Abstract
Diketopyrrolopyrroles (DPP) are a relatively new class of organic high-performance pigments. The present inhalation and particle characterization studies were performed to compare the effects of five DPP-based pigments (coarse and fine Pigment Red 254, coarse and fine meta-chloro DPP isomer and one form of mixed chlorinated DPP isomers) and compare it to coarse and fine inorganic Pigment Red 101. Wistar rats were exposed head-nose to atmospheres of the respective materials for 6 h/day on 5 consecutive days. Target concentrations were 30 mg/m3 as high dose for all compounds and selected based occupational exposure limits for respirable nuisance dust. Toxicity was determined after end of exposure and after 3-week recovery using broncho-alveolar lavage fluid (BALF) and microscopic examinations of the entire respiratory tract. Mixed chlorinated DPP isomers and coarse meta-chloro DPP isomer caused marginal changes in BALF, consisting of slight increases of polymorphonuclear neutrophils, and in case of coarse meta-chloro DPP increased MCP-1 and osteopontin levels. Mixed chlorinated DPP isomers, Pigment Red 254, and meta-chloro DPP caused pigment deposits and phagocytosis by alveolar macrophages, slight hypertrophy/hyperplasia of the bronchioles and alveolar ducts, but without evidence of inflammation. In contrast, only pigment deposition and pigment phagocytosis were observed after exposure to Pigment Red 101. All pigments were tolerated well and caused only marginal effects in BALF or no effects at all. Only minor effects were seen on the lung by microscopic examination. There was no evidence of systemic inflammation based on acute-phase protein levels in blood.
Keywords: Diketopyrrolopyrrole, inhalation toxicity, iron oxide, pigments, short-term inhalation toxicity test
Introduction
Diketopyrrolopyrroles (DPP) are a relatively new class of organic pigments and were discovered approximately 40 years ago (Farnum et al., 1974; Iqbal et al., 1998) and brought to the market in the 1980s. DPP are considered high-performance organic pigments, which mean overall high fastness properties (light, weather, solvent, heat). Due to these technical advantages DPP pigments are used in many industrial applications like dispersion paintings, general liquid industrial coatings, automotive coatings, high-quality printing inks, color filter applications for electronic materials as well as general plastics applications.
Application of particulate materials in general raise concerns about possible health effects, especially in occupational settings where materials are handled in their dust form and have not been embedded in a matrix. Among different exposure routes, inhalation is considered to be the exposure route of highest concern in humans. The present inhalation studies were performed to compare the effects of five DPP-based pigments (mixed chlorinated DPP isomers, fine Pigment Red 254, coarse Pigment Red 254, fine meta-chloro DPP and coarse meta-chloro DPP) and compare it to two inorganic, iron-oxide pigments (fine Pigment Red 101 and coarse Pigment Red 101). Red iron oxide pigments (Pigment Red 101) are used in large quantities for coloration of brick and roof tiles as well as colorants for wood stains, and coatings in general.
Usually, data from longer term inhalation toxicity studies (28- or 90-day studies) are used for a toxicological risk assessment. However, these studies are considerably resource-consuming and usually do not consider recovery or progression after the end of the exposure. Therefore, the present short-term inhalation study design was developed. It was optimized by incorporation of additional endpoints like measurement of pro-inflammatory cytokines in broncho-alveolar lavage fluid (BALF). Measurements of these parameters increase the predictivity of this short-term inhalation test for long-term effects, as shown by comparison of this study type with a 90-day inhalation study using titanium dioxide as a model compound (Ma-Hock et al., 2009a). It was shown that these results can be used as a basis for a quantitative risk assessment (Klein et al., 2012; Landsiedel et al., 2008,2010; Ma-Hock et al., 2007, 2009a, 2012; van Ravenzwaay et al., 2009).
Selection of concentrations was oriented on the occupational exposure limit for respirable nuisance dust in Germany, which was 3 mg/m3 until 2014 and is now lowered to 1.25 mg/m3 (density 2.5 g/cm3) (Federal Institute of Occupational Safety and Health, 2015).
Methods
General
The present animal studies were conducted according to the OECD Principles of Good Laboratory Practice (OECD, 1992) (except fine and coarse Pigment Red 101, which were conducted in a Good Laboratory Practice certified laboratory but not inspected), which principally meet the United States Environmental Protection Agency Good Laboratory Practice Standards [40 CFR Part 160 (FIFRA) and Part 792 (TSCA)]. The study was conducted referring to OECD Guideline 412 (OECD, 2009). Physico-chemical parameters were investigated at the department Material Physics, BASF SE. Methods follow in-house established operating procedures, and adhere to ISO standards where applicable. The accuracy is regularly checked against NIST-traceable standards.
Test materials and characterization
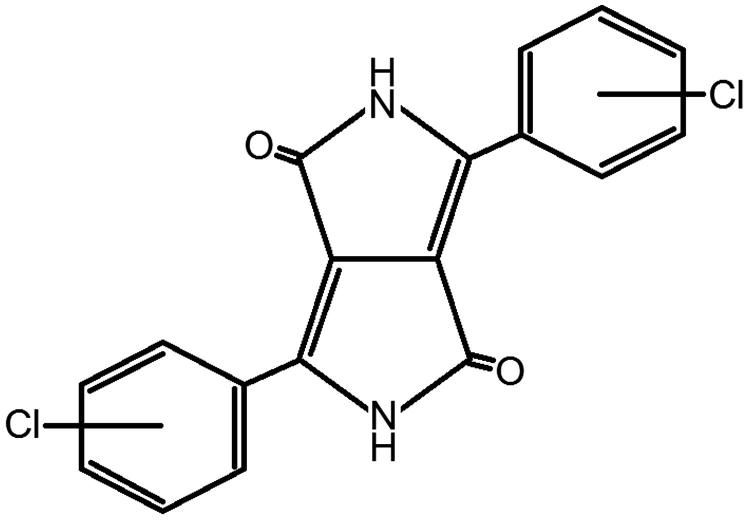
Details of the test materials are given in Table 1. The generic formula of the DPP pigments is shown in Figure 1.
Table 1. Test materials.
| Compound | CAS No. | Analytical characterization | Producer |
|---|---|---|---|
| Mixed chlorinated DPP isomers | 84632-67-7 and 88949-44-4 (isomers), 84632-65-5 (mixture) | Extractable fraction <0.4% | BASF Schweiz AG, Switzerland |
| Fine Pigment Red 254 | 84632-65-5 | Extractable fraction 0.5% | BASF Schweiz AG, Switzerland |
| Coarse Pigment Red 254 | 84632-65-5 | Extractable fraction 0.5% | BASF Schweiz AG, Switzerland |
| Coarse meta-chloro DPP | 84632-67-7 | Purity 98.02% | BASF Schweiz AG, Switzerland |
| Fine meta-chloro DPP | 84632-67-7 | Purity 98.04% | BASF Schweiz AG, Switzerland |
| Fine Pigment Red 101 | 1309-37-1 | Iron content 62 g/100 g | BASF SE, Germany |
| Coarse Pigment Red 101 | 1309-37-1 | Not characterized | Huntsman, Italy |
Figure 1.
Generic formula of the DPP pigments.
Characterization methods
Size (distribution), shape and representative image – dry
The primary size and shape were assessed using a transmission electron microscope (TEM) from FEI, Type Strata 400 DB, equipped with a field emission cathode (FEI, Hillsboro, OR). For TEM analysis, samples were wetted in ethanol, then gently spread on a sample holder and transferred into vacuum for TEM imaging. Analysis of the resulting images was done visibly. When possible the median diameter d50 was calculated.
Specific surface area
Specific surface area was determined by the Brunauer–Emmet–Teller (BET) method on 50–300 mg samples. First, samples were decontaminated under vacuum at 100 °C in case of the organic pigments and at 200 °C in case of the inorganic pigments. Nitrogen adsorption/desorption isotherms at 77 K were recorded at five pressures between 0 and 0.2 P/P0. The measurements were distributed between different instruments – Autosorb 6b (Quantachrome) or Tristar or ASAP2420 (both Micromeritics, Norcross, GA) – all adhering to the standard DIN 66131.
Composition
The composition of the organic pigments was investigated by oxidation and pyrolysis. To determine the content of C, H and N samples was exposed in duplicate to a stream of oxygen-containing helium. As detection reaction the reduction of NOX to N2 was used observed with Cu as reduction catalyst. Using a thermal conductivity detector (TCD, Elementar vario EL cube, Elementar Analysensysteme GmbH, Hanau, Germany), CO2, H2O and N2 were determined, which allows the quantification of C, H and N. The content of O in the organic pigments was analyzed in duplicate during pyrolysis in a stream of helium in which the conversion to CO with contact to carbon black was investigated. The resulting gas mixture was separated by chromatography and the content of CO was detected by a TCD (EuroVector EA3000, Milan, Italy). The content of O within the pigments was then calculated from the CO content. The content of Cl was analyzed during combustion in oxygen (Mitsubishi system AQF-100, Tokyo, Japan). The resulting gas mixture was absorbed into acetic acid and subsequent titration of chloride with silver nitrate delivered the Cl content in the organic pigments (Metrohm Ag Titrode). Contaminations were investigated by X-ray fluorescence (XRF).
Composition and crystallite phase of the inorganic pigments was determined by standard X-ray diffraction (XRD). For this, the samples were filled to sample holders and their surface was flattened using a glass plate. The Bruker D8 Advance Series II Diffractometer (Bruker, Billerica, MA) acquires the intensity as function of the diffraction angle with the Sol-X detector and variable slits (V20). The range of 2° < 2θ < 80° is scanned in steps width 0.02° (2θ) in intervals of 2.4 s.
Surface chemistry
The chemical composition of the materials’ surface was determined by X-ray Photoelectron Spectroscopy (XPS), PHI XPS 5500 system equipped with 300 W monochromatic Al Kα radiation. The pass energy for surveys was 117 eV (measurement time of 45 min) and for detailed spectra 23.5 eV (measurement time of 6 min). In this case, spectra evaluation was performed by CasaXPS 2.3.15. Information depth of XPS is specific to the surface 1–10 nm of flat bulk materials, depending on cross-sections of photoelectron reabsorption. Due to increased path lengths in rough surfaces, the XPS signal is even more specific to the surface for powders. Three measurements per sample were performed, each integrating over 0.5 mm2. The results in % indicate the relative concentration of elements.
Reactivity
Surface reactivity of the pigments was investigated by the FRAS assay (ferric reducing ability of serum) which measures depletion of antioxidants in serum due to oxidizing species/mechanisms. Even though the application of the FRAS assay for detecting the reactivity of (nano)materials is relatively new (Hsieh et al., 2013), it is very promising and was therefore used in a modified version to detect the reactivity of the pigments in this study. The pigment powders were mixed with human serum and incubated for 180 min at 37 °C and stirring at 400 rpm. Afterwards, the pigments were removed by centrifugation (14 000g, 150 min). The supernatant was mixed with an iron complex and incubated at 25 °C over 1 h at continuous stirring. During that time the iron complex is reduced by antioxidants present in the supernatant which can be measured spectrometrically at 593 nm afterwards. The resulting reactivity is given in Table 2 in the surface- and time-dependent unit μUFRAS/m2 h. The classification of the pigments to non-reactive, intermediate and reactive was benchmarked by the highly reactive and insoluble reference material Mn2O3, using threshold levels of <1%, 1–10% and >10% of the Mn2O3 reactivity, respectively.
Table 2. Physico-chemical parameters of the test materials.
| Pigment | Size in nm (d50) (TEM) | Surface in m2/g (BET) | Composition (elements in %) (oxidation, XRD, XRF) | Surface chemistry (elements in %) (XPS) | Reactivity (in μUFRAS/m2h) (FRAS) | Dissolution in water (ICP-MS, UV/Vis) | Dispersibility in DMEM-FCS (d50) (AUC-XLA) | Charge in mV (zeta potential) | Hydrophobicity (contact Angle) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mixed chlorinated DPP isomers | 39 | 62 | C 60.5, H 2.9, N 7.8, O 9.1, Cl 19.6; amorphous and crystalline; no contaminations >0.1% | C 74.8, O 8.1, N 8.3, Cl 8.9 | <1 ppm | 988 nm, AAN: 25 | N/A a | Hydrophobic (138°) | ||
| Fine Pigment Red 254 | 43 | 94 | C 60.9, H 2.8, N 7.8, O 9.0, Cl 19.9; no contaminations >0.1% Si, Br <0.1% | C 77.1, O 10.9, N 5.9, Cl 6.1 | Non-active (0.0006) | ≪1 ppm | 774 nm, AAN: 18 | −16 | Hydrophobic (135°) | |
| Coarse Pigment Red 254 | 233 | 16 | C 60.5, H 2.8, N 7.8, O 8.9, Cl 19.7; no contaminations >0.1% Si, Br <0.1% | C 79.4, O 10.0, N 5.1, Cl 5.2, | Non-active (0.0007) | <1 ppm | 352 nm, AAN: 1.5 | -41 | Hydrophobic (136°) | |
| Coarse meta-chloro DPP | 0.3–3 μm × 70–200 nm | 42 | C 60.4, H 2.9, N 7.8, O 9.1, Cl 19.8; no contaminations >0.1% | C 73.1, O 8.4, N 9.5, Cl 9, | Intermediate (0.0196) | ≪1 ppm | ≫1 μm, AAN > 10 | −11 | Hydrophobic (110°) | |
| Fine meta-chloro DPP | 30–400 nm × 10-50 nm | 64 | C 60.6, H 2.9, N 7.9, O 9.0, Cl 19.6; no contaminations >0.1% | C 73.6, O: 8.8, N 8.7, Cl 8.8 | Intermediate (0.0256) | ≪1 ppm | ≫1 μm, AAN > 33 | −12 | Hydrophobic (141°) | |
| Fine Pigment Red 101 | 15–130 nm× 4–21 nm | 107 | Fe2O3, hematite | O 54.2, Fe 27.2, C 15.7, P 1.6, Ca 0.9, S 0.2, F 0.1, Mg 0.1 | Intermediate (0.0372) | <1 ppm | >1 μm, AAN > 1000 | −27 | Hydrophilic (0°) | |
| Coarse Pigment Red 101 | 48–90 nm | 12 | predominantly Fe2O3, hematite; possibly also Fe3O4 magnetite | O 49.6, Fe 24.6, C 23.9, Na 1.9 | Intermediate (0.0200) | <1 ppm | >1 μm, AAN > 20 | −55 | Hydrophilic (0°) |
TEM: transmission electron microscopy; BET: Brunauer–Emmet–Teller; XRD: X-ray diffraction; XRF: X-ray fluorescence; XPS: X-ray photoelectron spectroscopy; FRAS: ferric reducing ability of serum; ICP-MS: inductively coupled plasma mass spectrometry; AUC-XLA: analytical ultracentrifuge; AAN: average agglomeration number.
Charge of mixed chlorinated DPP isomers is not detectable by the used measurement technique.
Solubility in water
The pigments were suspended in 20 ml water at 10 mg/ml for a fixed dissolution time of 24 h at 25 °C. The ensuing ultracentrifugation at 300 000g (in units of the gravitational acceleration g = 9.81 m/s2) for 10 h removes particles quantitatively down to about 2 nm diameter, as verified by analytical ultracentrifugation (AUC) of the remaining particulate content in the supernatant.
In case of the organic pigments, the supernatants were analyzed by UV/Vis spectroscopy (Ocean Optic USB4000 spectrometer, wavelength 200–800 nm; Ocean Optics, Dunedin, FL). For the inorganic pigments the content of metal ions in the supernatants was analyzed by inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7500a, Santa Clara, CA). Samples are introduced via a Scott type spray chamber with Meinhardt nebulizer (plasma power 1300 W, plasma gas 16.5 l/min, auxiliary gas 1.5 l/min). As internal standard, 45Sc (ICP-MS) was used. One gram sample was weighed in and diluted with 5% (v/v) HCl to a total volume of 10 ml. Calibration standards were measured at 0 and 10 mg/l. The results are given as the measured ppm levels of metal ions in the supernatant, which correspond to dissolution of 0.01% of the dispersed material. The limit of detection was 0.1 ppm, corresponding to 0.001% dissolution.
Dispersibility, size distribution in DMEM-FCS
Dispersibility of the pigments was measured in serum containing cell culture medium [DMEM-FCS, Dulbecco’s modified Eagle medium, low glucose, no pH indicators, mixed with 10% FCS (FBS Gold, defined fetal bovine serum, PAA Laboratories, Pasching, Austria)]. For the measurements an AUC machine (Beckman Ultracentrifuge type XLA with integrated absorption optics, Brea, CA) was used, as described in detail earlier (Walter et al., 2014). The samples were diluted to obtained concentrations of 1 mg/ml. Speed ramps from 1000 to 20 000 rpm were used to ensure complete coverage of the relevant measurement interval between 1 μm down to primary particles. The size distribution is evaluated by the freeware program Sedfit, with fitting model ls g(s*). The average agglomeration number (AAN) represents the ratio of the volume based median particle size (d50) determined by AUC to the median primary particle size determined by TEM (Wohlleben, 2012).
Surface charge
Zeta potential at pH 7 was determined by Laser Doppler Electrophoresis using Zetasizer Nano (Malvern, Worcestershire, UK) and a folded capillary cell (DTS 1060, Malvern). The samples were dispersed at room temperature in the background electrolyte solution (10 mmol/l KCl) and adjusted to the corresponding pH by 0.1 M NaOH or HCl. The concentration of the samples was adjusted to the signal intensity in the range of 3000–7000 kcps. The results are the average of two measurements. The instrument was calibrated with Malvern Standard DTS 1235. We recorded the electrophoretic mobility across the titration range of pH 3 to pH 10 to identify the iso-electric point where the electrophoretic mobility crosses zero. Using the dispersed size determined by AUC, mobility and pH 7, we extracted the zeta-potential reported in Table 2.
Hydrophobicity
The contact angle of the pigments was used as a measure for their hydrophobicity. For this, the pigment powders were prepared onto microscope glass slide which was analyzed in a Krüss DSA 100 (Hamburg, Germany) by using the static droplet method and water as a wetting agent. A minimum of three droplets was used for each pigment sample. Conventionally, the contact angle of 90° separates hydrophilic from hydrophobic materials.
Animals
Permission for animal studies was obtained from the local regulatory agencies, and all protocols were in compliance with the federal guidelines. The laboratories of BASF’s Experimental Toxicology and Ecology, where all the studies were performed, are AAALAC-certified. All procedures for animal care and exposure were conducted under the rule of the German Animal Welfare Act (1998). Male Wistar (strain Crl:WI (Han)) rats (7 weeks of age) were obtained from Charles River Laboratories (Sandhofer Weg, Sulzfeld, Germany) and were allowed free access to mouse/rat laboratory diet (Provimi Kliba SA, Kaiseraugst, Switzerland) and water. The animals were housed singly in mesh floored cages in accommodation maintained at 20–24 °C, with a relative humidity of 30–70%, a light/dark cycle of 06.00–18.00 h light and 18.00–06.00 h dark and were allowed to acclimatize to these conditions for approximately 2 weeks before commencement of the study.
Exposure regimen/test groups
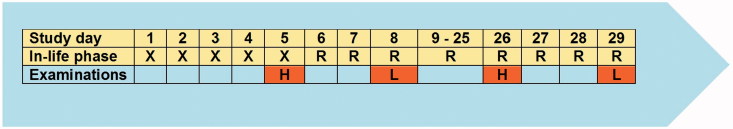
The exposure regimen is outlined in Figure 2. Groups of eight male Wistar rats were head-nose exposed to respirable dusts on 6 h/day, on 5 consecutive days (days 1–5). Target concentrations were 30 mg/m3 as high dose for all compounds. Additionally, 10 mg/m³ was tested with fine Pigment Red 101, and 3 and 10 mg/m³ were tested in case of the meta-chloro DPP isomers. Four concurrent control groups were exposed to conditioned air (one common control group was used for coarse and fine Pigment Red 254, coarse and fine Meta-chloro DPP and coarse and fine Pigment Red 101, respectively). On study day 5 and 26, three animals per group were sacrificed for histopathological examinations. On study day 8 and 29, the remaining five animals per group were sacrificed. The lungs of these animals were lavaged, and BALF was analyzed for markers indicative for injury of the bronchoalveolar region.
Figure 2.
Study design: X, head–nose exposure for 6 h/day on 5 consecutive days, R, post-exposure time; H, histology of selected organs; L, examination of blood and broncho-alveolar lavage fluid (BALF).
Generation of the test atmospheres
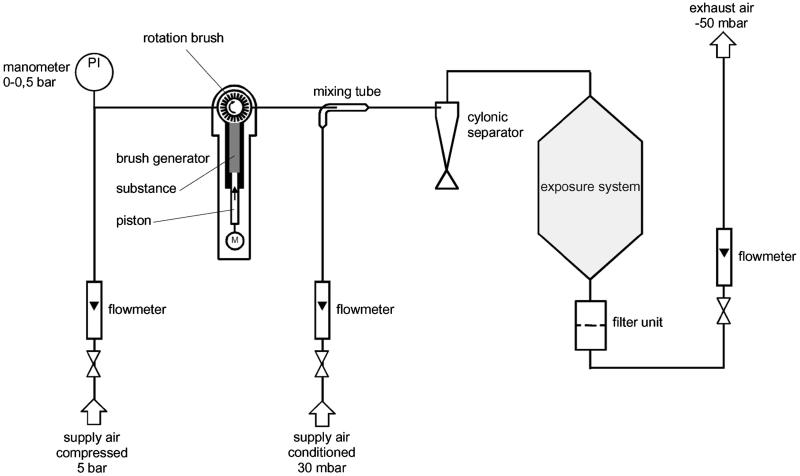
Brush dust generators (developed by the Technical University of Karlsruhe in cooperation with BASF, Germany) served for generation of test atmospheres. Generated dusts were mixed with compressed air (filtered air pressurized to about 6 bar, flow rate 1.5 ± 0.3 m3/h) in a glass tube, diluted with conditioned air (activated charcoal-filtered air, 22 ± 2 °C, 50 ± 20% relative humidity, flow rate 4.5 ± 0.3 m3/h) and passed via a cyclone into the inhalation system. The 90-l cylindrical stainless steel inhalation chamber was fed via a cone-shaped inlet at the top and exhausted at the opposite end. The desired inhalation chamber concentrations were achieved by withdrawing/exhausting and replacing a portion of the dust aerosol air with conditioned supply air immediately before entering the chamber (6 m3/h). Mean flow rate through the inhalation chamber, measured at exhaust air, was 5.4 ± 0.3 m3/h for all concentrations, that is, air was changed in the inhalation chambers about 67 times per hour. A schematic diagram of the inhalation system is shown in Figure 3.
Figure 3.
Schematic picture of the inhalation exposure system.
Monitoring and characterization of the test atmosphere
Compressed and conditioned supply air and exhaust air flow rates, chamber temperature and humidity were measured automatically with appropriate sensors/orifice plates; data were saved every 1 s and retained for analysis. To ensure the stability of the dust aerosols, the inhalation chambers were monitored continuously during exposure using scattered light photometers (VisGuard; Sigrist-Photometer AG, Ennetbürgen Switzerland; for details, see Ma-Hock et al. (2009b). To quantify the atmospheric dust concentration, gravimetric measurements of air samples taken adjacent to the animals’ breathing zone were performed (probe internal diameter 7 mm). A defined volume of sample air was drawn by vacuum pump across a binder-free glass-fiber filter paper (Macherey-Nagel MN 85/90 BF, diameter 4.7 cm, Düren, Germany).
Aerosol dust concentration was calculated as the increase in weight of the filter after sampling, divided by sample volume at test conditions (22 °C, atmospheric pressure, 50% relative humidity). As a rule, two samples were taken per exposure and concentration group. The duration of sampling was adjusted to the test substance concentration in the chamber to obtain a total sample weight of 1–5 mg. Thus, the volume of the air samples varied with the atmospheric concentration. To determine the mass median aerodynamic diameters (MMAD) (the calculated aerodynamic diameter, which divides the size distribution in half when measured by mass), cascade impactor measurements were performed with a stack sampler Marple 298 (New Star Environmental Inc., Roswell, GA). The effective aerodynamic cutoff diameters were 17.3, 11.9, 7.9, 4.8, 2.8, 1.3, 0.7 and 0.4 μm. To capture the particles < 0.4 μm, the impactor was equipped with a backup filter. The deposition on each impactor stage as well as on the backup filter was determined gravimetrically. Particle size distributions were calculated according to DIN 66141 and DIN 66161, that is linear regression of cumulative percent (probit values) versus logarithms of effective cutoff diameters. Particle size distributions measured by cascade impactor were expressed as MMAD and geometrical standard deviation (GSD). Additionally, a light-scattering spectrometer (WELAS 2000; Palas, Karlsruhe, Germany) was used for particle sizes from 0.25 to 9.7 μm (at least 10 repeats). In the submicrometer range (11–1083 nm), particle size distribution was measured with a Scanning Mobility Particle Sizer equipped with a condensation particle counter (SMPS + C) (Grimm Aerosol Technik GmbH, Ainring, Germany). Particles were classified by electrostatic fractionation of the different sized particles. Particle counts in each of the fractions were counted by condensation particle counter. The SMPS spectrometer uses electrical mobility to measure the particle size. This technique utilizes a bipolar charger to impact a known charge distribution on the aerosol sample, and classify particles according to their ability to traverse an electric field.
Animal exposure
During exposure, rats were restrained in glass tubes fixed to the inhalation chamber walls with their snouts projecting into the inhalation chamber (head/nose exposure). Overpressure was maintained inside the inhalation chamber to ensure that the aerosol in the animals’ breathing zone was not diluted by laboratory air. The exposure systems were kept under exhaust hoods in an air-conditioned room.
Clinical observations
Health status and cage-side clinical signs were checked at least once daily (on exposure days before, during and after exposure). Body weights were measured weekly.
Hematology, clinical chemistry and acute phase proteins
Before sacrifice, blood samples for hematology and clinical chemistry were taken from all animals designated for collection of BALF by retrobulbar venous plexus puncture under isoflurane (Isoba®, Essex GmbH, Munich, Germany) anesthesia. Hematology (ADVIA120 Instrument, Siemens, Fernwald, Germany) comprised red blood cell counts, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin content, mean corpuscular hemoglobin concentration, platelets, total white blood cell and differential blood cell counts. Prothrombin time was determined using a ball coagulometer (AMAX destiny plus model; Trinity biotech, Lemgo, Germany). Clinical chemistry parameters (Hitachi 917 or COBAS c501; Roche, Mannheim, Germany) included alanine aminotransferase (EC 2.6.1.2.), aspartate aminotransferase (EC 2.6.1.1.), alkaline phosphatase (EC 3.1.3.1.), γ-glutamyl transferase (EC 2.3.2.2.), sodium, potassium, chloride, inorganic phosphate, calcium, urea, creatinine, glucose, total bilirubin, total protein, albumin, globulins, triglycerides and cholesterol.
Acute phase proteins: Rat α2-macroglobulin and rat haptoglobin were measured with MTP-ELISA kits (rat α2-macroglobulin: Immmunology Consultants Laboratory Inc., Newberg, OR; cat. no. E-25A2M), rat haptoglobin (Immmunology Consultants Laboratory Inc.; cat. no. E-25HPT), measured with a Sunrise MTP Reader, Tecan AG, Männedorf, Switzerland, by using the Magellan Software provided by the instrument producer.
Broncho-alveolar lavage
To obtain BALF, animals were killed by exsanguination under Narcoren® anesthesia and the lungs lavaged twice with 6 ml (22 ml/kg body weight) physiological saline. The two washes were combined (an average of 11 ml of lavage fluid was recovered per animal) and aliquots of the combined washes were used for the determination of cytology, total protein concentration and enzyme activities, as well as mediators.
Total BALF cell counts were determined with an Advia 120 (Siemens Diagnostics) hematology analyzer. Counts of macrophages, polymorphonuclear neutrophils, lymphocytes, eosinophils, monocytes and atypical cells were performed on Wright-stained cytocentrifuge slide preparations as described by Warheit & Hartsky (1993). The differential cell count was evaluated manually by counting at least 400 BALF cells per sample. The following parameters were measured with a Hitachi 917 or COBAS c501 (Roche Diagnostics) reaction rate analyzer: total protein (turbidimetric method with Benzethonium chloride), LDH (EC 1.1.1.27; kinetic UV test, 340 nm, 37 °C acc. to IFCC), ALP (EC 3.1.3.1; kinetic color test, 450 nm, 37 °C acc. to IFCC), NAG (EC 3.2.1.30; color test, 580 nm, 37 °C) (Yakata et al., 1983) and GGT (EC 2.3.2.2, Szasz method) (kinetic color test, 415 nm, 37 °C acc. to IFCC) activities.
The mediators were measured with MTP ELISAs using a Sunrise MTP Reader, by using the Magellan Software provided by the instrument producer (Elshal & McCoy, 2006; Fulton et al., 1997; Kettman et al., 1998; Skogstrand et al., 2005). The following antigens were measured in BALF: Rat monocyte chemoattractant protein-1 (rat MCP-1) level was measured with an Instant ELISA produced by Bender MedSystems, Vienna, Austria (cat. no. BMS631INST). Rat cytokine-induced neutrophil chemoattractant-1 level (rat CINC-1/IL-8) was measured with an ELISA produced by R&D Systems Inc., Minneapolis, MN (Quantikine rat CINC-1, cat. no. RCN100). Macrophage colony stimulating factor (M-CSF) was measured with a Quantikine Mouse MCSF ELISA produced by R&D Systems Inc. (cat. no. MMC00). Rodent osteopontin was measured with an ELISA produced by R&D Systems, Inc. (Quantikine mouse osteopontin, cat. no. MOST00).
Pathology
Animals were euthanized by exsanguination under Narcoren® anesthesia. Gross necropsy was carried out. Lungs were instilled at a pressure of 20–30 cm of water. Weights of adrenal glands, brain with olfactory bulb, epididymides, heart, kidneys, liver, lungs, spleen, testes, thymus and thyroid glands were determined.
Brain, head (with oropharynx), larynx, lungs, mediastinal lymph nodes, and trachea were fixed in 4% buffered formaldehyde (corresponding to 10% formalin), paraffin embedded, sectioned and stained with hematoxylin–eosin for histopathology.
Light microscopic examination was performed on the respiratory tract comprising nasal cavity (four levels), larynx (three levels), trachea (longitudinal with carina), lung (five lobes), tracheobronchial and mediastinal lymph nodes.
Statistical analysis
In case of fine Pigment Red 101, coarse meta-chloro DPP and fine meta-chloro DPP Dunnett’s test (Dunnet, 1955, 1964) was used for simultaneous comparison of all concentration groups with the control group for body weights and body weight changes. In case of the other compounds, where only one concentration group was used, body weights and body weight gains were evaluated by comparison of the dose group with the control group using the student t-test (two-sided) for the hypothesis of equal means (Winer, 1971). Clinical pathology parameters in blood were analyzed by pair-wise comparison of the dose group with the control group was performed using Wilcoxon test (two-sided) for the equal medians. Clinical pathology parameters in BALF were analyzed by pair-wise comparison of the dose group with the control group was performed using Wilcoxon test (one-sided) for the equal medians. Statistical significance was defined as p ≤ 0.05 compared with the control group (Siegel, 1956).
Results
Characterization of test materials
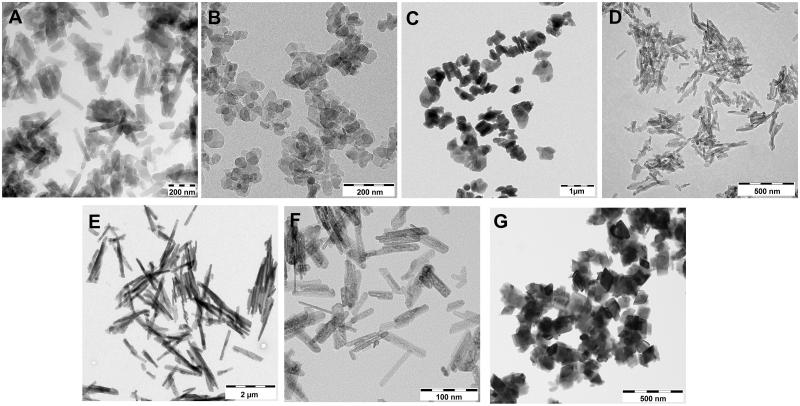
A comprehensive list of physico-chemical characteristics of the tested materials is given in Table 2. In addition, Figure 4 shows representative electron microscopy images, which clearly highlight the different structures of the organic pigments, with fine Pigment Red 254 and fine meta-chloro DPP showing much smaller particle sizes compared to coarse Pigment Red 254 and coarse meta-chloro DPP on a statistical based evaluation. In accordance, BET surfaces are smaller for coarse Pigment Red 254 and coarse meta-chloro DPP compared to fine Pigment Red 254 and fine meta-chloro DPP (16 m2/g versus 94 m2/g for DPP Reds and 42 m2/g versus 64 m2/g for DPP Oranges). According to the NanoDefine implementation of the EC nanodefinition, coarse Pigment Red 254 is clearly a non-nano-material, and coarse meta-chloro DPP is borderline-non-nano. In the case of the inorganic pigments, the combination of electron microscopy and BET surface ranks the rod-shaped fine Pigment Red 101 as a nanomaterial, and coarse Pigment Red 101 as a borderline-nano.
Figure 4.
Representative electron microscopy images of the five organic pigments. Mixed chlorinated DPP isomers (A), fine Pigment Red 254 (B), coarse Pigment Red 254 (C), fine meta-chloro DPP (D) and coarse meta-chloro DPP (E) and the two inorganic fine Pigment Red 101 (F) and coarse Pigment Red 101 (G).
The elemental composition and surface chemistry matches expectations and shows good agreement between fine and coarse versions of the pigments. Additionally, no contaminations >0.1% could be observed for all organic pigments (not investigated for the inorganic pigments). Fine Pigment Red 101 exists as hematite (Fe2O3), which is also mainly present in the case of coarse Pigment Red 101 which, however, possibly contains magnetite (Fe3O4) as well.
Reactivity in the FRAS assay showed no remarkable activity for all investigated pigments. They can be regarded as intermediate reactive or even non-active in the case of fine Pigment Red 254 and coarse Pigment Red 254.
In particle inhalation toxicity, the release of metal ions is considered a key mode of action. The solubility of the pigments was investigated after 24 h incubation in water. All pigments are highly stable under the tested conditions with a solubility <1 ppm. In biophysical interaction, all pigments showed low dispersability in the DMEM-FCS (serum containing cell culture media), except the coarse Pigment Red 254 whose dispersed size in DMEM-FCS indicates low agglomeration with AAN of ∼1.5.
At neutral pH, all pigments showed a negative surface charge. No difference was visible for the DPP Oranges; however for the Pigment Reds 254 a difference between fine (−16 mV) and coarse (−41 mV) was observed. The inorganic iron oxide pigments also showed different charges (−27 mV for fine Pigment Red 101, −55 mV for coarse Pigment Red 101).
The organic pigments are highly hydrophobic (132–138°), except of coarse meta-chloro DPP, which showed a less hydrophobic behavior (110°). As expected, the inorganic iron oxide pigments are clearly hydrophilic with a contact angle of 0°, which is a result of complete water wetting at the pigments’ surface.
Characterization of the test atmosphere
Results of gravimetric determination of aerosol concentrations and particle size distribution are summarized in Table 3. Measured concentrations of aerosols were close to the target concentrations. The MMAD of the particles in the aerosols were small enough to reach the lower respiratory tract of rats (the particles were respirable for rats).
Table 3. Results of gravimetric determination of test atmosphere concentration and particle size distribution.
| Mixed chlorinated DPP isomers | |||
| Target concentration (mg/m3) | 30 | ||
| Measured concentration (mg/m3±SD) | 31.49 ± 1.27 | ||
| MMAD (μm) | 0.7/0.6 | ||
| GSD | 2.6/2.8 | ||
| OPC | |||
| Count median diameter (μm) | 0.376 | ||
| Count concentration (N/cm3) | 94 423 | ||
| SMPS | |||
| Count median diameter (μm) | 0.292 | ||
| Count concentration (N/cm3) | 373 840 | ||
| Fine Pigment Red 254 | |||
| Target concentration (mg/m3) | 30 | ||
| Measured concentration (mg/m3±SD) | 29.86 ± 2.77 | ||
| MMAD (μm) | 1.1/0.6 | ||
| GSD | 2.5/3.2 | ||
| OPC | |||
| Count median diameter (μm) | 0.362 | ||
| SMPS | |||
| Count concentration (N/cm3) | 63 792 | ||
| Count median diameter (μm) | 0.255 | ||
| Coarse Pigment Red 254 | |||
| Target concentration (mg/m3) | 30 | ||
| Measured concentration (mg/m3±SD) | 31.34 ± 4.49 | ||
| MMAD (μm) | 0.7/0.7 | ||
| GSD | 2.9/2.5 | ||
| OPC | |||
| Count median diameter (μm) | 0.370 | ||
| Count concentration (N/cm3) | 191 421 | ||
| SMPS | |||
| Count median diameter (μm) | 0.255 | ||
| Count concentration (N/cm3) | 506 049 | ||
| Coarse meta-chloro DPP | |||
| Target concentration (mg/m3) | 3 | 10 | 30 |
| Measured concentration (mg/m3±SD) | 3.45±.23 | 10.54 ± 1.54 | 31.04 ± 3.11 |
| MMAD (μm) | 0.7/0.9 | 0.6/0.6 | 1.1/0.8 |
| GSD | 2.8/2.4 | 2.9/3.1 | 2.6/2.5 |
| OPC | |||
| Count median diameter (μm) | 0.498 | 0.605 | 0.589 |
| Count concentration (N/cm3) | 18 987 | 63 792 | |
| SMPS | |||
| Count median diameter (μm) | 0.366 | 0.409 | 0.359 |
| Count concentration (N/cm3) | 96 924 | 33 168 | 115 116 |
| Fine meta-chloro DPP | |||
| Target concentration (mg/m3) | 3 | 10 | 30 |
| Measured concentration (mg/m3±SD) | 3.55 ± 0.30 | 13.97 ± 1.58 | 32.00 ± 5.19 |
| MMAD (μm) | 1.1/1.1 | 0.7/0.7 | 0.9/0.9 |
| GSD | 2.8/2.6 | 3.1/2.8 | 2.7/2.7 |
| OPC | |||
| Count median diameter (μm) | 0.380 | 0.366 | 0.389 |
| Count concentration (N/cm3) | 6322 | 25 819 | 96 507 |
| SMPS | |||
| Count median diameter (μm) | 0.263 | 0.242 | 0.233 |
| Count concentration (N/cm3) | 35 004 | 178 400 | 506 463 |
| Fine Pigment Red 101 | |||
| Target concentration (mg/m3) | 10 | 30 | |
| Measured concentration (mg/m3±SD) | 11.3 ± 1.7 | 29.5 ± 6.5 | |
| MMAD (μm) | 3.0/2.3 | 2.1/2.8 | |
| GSD | 3.1/2.5 | 2.8/2.1 | |
| OPC | |||
| Count median diameter (μm) | 0.347 | 0.348 | |
| SMPS | |||
| Count concentration (N/cm3) | 9712 | 28 502 | |
| Count median diameter (μm) | 0.233 | 0.233 | |
| 77 062 | 156 058 | ||
| Coarse Pigment Red 101 | 30 | ||
| Target concentration (mg/m3) | 26.8 ± 3.9 | ||
| Measured concentration (mg/m3±SD) | 0.9/0.9 | ||
| MMAD (μm) | 3.6/4.9 | ||
| GSD | 0.292 | ||
| OPC | |||
| Count median diameter (μm) | 90 462 | ||
| Count concentration (N/cm3) | 0.198 | ||
| SMPS | |||
| Count median diameter (μm) | 833 882 | ||
| Count concentration (N/cm3) | 30 | ||
OPC: optical particle counter; SMPS: scanning mobility particle sizer.
Clinical observations and body weight
Inhalation of mixed chlorinated DPP isomers, fine Pigment Red 254, coarse Pigment Red 254, fine Pigment Red 101 or coarse Pigment Red 101 did not cause any adverse clinical signs. Red encrusted nose was observed in few animals exposed to coarse meta-chloro DPP and fine meta-chloro DPP. During the exposure period, substance-contaminated fur was observed after the daily exposure. The body weight development of exposed rats was comparable to control animals in all cases except after exposure to coarse Pigment Red 101, where body weights were slightly, but statistically significantly lower compared to controls.
Hematology, clinical chemistry and acute phase proteins in serum
In the studies of all seven compounds, no systemic toxicological relevant effects were observed regarding hematology, clinical chemistry and acute-phase protein levels (α2-macroglobulin and haptoglobin) in blood.
Total protein concentrations in rats exposed to mixed chlorinated DPP isomers were slightly lower due to lower globulin values at study day 8. Since no other changes occurred in clinical chemistry, this difference is considered not to be adverse. The same applies to marginally higher neurophil counts observed in rats exposed to 30 mg/m³ coarse meta-chloro DPP.
Broncho-alveolar lavage
In case of mediators, only parameters which were altered by exposure to the test substances are shown in the tables.
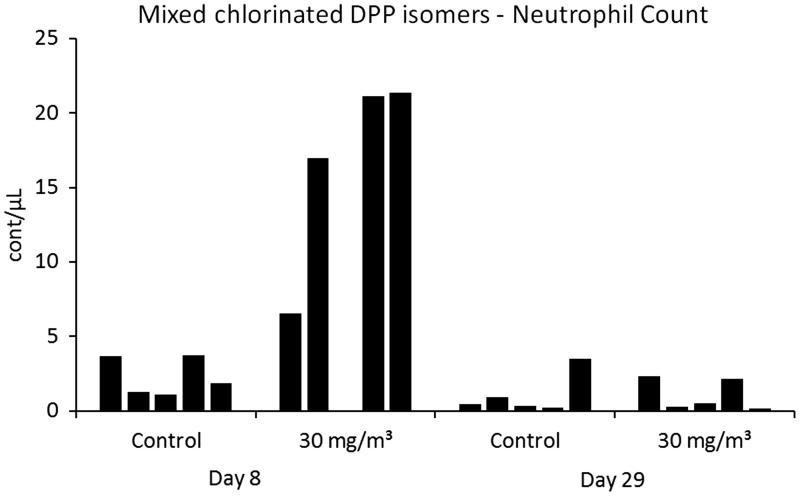
Mixed chlorinated DPP isomers
Differences of parameters measured in BALF when compared to the controls are shown in Table 4. Individual values are shown in Figure 5. On study day 8, a slight increase of polymorphonuclear neutrophils was observed. No changes in BALF cytology parameters were noted on study day 29. No treatment-related effects were observed by examination of protein concentration, enzyme activities and mediators in BALF.
Table 4. Cytology parameters in BALF after exposure to mixed chlorinated DPP isomers.
| Study day 8 | Study day 29 | |
|---|---|---|
| Concentration (mg/m3) | 30 | 30 |
| Polymorphonuclear Neutrophils | 7.0* | 1.0 |
Differences are expressed as x-fold ratios compared to the concurrent control group.
*Statistically significant, p < 0.05.
Figure 5.
Individual values of polymorphonuclear neutrophils in BALF. Only four animals were evaluated at 30 mg/m³.
Fine pigment red 254
No treatment-related effects were observed by examination of cytology, protein concentration, enzyme activities and mediators in BALF.
Coarse pigment red 254
No treatment-related effects were observed by examination of cytology, protein concentration, enzyme activities and mediators in BALF.
Coarse meta-chloro DPP
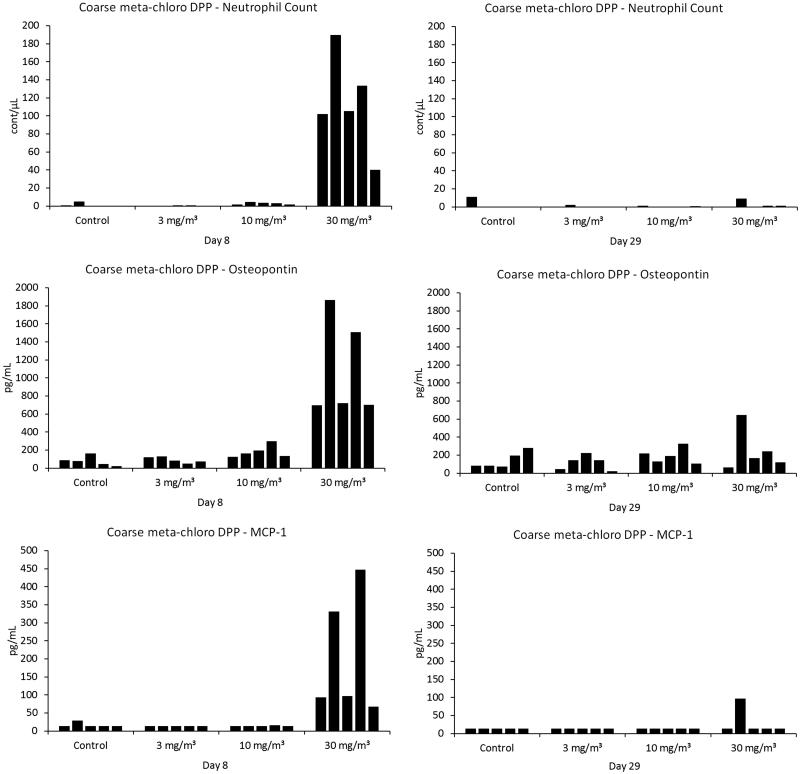
Differences of parameters measured in BALF when compared to the controls are shown in Tables 5–7. Individual values of polymorphonuclear neutrophils, osteopontin and MCP-1 are shown in Figure 6.
Table 5. Cytology parameters in BALF after exposure to coarse meta-chloro DPP.
| Study day 8 | Study day 29 | |||||
|---|---|---|---|---|---|---|
| Concentration (mg/m3) | 3 | 10 | 30 | 3 | 10 | 30 |
| Total cells | 0.8 | 0.8 | 2.3* | 0.9 | 1.2 | 0.9 |
| Polymorphonuclear neutrophils | 0.2 | 2.2 | 83.9* | 0.2 | 0.2 | 1.0 |
Differences are expressed as x-fold ratios compared to the concurrent control group.
*Statistically significant, p < 0.05.
Table 6. Protein concentration and enzyme activities in BALF after exposure to coarse meta-chloro DPP.
| Study day 8 | Study day 29 | |||||
|---|---|---|---|---|---|---|
| Concentration (mg/m3) | 3 | 10 | 30 | 3 | 10 | 30 |
| Total protein | 1.2 | 0.8 | 2.1* | 1.2 | 1.1 | 1.1 |
| GGT | 1.6 | 1.0 | 3.8* | 4.8 | 2.4 | 5.8 |
| LDH | 1.5 | 1.0 | 3.1* | 0.9 | 1.2 | 1.2 |
| ALP | 1.2 | 1.1 | 2.0* | 1.0 | 1.2 | 1.5* |
Differences are expressed as x-fold ratios compared to the concurrent control group.
*Statistically significant, p < 0.05.
Table 7. Mediators in BALF after exposure to coarse meta-chloro DPP.
| Study day 8 | Study day 29 | |||||
|---|---|---|---|---|---|---|
| Concentration (mg/m3) | 3 | 10 | 30 | 3 | 10 | 30 |
| MCP-1 | 0.8 | 0.9 | 12.2* | 1.0 | 1.0 | 2.0 |
| CINC-1/IL-8 | 1.3 | 1.3 | 2.2* | 1.0 | 1.2 | 1.7 |
| Osteopontin | 1.2 | 2.3* | 13.9* | 0.8 | 1.4 | 1.7 |
Differences are expressed as x-fold ratios compared to the concurrent control group.
*Statistically significant, p < 0.05.
Figure 6.
Individual values of polymorphonuclear neutrophils, osteopontin and MCF-1 in BALF.
At study day 8, relative neutrophil count was biologically relevantly increased in rats exposed to 30 mg/m³. Total cell count was also slightly increased. Neutrophil counts were in the normal range on study day 29.
At study day 8 total protein as well as γ-glutamyl transferase, lactate dehydrogenase and alkaline phosphatase values were marginally higher in males exposed to 30 mg/m³, but these differences were regarded as not toxicologically relevant. No differences between the groups were observed on study day 29.
Increased MCP-1 and osteopontin levels were observed at 30 mg/m³. Marginally higher CINC-1/IL-8 levels were present in this group. No differences between the groups were observed on study day 29.
Fine meta-chloro DPP
Differences of parameters measured in BALF when compared to the controls are shown in Table 8. No treatment-related effects were observed by examination of cytology, protein concentration and enzyme activities in BALF. At study day 8, in rats exposed to 30 mg/m³ higher IL-8 and osteopontin levels and at study day 29, still higher IL-8 levels compared to controls were measured. Since the values were only marginally higher than the historical control range of the rat strain used, these differences were regarded as not adverse.
Table 8. Mediators in BALF after exposure to fine meta-chloro DPP.
| Study day 8 | Study day 29 | |||||
|---|---|---|---|---|---|---|
| Concentration (mg/m3) | 3 | 10 | 30 | 3 | 10 | 30 |
| CINC-1/IL-8 | 0.9 | 1.2 | 2.9** | 0.8 | 1.2 | 2.8* |
| Osteopontin | 0.7 | 0.8 | 2.4* | 2.1 | 2.8 | 0.9 |
Differences are expressed as x-fold ratios compared to the concurrent control group.
*Statistically significant, p < 0.05; **statistically significant, p < 0.01.
Fine pigment red 101
No treatment-related effects were observed by examination of cytology, protein concentration, enzyme activities and mediators in BALF.
Coarse pigment red 101
No treatment-related effects were observed by examination of cytology, protein concentration, enzyme activities and mediators in BALF.
Organ weights
A summary of organ weight changes is given in Table 9. No compound-related effects on organ weights were observed after inhalation of mixed chlorinated DPP isomers, fine Pigment Red 254, coarse Pigment Red 101 and fine meta-chloro DPP. Adrenal weights were increased after exposure to coarse Pigment Red 254 (absolute weights: 22%; relative weights: 28%). Thymus weights were decreased by approximately 30% in rats exposed to 30 mg/m³ coarse meta-chloro DPP after the end of exposure. These changes were regarded as treatment-related. Lung weights were slightly increased after exposure to 30 mg/m³ fine Pigment Red 101 at study day 26 (absolute weights: 24%; relative weights: 23%). No histopathological correlate was found that could explain these changes, which was therefore considered not to be treatment-related.
Table 9. Summary of organ weights (three animals per group, day 5).
| Compound/organ | Coarse Pigment Red 254 | Coarse meta-chloro DPP | ||||
|---|---|---|---|---|---|---|
| Concentration (mg/m³) | 0 | 30 | 0 | 3 | 10 | 30 |
| Adrenal gland | ||||||
| Absolute (mg) | 57.3 | 70.0 | ||||
| Relative (%) | 0.023 | 0.03 | ||||
| Thymus | ||||||
| Absolute (mg) | 435.7 | 395.3 | 329.3 | 279.3 | ||
| Relative (%) | 0.177 | 0.158 | 0.138 | 0.121 | ||
Macroscopic examination
A slight orange red discoloration was observed in the lungs of all treated animals of mixed chlorinated DPP isomers, fine Pigment Red 254 and coarse Pigment Red 254.
After 3-week exposure free period, an orange red discoloration of the lungs was still present in the animals exposed to fine Pigment Red 254 and coarse Pigment Red 254. In the lungs of two animals exposed to coarse Pigment Red 101 a light gray discoloration was observed. Mediastinal lymph nodes were enlarged one animal exposed to 10 and 30 mg/m³ coarse meta-chloro DPP each.
Microscopic examination
A summary of the incidence of microscopic findings is given in Tables 10 (day 5) and 11 (day 26).
Table 10. Incidence of treatment-related microscopic findings – day 5.
| Examined organs/microscopic findings | Mixed chlorinated DPP isomers | Fine Pigment Red 254 | Coarse Pigment Red 254 | Fine meta-chloro DPP | Coarse meta-chloro DPP | Fine Pigment Red 101 | Coarse Pigment Red 101 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (mg/m³) | 30 | 30 | 30 | 3 | 10 | 30 | 3 | 10 | 30 | 10 | 30 | 30 |
| No. of animals/group | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Lung | ||||||||||||
| Pigment-laden macrophages | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Hypertrophy/hyperplasia, epithelial, terminal bronchioles | 3 | 3 | 3 | 0 | 2 | 3 | 0 | 3 | 3 | 0 | 0 | 0 |
| Pigment-laden macrophages and/or particles in BALT | 0 | 0 | 3 | 1 | 1 | 3 | 0 | 0 | 3 | 3 | 3 | 3 |
| Mediastinal/tracheobronchial lymph nodes | a | |||||||||||
| Pigment-laden macrophages and/or particles | 3 | 3 | 0 | 0 | 0 | 2 | 0 | 0 | 3 | 3 | 3 | |
| Nasal cavity | a | |||||||||||
| Pigment-laden macrophages/pigment particles, luminal | 2 | 3 | 3 | 0 | 2 | 2 | 0 | 0 | 3 | 0 | 0 | |
| Larynx | a | |||||||||||
| Pigment-laden macrophages/pigment particles, luminal | 0 | 1 | 2 | 0 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | |
| Trachea | a | |||||||||||
| Pigment particles, luminal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | |
Not examined.
Table 11. Incidence of treatment-related microscopic findings – day 26.
| Examined organs/microscopic findings | Mixed chlorinated DPP isomers | Fine Pigment Red 254 | Coarse Pigment Red 254 | Fine meta-chloro DPPa | Coarse meta-chloro DPP | Fine Pigment Red 101 | Coarse Pigment Red 101 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (mg/m³) | 30 | 30 | 30 | 3 | 10 | 30 | 3 | 10 | 30 | 10 | 30 | 30 |
| No. of animals/test group | 3 | 3 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 3 | 3 | 3 |
| Lung | ||||||||||||
| Pigment-laden macrophages, decreased (compared to day 5) | 3 | 3 | 3 | 3 | 3 | 2 | 0 | 0 | 3 | 3 | 3 | 3 |
| Pigment-laden macrophages and/ or particles in BALT | 3 | 3 | 3 | 3 | 3 | 1 | 0 | 0 | 3 | 3 | 3 | 3 |
| Mediastinal/tracheobronchial lymph nodes | ||||||||||||
| Pigment-laden macrophages/pigment particles | 3 | 3 | 2 | 0 | 3 | 2 | 0 | 3 | 3 | 2 | 3 | 2 |
| Lymphoreticular hyperplasia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Nasal cavity | a | a | a | a | a | a | a | a | a | |||
| Pigment particles, luminal | 0 | 1 | 0 | |||||||||
| Larynx | a | a | a | a | a | a | ||||||
| Pigment-laden macrophages/pigment particles, luminal | 0 | 0 | 0 | 0 | 2 | 0 | ||||||
| Trachea | a | a | a | a | a | a | a | a | a | |||
| Pigment-laden macrophages/pigment particles, luminal | 0 | 0 | 0 | |||||||||
Not examined.
Mixed chlorinated DPP isomers
On study day 5, the test substance was visualized in the lungs of all animals, as fine orange particles contained in the cytoplasm of macrophages. These pigment-laden macrophages were slightly increased in number, when compared with the number of normal alveolar macrophages in control animals. They were predominantly present in the lumen of terminal bronchioles, alveolar ducts and adjacent alveoli. Slight epithelial hypertrophy and/or hyperplasia was primarily found at the level of the terminal bronchioles and alveolar ducts. This finding was characterized by an increased size and number of bronchiolar epithelial cells, accompanied by a slight cytoplasmic basophilia. Similar findings were seen also in some bronchioles, which were located more proximal. In addition, in alveolar ducts, the septa were slightly thickened.
Minimal number of pigment-laden macrophages with minimal amount of pigment particles was observed in the mediastinal and tracheobronchial lymph nodes. No compound-related findings were observed in the nasal cavity, larynx and trachea other than luminal free pigment particles in the nasal cavity.
After a 3-week recovery period, the number of pigment-laden macrophages decreased but was still minimally higher than in the control group, with a tendency to accumulate in the lumen of the bronchiole-alveolar junction. At the same time, the pigment-laden macrophages increased in number in the bronchus-associated lymphoid tissue (BALT) and in mediastinal and tracheobronchial lymph nodes, suggesting an ongoing clearance of the test substance from the lung to the regional lymph nodes. The epithelial hypertrophy and/or hyperplasia in bronchioles and terminal bronchioles and alveolar ducts completely regressed. No treatment-related findings were observed in the nasal cavity, larynx and trachea.
Fine and coarse pigment red 254
On study day 5, the test substance was found in alveolar macrophages as brownish-orange (fine Pigment Red 254) or dark brown (coarse Pigment Red 254) pigment particles. These pigment-laden macrophages were moderate in number. They were predominantly present in the lumen of terminal bronchioles, alveolar ducts and adjacent alveoli. Occasional free pigment particles were found in alveoli and bronchioles. In animals exposed to coarse Pigment Red 254, a minimal number of pigment-laden macrophages were observed in the BALT. A minimal epithelial hypertrophy and/or hyperplasia was observed at the level of terminal bronchioles and alveolar ducts and in more proximal located bronchioles in animals treated with both fine and coarse Pigment Red 254. This finding was characterized by an increased size and number of bronchiolar epithelial cells, accompanied by a slight cytoplasmic basophilia.
Minimal or no pigment-laden macrophages were observed in the mediastinal and tracheobronchial lymph nodes of animals exposed to fine and coarse Pigment Red 254, respectively. No compound-related findings were observed in the nasal cavity, larynx and trachea other than minimal free pigment particles in the lumina of nasal cavity and larynx.
After the recovery period, the lung of all males showed a decrease in the number of pigment-laden macrophages (from moderate to slight). They showed a marked tendency to accumulate but not to aggregate in the bronchiolo-alveolar junction (terminal bronchiole, alveolar ducts and adjacent alveoli). The hypertrophy and/or hyperplasia in terminal bronchioles and alveolar ducts had fully regressed. The BALT of all males showed minimal number of pigment-laden macrophages. Simultaneously, a minimal increase in the number of pigment-laden macrophages in the regional mediastinal and tracheobronchial lymph nodes (minimal to slight) reflected an ongoing clearance function. No relevant findings other than free pigment particles were observed in the nasal cavity. The larynx and trachea showed no treatment-related changes.
Coarse and fine meta-chloro DPP
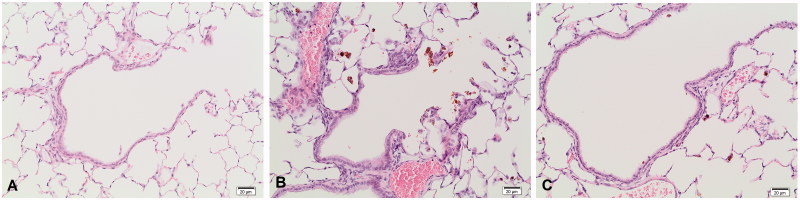
On study day 5, pigment-laden macrophages (containing orange-colored granules) were observed in the whole lung parenchyma, with a tendency to concentrate in the lumen of the bronchiolo-alveolar junction. Pigment-laden macrophages showed a dose-dependent increase in number and amount of pigment storage, starting from minimal at 3 mg/m³ up to moderate at 30 mg/m³. The bronchiolar epithelium from medium size bronchioles to terminal bronchioles showed hypertrophy ranging from minimal at 10 mg/m³ to slight at 30 mg/m³ (Figure 7). Furthermore, a minimal to slight number of pigment-laden macrophages was present in the BALT at 30 mg/m³.
Figure 7.
Histopathological findings in the lung of animals treated with coarse meta-chloro DPP pigment (30 mg/m³) after 5 days (A and B) and after a 3-week recovery period (C); H&E, 200×. (A) Terminal bronchiole of a control animal. (B) Epithelial hypertrophy of the terminal bronchiole and increased number of pigment-laden macrophages in a treated animal. (C) Regression of the epithelial hypertrophy of the terminal bronchiole and reduction of the number of pigment-laden macrophages.
In the upper respiratory tract (nasal cavity, larynx and trachea) of animals exposed to coarse or fine meta-chloro DPP, minimal to slight numbers of pigment free granules and/or pigment-laden macrophages were observed in the lumina. At 30 mg/m³, minimal to slight number of pigment-laden macrophages was noted in the mediastinal and tracheobronchial lymph nodes for both substances.
After a 3-week exposure-free period, all animals at 30 mg/m³ coarse and fine meta-chloro DPP showed a decrease in the number of pigment-laden macrophages in the lung with a tendency to accumulate in the bronchiolo-alveolar junction. In the BALT of animals at 30 mg/m3 pigment-laden macrophages tended to increase and the hypertrophy of the bronchiolar epithelium seen after 4 days exposure at 10 and 30 mg/m3 regressed completely. The enlargement of the mediastinal lymph nodes observed with the coarse pigment correlated with slight lympho-reticular hyperplasia in the paracortex.
In the regional lymph nodes, the pigment-laden macrophages tended to increase in number for both substances at 10 and 30 mg/m3. In some animals at 10 and 30 mg/m3 coarse meta-chloro DPP, pigment-macrophages formed aggregates.
Fine and coarse pigment red 101
On study day 5, in the lungs of all groups exposed to fine and coarse Pigment Red 101 there was a minimal to mild increase in pigment-laden macrophages which contained brown-red (fine Pigment Red 101) or dark (coarse Pigment Red 101) particles within their cytoplasm. In all treated animals, these particles could also be observed within the BALT.
After the 3-week recovery period, the numbers of alveolar macrophages were lower compared to the final sacrifice animals but still minimally increased compared to control animals exposed to fine and coarse Pigment Red 101 at 30 mg/m³. Pigment-laden macrophages with few pigment particles were still observable in all treated animals. All treated animals showed minimal to mild numbers of particles within the BALT. All these findings were regarded to be treatment-related and adaptive.
On study day 5, there were single particles within macrophages in the tracheobronchiolar and mediastinal lymph nodes of some animals exposed to coarse Pigment Red 101, which were of comparable nature as those observed in the lungs. After 3 weeks exposure-free period, there was an increase in number of animals, showing these single black particles within the lymph nodes with a combined incidence (tracheobronchial and mediastinal lymph nodes) of 0, 1, 3 and 4 animals in the control, 10 mg/m³ fine Pigment Red 101 group, 30 mg/m³ fine Pigment Red 101 group and the 30 mg/m³ coarse Pigment Red 101 group, respectively.
Discussion
During the exposure period, the target concentrations were maintained constant and stable. Particle size distribution measurement demonstrated very high fraction of respirable particles. Concerning clinical observation, body weight development, hematology and clinical chemistry no systemic treatment-related, adverse effects were observed up to an atmospheric concentration of 30 mg/m3 in all tested pigments. There was no evidence for systemic inflammation in non-local organs based on the measured acute phase proteins, alpha-macroglobulin and haptoglobin.
Likewise, examination of BALF did not reveal any evidence for adverse effects after exposure of rats to fine Pigment Red 254, coarse Pigment Red 254, fine meta-chloro DPP, fine Pigment Red 101 and coarse Pigment Red 101. After exposure to 30 mg/m³ mixed chlorinated DPP isomers, an initial inflammatory reaction by an increase in polymorphonuclear cell counts (approx. sevenfold) was observed. No other compound-related changes in BALF parameters were observed. More pronounced changes in BALF parameters occurred after exposure to 30 mg/m³ coarse meta-chloro DPP. Slight increases of total cell counts and neutrophil counts were observed. Additionally, MCP-1 and osteopontin concentrations were higher than in the controls. Both mediators are released from several kinds of cells (monocytes, endothelial cells, smooth muscle cells, etc.) with the aim to attract and stimulate macrophages. The inflammation was restricted to the lung. All altered parameters were in the normal range after three weeks exposure free period.
The weight increase of the adrenal glands after exposure to 30 mg/m3 coarse Pigment Red 254 and the thymus weight decrease after exposure to 30 mg/m³ coarse meta-chloro DPP was attributed most likely to stress. The weight increase of the lungs in fine Pigment Red exposed animals was not regarded to be treatment-related as in histopathology no finding was observed that could explain the weight increase.
Histopathological examinations of animals exposed to all pigments revealed the presence of the test substance in the lungs, which correlated in general with the discoloration noted at gross pathology. All pigments induced similar treatment-related changes of low severity at the bronchiolo-alveolar junction, characterized by a concentration of pigment-laden macrophages at this site accompanied by local reactive epithelial hypertrophy and/or hyperplasia. This epithelial change extended more or less proximally (bronchioles) or distally (alveolar ducts and adjacent alveoli) depending on the substance without showing cell injury or inflammation. However, in combination with the BALF results, indicative of inflammation and the decrease thymus weights suggestive of stress, these findings altogether suggested that coarse meta-chloro DPP at 30 mg/m3 evoked the strongest effects, followed by the mixed chlorinated DPP, which revealed in the BALF marginal inflammatory changes. In case of the fine and coarse Pigment Red 254, not only hypertrophy but also hyperplasia of the epithelial lining at terminal bronchioles, alveolar ducts and adjacent alveoli was seen. Although these findings were quantitatively minimal, they most likely implied a subtle cellular injury and regeneration, which is considered a potentially adverse effect. In case of the fine meta-chloro DPP pigment, only hypertrophy was noted and was therefore assessed as adaptive. Fine and coarse Pigment Red 101 induced only an increase in the number of macrophages but no epithelial reactions after 5 days exposure. The presence of pigment-laden macrophages in the BALT and regional lymph nodes reflected a physiological clearance function. No treatment-related changes were observed in the upper respiratory tract (nasal cavity, larynx and trachea) in any substance.
At the end of the 3-week recovery period, histopathology revealed a full regression of the hypertrophy and/or hyperplasia in bronchioles, terminal bronchioles and alveolar ducts. In addition, a minimal decrease in the number of luminal pigment-laden macrophages was noted. At the same time a minimal increase in the number of pigment-laden macrophages in the BALT and indicative of a continuing clearance of the test substance from the lung to the regional lymph nodes.
Regarding the severity of effects, the alterations after exposure to the coarse meta-chloro DPP isomer were more pronounced compared to the other compounds, but most interestingly also when compared to fine meta-chloro DPP. Based on the lower dose in surface metrics for coarse meta-chloro DPP, one would have expected the opposite if surface-mediated effects dominate. Instead, the finding that the microscale material seems to be slightly more potent than the nano-size material may be associated with the needle-like crystal form of the microscale material.
The results can be further interpreted in the frame of the recent schemes for grouping and read-across of nanomaterials. In the ECETOC grouping scheme (Arts et al., 2016), all physical–chemical parameters selected for tier 1 and tier 2 indicate an assignment of the DPP materials to Main Group 3 (passive nanomaterials), with the following peculiarities:
One material (coarse Pigment Red 254) is relatively well dispersible and actually has an AAN in serum-containing medium below the cutoff of 3; nonetheless, this material is not considered “potentially mobile” by the ECETOC tier 2 because it has an absolute diameter far above the cutoff at 100 nm. This second criterion of the dispersability screening hence proves useful to prevent false positives in this case.Another material (coarse meta-chloro DPP) has clearly an aspect ratio above 3 with a length range 0.3–3 μm and a diameter range 70–200 nm, and straight rod shape. Yet, it is not considered as Main Group 2 (high aspect ratio nanomaterials) because the maximum length remains below the WHO cutoff at 5 μm. Considering that the effects in short-term inhalation screening are still very mild, although higher than the nanomaterial, both forms belong into the same Main Group 3 (passive nanomaterials).
Although no deposition in the pleura was observed, it remains open if the non-nano straight rods elicit the telltale chronic effects of stiff long fibers, because the study duration was too short to detect pathological changes on the pleura, and persistence in vivo is not known.
Finally, it is worthwhile to consider the short-term inhalation test results under the perspective of the additional physical–chemical parameters that are proposed as grouping and read-across criteria by RIVM (Sellers et al., 2015) and in simplified form by the MARINA project (Oomen et al., 2015). Materials that clearly belong into the same inhalation hazard group have very different zeta potentials, ranging from −11 to −55 mV. Charge does not seem to be a useful criterion for grouping under the present perspective. Furthermore, the degree of hydrophobicity is no useful screening parameter for materials with a potential to translocate: The most hydrophilic materials of the present study (iron oxides) show no free isolated particles in physiological medium; vice versa, the only material that does disperse with some isolated particles would not have been recognized by either hydrophobicity or charge parameters. This finding extends to further materials, such as surface-coated silica materials, for which the correlation between corona adsorption phenomena and short-term inhalation test results was just recently published (Wohlleben et al., 2016). In conclusion, tier 2 screenings cannot use intrinsic physical–chemical properties, but must devise system-dependent properties with higher relevance for the in vivo situation, such as the dispersability criterion (Arts et al., 2016).
Conclusion
In the present study, five diketopyrrolopyrrole-based pigments (mixed chlorinated DPP isomers, fine and coarse Pigment Red 254, fine and coarse meta-chloro DPP) and two iron oxide pigments (fine and coarse Pigment Red 101) were tested in a short-term inhalation test protocol over 5 days at a concentration up to 30 mg/m³ air. All pigments were tolerated well and caused only marginal effects in BALF (mixed chlorinated DPP isomers, coarse meta-chloro DPP) or no effects at all. Likewise, only minor effects were seen in the lung after exposure to mixed chlorinated DPP isomers, fine Pigment Red 254, coarse Pigment Red 254, coarse meta-chloro DPP and fine meta-chloro DPP. Beside pigment deposits and phagocytosis by an increased number of alveolar macrophages, these findings consisted of minimal to slight epithelial hypertrophy and/or hyperplasia predominantly localized at the bronchiolo-alveolar junction without evidence of inflammation and fully recovered after a 3-week free exposure period. In contrast, only pigment deposition and pigment phagocytosis were observed after exposure to fine and coarse Pigment Red 101, without any microscopically visible adaptive reaction of the bronchioles.
The “no observed adverse effect concentration” was established at 10 mg/m³ for coarse meta-chloro DPP, and at 30 mg/m³ air for fine meta-chloro DPP, fine and coarse Pigment Red 101. Only marginal effects occurred after exposure to 30 mg/m³ mixed chlorinated DPP isomers, fine and coarse Pigment Red 254.
Acknowledgements
The authors thank Elke Wittmer, Ernst Bohrer, Stefan Rath, Sarah Koppenhagen, Annette Knecht, Sabine Löffler, Irmgard Weber, Jeanette Vogt, Ulrich Flörchinger and the Pathology team, for their excellent technical assistance in performing the inhalation study, the clinical and histopathological examinations, and Jasmin Athas (lab work on dispersibility and contact angle) and Arnaud Gandon (lab work and assay development of FRAS assay).
Declaration of interest
The authors are employees of BASF SE, a company producing and marketing DPP pigments and iron oxide. This work was sponsored by BASF SE.
References
- Arts JHE, Irfan M-A, Keene AM, et al. Case studies putting the decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping) into practice. Regul Toxicol Pharmacol. 2016;71:S1–27. doi: 10.1016/j.yrtph.2015.11.020. [DOI] [PubMed] [Google Scholar]
- Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. JASA. 1955;50:1096–121. [Google Scholar]
- Dunnett CW. New tables for multiple comparisons with a control. Biometrics. 1964;20:482–91. [Google Scholar]
- Elshal MF, McCoy JP., Jr. Multiplex bead array assays: performance evaluation and comparison of sensitivity to ELISA. Methods. 2006;38:317–23. doi: 10.1016/j.ymeth.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton RJ, McDade RL, Smith PL, et al. Advanced multiplexed analysis with the FlowMetrix system. Clin Chem. 1997;43:1749–56. [PubMed] [Google Scholar]
- Federal Institute for Occupational Safety and Health . 2015. http://www.baua.de/de/Themen-von-A-Z/Gefahrstoffe/TRGS/pdf/TRGS-900.pdf;jsessionid=1CBFD74AB182E289E5E3B63AD8E1EAB4.1_cid333?__blob=publicationFile&v=20 [Google Scholar]
- Farnum DG, Mehta G, Moore GGI, Siegal FP. Attempted reformatski reaction of benzonitrile, 1,4-diketo-3,6-diphenylpyrollo(3,4-c)pyrrole. A lactam analogue of pentalene. Tetrahedron Lett. 1974;29:2549–52. [Google Scholar]
- Hsieh SF, Bello D, Schmidt DF, et al. Mapping the biological oxidative damage of engineered nanomaterials. Small. 2013;9:1853–65. doi: 10.1002/smll.201201995. [DOI] [PubMed] [Google Scholar]
- Iqbal A, Jost M, Kirchmayr R, et al. The synthesis and properties of 1,4-diketo-pyrrolo(3,4-C)pyrroles. Bull Soc Chim Belg. 1998;97:615–44. [Google Scholar]
- Kettman JR, Davies T, Chandler D, et al. Classification and properties of 64 multiplexed microsphere sets. Cytometry. 1998;33:234–43. [PubMed] [Google Scholar]
- Klein CL, Wiench K, Wiemann M, et al. Hazard identification of inhaled nanomaterials: making use of short-term inhalation studies. Arch Toxicol. 2012;86:1137–51. doi: 10.1007/s00204-012-0834-2. [DOI] [PubMed] [Google Scholar]
- Landsiedel R, Ma-Hock L, Kroll A, et al. Testing metal-oxide nanomaterials for human safety. Adv Mater. 2010;22:2602–27. doi: 10.1002/adma.200902658. [DOI] [PubMed] [Google Scholar]
- Landsiedel R, Wiench K, Wohlleben W. Geeignete Methoden zur Prüfung der Sicherheit von Nanomaterialien. Chem Ing Tech. 2008;80:1–11. [Google Scholar]
- OECD . 1992. [Google Scholar]
- OECD . 2009. [Google Scholar]
- Oomen AG, Bleeker EAJ, Bos PMJ, et al. Grouping and read-across approaches for risk assessment of nanomaterials. Int J Environ Res Public Health. 2015;12:13415–34. doi: 10.3390/ijerph121013415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma-Hock L, Gamer AO, Landsiedel R, et al. Generation and characterization of test atmospheres with nanomaterials. Inhal Toxicol. 2007;19:833–48. doi: 10.1080/08958370701479190. [DOI] [PubMed] [Google Scholar]
- Ma-Hock L, Burkhardt S, Strauss V, et al. Development of a short-term inhalation test in the rat using nano-titanium dioxide as a model substance. Inhalation Toxicol. 2009a;21:102–18. doi: 10.1080/08958370802361057. [DOI] [PubMed] [Google Scholar]
- Ma-Hock L, Treumann S, Strauss V, et al. Inhalation toxicity of multiwall carbon nanotubes in rats exposed for 3 months. Toxicol Sci. 2009b;112:468–81. doi: 10.1093/toxsci/kfp146. [DOI] [PubMed] [Google Scholar]
- Ma-Hock L, Landsiedel R, Wiench K, et al. Short-term rat inhalation study with aerosols of acrylic ester-based polymer dispersions containing a fraction of nanoparticles. Int J Toxicol. 2012;31:46–57. doi: 10.1177/1091581811424778. [DOI] [PubMed] [Google Scholar]
- Sellers K, Deleebeeck NME, Messiaen M, et al. Grouping nanomaterials: a strategy towards grouping and read-across. RIVM Report 2015-0061. Bilthoven: National Institute for Public Health and the Environment; 2015. [Google Scholar]
- Siegel S. Non-parametric statistics for the behavioural sciences. New York: McGraw-Hill; 1956. [Google Scholar]
- Skogstrand K, Thorsen P, Norgaard-Pedersen B, et al. Simultaneous measurement of 25 inflammatory markers and neurotrophins in neonatal dried blood spots by immunoassay with Xmap technology. Clin Chem. 2005;51:1854–66. doi: 10.1373/clinchem.2005.052241. [DOI] [PubMed] [Google Scholar]
- Van Ravenzwaay B, Landsiedel R, Fabian E, et al. Comparing fate and effects of three particles of different surface properties: nano-TiO(2), pigmentary TiO(2) and quartz. Toxicol Lett. 2009;186:152–9. doi: 10.1016/j.toxlet.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Walter J, Löhr K, Karabudak E, et al. Multidimensional analysis of nanoparticles with highly disperse properties using multiwavelength analytical ultracentrifugation. ACS Nano. 2014;8:8871–86. doi: 10.1021/nn503205k. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Hartsky MA. Role of alveolar macrophage chemotaxis and phagocytosis in pulmonary clearance responses to inhaled particles: comparisons among rodent species. Microsc Res Techn. 1993;26:412–22. doi: 10.1002/jemt.1070260509. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical principles in experimental design. 2nd ed. New York: McGraw-Hill; 1971. [Google Scholar]
- Wohlleben W. Validity range of centrifuges for the regulation of nanomaterials: from classification to as-tested coronas. J Nanopart Res. 2012;14:1300. doi: 10.1007/s11051-012-1300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlleben W, Driessen MD, Raesch S, et al. Influence of agglomeration and specific lung lining lipid/protein interaction on short-term inhalation toxicity. Nanotoxicology. 2016;10:970–80. doi: 10.3109/17435390.2016.1155671. [DOI] [PubMed] [Google Scholar]
- Yakata M, Sugita O, Sakai T, et al. Urinary enzyme determination and its clinical significance C. Enzyme derived from the kidney tubular epithelium-N-acetyl-beta-glucosaminidase. 4. Preclinical evaluation of the urinary NAG activity and changes in renal diseases. Rinsho Byori. 1983;56:90–101. [PubMed] [Google Scholar]