2/50 (4%), 2/50 (4%), 5/50 (10%) | 3.2% ± 3.4% (range 0–8%) | Due to the variability of control TVMs in dermal studies and the fact that the high-dose incidence was only one tumor outside the control range, this response was considered equivocal evidence of carcinogenicity. |
TR: NTP Technical Report; M: Male; F: Female; TVM: Tunica vaginalis mesothelioma.
a = Tumor incidences arranged starting with controls and progressing through increased doses.
Conclusions
TVMs are rarely seen in male control F344 rats making detection of increases relatively easy to assess. Even in studies with up to a 90% incidence of TVM in male F344 rats, there was never a mesothelioma response in females, including the cytembena study (NTP TR207) where the route of administration was intraperitoneal and accompanied by peritoneal inflammation in both sexes. Furthermore, human TVMs are found only in the scrotal sac, and bear a more invasive and metastatic potential when compared to TVM in the F344 rat. Moreover, F344 rat TVM is considered a secondary event to the common LCT, and the rarity of LCTs in humans suggests that a different mechanism underlies development of this tumor in humans. This supports the contention that the TVM response is a rat strain and gender-specific response characteristic of the F344 rat without significant relevance to human health.
Perspective on the legacy of the F344/N rat
Cancer bioassays conducted in F344 rats have been used by the NTP since 1978 for hazard identification. In this context, some have regarded a positive tumor response as evidence of potential carcinogenic hazard at any site in humans. In our present evaluation, we have assessed the relevance of MNCL, LCTs and TVM to human cancer risk based on tissue site-concordance. The topic of tissue site-concordance between tumor responses in rodents and expected tumor responses in humans has long been contested in the literature and been the topic of scientific debate. While we cannot easily resolve this debate here, we point out our rationale for favoring site-concordance in our assessment of the human relevance of F344 rat MNCL, LCT and TVM bioassay responses.
When evaluating risk assessment, a higher level of confidence has generally been given to animal data when there is site-concordance with tumor data (EPA 2005). This higher level of confidence derives from experimental evidence indicating “that there are more physiological, biochemical and metabolic similarities between laboratory animals and humans than there are differences” (Rall et al. 1987). Tissue site concordance between animals and humans exposed to the same xenobiotics has been shown for many chemicals including aflatoxins B1, vinyl chloride, some arsenicals, asbestos, benzene and diethylstilbesterol among others (Tomatis et al. 1989; Goodman & Wilson 1991). Even though target sites for carcinogenesis are often concordant between animals and humans (Tomatis et al. 1989; Huff 1994), admittedly concordance by site is not universal. Outcomes in any given animal study are influenced by multiple factors, including experimental design, exposure levels, species, strain, sex, route of exposure, duration of exposure, metabolism, diet, pathology, DNA reactivity of the test agent and mode of action leading to a cancer response (Maronpot et al. 2004). Yet there are enough examples of tissue site concordance to favor its use in assessing human carcinogenic risk. Furthermore, a fundamental premise for the contemporary approach of employing the mode of action/human relevance framework to assess carcinogenic hazard to humans is based on tissue site concordance between animals and humans (Cohen et al. 2004; EPA 2005) and even the premise of toxicokinetic and toxicodynamic modeling is based on tissue site concordance (EPA 2005). Consequently, we have assessed the human relevance of F344 rat MNCL, LCT and TVM based on tissue site-concordance since there is no biologically compelling reason to expect that any potential mechanisms inducing these specific tumors in F344 rats would lead to human cancer at an entirely different tissue site.
Unfortunately, it became clear from the NTP experience over time, as highlighted in our review, that the F344/N rat strain harbors significant health issues which critically affect the integrity of certain organs, as well as the life-span of these animals. These and other health issues in this rat strain led to the NTP decision to switch to a more appropriate strain of rats for their toxicity and carcinogenicity studies (King-Herbert et al. 2010). Specifically, we highlight three cancers that are uniquely common in the F344/N rat strain. MNCL and LCT occur with a high background incidence and for MNCL the high background incidence is extremely variable. The very high spontaneous incidence of LCT prevents the use of this strain to reliably predict a potential testicular carcinogenic effect. TVM occurs rarely in rat carcinogenicity studies but it is unique to the F344 rat with a biologically plausible association with the high background incidence of LCT. In addition, we have provided documentation from contemporary literature indicating important pathobiological differences between these F344 rat tumors and similar target tissue-tumor responses in humans. We believe that rodent tumor responses are potentially relevant to human health risk unless there are good reasons to believe this is not the case.
For concluding on the relevance of study findings to humans, two main factors are considered: first, whether the observed tumor is related to the chemical exposure and second, whether the chemically induce tumor in the rodent model is relevant for human risk assessment. As discussed in depth previously in this review, we summarize below how data for these tumor types relate to these main factors.
For MNCL, there are statistical considerations for determining whether or not changes in incidence are related to chemical exposure as well as considerations of whether chemically induced alterations in this tumor type are relevant to humans. First, for determining whether a chemical does influence incidence of this tumor type it is important to compare values to the appropriate historical control values and to use more stringent statistical criteria such as those outlined in Thomas et al. (2007) (p < 0.01 and p < 0.005 for a trend test) for tumor responses assessed against a high background frequency as for MNCL. The etiology of the most similar human LGL leukemia (ANKCL) to F344 rat MNCL is related to infection with Epstein–Barr virus, and is not associated with drug or chemical exposure. Though the specific mode of action for the F344 rat MNCL is not known, it is not associated with a viral etiology. There is a qualitative difference in how human ANKCL leukemia and F344 rat MNCL are initiated; therefore, despite some commonalities between the pathologies of these tumors, MNCL is not a model for human LGL leukemia. In addition, there is no evidence for a genotoxic mechanism of action for MNCL induction; rather it is due to a yet unknown secondary mechanism. These data indicate there is not a concern for prediction of a site-concordant or non-concordant human relevant tumor type.
For the LCTs, the high background incidence makes it difficult to determine if the tumor incidence observed in the F344 rat is related to chemical exposure. The mode of action for the induction of the LCTs is unique to F344 rats. There is no equivalent mode of action in humans; therefore, non-genotoxic chemicals operating through this mode of action in F344 rats would not influence human carcinogenicity incidence.
Development of TVM in F344 rats is strain- and gender-specific and closely tied to the presence of LCTs. Since induction of LCTs in F344 rats is not relevant to human cancer risk, the development of TVMs in this rat strain is also not relevant to human cancer risk.
Given the material presented in this retrospective review, we contend that MNCL, LCT, and TVM responses in F344 rat carcinogenicity studies lack relevance in predicting human carcinogenicity.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the comments and suggestions of the four reviewers selected by the Editor and unknown to the authors.
Declaration of interest
Dr Robert R. Maronpot: I retired from the National Institute of Environmental Health Sciences and National Toxicology Program in 2007 and have been a private consultant since that time. It was my idea to prepare this manuscript with my colleagues and ExxonMobil agreed to compensate us for our time spent researching and writing the paper. I have no responsibility for or any financial interest in any of the chemicals mentioned in this manuscript and no financial interests in any way to ExxonMobil.
Dr Abraham Nyska: I am an adjunct faculty member of the Sackler School of Medicine, Tel Aviv University and Timrat, Israel and agreed to participate in preparation of this manuscript at the invitation of Dr Robert R. Maronpot. I have no financial interest in Exxon Mobil and am not consulting elsewhere with Exxon Mobil on other projects. My participation in writing this review was not influenced by Exxon Mobil in any way.
Dr Yuval Ramot: I am a faculty member of Hadassah-Hebrew University Medical Center, Jerusalem, Israel and agreed to participate in preparation of this manuscript at the invitation of Dr Robert R. Maronpot. I have no financial interest in Exxon Mobil and am not consulting elsewhere with Exxon Mobil on other projects. My participation in writing this review was not influenced by Exxon Mobil in any way.
Dr Jennifer E. Foreman: I am employed by ExxonMobil Biomedical Sciences, Inc., a separately incorporated, wholly owned affiliate of Exxon Mobil Corporation. I agreed to participate in preparation of this manuscript at the invitation of Dr Robert R. Maronpot.
The authors alone are responsible for the preparation and content of this article.
Notes
Note: MNCL is now considered a large granular lymphocytic (LGL) leukemia but has traditionally been called MNCL in NTP bioassays. We will continue to refer to it as MNCL to avoid confusion with listings in the NTP database.
A review of NTP MNCL studies with respect to human health risk was previously published (Thomas et al. 2007). This study includes the studies covered in that 2007 publication plus six additional F344 rat studies with a MNCL response completed prior to the NTP decision to no longer use the F344 rat.
References
- Abe K, Kato N, Miki K, Nimura S, Suzuki M, Kiyota H, Onodera S, Oishi Y. Malignant mesothelioma of testicular tunica vaginalis. Int J Urol. 2002;9:602–603. doi: 10.1046/j.1442-2042.2002.00521.x. [DOI] [PubMed] [Google Scholar]
- Albers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Fizazi K, Horwich A, Laguna MP. EAU guidelines on testicular cancer: 2011 update. Eur Urol. 2011;60:304–319. doi: 10.1016/j.eururo.2011.05.038. [DOI] [PubMed] [Google Scholar]
- Alekshun TJ, Tao J, Sokol L. Aggressive T-cell large granular lymphocyte leukemia: a case report and review of the literature. Am J Hematol. 2007;82:481–485. doi: 10.1002/ajh.20853. [DOI] [PubMed] [Google Scholar]
- Amin KM, Litzky LA, Smythe WR, Mooney AM, Morris JM, Mews DJ, Pass HI, Kari C, Rodeck U, Rauscher FJ, III, et al. Wilms' tumor 1 susceptibility (WT1) gene products are selectively expressed in malignant mesothelioma. Am J Pathol. 1995;146:344–356. [PMC free article] [PubMed] [Google Scholar]
- Attanoos RL, Gibbs AR. Pathology of malignant mesothelioma. Histopathology. 1997;30:403–418. doi: 10.1046/j.1365-2559.1997.5460776.x. [DOI] [PubMed] [Google Scholar]
- Attanoos RL, Griffin A, Gibbs AR. The use of immunohistochemistry in distinguishing reactive from neoplastic mesothelium. A novel use for desmin and comparative evaluation with epithelial membrane antigen, p53, platelet-derived growth factor-receptor, P-glycoprotein and Bcl-2. Histopathology. 2003;43:231–238. doi: 10.1046/j.1365-2559.2003.01686.x. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay A, Bhattacharya S, Konar K. Preoperative cytological diagnosis of malignant mesothelioma of tunica vaginalis. Diagn Cytopathol. 2015;43:850–854. doi: 10.1002/dc.23325. [DOI] [PubMed] [Google Scholar]
- Barbera V, Rubino M. Papillary mesothelioma of the tunica vaginalis. Cancer. 1957;10:183–189. doi: 10.1002/1097-0142(195701/02)10:1<183::aid-cncr2820100127>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Ben-Eliyahu S, Page GG, Yirmiya R, Shakhar G. Evidence that stress and surgical interventions promote tumor development by suppressing natural killer cell activity. Int J Cancer. 1999;80:880–888. doi: 10.1002/(sici)1097-0215(19990315)80:6<880::aid-ijc14>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Bisceglia M, Dor DB, Carosi I, Vairo M, Pasquinelli G. Paratesticular mesothelioma. Report of a case with comprehensive review of literature. Adv Anat Pathol. 2010;17:53–70. doi: 10.1097/PAP.0b013e3181c66fbc. [DOI] [PubMed] [Google Scholar]
- Boorman G, Chapin R, Mitsumori K. Testis and epididymis. In: Boorman G, Eustis S, Elwell M, Montgomery C, MacKenzie W, editors. Pathology of the Fischer rat. Reference and Atlas. New York: Academic Press; 1990. pp. 405–418. [Google Scholar]
- Boyum J, Wasserman NF. Malignant mesothelioma of the tunica vaginalis testis: a case illustrating Doppler color flow imaging and its potential for preoperative diagnosis. J Ultrasound Med. 2008;27:1249–1255. doi: 10.7863/jum.2008.27.8.1249. [DOI] [PubMed] [Google Scholar]
- Caldwell DJ. Review of mononuclear cell leukemia in F-344 rat bioassays and its significance to human cancer risk: a case study using alkyl phthalates. Regul Toxicol Pharmacol. 1999;30:45–53. doi: 10.1006/rtph.1999.1305. [DOI] [PubMed] [Google Scholar]
- Cameron TP, Hickman RL, Kornreich MR, Tarone RE. History, survival, and growth patterns of B6C3F1 mice and F344 rats in the National Cancer Institute Carcinogenesis Testing Program. Fundam Appl Toxicol. 1985;5:526–538. doi: 10.1016/0272-0590(85)90100-9. [DOI] [PubMed] [Google Scholar]
- Chan J, Jaffe E, Ralfkiaer E, Ko JH. Aggressive NK-cell leukemia. In: Swerdlow S, Campo E, Harris N, Jaffe E, Pileri S, Stein H, Thiele J, Vardiman J, editors. World Health Organization classification of tumours of hematopoietic and lymphoid tissues. Lyon: IARC Press; 2008b. pp. 276–277. [Google Scholar]
- Chan W, Foucar K, Morice W, Catovsky D. T-cell large granular lymphocytic leukemia. In: Swerdlow S, Campo E, Harris N, Jaffe E, Pileri S, Stein H, Thiele J, Vardiman J, editors. World Health Organization classification of tumours of hematopoietic and lymphoid tissues. Lyon: IARC Press; 2008a. pp. 272–273. [Google Scholar]
- Chekol SS, Sun CC. Malignant mesothelioma of the tunica vaginalis testis: diagnostic studies and differential diagnosis. Arch Pathol Lab Med. 2012;136:113–117. doi: 10.5858/arpa.2010-0550-RS. [DOI] [PubMed] [Google Scholar]
- Chen JL, Hsu YH. Malignant mesothelioma of the tunica vaginalis testis: a case report and literature review. Kaohsiung J Med Sci. 2009;25:77–81. doi: 10.1016/S1607-551X(09)70044-0. [DOI] [PubMed] [Google Scholar]
- Cheung MM, Chan JK, Wong KF. Natural killer cell neoplasms: a distinctive group of highly aggressive lymphomas/leukemias. Semin Hematol. 2003;40:221–232. doi: 10.1016/s0037-1963(03)00136-7. [DOI] [PubMed] [Google Scholar]
- Cheville JC. Classification and pathology of testicular germ cell and sex cord-stromal tumors. Urol Clin North Am. 1999;26:595–609. doi: 10.1016/s0094-0143(05)70201-9. [DOI] [PubMed] [Google Scholar]
- Churg A. Paratesticular mesothelial proliferations. Semin Diagn Pathol. 2003;20:272–278. doi: 10.1053/j.semdp.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Klaunig J, Meek ME, Hill RN, et al. Evaluating the human relevance of chemically induced animal tumors. Toxicol Sci. 2004;78:181–186. doi: 10.1093/toxsci/kfh073. [DOI] [PubMed] [Google Scholar]
- Coleman GL, Barthold W, Osbaldiston GW, Foster SJ, Jonas AM. Pathological changes during aging in barrier-reared Fischer 344 male rats. J Gerontol. 1977;32:258–278. doi: 10.1093/geronj/32.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JC, Klinefelter GR, Hardisty JF, Sharpe RM, Foster PM. Rodent Leydig cell tumorigenesis: a review of the physiology, pathology, mechanisms, and relevance to humans. Crit Rev Toxicol. 1999;29:169–261. doi: 10.1080/10408449991349203. [DOI] [PubMed] [Google Scholar]
- Creasy D, Bube A, de Rijk E, Kandori H, Kuwahara M, Masson R, Nolte T, Reams R, Regan K, Rehm S, et al. Proliferative and nonproliferative lesions of the rat and mouse male reproductive system. Toxicol Pathol. 2012;40:40S–121S. doi: 10.1177/0192623312454337. [DOI] [PubMed] [Google Scholar]
- Davey FR, Moloney WC. Postmortem observations on Fischer rats with leukemia and other disorders. Lab Invest. 1970;23:327–334. [PubMed] [Google Scholar]
- Dieter MP, Jameson CW, French JE, Gangjee S, Stefanski SA, Chhabra RS, Chan PC. Development and validation of a cellular transplant model for leukemia in Fischer rats: a short-term assay for potential anti-leukemic chemicals. Leuk Res. 1989;13:841–849. doi: 10.1016/0145-2126(89)90098-2. [DOI] [PubMed] [Google Scholar]
- Dieter MP, Jameson CW, Maronpot RR, Langenbach R, Braun AG. The chemotherapeutic potential of glycol alkyl ethers: structure-activity studies of nine compounds in a Fischer-rat leukemia transplant model. Cancer Chemother Pharmacol. 1990;26:173–180. doi: 10.1007/BF02897195. [DOI] [PubMed] [Google Scholar]
- Dieter MP, Maronpot RR, French JE. Comparison of the morphology and enzyme activity of mononuclear cells from Fischer 344 rats with either spontaneous or transplanted leukemia. Cancer Res. 1985;45:4301–4307. [PubMed] [Google Scholar]
- Dunnick JK, Eustis SL, Huff JE, Haseman JK. Two-year toxicity and carcinogenicity studies of ampicillin trihydrate and penicillin VK in rodents. Fundam Appl Toxicol. 1989;12:252–257. doi: 10.1016/0272-0590(89)90042-0. [DOI] [PubMed] [Google Scholar]
- Dunning WF, Curtis MR. The respective roles of longevity and genetic specificity in the occurrence of spontaneous tumors in the hybrids between two inbred lines of rats. Cancer Res. 1946;6:61–81. [PubMed] [Google Scholar]
- Dunning WF, Curtis MR. A transplantable acute leukemia in an inbred line of rats. J Natl Cancer Inst. 1957;19:845–853. [PubMed] [Google Scholar]
- Elwell MR, Dunnick JK, Hailey JR, Haseman JK. Chemicals associated with decreases in the incidence of mononuclear cell leukemia in the Fischer rat. Toxicol Pathol. 1996;24:238–245. doi: 10.1177/019262339602400212. [DOI] [PubMed] [Google Scholar]
- EPA . Guidelines for carcinogen risk assessment. 2005. [Google Scholar]
- Erdogan S, Acikalin A, Zeren H, Gonlusen G, Zorludemir S, Izol V. Well-differentiated papillary mesothelioma of the tunica vaginalis: a case study and review of the literature. Korean J Pathol. 2014;48:225–228. doi: 10.4132/KoreanJPathol.2014.48.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas LM, Szekely JG, Pusztai C, Baki M. High frequency of metastatic Leydig cell testicular tumours. Oncology. 2000;59:118–121. doi: 10.1159/000012147. [DOI] [PubMed] [Google Scholar]
- Fernandez GC, Tardaguila F, Rivas C, Trinidad C, Pesqueira D, Zungri E, San Miguel P. Case report: MRI in the diagnosis of testicular Leydig cell tumour. Br J Radiol. 2004;77:521–524. doi: 10.1259/bjr/31653779. [DOI] [PubMed] [Google Scholar]
- Friedman MA, Dulak LH, Stedham MA. A lifetime oncogenicity study in rats with acrylamide. Fundam Appl Toxicol. 1995;27:95–105. doi: 10.1093/toxsci/27.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CH. Morphologic classification and incidence of hematopoietic neoplasms in the Sprague-Dawley rat. Toxicol Pathol. 1988;16:451–457. doi: 10.1177/019262338801600405. [DOI] [PubMed] [Google Scholar]
- Frith CH, Ward JM, Chandra M. The morphology, immunohistochemistry, and incidence of hematopoietic neoplasms in mice and rats. Toxicol Pathol. 1993;21:206–218. doi: 10.1177/019262339302100213. [DOI] [PubMed] [Google Scholar]
- Gana BM, Windsor PM, Lang S, Macintyre J, Baxby K. Leydig cell tumour. Br J Urol. 1995;75:676–678. doi: 10.1111/j.1464-410x.1995.tb07435.x. [DOI] [PubMed] [Google Scholar]
- Garriga V, Serrano A, Marin A, Medrano S, Roson N, Pruna X. US of the tunica vaginalis testis: anatomic relationships and pathologic conditions. Radiographics. 2009;29:2017–2032. doi: 10.1148/rg.297095040. [DOI] [PubMed] [Google Scholar]
- Gentile TC, Uner AH, Hutchison RE, Wright J, Ben-Ezra J, Russell EC, Loughran TP., Jr. CD3+, CD56+ aggressive variant of large granular lymphocyte leukemia. Blood. 1994;84:2315–2321. [PubMed] [Google Scholar]
- Gerris J, Schoysman R. Tunica vaginalis fluid (TVF): hormonal composition. Assessment of testosterone, 5 alpha-dihydrotestosterone, delta 4-androstenedione, dehydroepiandrosteronesulphate, estradiol, LH, FSH and PRL. Acta Eur Fertil. 1984;15:205–216. [PubMed] [Google Scholar]
- Gerwin BI, Lechner JF, Reddel RR, Roberts AB, Robbins KC, Gabrielson EW, Harris CC. Comparison of production of transforming growth factor-beta and platelet-derived growth factor by normal human mesothelial cells and mesothelioma cell lines. Cancer Res. 1987;47:6180–6184. [PubMed] [Google Scholar]
- Giannarini G, Dieckmann KP, Albers P, Heidenreich A, Pizzocaro G. Organ-sparing surgery for adult testicular tumours: a systematic review of the literature. Eur Urol. 2010;57:780–790. doi: 10.1016/j.eururo.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Giwercman A, Grindsted J, Hansen B, Jensen OM, Skakkebaek NE. Testicular cancer risk in boys with maldescended testis: a cohort study. J Urol. 1987;138:1214–1216. doi: 10.1016/s0022-5347(17)43553-1. [DOI] [PubMed] [Google Scholar]
- Goel A, Agrawal A, Gupta R, Hari S, Dey AB. Malignant mesothelioma of the tunica vaginalis of the testis without exposure to asbestos. Cases J. 2008;1:310. doi: 10.1186/1757-1626-1-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M, Merlani P, Egger JF, Pugin F, Morel P. Hemorrhagic shock caused by rupture of an intra-abdominal leydig cell tumour: case report. Case Rep Gastroenterol. 2007;1:53–58. doi: 10.1159/000107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman D, Boorman G, Strandberg J. Selection and use of the B6C3F1 mouse and F344 rat in long-term assays for carcinogenicity. In: Millman H, Weisburger E, editors. Handbook of carcinogen testing. New Jersey: Noyes Publications; 1985. pp. 282–320. [Google Scholar]
- Goodman DG, Ward JM, Squire RA, Chu KC, Linhart MS. Neoplastic and nonneoplastic lesions in aging F344 rats. Toxicol Appl Pharmacol. 1979;48:237–248. doi: 10.1016/0041-008x(79)90029-2. [DOI] [PubMed] [Google Scholar]
- Goodman G, Wilson R. Predicting the carcinogenicity of chemicals in humans from rodent bioassay data. Environ Health Perspect. 1991;94:195–218. doi: 10.1289/ehp.94-1567942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guney N, Basaran M, Karayigit E, Muslumanoglu A, Guney S, Kilicaslan I, Gulbarut S. Malignant mesothelioma of the tunica vaginalis testis: a case report and review of the literature. Med Oncol. 2007;24:449–452. doi: 10.1007/s12032-007-0010-3. [DOI] [PubMed] [Google Scholar]
- Hamm M, Rupp C, Rottger P, Rathert P. [Malignant mesothelioma of the tunica vaginalis testis]. Chirurg. 1999;70:302–305. doi: 10.1007/s001040050648. [DOI] [PubMed] [Google Scholar]
- Harada T, Maronpot RR, Morris RW, Boorman GA. Effects of mononuclear cell leukemia on altered hepatocellular foci in Fischer 344 rats. Vet Pathol. 1990;27:110–116. doi: 10.1177/030098589002700206. [DOI] [PubMed] [Google Scholar]
- Hart DN, Baker BW, Inglis MJ, Nimmo JC, Starling GC, Deacon E, Rowe M, Beard ME. Epstein-Barr viral DNA in acute large granular lymphocyte (natural killer) leukemic cells. Blood. 1992;79:2116–2123. [PubMed] [Google Scholar]
- Haseman JK. A reexamination of false-positive rates for carcinogenesis studies. Fundam Appl Toxicol. 1983;3:334–339. doi: 10.1016/s0272-0590(83)80148-1. [DOI] [PubMed] [Google Scholar]
- Haseman JK, Hailey JR, Morris RW. Spontaneous neoplasm incidences in Fischer 344 rats and B6C3F1 mice in two-year carcinogenicity studies: a National Toxicology Program update. Toxicol Pathol. 1998;26:428–441. doi: 10.1177/019262339802600318. [DOI] [PubMed] [Google Scholar]
- Haseman JK, Huff JE, Rao GN, Arnold JE, Boorman GA, McConnell EE. Neoplasms observed in untreated and corn oil gavage control groups of F344/N rats and (C57BL/6N X C3H/HeN)F1 (B6C3F1) mice. J Natl Cancer Inst. 1985;75:975–984. doi: 10.1093/jnci/75.5.975. [DOI] [PubMed] [Google Scholar]
- Haseman JK, Ney E, Nyska A, Rao GN. Effect of diet and animal care/housing protocols on body weight, survival, tumor incidences, and nephropathy severity of F344 rats in chronic studies. Toxicol Pathol. 2003;31:674–681. doi: 10.1080/01926230390241927. [DOI] [PubMed] [Google Scholar]
- Haseman JK, Rao GN. Effects of corn oil, time-related changes, and inter-laboratory variability on tumor occurrence in control Fischer 344 (F344/N) rats. Toxicol Pathol. 1992;20:52–60. doi: 10.1177/019262339202000107. [DOI] [PubMed] [Google Scholar]
- Haseman JK, Young E, Eustis SL, Hailey JR. Body weight-tumor incidence correlations in long-term rodent carcinogenicity studies. Toxicol Pathol. 1997;25:256–263. doi: 10.1177/019262339702500302. [DOI] [PubMed] [Google Scholar]
- Hassan R, Alexander R. Nonpleural mesotheliomas: mesothelioma of the peritoneum, tunica vaginalis, and pericardium. Hematol Oncol Clin North Am. 2005;19:1067–1087. doi: 10.1016/j.hoc.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Hasserjian RP, Harris NL. NK-cell lymphomas and leukemias: a spectrum of tumors with variable manifestations and immunophenotype. Am J Clin Pathol. 2007;127:860–868. doi: 10.1309/2F39NX1AL3L54WU8. [DOI] [PubMed] [Google Scholar]
- Hellman S, Moloney WC, Meissner WA. Paradoxical effect of radiation on tumor incidence in the rat: implications for radiation therapy. Cancer Res. 1982;42:433–436. [PubMed] [Google Scholar]
- Hendry J, Fraser S, White J, Rajan P, Hendry DS. Retroperitoneal lymph node dissection (RPLND) for malignant phenotype Leydig cell tumours of the testis: a 10-year experience. Springerplus. 2015;4:20. doi: 10.1186/s40064-014-0781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga K, Fujii T. Carcinogenicity testing of acetaminophen in F344 rats. Jpn J Cancer Res. 1985;76:79–85. [PubMed] [Google Scholar]
- Hoei-Hansen CE, Holm M, Rajpert-De Meyts E, Skakkebaek NE. Histological evidence of testicular dysgenesis in contralateral biopsies from 218 patients with testicular germ cell cancer. J Pathol. 2003;200:370–374. doi: 10.1002/path.1372. [DOI] [PubMed] [Google Scholar]
- Huff JE. Chemicals causally associated with cancers in humans and laboratory animals. A perfect concordance. In: Waalkes MP, Ward JM, editors. Carcinogenesis. New York: Raven Press; 1994. pp. 25–37. [Google Scholar]
- Hursting SD, Switzer BR, French JE, Kari FW. The growth hormone: insulin-like growth factor 1 axis is a mediator of diet restriction-induced inhibition of mononuclear cell leukemia in Fischer rats. Cancer Res. 1993;53:2750–2757. [PubMed] [Google Scholar]
- Hursting SD, Switzer BR, French JE, Kari FW. Inhibition of rat mononuclear cell leukemia by corn oil gavage: in vivo, in situ and immune competence studies. Carcinogenesis. 1994;15:193–199. doi: 10.1093/carcin/15.2.193. [DOI] [PubMed] [Google Scholar]
- Husain AN, Colby T, Ordonez N, Krausz T, Attanoos R, Beasley MB, Borczuk AC, Butnor K, Cagle PT, Chirieac LR, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2013;137:647–667. doi: 10.5858/arpa.2012-0214-OA. [DOI] [PubMed] [Google Scholar]
- Ikegami Y, Kawai N, Tozawa K, Hayashi Y, Kohri K. Malignant mesothelioma of the tunica vaginalis testis related to recent asbestos. Int J Urol. 2008;15:560–561. doi: 10.1111/j.1442-2042.2008.02036.x. [DOI] [PubMed] [Google Scholar]
- Ilgren E. Mesotheliomas of animals: a comprehensive tabular compendium of the world's literature. Oxford: CRC Press; 1993. [Google Scholar]
- Ilgren EB, Wagner JC. Background incidence of mesothelioma: animal and human evidence. Regul Toxicol Pharmacol. 1991;13:133–149. doi: 10.1016/0273-2300(91)90018-q. [DOI] [PubMed] [Google Scholar]
- Imai K, Yoshimura K, Yamaguchi K, Matsui E, Isaka H, Hashimoto K, Boorman G. Effects of dietary restriction on age-associated pathologic changes in F-344 rats. J Toxicol Pathol. 1990;3:209–221. [Google Scholar]
- Inbar S, Neeman E, Avraham R, Benish M, Rosenne E, Ben-Eliyahu S. Do stress responses promote leukemia progression? An animal study suggesting a role for epinephrine and prostaglandin-E2 through reduced NK activity. PLoS One. 2011;6:e19246. doi: 10.1371/journal.pone.0019246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson P, Bergmann A, Rahmann S, Wohlers I, Scholtysik R, Przekopowitz M, Seifert M, Tschurtschenthaler G, Webersinke G, Jager U, et al. Recurrent alterations of TNFAIP3 (A20) in T-cell large granular lymphocytic leukemia. Int J Cancer. 2015;138:121–124. doi: 10.1002/ijc.29697. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Gorzinski SJ, Bodner KM, Campbell RA, Wolf CH, Friedman MA, Mast RW. Chronic toxicity and oncogenicity study on acrylamide incorporated in the drinking water of Fischer 344 rats. Toxicol Appl Pharmacol. 1986;85:154–168. doi: 10.1016/0041-008x(86)90109-2. [DOI] [PubMed] [Google Scholar]
- Jones MA, Young RH, Scully RE. Malignant mesothelioma of the tunica vaginalis. A clinicopathologic analysis of 11 cases with review of the literature. Am J Surg Pathol. 1995;19:815–825. doi: 10.1097/00000478-199507000-00010. [DOI] [PubMed] [Google Scholar]
- Karpe B, Fredricsson B, Svensson J, Ritzen EM. Testosterone concentration within the tunica vaginalis of boys and adult men. Int J Androl. 1982;5:549–556. doi: 10.1111/j.1365-2605.1982.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Kato R, Matsuda Y, Maehana T, Miyao N, Konishi Y, Kon S. Intrascrotal malignant mesothelioma diagnosed after surgery for hydrocele testis: a case report. Hinyokika Kiyo. 2012;58:45–48. [PubMed] [Google Scholar]
- Kato Y, Tsuta K, Seki K, Maeshima AM, Watanabe S, Suzuki K, Asamura H, Tsuchiya R, Matsuno Y. Immunohistochemical detection of GLUT-1 can discriminate between reactive mesothelium and malignant mesothelioma. Mod Pathol. 2007;20:215–220. doi: 10.1038/modpathol.3800732. [DOI] [PubMed] [Google Scholar]
- Kawa-Ha K, Ishihara S, Ninomiya T, Yumura-Yagi K, Hara J, Murayama F, Tawa A, Hirai K. CD3-negative lymphoproliferative disease of granular lymphocytes containing Epstein-Barr viral DNA. J Clin Invest. 1989;84:51–55. doi: 10.1172/JCI114168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Young RH, Scully RE. Leydig cell tumors of the testis. A clinicopathological analysis of 40 cases and review of the literature. Am J Surg Pathol. 1985;9:177–192. doi: 10.1097/00000478-198503000-00002. [DOI] [PubMed] [Google Scholar]
- King-Herbert A, Thayer K. NTP workshop: animal models for the NTP rodent cancer bioassay: stocks and strains – should we switch? Toxicol Pathol. 2006;34:802–805. doi: 10.1080/01926230600935938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Herbert AP, Sills RC, Bucher JR. Commentary: update on animal models for NTP studies. Toxicol Pathol. 2010;38:180–181. doi: 10.1177/0192623309356450. [DOI] [PubMed] [Google Scholar]
- Kivipensas P, Bjorkqvist AM, Karhu R, Pelin K, Linnainmaa K, Tammilehto L, Mattson K, Kallioniemi QP, Knuutila S. Gains and losses of DNA sequences in malignant mesothelioma by comparative genomic hybridization. Cancer Genet Cytogenet. 1996;89:7–13. doi: 10.1016/0165-4608(95)00358-4. [DOI] [PubMed] [Google Scholar]
- Kodell RL, Blackwell BN, Bucci TJ, Greenman DL. Cause-of-death assignment at the National Center for Toxicological Research. Toxicol Pathol. 1995;23:241–247. doi: 10.1177/019262339502300301. [DOI] [PubMed] [Google Scholar]
- Kusewitt D, Hahn F, Pickerell J. Hematologic and serum chemical characteristics of mononuclear leukemia in Fischer 344 rats. Lab Anim Sci. 1982;32:275–277. [PubMed] [Google Scholar]
- Lafferty JS, Kamendulis LM, Kaster J, Jiang J, Klaunig JE. Subchronic acrylamide treatment induces a tissue-specific increase in DNA synthesis in the rat. Toxicol Lett. 2004;154:95–103. doi: 10.1016/j.toxlet.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Lagana SM, Taub RN, Borczuk AC. Utility of glucose transporter 1 in the distinction of benign and malignant thoracic and abdominal mesothelial lesions. Arch Pathol Lab Med. 2012;136:804–809. doi: 10.5858/arpa.2011-0219-OA. [DOI] [PubMed] [Google Scholar]
- Le Cornet C, Lortet-Tieulent J, Forman D, Béranger R, Flechon A, Fervers B, Schüz J, Bray F. Testicular cancer incidence to rise by 25% by 2025 in Europe? Model-based predictions in 40 countries using population-based registry data. Eur J Cancer. 2014;50:831–839. doi: 10.1016/j.ejca.2013.11.035. [DOI] [PubMed] [Google Scholar]
- Lee AF, Gown AM, Churg A. IMP3 and GLUT-1 immunohistochemistry for distinguishing benign from malignant mesothelial proliferations. Am J Surg Pathol. 2013;37:421–426. doi: 10.1097/PAS.0b013e31826ab1c0. [DOI] [PubMed] [Google Scholar]
- Lee S, Illei PB, Han JS, Epstein JI. Florid mesothelial hyperplasia of the tunica vaginalis mimicking malignant mesothelioma: a clinicopathologic study of 12 cases. Am J Surg Pathol. 2014;38:54–59. doi: 10.1097/PAS.0b013e31829ab20e. [DOI] [PubMed] [Google Scholar]
- Libé R, Fratticci A, Lahlou N, Jornayvaz FR, Tissier F, Louiset E, Guibourdenche J, Vieillefond A, Zerbib M, Bertherat J. A rare cause of hypertestosteronemia in a 68-year-old patient: a Leydig cell tumor due to a somatic GNAS (guanine nucleotide-binding protein, alpha-stimulating activity polypeptide 1)-activating mutation. J Androl. 2012;33:578–584. doi: 10.2164/jandrol.111.013441. [DOI] [PubMed] [Google Scholar]
- Lindsey J. Historical foundations. In: Baker H, Lindsey J, Weisbroth S, editors. The laboratory rat. Vol. 1. Biology and diseases. New York: Academic Press; 1979. pp. 2–33. [Google Scholar]
- Lington AW, Bird MG, Plutnick RT, Stubblefield WA, Scala RA. Chronic toxicity and carcinogenic evaluation of diisononyl phthalate in rats. Fundam Appl Toxicol. 1997;36:79–89. doi: 10.1006/faat.1997.2286. [DOI] [PubMed] [Google Scholar]
- Lipman RD, Dallal GE, Bronson RT. Effects of genotype and diet on age-related lesions in ad libitum fed and calorie-restricted F344, BN, and BNF3F1 rats. J Gerontol A Biol Sci Med Sci. 1999;54:B478–B491. doi: 10.1093/gerona/54.11.b478. [DOI] [PubMed] [Google Scholar]
- Losco PE, Ward JM. The early stage of large granular lymphocyte leukemia in the F344 rat. Vet Pathol. 1984;21:286–291. doi: 10.1177/030098588402100304. [DOI] [PubMed] [Google Scholar]
- Maizlin ZV, Belenky A, Kunichezky M, Sandbank J, Strauss S. Leydig cell tumors of the testis: gray scale and color Doppler sonographic appearance. J Ultrasound Med. 2004;23:959–964. doi: 10.7863/jum.2004.23.7.959. [DOI] [PubMed] [Google Scholar]
- Maqdasy S, Bogenmann L, Batisse-Lignier M, Roche B, Franck F, Desbiez F, Tauveron I. Leydig cell tumor in a patient with 49,XXXXY karyotype: a review of literature. Reprod Biol Endocrinol. 2015;13:72. doi: 10.1186/s12958-015-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinaccio A, Binazzi A, Di Marzio D, Scarselli A, Verardo M, Mirabelli D, Gennaro V, Mensi C, Merler E, De Zotti R, et al. Incidence of extrapleural malignant mesothelioma and asbestos exposure, from the Italian national register. Occup Environ Med. 2010;67:760–765. doi: 10.1136/oem.2009.051466. [DOI] [PubMed] [Google Scholar]
- Maronpot RR, Flake G, Huff J. Relevance of animal carcinogenesis findings to human cancer predictions and prevention. Toxicol Pathol. 2004;32:40–48. doi: 10.1080/01926230490425003. [DOI] [PubMed] [Google Scholar]
- Maronpot RR, Thoolen RJ, Hansen B. Two-year carcinogenicity study of acrylamide in Wistar Han rats with in utero exposure. Exp Toxicol Pathol. 2015;67:189–195. doi: 10.1016/j.etp.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Maronpot RR, Zeiger E, McConnell EE, Kolenda-Roberts H, Wall H, Friedman MA. Induction of tunica vaginalis mesotheliomas in rats by xenobiotics. Crit Rev Toxicol. 2009;39:512–537. doi: 10.1080/10408440902969430. [DOI] [PubMed] [Google Scholar]
- Masoudi JF, Van Arsdalen K, Rovner ES. Organ-sparing surgery for bilateral leydig cell tumor of the testis. Urology. 1999;54:744. doi: 10.1016/s0090-4295(99)00227-7. [DOI] [PubMed] [Google Scholar]
- Mensi C, Pellegatta M, Sieno C, Consonni D, Riboldi L, Bertazzi PA. Mesothelioma of tunica vaginalis testis and asbestos exposure. BJU Int. 2012;110:533–537. doi: 10.1111/j.1464-410X.2012.10932.x. [DOI] [PubMed] [Google Scholar]
- Moloney WC, Boschetti AE, King V. Observations on leukemia in Wistar Furth rats. Cancer Res. 1969;29:938–946. [PubMed] [Google Scholar]
- Moloney WC, Boschetti AE, King VP. Spontaneous leukemia in Fischer rats. Cancer Res. 1970;30:41–43. [PubMed] [Google Scholar]
- Moloney WC, King VP. Reduction of leukemia incidence following splenectomy in the rat. Cancer Res. 1973;33:573–574. [PubMed] [Google Scholar]
- Morris JE, Sasser LB, Miller DL, Dagle GE, Rafferty CN, Ebi KL, Anderson LE. Clinical progression of transplanted large granular lymphocytic leukemia in Fischer 344 rats exposed to 60 Hz magnetic fields. Bioelectromagnetics. 1999;20:48–56. doi: 10.1002/(sici)1521-186x(1999)20:1<48::aid-bem7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Nolte T, Rittinghausen S, Kellner R, Karbe E, Kittel B, Rinke M, Deschl U. RITA-Registry of Industrial Toxicology Animal data: the application of historical control data for Leydig cell tumors in rats. Exp Toxicol Pathol. 2011;63:645–656. doi: 10.1016/j.etp.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Nyska A, Hester SD, Cooper RL, Goldman JM, Stoker TE, House D, Wolf DC. Single or group housing altered hormonal physiology and affected pituitary and interstitial cell kinetics. J Toxicol Sci. 2002;27:449–457. doi: 10.2131/jts.27.449. [DOI] [PubMed] [Google Scholar]
- Nyska A, Leininger JR, Maronpot RR, Haseman JK, Hailey JR. Effect of individual versus group caging on the incidence of pituitary and Leydig cell tumors in F344 rats: proposed mechanism. Med Hypotheses. 1998;50:525–529. doi: 10.1016/s0306-9877(98)90276-6. [DOI] [PubMed] [Google Scholar]
- Ohshima K, Suzumiya J, Kanda M, Kato A, Kikuchi M. Integrated and episomal forms of Epstein-Barr virus (EBV) in EBV associated disease. Cancer Lett. 1998;122:43–50. doi: 10.1016/s0304-3835(97)00368-6. [DOI] [PubMed] [Google Scholar]
- Ozyavuz R, Ozen HA, Gedikoglu G, Ozgu IH, Sahin A, Tekgul S, Remzi D. Leydig cell tumour of the testis: presentation of two cases. Int Urol Nephrol. 1993;25:385–388. [PubMed] [Google Scholar]
- Pacheco AJ, Torres JL, de la Guardia FV, Arrabal Polo MA, Gomez AZ. Intraparenchymatous adenomatoid tumor dependent on the rete testis: a case report and review of literature. Indian J Urol. 2009;25:126–128. doi: 10.4103/0970-1591.45551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JT, Jr, Greenberg SD, Buffler PA. Non-asbestos-related malignant mesothelioma. A review. Cancer. 1984;54:951–960. doi: 10.1002/1097-0142(19840901)54:5<951::aid-cncr2820540536>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Plas E, Riedl CR, Pfluger H. Malignant mesothelioma of the tunica vaginalis testis: review of the literature and assessment of prognostic parameters. Cancer. 1998;83:2437–2446. doi: 10.1002/(sici)1097-0142(19981215)83:12<2437::aid-cncr6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Poullot E, Zambello R, Leblanc F, Bareau B, De March E, Roussel M, Boulland ML, Houot R, Renault A, Fest T, et al. Chronic natural killer lymphoproliferative disorders: characteristics of an international cohort of 70 patients. Ann Oncol. 2014;25:2030–2035. doi: 10.1093/annonc/mdu369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice DE, Siegel RA, Donatsch P, Qureshi S, Ettlin RA. Mesulergine induced Leydig cell tumours, a syndrome involving the pituitary-testicular axis of the rat. Arch Toxicol Suppl. 1992;15:197–204. doi: 10.1007/978-3-642-77260-3_27. [DOI] [PubMed] [Google Scholar]
- Purdue MP, Devesa SS, Sigurdson AJ, McGlynn KA. International patterns and trends in testis cancer incidence. Int J Cancer. 2005;115:822–827. doi: 10.1002/ijc.20931. [DOI] [PubMed] [Google Scholar]
- Rajan V, Nandhakumar R, Shanmugasundaram S, Ravi R, Natarajan S, Mohan G, Nanjundappan PM. Paratesticular malignant mesothelioma – a rare case presentation. Indian J Surg. 2013;75:174–176. doi: 10.1007/s12262-012-0573-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall DP, Hogan MD, Huff JE, Schwetz BA, Tennant RW. Alternatives to using human experience in assessing health risks. Annu Rev Public Health. 1987;8:355–385. doi: 10.1146/annurev.pu.08.050187.002035. [DOI] [PubMed] [Google Scholar]
- Rao G, Boorman G. History of the F344 rat. In: Boorman G, Eustis S, Elwell M, Montgomery C, MacKenzie W, editors. Pathology of the Fischer rat. Reference and Atlas. New York: Academic Press; 1990. pp. 5–8. [Google Scholar]
- Rao GN, Haseman JK. Influence of corn oil and diet on body weight, survival, and tumor incidences in F344/N rats. Nutr Cancer. 1993;19:21–30. doi: 10.1080/01635589309514233. [DOI] [PubMed] [Google Scholar]
- Rao GN, Haseman JK, Grumbein S, Crawford DD, Eustis SL. Growth, body weight, survival, and tumor trends in F344/N rats during an eleven-year period. Toxicol Pathol. 1990;18:61–70. doi: 10.1177/019262339001800109. [DOI] [PubMed] [Google Scholar]
- Reynolds CW. Large granular lymphocyte (LGL) lymphoproliferative diseases: naturally cytotoxic tumors in man and experimental animals. Crit Rev Oncol Hematol. 1985;2:185–208. doi: 10.1016/s1040-8428(85)80002-0. [DOI] [PubMed] [Google Scholar]
- Reynolds CW, Bere EW, Jr, Ward JM. Natural killer activity in the rat. III. Characterization of transplantable large granular lymphocyte (LGL) leukemias in the F344 rat. J Immunol. 1984;132:534–540. [PubMed] [Google Scholar]
- Reynolds CW, Bonyhadi M, Herberman RB, Young HA, Hedrick SM. Lack of gene rearrangement and mRNA expression of the beta chain of the T cell receptor in spontaneous rat large granular lymphocyte leukemia lines. J Exp Med. 1985;161:1249–1254. doi: 10.1084/jem.161.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskova A, Thula R, Chan G. Aggressive natural killer-cell leukemia: report of five cases and review of the literature. Leuk Lymphoma. 2004;45:2427–2439. doi: 10.1080/10428190400004513. [DOI] [PubMed] [Google Scholar]
- Ryder J, Wang X, Bao L, Gross SA, Hua F, Irons RD. Aggressive natural killer cell leukemia: report of a Chinese series and review of the literature. Int J Hematol. 2007;85:18–25. doi: 10.1532/IJH97.A10612. [DOI] [PubMed] [Google Scholar]
- Sacksteder MR. Occurrence of spontaneous tumors in the germfree F344 rat. J Natl Cancer Inst. 1976;57:1371–1373. doi: 10.1093/jnci/57.6.1371. [DOI] [PubMed] [Google Scholar]
- Sass B, Rabstein LS, Madison R, Nims RM, Peters RL, Kelloff GJ. Incidence of spontaneous neoplasms in F344 rats throughout the natural life-span. J Natl Cancer Inst. 1975;54:1449–1456. doi: 10.1093/jnci/54.6.1449. [DOI] [PubMed] [Google Scholar]
- Sasser LB, Morris JE, Miller DL, Rafferty CN, Ebi KL, Anderson LE. Exposure to 60 Hz magnetic fields does not alter clinical progression of LGL leukemia in Fischer rats. Carcinogenesis. 1996;17:2681–2687. doi: 10.1093/carcin/17.12.2681. [DOI] [PubMed] [Google Scholar]
- Schurkes C, Brock W, Abel J, Unfried K. Induction of 8-hydroxydeoxyguanosine by man-made vitreous fibres and crocidolite asbestos administered intraperitoneally in rats. Mutat Res. 2004;553:59–65. doi: 10.1016/j.mrfmmm.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Segura-Gonzalez M, Urias-Rocha J, Castelan-Pedraza J. Malignant mesothelioma of the tunica vaginalis: a rare neoplasm-case report and literature review. Clin Genitourin Cancer. 2015;13:e401–e405. doi: 10.1016/j.clgc.2015.05.009. [DOI] [PubMed] [Google Scholar]
- Semenzato G, Marino F, Zambello R. State of the art in natural killer cell malignancies. Int J Lab Hematol. 2012;34:117–128. doi: 10.1111/j.1751-553X.2011.01374.x. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, McNeilly AS. The effect of induced hyperprolactinaemia on Leydig cell function and LH-induced loss of LH-receptors in the rat testis. Mol Cell Endocrinol. 1979;16:19–27. doi: 10.1016/0303-7207(79)90003-0. [DOI] [PubMed] [Google Scholar]
- Shiga A, Narama I. Hepatic lesions caused by large granular lymphocyte leukemia in Fischer 344 rats: similar morphologic features and morphogenesis to those of nodular regenerative hyperplasia (NRH) in the human liver. Toxicol Pathol. 2015;43:852–864. doi: 10.1177/0192623315578719. [DOI] [PubMed] [Google Scholar]
- Shimokawa I, Yu BP, Higami Y, Ikeda T, Masoro EJ. Dietary restriction retards onset but not progression of leukemia in male F344 rats. J Gerontol. 1993;48:B68–B73. doi: 10.1093/geronj/48.2.b68. [DOI] [PubMed] [Google Scholar]
- Shipp A, Lawrence G, Gentry R, McDonald T, Bartow H, Bounds J, Macdonald N, Clewell H, Allen B, Van Landingham C. Acrylamide: review of toxicity data and dose-response analyses for cancer and noncancer effects. Crit Rev Toxicol. 2006;36:481–608. doi: 10.1080/10408440600851377. [DOI] [PubMed] [Google Scholar]
- Skakkebaek NE, Holm M, Hoei-Hansen C, Jorgensen N, Rajpert-De Meyts E. Association between testicular dysgenesis syndrome (TDS) and testicular neoplasia: evidence from 20 adult patients with signs of maldevelopment of the testis. APMIS. 2003;111:1–9. doi: 10.1034/j.1600-0463.2003.11101031.x. [DOI] [PubMed] [Google Scholar]
- Sokol L, Loughran TP., Jr. Large granular lymphocyte leukemia. Oncologist. 2006;11:263–273. doi: 10.1634/theoncologist.11-3-263. [DOI] [PubMed] [Google Scholar]
- Song SY, Kim WS, Ko YH, Kim K, Lee MH, Park K. Aggressive natural killer cell leukemia: clinical features and treatment outcome. Haematologica. 2002;87:1343–1345. [PubMed] [Google Scholar]
- Stefanski S, Elwell M, Stromberg P. Spleen, lymph nodes, and thymus. In: Boorman G, Eustis S, Elwell M, Montgomery C, MacKenzie W, editors. Pathology of the Fischer rat. Reference and Atlas. New York: Academic Press; 1990. pp. 369–394. [Google Scholar]
- Stefanski SA, Greenwell A, Merrick BA, Brown TT, Reynolds SH. Proliferating cell nuclear antigen staining of Fischer-344/N rat spleens affected by large granular lymphocyte leukemia. Toxicol Pathol. 1995;23:1–6. doi: 10.1177/019262339502300101. [DOI] [PubMed] [Google Scholar]
- Steinbach T, Maronpot R, Hardisty J. Human relevance of rodent Leydig cell tumors. In: Harbison R, Bourgeois M, Johnson G, editors. Hamilton & Hardy's industrial toxicology. New York: John Wiley & Sons; 2015. pp. 1189–1196. [Google Scholar]
- Steinway SN, LeBlanc F, Loughran TP. The pathogenesis and treatment of large granular lymphocyte leukemia. Blood Rev. 2014;28:87–94. doi: 10.1016/j.blre.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg PC, Grants IS, Kociba GJ, Krakowka GS, Mezza LE. Serial syngeneic transplantation of large granular lymphocyte leukemia in F344 rats. Vet Pathol. 1990;27:404–410. doi: 10.1177/030098589902700604. [DOI] [PubMed] [Google Scholar]
- Stromberg PC, Rojko JL, Vogtsberger LM, Cheney C, Berman R. Immunologic, biochemical, and ultrastructural characterization of the leukemia cell in F344 rats. J Natl Cancer Inst. 1983d;71:173–181. [PubMed] [Google Scholar]
- Stromberg PC, Vogtsberger LM. Pathology of the mononuclear cell leukemia of Fischer rats. I. Morphologic studies. Vet Pathol. 1983a;20:698–708. doi: 10.1177/030098588302000605. [DOI] [PubMed] [Google Scholar]
- Stromberg PC, Vogtsberger LM, Marsh LR. Pathology of the mononuclear cell leukemia of Fischer rats. III. Clinical chemistry. Vet Pathol. 1983c;20:718–726. doi: 10.1177/030098588302000607. [DOI] [PubMed] [Google Scholar]
- Stromberg PC, Vogtsberger LM, Marsh LR, Wilson FD. Pathology of the mononuclear cell leukemia of Fischer rats. II. Hematology. Vet Pathol. 1983b;20:709–717. doi: 10.1177/030098588302000606. [DOI] [PubMed] [Google Scholar]
- Stromberg PC, Vogtsberger LM, McMurray DN, Marsh LR, Kotur MS, Brown CA. Behavior of transplanted large granular lymphocyte leukemia in Fischer 344 rats. Lab Invest. 1985;53:200–208. [PubMed] [Google Scholar]
- Suzuki R, Suzumiya J, Nakamura S, Aoki S, Notoya A, Ozaki S, Gondo H, Hino N, Mori H, Sugimori H, et al. Aggressive natural killer-cell leukemia revisited: large granular lymphocyte leukemia of cytotoxic NK cells. Leukemia. 2004;18:763–770. doi: 10.1038/sj.leu.2403262. [DOI] [PubMed] [Google Scholar]
- Taheri ZM, Mehrafza M, Mohammadi F, Khoddami M, Bahadori M, Masjedi MR. The diagnostic value of Ki-67 and repp86 in distinguishing between benign and malignant mesothelial proliferations. Arch Pathol Lab Med. 2008;132:694–697. doi: 10.5858/2008-132-694-TDVOKA. [DOI] [PubMed] [Google Scholar]
- Takeda M, Kasai T, Enomoto Y, Takano M, Morita K, Kadota E, Iizuka N, Maruyama H, Nonomura A. Genomic gains and losses in malignant mesothelioma demonstrated by FISH analysis of paraffin-embedded tissues. J Clin Pathol. 2012;65:77–82. doi: 10.1136/jclinpath-2011-200208. [DOI] [PubMed] [Google Scholar]
- Tanigawa H, Onodera H, Maekawa A. Spontaneous mesotheliomas in Fischer rats – a histological and electron microscopic study. Toxicol Pathol. 1987;15:157–163. doi: 10.1177/019262338701500205. [DOI] [PubMed] [Google Scholar]
- Testa JR, Cheung M, Pei J, Below JE, Tan Y, Sementino E, Cox NJ, Dogan AU, Pass HI, Trusa S, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43:1022–1025. doi: 10.1038/ng.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J, Haseman JK, Goodman JI, Ward JM, Loughran TP, Jr, Spencer PJ. A review of large granular lymphocytic leukemia in Fischer 344 rats as an initial step toward evaluating the implication of the endpoint to human cancer risk assessment. Toxicol Sci. 2007;99:3–19. doi: 10.1093/toxsci/kfm098. [DOI] [PubMed] [Google Scholar]
- Thurman JD, Bucci TJ, Hart RW, Turturro A. Survival, body weight, and spontaneous neoplasms in ad libitum-fed and food-restricted Fischer-344 rats. Toxicol Pathol. 1994;22:1–9. doi: 10.1177/019262339402200101. [DOI] [PubMed] [Google Scholar]
- Tomatis L, Aitio A, Wilbourn J, Shuker L. Human carcinogens so far identified. Jpn J Cancer Res. 1989;80:795–807. doi: 10.1111/j.1349-7006.1989.tb01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trabert B, Zugna D, Richiardi L, McGlynn KA, Akre O. Congenital malformations and testicular germ cell tumors. Int J Cancer. 2013;133:1900–1904. doi: 10.1002/ijc.28207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triola MF. Elementary statistics. 11th ed. Boston: Pearson Education; 2010. [Google Scholar]
- Tsitouridis I, Maskalidis C, Panagiotidou D, Kariki EP. Eleven patients with testicular leydig cell tumors: clinical, imaging, and pathologic correlation. J Ultrasound Med. 2014;33:1855–1864. doi: 10.7863/ultra.33.10.1855. [DOI] [PubMed] [Google Scholar]
- Turek FW, Desjardins C. Development of Leydig cell tumors and onset of changes in the reproductive and endocrine systems of aging F344 rats. J Natl Cancer Inst. 1979;63:969–975. [PubMed] [Google Scholar]
- Turturro A, Blank K, Murasko D, Hart R. Mechanisms of caloric restriction affecting aging and disease. Ann N Y Acad Sci. 1994;719:159–170. doi: 10.1111/j.1749-6632.1994.tb56827.x. [DOI] [PubMed] [Google Scholar]
- Ulbright TM, Srigley JR, Hatzianastassiou DK, Young RH. Leydig cell tumors of the testis with unusual features: adipose differentiation, calcification with ossification, and spindle-shaped tumor cells. Am J Surg Pathol. 2002;26:1424–1433. doi: 10.1097/00000478-200211000-00004. [DOI] [PubMed] [Google Scholar]
- Unluer E, Ozcan D, Altin S. Malignant Leydig cell tumour of the testis: a case report and review of the literature. Int Urol Nephrol. 1990;22:455–460. doi: 10.1007/BF02549777. [DOI] [PubMed] [Google Scholar]
- Versnel MA, Hagemeijer A, Bouts MJ, van der Kwast TH, Hoogsteden HC. Expression of c-sis (PDGF B-chain) and PDGF A-chain genes in ten human malignant mesothelioma cell lines derived from primary and metastatic tumors. Oncogene. 1988;2:601–605. [PubMed] [Google Scholar]
- Vukina J, Chism DD, Sharpless JL, Raynor MC, Milowsky MI, Funkhouser WK. Metachronous bilateral testicular Leydig-like tumors leading to the diagnosis of congenital adrenal hyperplasia (adrenogenital syndrome). Case Rep Pathol. 2015;2015:459318. doi: 10.1155/2015/459318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J, Rehm S, Reynolds C. Tumours of the hematopoietic system. In: Turusov V, Mohr U, editors. Pathology of tumours in laboratory animals. Vol. 1. Tumours of the rat. Lyon: International Agency for Research on Cancer; 1990. pp. 625–657. [Google Scholar]
- Ward JM, Reynolds CW. Large granular lymphocyte leukemia. A heterogeneous lymphocytic leukemia in F344 rats. Am J Pathol. 1983;111:1–10. [PMC free article] [PubMed] [Google Scholar]
- Weisburger EK. History of the bioassay program of the National Cancer Institute. Prog Exp Tumor Res. 1983;26:187–201. doi: 10.1159/000407260. [DOI] [PubMed] [Google Scholar]
- Weisburger JH, Rivenson A, Reinhardt J, Braley J, Pittman B, Zang E. On the occurrence of Leydig cell tumors in the F344 rat. Cancer Lett. 2002;182:213–216. doi: 10.1016/s0304-3835(01)00865-5. [DOI] [PubMed] [Google Scholar]
- Winstanley AM, Landon G, Berney D, Minhas S, Fisher C, Parkinson MC. The immunohistochemical profile of malignant mesotheliomas of the tunica vaginalis: a study of 20 cases. Am J Surg Pathol. 2006;30:1–6. doi: 10.1097/01.pas.0000178094.07513.71. [DOI] [PubMed] [Google Scholar]
- Woodward PJ, Schwab CM, Sesterhenn IA. From the archives of the AFIP: extratesticular scrotal masses: radiologic-pathologic correlation. Radiographics. 2003;23:215–240. doi: 10.1148/rg.231025133. [DOI] [PubMed] [Google Scholar]
- Woodward PJ, Sohaey R, O'Donoghue MJ, Green DE. From the archives of the AFIP: tumors and tumorlike lesions of the testis: radiologic-pathologic correlation. Radiographics. 2002;22:189–216. doi: 10.1148/radiographics.22.1.g02ja14189. [DOI] [PubMed] [Google Scholar]
- Xiao SY, Rizzo P, Carbone M. Benign papillary mesothelioma of the tunica vaginalis testis. Arch Pathol Lab Med. 2000;124:143–147. doi: 10.5858/2000-124-0143-BPMOTT. [DOI] [PubMed] [Google Scholar]
- Yen CH, Lee CT, Su CJ, Lo HC. Malignant mesothelioma of the tunica vaginalis testis: a malignancy associated with recurrent epididymitis? World J Surg Oncol. 2012;10:238. doi: 10.1186/1477-7819-10-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Jing W, Ouyang J, Zeng H, George SK, Liu Z. Six cases of aggressive natural killer-cell leukemia in a Chinese population. Int J Clin Exp Pathol. 2014;7:3423–3431. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.