Abstract
The whooping cough agent Bordetella pertussis regulates the production of its virulence factors by the BvgA/S system. Phosphorylated BvgA activates the virulence-activated genes (vags) and represses the expression of the virulence-repressed genes (vrgs) via the activation of the bvgR gene. In modulating conditions, with MgSO4, the BvgA/S system is inactive, and the vrgs are expressed. Here, we show that the expression of almost all vrgs depends on RisA, another transcriptional regulator. We also show that some vags are surprisingly no longer modulated by MgSO4 in the risA− background. RisA also regulates the expression of other genes, including chemotaxis and flagellar operons, iron-regulated genes, and genes of unknown function, which may or may not be controlled by BvgA/S. We identified RisK as the likely cognate RisA kinase and found that it is important for expression of most, but not all RisA-regulated genes. This was confirmed using the phosphoablative RisAD60N and the phosphomimetic RisAD60E analogues. Thus the RisA regulon adds a new layer of complexity to B. pertussis virulence gene regulation.
Whooping cough or pertussis is a life-threatening respiratory disease and remains one of the major causes of infant mortality despite a global vaccination coverage of >85%. It represents today the most prevalent vaccine-preventable disease in infants1, thereby illustrating the shortcomings of current vaccination programs. The development of improved vaccines will certainly benefit from a more thorough understanding of the biology of Bordetella pertussis, the main etiologic agent of whooping cough.
B. pertussis produces a large array of bona fide virulence factors whose production is under the transcriptional control of the two-component system BvgA/S (for review see ref. 2). BvgS is the transmembrane sensor component of the system, which, after a complex cascade of autophosphorylation finally transfers a phosphate group to the cytoplasmic transcriptional regulator BvgA. The BvgA/S system is functional by default, but can be turned off by growth at low temperatures or in the presence of sulphate or nicotinic acid, a process referred to as antigenic or phenotypic modulation3,4. During the Bvg+ or virulence phase, BvgA is phosphorylated and triggers the transcription of the genes coding for adhesins, toxins and other virulence factors. These genes are collectively called virulence-activated genes (vags). By contrast, in the Bvg− or avirulence phase, BvgA is not phosphorylated, and the vags are not expressed. Instead, another set of genes, collectively called virulence-repressed genes (vrgs), is expressed in the Bvg− phase5.
Two genes were demonstrated to be involved in the regulation of vrgs: bvgR and risA6,7. RisA is a member of the OmpR family of two-component response regulators and was shown to be required for maximal expression of at least some vrgs7,8. The risA gene is adjacent to risS coding for the putative sensor kinase of the RisA/S two-component system. Whereas for the closely related animal pathogen Bordetella bronchiseptica, risA and risS are required for resistance of this organism to oxidative stress and for in vivo persistence9, risS is a pseudogene in the genome of all B. pertussis isolates examined so far8. These observations suggest that in B. pertussis RisA may be an orphan response regulator, that its activity is independent of phosphorylation or that it is phosphorylated by an as yet unidentified kinase distinct from RisS.
In this study we characterized the RisA regulon by whole-genome transcriptomic analysis, identified the likely RisA kinase RisK and examined the role of RisA phosphorylation and of BvgR in RisA activity in B. pertussis.
Results
Transcriptomic profiling of B. pertussis BPSM grown in modulating and non-modulating conditions
To identify the full set of vag and vrg genes of B. pertussis BPSM, the organism was grown in the presence or absence of 50 mM MgSO4 and then subjected to microarray analyses covering 3552 open reading frames. The threshold to identify genes that were differentially transcribed was set at a 4-fold difference in transcript abundance between bacteria grown under modulating and non-modulating growth conditions (Fig. 1A, blue circle and Table S1). The genes more abundantly transcribed in non-modulating conditions than in modulating conditions include the well-known vags, such as the ptx/ptl operon, fhaB, bvgA/S, bvgR, prn, tcfA, vag8, sphB1, brkA, fim2, fimABC and some genes involved in siderophore production, whereas the genes more abundantly transcribed in modulating conditions than in non-modulating conditions include the vrgs, such as fim3, genes encoding the putative capsule, vrg6 and many genes with unknown function (Fig. 1A, orange circle, see also Fig. S1). These data are in agreement with previously reported transcriptomic profiles of B. pertussis grown in modulating versus non-modulating conditions10.
Figure 1. Transcriptome comparisons of different strains in modulating and non-modulating conditions.
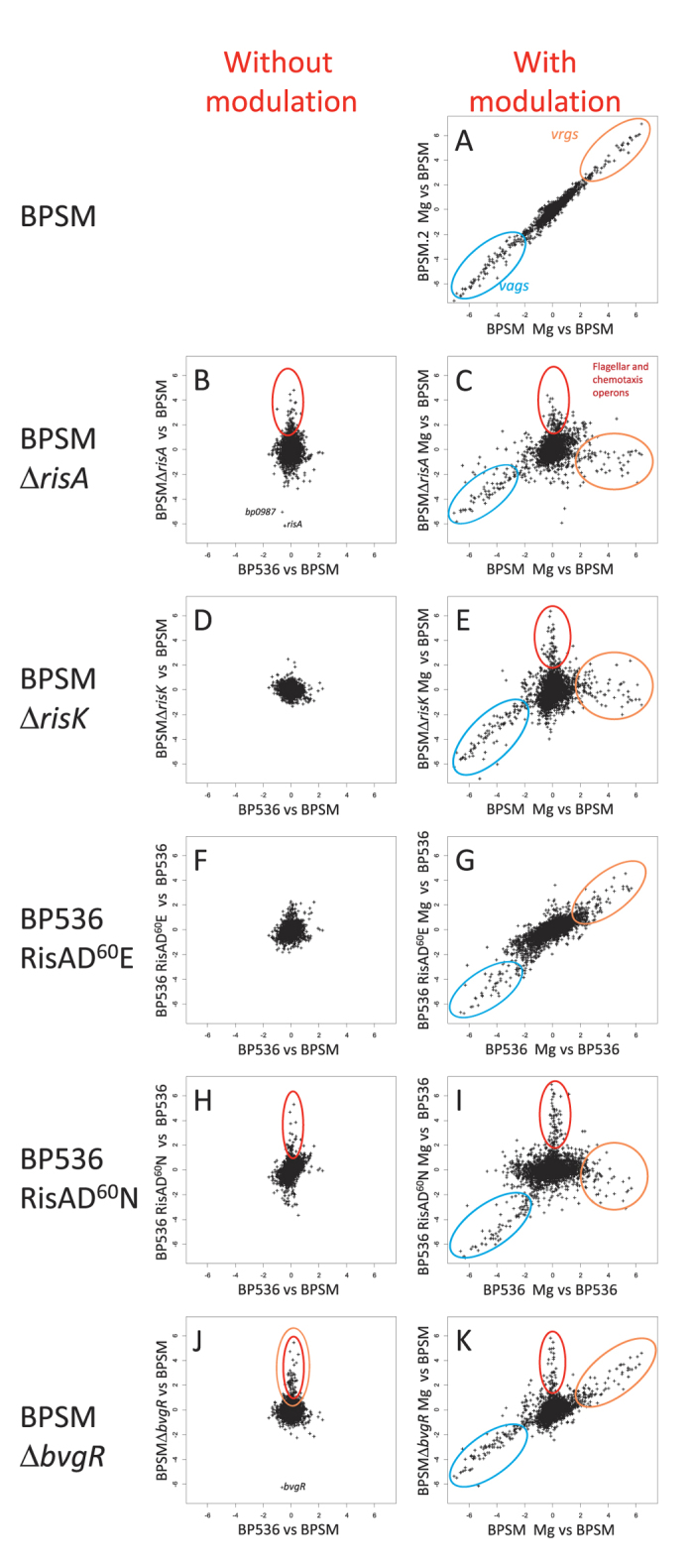
DNA array gene expression ratios, expressed as log2 ratios, between the mutant and parental strains in modulating and non-modulating conditions are depicted in the scatter plots, each point of which represents one gene. In non-modulating conditions, the ratio between a non-modulated mutant strain and its parental strain (y axis) is plotted against the ratio between two non-modulated parental strains (x axis). In modulating conditions, the ratio between a modulated mutant strain and its non-modulated parental strain (y axis) is plotted against the ratio between the modulated and the non-modulated parental strain (x axis). For the RisAD60E and RisAD60N mutations the parental strain background is BP536, while for the other strains the parental strain is BPSM. Coloured circles highlight genes of interest; blue for the vags, orange for the vrgs and red for genes related to the flagellar and chemotaxis operons. 50 mM MgSO4 was used as the modulating condition.
The RisA regulon in non-modulating conditions
To characterize the RisA regulon, we constructed a risA-deficient mutant in the B. pertussis BPSM background. The mutant strain, named BPSM∆RisA, carries a 735-bp internal deletion within the risA gene. A comparison of the transcriptome of BPSM with that of BPSM∆RisA, both grown in non-modulating conditions, identified 53 genes differentially regulated in BPSM∆RisA compared to BPSM. Among these 53 genes, 22 genes were less abundantly transcribed and 31 genes had higher transcript levels in BPSM∆RisA as compared to BPSM (Figs 1B and 2 and Table S2). Approximately half of the genes with decreased transcript abundance in BPSM∆RisA are vrgs (labelled in red in Fig. 2). They include tviD (capsular operon), osmB, vrg-6, bfrG but also bipA, while the remaining genes were not identified as BvgA/S-regulated genes. With the exception of bp0987, the gene with the strongest decrease in transcript abundance in BPSM∆RisA (42 fold), all the other genes showed only a 5-fold decrease in transcript abundance (Fig. 1B). Among the 31 genes presenting more transcripts in BPSMΔRisA than in BPSM, none could be identified as BvgA/S-regulated genes. However, many of them belong to the flagellar and chemotaxis operons (Fig. 2). These data suggest that under non-modulating conditions RisA does not regulate vag expression but modestly regulates the expression of many vrgs in addition to non-BvgA/S regulated genes in B. pertussis.
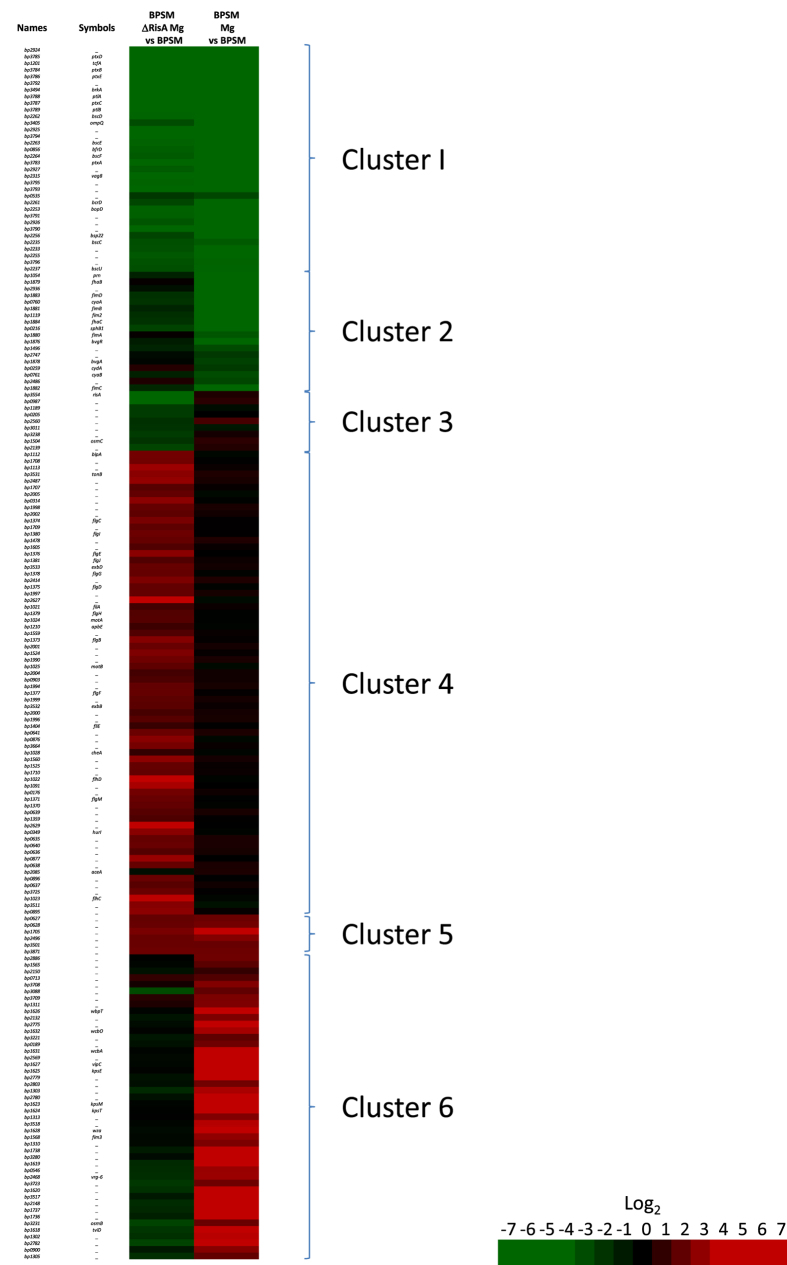
Figure 2. Heat map of RisA-regulated gene expression in non-modulated BPSM.
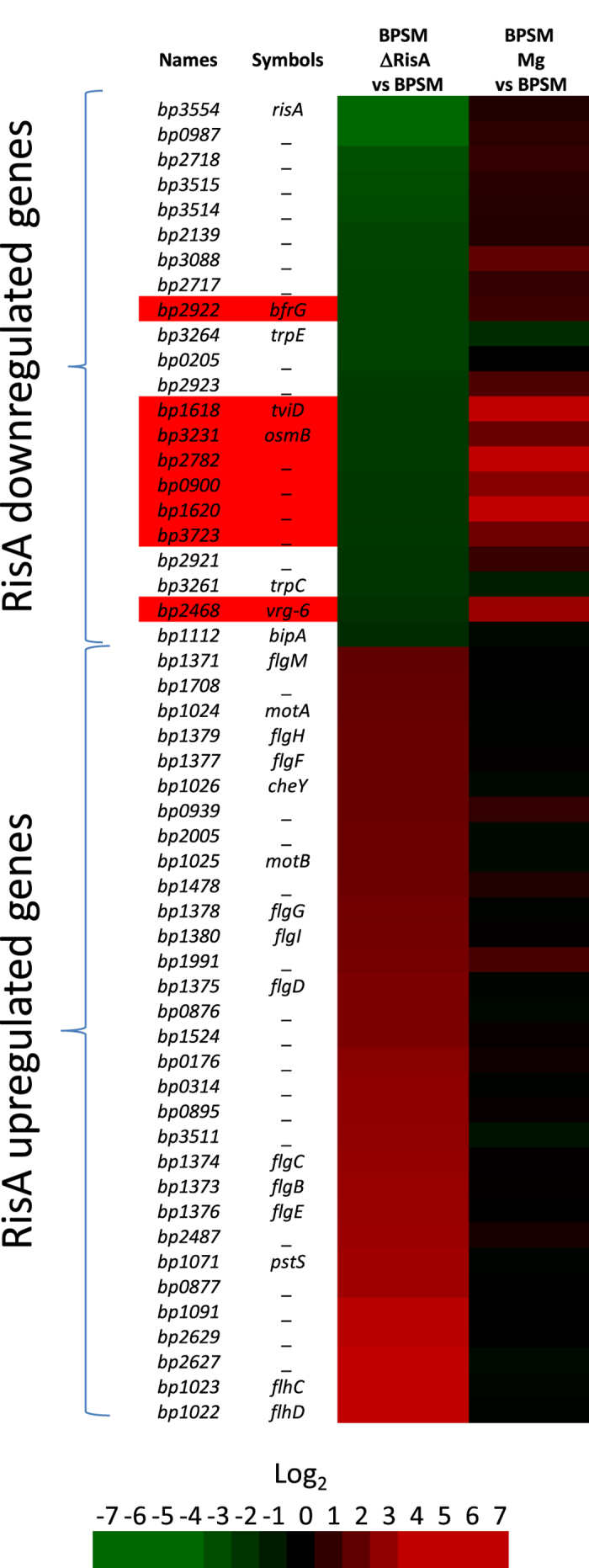
Rows correspond to array probes. The names and symbols in the left column correspond to the Tohama I Sanger Centre annotation. BPSMΔRisA vs BPSM corresponds to the ratios between BPSMΔRisA and BPSM used as reference, both cultivated in non-modulating conditions. BPSM Mg vs BPSM corresponds to the ratios between BPSM cultivated in the presence of 50 mM MgSO4 and non-modulated BPSM. The indicated ratios are the means of all the experiments. Data are centred from the first column between genes less transcribed (top) to more transcribed (bottom) in BPSMΔRisA compared to BPSM. Red, increased transcript abundance; green, decreased transcript abundance; black, no significant change in transcript abundance; the level of transcript abundance is defined by the coloured Log2 scale shown on the bottom of the figure.
RisA regulation in modulating conditions
The modest regulation of the vrgs by RisA under non-modulating conditions may be enhanced when the bacteria are grown in modulating conditions. Therefore, we compared the transcriptional profiles of BPSMΔRisA and BPSM grown under modulating conditions (in the presence of 50 mM MgSO4) to the transcriptional profile of BPSM grown under non-modulating conditions (Fig. 1C). Six different gene clusters were identified (Fig. 3 and Table S3). The first cluster is composed of many vags, which were less transcribed in both modulated BPSM and modulated BPSMΔRisA as compared to non-modulated BPSM, arguing that RisA is not involved in the regulation of most vags, as already proposed by Stenson et al.8. However, surprisingly, the second cluster is also composed of vags, but they were less transcribed in modulated BPSM than in modulated BPSMΔRisA (Fig. 3). These include the gene coding for pertactin (prn), whose transcripts are less abundant in modulated BPSM (fold change of −89.26) than in non-modulated BPSM. In contrast, in modulated BPSMΔRisA, prn shows only a 2.34 fold change as compared to non-modulated BPSM (Fig. S2). Other genes that fall in cluster 2 include fhaB, genes involved in fimbrial biogenesis and the adenylate cyclase toxin gene. Thus, for some vags the repression by modulation appears to depend on RisA.
Figure 3. Heat map of RisA-regulated gene expression in modulating conditions.
Rows correspond to array probes. The names and symbols in the left column correspond to the Tohama I Sanger Centre annotation. BPSMΔRisA Mg vs BPSM corresponds to the ratios between BPSMΔRisA cultivated in the presence of 50 mM MgSO4 and BPSM cultivated in non-modulating conditions. BPSM Mg vs BPSM corresponds to the ratios between BPSM cultivated in the presence of 50 mM MgSO4 and BPSM cultivated in non-modulating conditions. The indicated ratios are the means of all the experiments. Red, increased transcript abundance; green, decreased transcript abundance; black, no significant change in transcript abundance; the level of transcript abundance is defined by the coloured Log2 scale.
The third cluster is composed of genes, such as osmC and 7 other genes of unknown function, whose transcripts were less abundant in modulated BPSMΔRisA, but not in modulated BPSM, as compared to non-modulated BPSM. The fourth cluster contains genes that were more abundantly transcribed in modulated BPSMΔRisA than in modulated BPSM (Fig. 3). They comprise genes of the chemotaxis and flagellar operons (Fig. 1C red circle; Fig. S3, labelled in yellow), iron-regulated genes (Fig. S3, labelled in blue) and genes coding for a putative type II secretion system (Fig. S3, labelled in green). Among the genes involved in iron acquisition, some, such as bfrD, are regulated by BvgA/S11, whereas others, such as tonB, exbB and exbD, are not. The expression of these genes has been shown to be increased by iron starvation12,13,14.
The fifth cluster is composed of genes that were transcribed at higher levels in both modulated BPSMΔRisA and modulated BPSM as compared to non-modulated BPSM (Fig. 3). Only 6 genes fall in this cluster (bp0627, bp0628, bp1704, bp2496, bp3501, bp3871), all of unknown function. Based on their expression in modulated BPSM, these genes would be classified as vrgs. However, their expression does not appear to require RisA. The sixth cluster is composed of genes that were much more transcribed in modulated BPSM but not in modulated BPSMΔRisA, compared to non-modulated BPSM (Fig. 3) and contains most of the vrgs. Thus, with the exception of the 6 genes in cluster 5, the expression of all the vrgs depends on functional RisA.
Identification of RisK (BP3223) as the putative kinase of RisA
Since RisA is required for the expression of most of the vrgs, as well as for a set of genes that do not appear to be modulated by MgSO4 (Fig. 3, cluster 4), we further investigated the regulation mechanism of RisA and examined the requirement for its phosphorylation in its activity. To address this issue, we first set out to identify the main kinase involved in RisA phosphorylation. The B. pertussis genome contains 17 genes coding for putative two-component system kinases. One of them is risS, a gene separated from risA by only 4 bp and thus likely co-transcribed with risA within the same operon. However, the deletion of risS does not perturb the activity of RisA8. In addition, risS is a pseudogene in B. pertussis, in contrast to B. bronchiseptica8, which makes it unlikely that RisS is the cognate RisA kinase in B. pertussis.
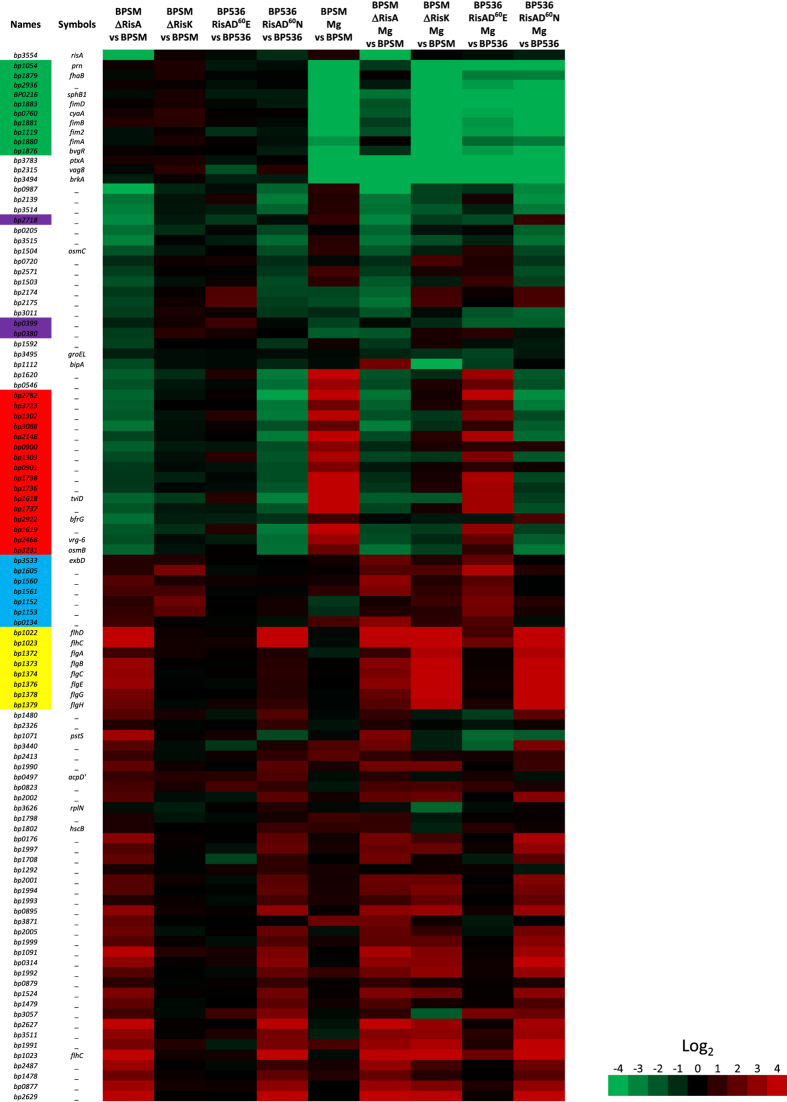
To search for an alternative RisA kinase, we made use of the concept of co-evolving residue pairs to predict the interacting partner of RisA (http://biohealth.snu.ac.kr/cgi-bin/platcom/tcs/intro.cgi). Among all intact putative B. pertussis histidine kinases, RisA was predicted to interact most strongly with the bp3223 gene product that we therefore propose to call RisK. We deleted the risK gene from the BPSM chromosome and analysed the transcriptomic profiles of BPSM∆RisK in modulating and non-modulating conditions. In non-modulating conditions, BPSMΔRisK presented a transcriptomic profile almost identical to that of BPSM and slightly different from that of BPSM∆RisA (Figs 1D and 4 and Table S4). Under modulating conditions, BPSM∆RisK presented a transcriptional profile similar to that of BPSMΔRisA (Figs 1E and 4), indicating that RisA requires the presence of RisK to express its full transcriptional activities. Notable exceptions include some of the vags (Fig. 4, genes in green) and bipA, which were more transcribed in modulated BPSMΔRisA than in modulated BPSM∆RisK.
Figure 4. Role of RisA phosphorylation in gene expression in modulated or non-modulated B. pertussis.
Rows correspond to array probes. The names and symbols in the left column correspond to the Tohama I Sanger Centre annotation. BPSMΔRisA vs BPSM corresponds to the ratios between BPSMΔRisA and BPSM, both cultivated in non-modulating conditions. BPSMΔRisK vs BPSM corresponds to the ratios between BPSMΔRisK and BPSM, both cultivated in non-modulating conditions. BP536 RisAD60E vs BP536 corresponds to the ratios between BP536 RisAD60E and BP536, both cultivated in non-modulating conditions. BP536 RisAD60N vs BP536 corresponds to the ratios between BP536 RisAD60N and BP536, both cultivated in non-modulating conditions. BPSM Mg vs BPSM corresponds to the ratios between BPSM cultivated in the presence of 50 mM MgSO4 and BPSM cultivated in non-modulating conditions. BPSMΔRisA Mg vs BPSM corresponds to the ratios between BPSMΔRisA cultivated in the presence of 50 mM MgSO4 and BPSM cultivated in non-modulating conditions. BPSMΔRisK Mg vs BPSM corresponds to the ratios between BPSMΔRisK cultivated in the presence of 50 mM MgSO4 and BPSM cultivated in non-modulating conditions. BP536 RisAD60E Mg vs BP536 corresponds to the ratios between BP536 RisAD60E cultivated in the presence of 50 mM MgSO4 and BP536 cultivated in non-modulating conditions. BP536 RisAD60N Mg vs BP536 corresponds to the ratios between BP536 RisAD60N cultivated in the presence of 50 mM MgSO4 and BP536 cultivated in non-modulating conditions. The indicated ratios are the means of all the experiments. Red, increased transcript abundance; green, decreased transcript abundance; black, no significant change in transcript abundance; the level of transcript abundance is defined by the coloured Log2 scale shown on the right.
In addition, RisK appears to play a minor role in regulating the expression levels of the RisA-dependent vrgs in non-modulating conditions, whereas in modulating conditions, RisK is absolutely required for the upregulation of the expression of these genes (Fig. 4, genes in red). Some of the non-vrg RisA-repressed genes (Fig. 3, cluster 4 and Fig. 4, genes in blue) were more abundantly transcribed in modulated BPSMΔRisA than in modulated BPSMΔRisK, whereas others (Fig. 4, genes in yellow) were more transcribed in both modulated BPSMΔRisA and BPSMΔRisK as compared to non-modulated BPSM. Hence, in modulating conditions, some genes require RisK for their RisA-mediated repression, whereas for other genes the involvement of RisK in regulation appears to be dispensable.
To confirm the results obtained by the microarray experiments, quantitative RT-PCR analyses were performed on several selected genes, covering all 6 identified clusters on Fig. 3 in BPSM, BPSMΔRisA and BPSM∆RisK grown in modulating or non-modulating conditions. In all cases the quantitative RT-PCR results confirmed the data obtained by DNA microarray (Fig. S4).
Transcriptomic profiles of B. pertussis RisAD60E and RisAD60N
To further deepen our understanding of the role of RisA phosphorylation in transcriptional activation, we used B. pertussis mutant strains producing phosphomimetic or phosphoablative RisA derivatives. Phosphorylation of two-component response regulators usually occurs at a conserved aspartate residue. Asp-60 of RisA is the most conserved aspartate residue in the RisA/OmpR family of response regulators and is therefore likely to be the site of RisA phosphorylation8. RisA was thus genetically replaced by the phosphomimetic RisAD60E or the phosphoablative RisAD60N analogue, and we compared the transcriptional profiles of the RisAD60E- or RisAD60N-producing strains to those of the parental strain in modulating and non-modulating conditions.
The phosphomimetic RisAD60E mutant presented a transcriptomic profile almost identical to that of its parental strain, both in modulating and in non-modulating conditions, suggesting that the RisAD60E protein is fully functional (Figs 1F,G and 4 and Table S4). In contrast, the phosphoablative RisAD60N mutant showed many differences with the parental strain, and its transcriptomic profile was similar, but not identical to that of the RisA-deficient mutant (Figs 1H,I and 4), suggesting that the loss of phosphorylation leads to a strong but not complete loss of RisA functions. These observations suggest that some of the RisA-dependent genes do not require phosphorylation of RisA. In non-modulating conditions, genes that are less transcribed in the absence of RisA, but not in the case of its replacement with RisAD60N, include genes coding for a putative glycosyl transferase and for a biotin synthase (labelled in purple on Fig. 4). In modulating conditions, all the vags identified in cluster 2 of Fig. 3 (labelled in green in Fig. 4), produced lower levels of transcripts in the RisAD60N-producing strain than in BPSMΔRisA. These data suggest that phosphorylated RisA is not required to regulate these genes and confirm that, in addition to modulation, a second level of regulation of these genes is RisA-dependent.
Some genes of the chemotaxis, flagellar and iron acquisition operons (labelled in yellow and blue, respectively, in Fig. 4) appear to be differentially regulated between the RisAD60N-producing strain and BPSMΔRisA. Finally, with the exception of the 6 genes of cluster 5 (Fig. 3), the expression of the vrgs appears to require phosphorylated RisA, as their transcripts were less abundant in the modulated RisAD60N mutant than in the modulated parental strain (Fig. 4, genes highlighted in red). The transcriptome of the RisAD60N mutant was very similar to that of BPSM∆RisK, suggesting that phosphorylation of RisA is required for the transcription of most genes belonging to the RisA regulon. However, the transcription of some of them does not require RisA phosphorylation. Furthermore, the effects of the loss of regulation by RisA through the absence of its phosphorylation were enhanced in modulating conditions.
RisA-mediated regulation in recent clinical B. pertussis isolates
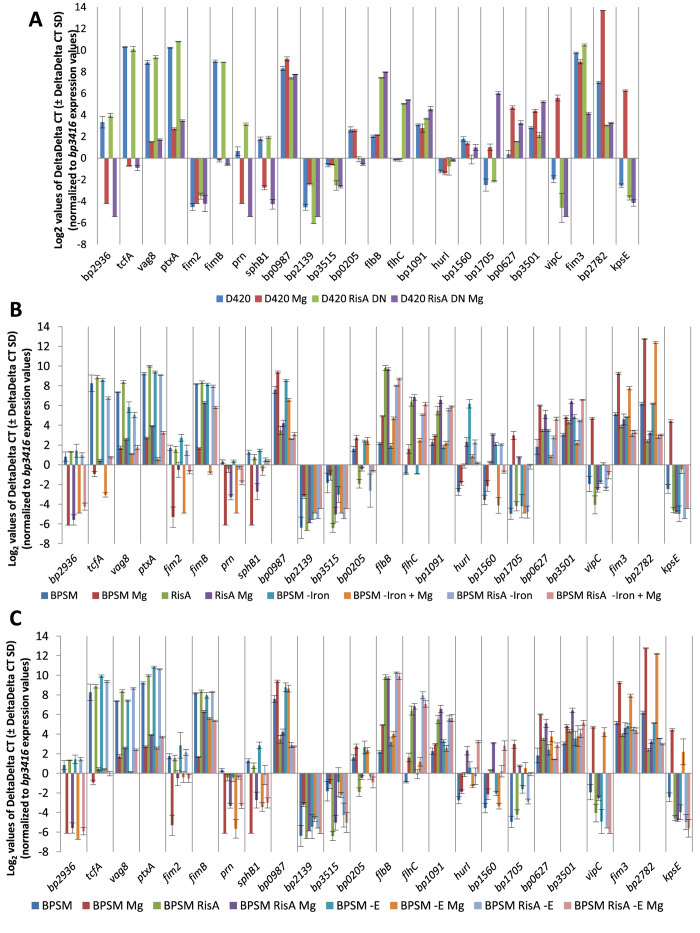
Since the above studies were all done with derivatives of the TohamaI laboratory strain, it was important to determine whether a similar role of RisA could be observed in more recent clinical isolates. We therefore attempted to construct risA mutants in several clinical isolates, including the highly virulent B. pertussis D420 strain15. Although several attempts to construct risA-deletion derivatives of these clinical strains were unsuccessful, we were able to obtain a D420 derivative in which RisA was replaced by the phosphoablative analogue RisAD60N. Using quantitative RT-PCR we compared the transcription of the set of representative genes for each cluster presented in figure S4 in the D420 RisAD60N mutant with that of the parent strain, both grown in modulating and in non-modulating conditions. As shown in Fig. 5A, the transcriptional profile of these genes in the D420 background was very similar to the profile seen in the TohamaI derivatives (Fig. S4). Notable exceptions include the fim2 and fim3 genes. Since D420 does not produce serotype 2 fimbriae15, fim2 was not expressed in D420, as expected, in contrast to the TohamaI derivatives. The fim3 gene was highly transcribed in non-modulated D420, non-modulated D420 producing RisAD60N and in modulated D420 but was less transcribed in the modulated D420 RisAD60N mutant, indicating that fim3 is not a vrg in D420 in contrast to BPSM (see Fig. 3), but has maintained RisA-mediated regulation in modulating conditions.
Figure 5.
(A) Quantitative RT-PCR analysis of genes in D420 and D420 RisAD60N. D420 (blue), MgSO4 modulated D420 (red), D420 RisAD60N (green) and MgSO4 modulated D420 RisAD60N (violet). (B) Quantitative RT-PCR analysis of genes in iron depleted conditions. BPSM (blue), MgSO4 modulated BPSM (red), BPSM RisA (green), MgSO4 modulated BPSM RisA (violet), iron depleted BPSM (cyan), iron depleted MgSO4 modulated BPSM (orange), iron depleted BPSM RisA (light purple) and iron depleted MgSO4 modulated BPSM RisA (pink). (C) Quantitative RT-PCR analysis of genes in glutamate depleted conditions. BPSM (blue), MgSO4 modulated BPSM (red), BPSM RisA (green), MgSO4 modulated BPSM RisA (violet), glutamate depleted BPSM (cyan), glutamate depleted MgSO4 modulated BPSM (orange), glutamate depleted BPSM RisA (light purple) and glutamate depleted MgSO4 modulated BPSM RisA (pink). The values represent the Log2 of mean expression of each gene from mid exponential cultures, normalized to bp3416 using the 2ΔΔCt method expressed relative to the expression in D420 for (a) or BPSM for (b,c). The error bars represent the ∆∆Ct S.D. 50 mM MgSO4 was used as the modulating condition.
RisA-mediated regulation in iron and in glutamate depleted conditions
Since RisA is involved in the regulation of expression of several iron-regulated genes (Fig. S3, labelled in blue), we investigated the role of iron on the expression of the set of representative genes in Fig. 5A by qRT-PCR. BPSM and BPSM∆RisA were therefore grown in iron depleted conditions with or without 50 mM MgSO4. As expected, bp1560 and hurI showed a higher transcript abundance in iron depleted conditions than in iron replete conditions, with respective 50.20 and 490.29 fold changes (Fig. 5B). However, the transcriptional profile of the remaining genes in iron depleted conditions was very similar to that seen in iron replete conditions in both BPSM and BPSM∆RisA, arguing that the iron depletion did not modify the regulatory properties of RisA.
Furthermore, it has recently been shown that the depletion of glutamate in the growth medium may also have a major impact on gene regulation in B. pertussis16. We therefore analyzed by qRT-PCR the transcription of the set of representative genes described above in BPSM and BPSM∆RisA, grown in glutamate depleted conditions with and without 50 mM MgSO4. As previously shown17, glutamate starvation led to increased transcript abundance of hurI, with a 9.96 fold change (Fig. 5C). However, the transcriptional profile of the remaining subset of genes in glutamate-depleted conditions was generally identical to the profile seen in glutamate replete conditions in both BPSM and BPSM∆RisA (Fig. 5C), indicating that, while glutamate depletion has an effect on the transcription of some B. pertussis genes, it does not modify the global pattern of regulation of RisA.
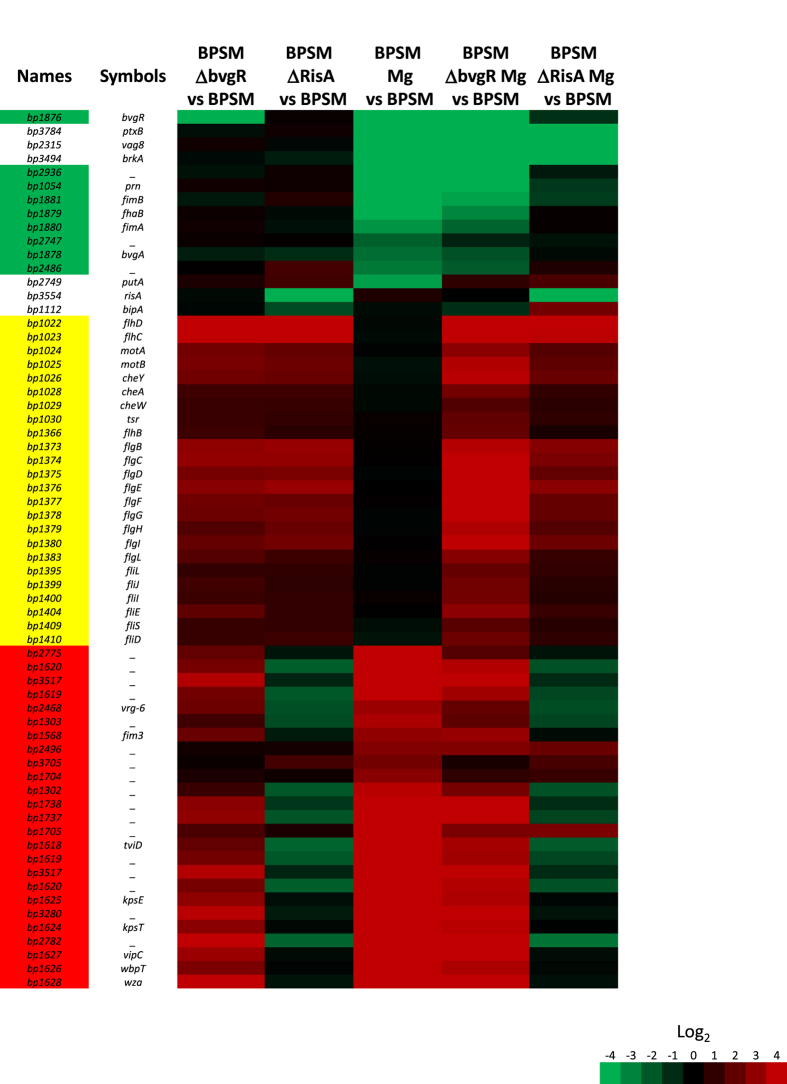
The role of BvgR in RisA-regulated gene expression
Since expression of the vrgs depends on RisA but is also regulated by BvgR and can be modulated by the presence of MgSO4, which itself results in reduced bvgR expression, we investigated the link between modulation, bvgR expression and RisA-dependent transcription. Therefore, the transcriptomic profile of a bvgR-deficient strain was compared to that of its parental strain and of the risA-deficient strain, all of which were grown under modulating and non-modulating conditions. In non-modulating conditions, the transcripts of most vrgs were more abundant in BPSM∆BvgR than in BPSM (Fig. 1J,K, labelled in red in Fig. 6 and Table S5), confirming the role of BvgR in repressing the vrgs. As expected, in modulating conditions the vags were less transcribed in both BPSM and BPSM∆BvgR (labelled in green in Fig. 6), whereas the vrgs were more transcribed (labelled in red in Fig. 6) as compared to non-modulated BPSM.
Figure 6. BvgR-regulated gene expression in modulated and non-modulated BPSM and BPSMΔRisA.
Rows correspond to array probes. The names and symbols in the left column correspond to the Tohama I Sanger Centre annotation. BPSMΔBvgR vs BPSM corresponds to the ratios between BPSMΔBvgR and BPSM, both cultivated in non-modulating conditions. BPSMΔRisA vs BPSM corresponds to the ratios between BPSMΔRisA and BPSM, both cultivated in non-modulating conditions. BPSM Mg vs BPSM corresponds to the ratios between BPSM cultivated in the presence of 50 mM MgSO4 and BPSM cultivated in non-modulating conditions. BPSMΔBvgR Mg vs BPSM corresponds to the ratios between BPSMΔBvgR cultivated in the presence of 50 mM MgSO4 and BPSM cultivated in non-modulating conditions. BPSMΔRisA Mg vs BPSM corresponds to the ratios between BPSMΔRisA cultivated in the presence of 50 mM MgSO4 and BPSM cultivated in non-modulating conditions. The indicated ratios are the means of all the experiments. Red, increased transcript abundance; green, decreased transcript abundance; black, no significant change in transcript abundance; the level of transcript abundance is defined by the coloured Log2 scale shown on the right.
Additionally, all the genes related to the flagellar and chemotaxis operons (labelled in yellow in Fig. 6) were also more transcribed in BPSM∆BvgR compared to BPSM in modulating and non-modulating conditions. The comparison of the transcriptional profiles of modulated versus non-modulated BPSM∆BvgR with those of BPSM∆RisA grown in the same conditions indicated that the expression level of most of the chemotaxis and flagellar genes is lower in BPSMΔRisA than in BPSMΔBvgR, especially in modulating conditions. The transcripts of the flagellum-related genes bp1022 and bp1023 were more abundant in modulated and non-modulated BPSMΔRisA and BPSM∆BvgR than in non-modulated BPSM. They code for the transcriptional activators FlbB and FlhC, respectively, suggesting that RisA is involved in the repression of these two regulators and therefore may indirectly affect the expression of the remaining genes of the flagellar and chemotaxis operons.
Discussion
In this study we used microarray analysis to investigate the role of the risA, risK and bvgR genes in the global B. pertussis transcriptomic regulation in both modulating and non-modulating conditions. The vast majority of the genes affected by the risA, risK or bvgR mutations are all members of the RisA regulon. RisA is a member of the two-component system transcriptional regulator family. However, its putative cognate kinase RisS encoded by the gene located immediately downstream of risA is not functional in B. pertussis because of a frame shift in risS. Instead, we found that the RisK kinase is required for the full regulatory activities of RisA, strongly suggesting that RisK is its cognate kinase. This was confirmed biochemically by some of us using the Phos-Tag technology, showing that the phosphorylation state of RisA is altered in the risK-deficient strain (Chen et al., submitted).
The transcriptomic profile of BPSM∆RisK, the strain that lacks risK, was nearly identical to that of BPSM∆RisA, suggesting that phosphorylation is important for RisA function. However, the expression of some genes was affected by the deletion of risA but not by the deletion of risK. Since the phosphoablative RisAD60N variant presented the same transcriptional profile as the risK-deletion mutant, we conclude that the genes differentially regulated in the risA and risK mutants are regulated by non-phosphorylated RisA, rather than through phosphorylation via cross-talk with another histidine kinase.
The risK gene is co-transcribed with the immediate upstream bp3222 gene, a member of the ompR gene family, located within the same bicistronic operon on the B. pertussis chromosome. This gene arrangement is typical for operons that code for two-component systems and suggests that RisK may also be a cognate kinase of the OmpR-like protein and that therefore risK deletion may have an impact on the regulatory function of BP3222. However, since the transcriptional profile of BPSM∆RisK was found to be nearly identical to that of the RisAD60N mutant, it is likely that RisK exclusively acts on RisA. We found that the transcriptome of a bp3222–deficient BPSM derivative was nearly identical to that of BPSM (data not shown). The few genes that were less transcribed in the bp3222–deficient strain compared to BPSM, were not thus affected in the risK–deficient strain, suggesting either that their expression did not require BP3222 phosphorylation, or that BP3222 is phosphorylated by a kinase different from RisK. In either case, these observations indicate that the transcriptomic profile observed for the risK mutant is mediated through the loss of RisA phosphorylation rather than through an effect on the OmpR-like protein.
Many of the genes that are under the control of RisA are vrgs. The risA deletion affects even the basal level of most vrgs in non-modulating conditions, indicating that RisA is required for vrg expression even without modulation (Figs 2 and 3). In modulating conditions, the effect of the risA deletion is stronger than in non-modulating conditions, as expected. However, the expression of some vrgs was not affected by the risA deletion in non-modulating conditions. These include bp0874 (vir-18) and fim3. Thus, RisA may act differentially on a subset of vrgs. Alternatively, the basal level of transcription of these genes in non-modulating conditions may be too low to detect a difference between BPSM and BPSM∆RisA. Among the 71 overexpressed genes in modulated versus non-modulated BPSM and therefore identified as vrgs, as confirmed by using a ΔBvgA mutant (data not shown), the expression of only 6 was independent of RisA. These 6 RisA-independent vrgs may have evolved from the other vrgs to become independent of RisA-mediated transcriptional activation or may have additional regulatory systems that override the lack of RisA.
The vrgs are also regulated by BvgR, as the presence of this protein represses vrg expression. However, binding of BvgR to vrg operator sites has not been observed, suggesting that BvgR does not act as a transcriptional repressor. Instead, BvgR contains a conserved EAL sequence found in diguanylate phosphodiesterases that are involved in the degradation of c-di-GMP. Additionally, RisA was shown recently to interact with c-di-GMP (Warfel et al., in preparation). Thus, BvgR may participate in the control of intracellular levels of c-di-GMP, a secondary messenger that might regulate the activity of RisA, as has already been shown for other two-component response regulators (for review, see ref. 18).
We found that the expression of the vrgs is strongly enhanced in the bvgR-deficient strain BPSM∆BvgR, even in non-modulating conditions. This observation suggests that, in order to be fully functional, RisA requires the presence of c-di-GMP, the concentration of which is reduced by the presence of BvgR. Our results also suggest that the expression of some RisA-dependent genes requires phosphorylated RisA but not c-di-GMP, whereas another subset of genes does not require phosphorylated RisA but requires the presence of c-di-GMP.
Interestingly, we have also detected genes that were more abundantly transcribed in BPSM∆RisA than in BPSM in non-modulating conditions. They mainly belong to the flagellar and chemotaxis operons. These genes were not identified as vrgs in B. pertussis, while they have previously been described as vrgs in B. bronchiseptica19,20. In addition, they were also shown to be regulated by c-di-GMP21. It remains to be investigated whether they are directly regulated by RisA acting as a repressor, or indirectly involving a regulation intermediate that has yet to be identified. The deletion of bvgR resulted in a transcriptomic profile for these genes that is similar to that observed for BPSM∆RisA, with the exception of flhC and flhD, encoding putative transcriptional regulators. The flagellar genes were also more strongly transcribed in the ∆bvgR background compared to the ∆risA background in modulating conditions (see Fig. 6), suggesting that c-di-GMP acts at two different levels in the regulation of flagellar gene expression. Hence, in addition to acting with RisA, c-di-GMP may also act on the FlhC and FlhD regulators. A similar mechanism has been shown for the B. bronchiseptica flagellum operon21, where in modulating conditions or in the absence of BvgR, the basal activity of the FlbB and FlhC regulators is enhanced, resulting in a higher expression of the remaining genes related to the flagellar and chemotaxis operons.
Several genes related to iron acquisition systems were also more transcribed in modulated BPSM∆RisA than in modulated BPSM. It is not known whether RisA acts directly on the expression of these genes or whether it modifies the bacterial perception of the environmental iron concentration. However, not all the genes that were demonstrated to be more transcribed in iron starvation conditions are regulated by RisA. RisA-independent iron-regulated genes include the operon coding for alcaligin biosynthesis (bp2456-2461)12,13,14. We also found that iron depletion does not perturb the general pattern of RisA regulation.
Surprisingly, in the RisA-deficient background 18 vags lost their repression mediated by the addition of MgSO4 (Fig. 4), whereas they were strongly repressed by the addition of MgSO4 in BPSM. In contrast, the expression of all other vags was still repressed in modulated BPSM∆RisA, similarly to modulated BPSM. Several of the former vags, like prn and fhaB, belong to the class of early vags, while others, like cyaA, belong to the class of late vags, suggesting that RisA may act on various kinds of vags involved in the pathogenesis at different times during infection22. RisA phosphorylation was not required to suppress the modulatory effect of MgSO4 of these vags, while, in contrast, RisA phosphorylation was required for the transcription of all the vrgs in modulating conditions.
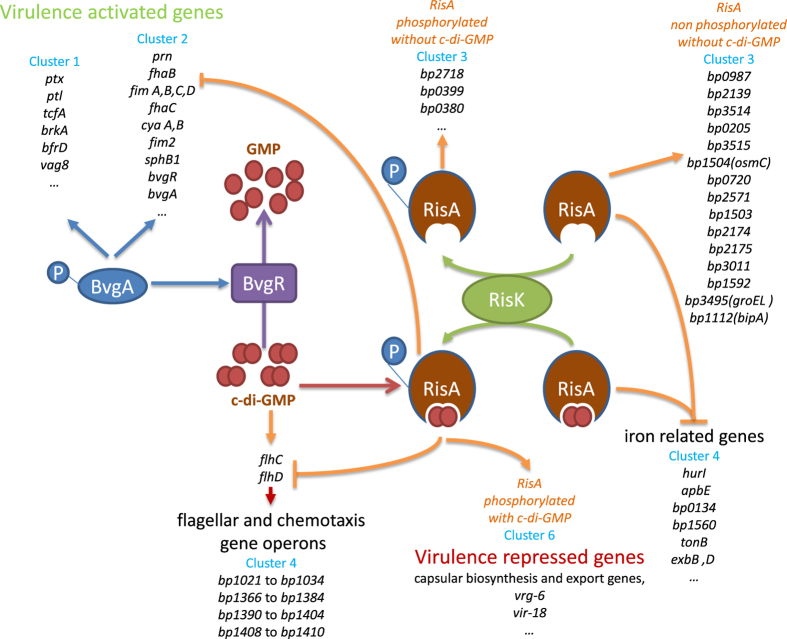
In conclusion, the data presented here prompted us to propose a model integrating the roles of bvgA/S, bvgR, risA and risK in the regulation of B. pertussis virulence genes (Fig. 7). According to this model, in non-modulating conditions, BvgA is phosphorylated by BvgS and activates the vags, including bvgR. Expression of bvgR leads to the degradation of intracellular c-di-GMP. RisA is the transcriptional activator of most of the vrgs, but in the absence of c-di-GMP RisA is not able to induce the expression of these genes, but can nevertheless activate the basal level of vrg expression and repress the expression of the flagellar and chemotaxis genes and of genes involved in iron acquisition. In modulating conditions, BvgS is not active and does not phosphorylate BvgA. Non-phosphorylated BvgA does not activate the expression of bvgR. Therefore, the concentration of c-di-GMP increases. The cofactor c-di-GMP binds to phosphorylated and non-phosphorylated RisA participating in the induction or repression of the RisA regulon, including the vrgs, the RisA-regulated vags and other genes of unknown function.
Figure 7. Schematic representation of the RisA regulatory network in B. pertussis.
Clusters shown in this figure are the same as those identified in Fig. 3. In non-modulating conditions phosphorylated BvgA induces the expression of vags (clusters 1 and 2), including bvgR, thereby leading to the degradation of c-di-GMP into GMP. In the absence of c-di-GMP RisK-phosphorylated RisA is able to activate many genes of cluster 3. A subset of cluster 3 genes are also activated by non-phosphorylated RisA in the absence of c-di-GMP. Under modulating conditions, and in the absence of BvgR, binding of c-di-GMP to RisA phosphorylated by RisK leads to the expression of vrgs (cluster 6), but also to the repression of flagellar and chemotaxis genes (cluster 4). Unphosphorylated RisA, in the presence or absence of c-di-GMP, inhibits the expression of other cluster 4 genes, including iron-regulated genes. Phosphorylated c-di-GMP-associated RisA is also able to repress the expression of the vags belonging to cluster 2.
Although the role of the RisA regulon in the pathogenesis of pertussis is not yet known, RisA-mediated regulation may perhaps be required to allow the bacteria to adapt to different phases during the infectious cycle. In addition, fine-tuning of adhesin production may contribute to transmission and/or the colonization of a specific niche in the respiratory tract. Furthermore, we have analysed the RisA regulon in standard in vitro growth conditions, in iron depleted conditions and in glutamate depleted conditions in the absence or presence of 50 mM MgSO4. It remains to be investigated whether the RisA-dependent transcriptome may vary in different growth conditions or during infection. We cannot exclude the possibility that under different growth conditions, additional members of the RisA regulon might be identified.
Methods
Construction of B. pertussis mutant Strains
The B. pertussis strains used in this study were derived from Tohama I derivatives BPSM23 or BP53624, or from the clinical isolate D42015. B. pertussis BPSM∆RisA, BPSMΔRisK and BPSMΔBvgR were obtained by homologous recombination using either pSS1129 or pJQ200 mp18 rpsL as allelic exchange vectors25,26,27. The recombinant plasmids were introduced into B. pertussis by conjugation via Escherichia coli SM1028.
BPSMΔRisA carries a 735 bp internal deletion in the risA gene (BP3554). It was obtained as follows. Two 800-bp DNA fragments flanking the region to be deleted were obtained by PCR using the BPSM genomic DNA as template and the oligonucleotide pairs 5′-GAATTCGCGGCCACGCCGCCGCCATCCCGCCAGGCC-3′ and 5′-TCTAGAGGCCGGAAATGTAACAGTGA-3′ and 5′-TCTAGACCTAATGGCCCGCCCCGGGC-3′ and 5′-AAGCTTCGCCAGCGGCGTGCACAGGTCGTGTGAAATGCC-3′ as primers. The resulting EcoRI-XbaI and XbaI-HindIII fragments were then introduced into EcoRI-HindIII-digested pJQ200 mp18 rpsL, yielding pJQ∆RisA. This construct was used for allelic exchange in BPSM, yielding BPSMΔRisA, in which risS is located directly downstream of the risA/S promoter.
BPSMΔRisK carries a 1392-bp internal deletion in the risK gene (BP3223). It was obtained as follows. Two 400-bp DNA fragments flanking the region to be deleted were obtained by PCR using the BPSM genomic DNA as template and the oligonucleotide pairs 5′-TATAAAGCTTACGACTACCTCGGCAAGCCCTT-3′ and 5′-TATACTCGAGGCGGAGCAGTTTCATCAGGG-3′ and 5′-TATACTCGAGCCGCTTGCGAAGGCTTGACC-3′ and 5′-TATAGGATCCTGGAGCAATACGGCCCACCT-3′ as primers. The resulting HindIII-XhoI and XhoI-BamHI fragments were successively introduced into the HindIII-BamHI sites of pSS1129, yielding pSS1129 BP3223. This construct was used for allelic exchange in BPSM, yielding BPSMΔRisK. The internal deletion in the risK gene led to a truncated RisK protein constituted by the first and last five amino acids of the original RisK protein.
BPSMΔBvgR carries a 804-bp internal deletion in the bvgR gene (BP1876). It was obtained as follows. Two 470-bp DNA fragments flanking the region to be deleted were obtained by PCR using the BPSM genomic DNA as template and the oligonucleotide pairs 5′-TATAAAGCTTCAATCCGCGCCATCCAGGTC-3′ and 5′-TATACTCGAGAGCCTCGAAGCTGCTGCGAG-3′ and 5′-TATACTCGAGCGCCGCGAGATGCCGCCCAA-3′ and 5′-TATAGGATCCCGCGCCGGCCACGGACGACG-3′ as primers. The resulting HindIII-XhoI and XhoI-BamHI fragments were successively introduced into the HindIII-BamHI sites of pSS1129, yielding pSS1129 BvgR. This construct was used for allelic exchange in BPSM, yielding BPSMΔBvgR. The internal deletion in the bvgR gene led to a truncated BvgR protein constituted by the first 14 and last 9 amino acids of the original BvgR protein.
Strains QC3296 (RisAD60N) and BP1942 (RisAD60E) were constructed as follows. Plasmid pSS4894 (Chen et al., unpublished results) was used as the allelic exchange vector for the construction of pSS5085 (pSS4894::ΔBP-risA), pSS5085.5 (pSS4894::BP-risAD60N), and pSS5086 (pSS4894::BP-risAD60E). For each, fragments comprising sequences flanking the mutation were synthesized using PCR amplification with BP536 chromosomal DNA as a template.
For pSS5085, the upstream fragment was amplified with primers 5′-TATAGGTCTCCGGCCGCGGTGGTGAAGGCCACCTTGTC-3′ and 5′-TATAGGTCTCCGGGTTTTGCGTGTTCATGGCCGGAAATGTAACAGTG-3′. The downstream fragment was amplified with primers 5′-TATAGGTCTCAACCCGGATGGCGGCAGTTGACCTAATG-3′ and 5′-TATAGGTCTCGGATCCGATCTGGCCGAGGTCCTCGTCGATG-3′. Both fragments were digested with the type II-S restriction enzyme BsaI to create cohesive ends compatible with NotI, BamHI or unique, compatible cohesive ends in the vicinity of the deletion endpoint. The two digested fragments were ligated together with pSS4984 digested with NotI and BamHI, transformed, and screened to create pSS5085. For pSS5085.5 the upstream fragment was amplified with primers 5′-TATAGGTCTCCGGCCGCGGTGGTGAAGGCCACCTTGTC-3′ and 5′-TATAGGTCTCCATCAGGTTGAGAACCAGCAGGTCAAAGTG-3′. The downstream fragment was amplified with primers 5-TATAGGTCTCGGATCCGATCTGGCCGAGGTCCTCGTCGATG-3′ and 5′-TATAGGTCTCCTGATGCTGCCGGGCGAGGATGGCCTGTCGATC-3′. For pSS5086 synthesis was similar, with the exception that primer 5′-TATAGGTCTCCATCAGTTCGAGAACCAGCAGGTCAAAGTG-3′ was used, such that the D60E, rather than the D60N, mutation was incorporated.
Transfer of pSS5085 to B. pertussis BP536 was accomplished by conjugation following its transformation into the dap E. coli strain RHO3, with selection on LB supplemented with gentamicin and DAP. Prior to mating, the recipient BP536 was grown on BG agar plus streptomycin and 50 mM MgSO4. Mating was performed by swabbing the E. coli donor strain together with the B. pertussis recipient strain on BG agar plus MgSO4 and DAP. After incubation at 37 °C for 3 hours, bacteria were recovered by swabbing and re-swabbed onto BG agar plus gentamicin (100 μg/ml), streptomycin (50 μg/ml) and 50 mM MgSO4 to select for transfer and integration by a single crossover. MgSO4 was included to maintain repression of I-SceI synthesis from the pSS4894 vector and the higher level of gentamicin was used to reduce background typically observed under modulating conditions. Discrete colonies arising on these selection plates were then restreaked onto BG agar lacking MgSO4 to induce the synthesis of I-SceI enzyme driven by Pptx, the subsequent cleavage of the integrated pSS4894 vector, and the stimulation of homologous recombination to repair the resulting double stranded cleavage, resulting in loss of the vector and either incorporation of the mutant allele or a return to the wild-type allele. Colonies that arose on these plates were screened by PCR for incorporation of the deletion allele and also for sensitivity to gentamicin to verify loss of the plasmid vector. In this way strain BP1928 was created. In a similar way, the D60N and D60E mutations were incorporated into the BP536 genetic background using pSS5085.5 and pSS5086 as allelic exchange constructs. However, in these crosses BP1928 was used as the recipient, in order to be able to distinguish the resident deletion allele from the incoming D60 substitution alleles by PCR in the screening step. In this way the strains QC3296 (risAD60N) and BP1942 (risAD60E) were created. Strain QC4470 (B. pertussis D420, risAD60N) was constructed as follows QC4470 was created by allelic exchange with the plasmid pQC2266 in B. pertussis strain D420. This plasmid was created by cloning a 1 kb gene fragment containing sequences flanking the D60N mutation as well as synonymous changes to introduce a SmaI restriction site nearby. The latter was included to facilitate screening of recombinants. The fragment was synthesized as a gBlock (Integrated DNA Technologies, Inc.) with flanking BamHI and NotI sites and was cloned, following digestion, into the same sites of pSS4894. The sequence of the fragment is given in Table S6. Transfer of pQC2266 to D420 was accomplished by conjugation following its transformation into the dap E. coli RHO3, with selection on LB supplemented with gentamicin and DAP. To maintain modulation in the first stages of this allelic exchange, The recipient D420 was grown on BG agar containing 20 mM MgSO4 and mating was performed on BG with 20 mM MgSO4 plus DAP. Mating was for 3 hours at 37 °C after which exconjugants were selected after reswabbing on BG agar containing 20 mM MgSO4, and gentamicin, but without DAP. Discrete colonies arising on these selection plates were then restreaked onto BG agar lacking MgSO4 to allow the cross out of the plasmid vector. Colonies that arose on these plates were screened by sensitivity to gentamicin and PCR of the risA region followed by SmaI digestion of the resulting product. The DNA sequence of the risA locus in the final strain QC4470 was verified by sequencing the PCR product.
All B. pertussis strains were grown on Bordet Gengou agar (BG), in liquid modified Stainer-Scholte medium as described by Locht et al.29, in liquid iron-depleted Stainer-Scholte medium as described by Alvarez Hayes et al.13 or in liquid modified Stainer-Scholte medium containing 20% of the standard glutamate concentration, as described by Hanawa et al.16. The culture media were supplemented with 100 mg/ml streptomycin and 50 mg/ml magnesium sulphate where appropriate.
Microarray production and analysis
Long oligonucleotide probes were designed on the sequences of the 3552 open reading frames, including all coding sequences, except those of the transposases in IS elements, of B. pertussis Tohama I genome using OligoArray v2.130. Oligonucleotides were synthesized by Sigma-Aldrich and spotted on Nexterion AL slides (Schott Nexterion) in 1× SciSpot-AM buffer (Scienion) using a Q-Array II spotter (Genetix). Total RNA was extracted from bacterial pellets harvested from two or three individual cultures for each mutant strain, using TriReagent (Ambion) following manufacturer’s instructions. For each sample, 5 μg of total RNA was reverse transcribed with 400 units of SuperScript III (Invitrogen) in the presence of 100 μM Cy3-dCTP or Cy5-dCTP (GE) and 300 mM of random hexanucleotides (Roche). The labelled cDNA was treated with 1 M NaOH to degrade the RNA and then purified on the Qiaquick PCR purification kit (Qiagen). Hybridization was performed in 40% formamide, 5× Denhardt’s solution, 0.1% SDS, 1 mM Sodium pyrophosphate and 5× SSC during 14–16 h at 52 °C under agitation. Slides were then washed sequentially in 2× SSC/0.2% SDS during 5 min, 0.5× SSC during 10 min, 0.05× SSC during 5 min and 0.01× SSC during 1 min before drying. Hybridized slides were scanned using an Innoscan 700 (Innopsys) microarray scanner and analysed with Mapix v3.1 (Innopsys). For normalisation and differential expression analyses the LIMMA package (Linear Models for Microarray Data)31, running under the statistical language R v2.11.1, was used. Statistically significant regulation was identified using moderated t-statistic with empirical Bayes shrinkage of the standard errors32. Because of multiple testing, obtained P-values were corrected using the Benjamini & Hochberg method to control false discovery rates33. The expression data shown corresponding to each of the 3552 open reading frames were calculated by a means of 2 to 4 individual cultures that were each analysed in 4 technical replicates. All the microarray data are under GEOarchive GSE77754.
Generation of cDNA
RNA was extracted from bacterial pellets of mid exponential phase cultures grown in Stainer-Scholte medium, using TriReagent (Ambion) following the manufacturer’s instructions. For each sample, 5 μg of total RNA was reverse transcribed with 400 units of SuperScript III (Invitrogen) and 300 mM of random hexanucleotides (Roche). The cDNA products were treated with 1 M NaOH to degrade the RNA and then purified on the Qiaquick PCR purification kit (Qiagen).
Quantitative real-time polymerase chain reaction
Polymerase chain reaction (PCR) was performed in an Roche LightCycler® 480 Instrument II using 10 μl of 2× Master Mix SybrGreen (Roche), 1 μl of cDNA product (30 ng), 1 μl of 5 μM forward+ reverse primer mix and water to reach a final reaction volume of 20 μl. The primers used are presented in Supplementary Table S6. A 15-min cycle at 95 °C was followed by 40 cycles of 15 s at 95 °C, 8 s at 64 °C and 1 min at 72 °C. At completion of the PCR run, a dissociation curve from 55 °C to 95 °C was run to determine that a single product was generated. The efficiency for each primer pair was determined by serial dilution. The expression of the housekeeping gene bp3416 was used as reference to normalize the expression of the genes of interest. The experiments were done 3 times with at least 4 technical replicates for each measurement.
Additional Information
How to cite this article: Coutte, L. et al. The multifaceted RisA regulon of Bordetella pertussis. Sci. Rep. 6, 32774; doi: 10.1038/srep32774 (2016).
Supplementary Material
Acknowledgments
We thank Dr Raymond Pierce for carefully reading the manuscript.
Footnotes
Author Contributions L.C., D.H. and C.L. designed the conceptual framework of this study. L.C., L.H. and S. Slupek carried out the experiments. L.C., R.A., D.H. and C.L. analysed the data. T.J.M., Q.C. and S. Stibitz substantially contributed to the discussion and provided important unpublished information. L.C., Q.C. and S. Stibitz constructed the mutated strains, and L.C. and C.L. wrote the paper.
References
- World Health Organisation. WHO vaccine-preventable diseases: monitoring system, 2016 global summary. http://apps.who.int/immunization_monitoring/globalsummary/Date of access: 02/08/2016.
- Stibitz S. The bvg regulon in Bordetella Molecular Microbiology. (ed. Locht C.) 47–67 (Horizon Bioscience, 2007).
- Lacey B. W. Antigenic modulation of Bordetella pertussis. J Hyg 31, 423–434 (1960). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton A. R. & Weiss A. A. Environmental regulation of expression of virulence determinants in Bordetella pertussis. J. Bacteriol 171, 6206–6212 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp S. & Mekalanos J. J. Two trans-acting regulatory genes (vir and mod) control antigenic modulation in Bordetella pertussis. J. Bacteriol 170, 5059–5066 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel T. J. & Stibitz S. Identification of a locus required for the regulation of bvg-repressed genes in Bordetella pertussis. J. Bacteriol 177, 2727–2736 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croinin T. O., Grippe V. K. & Merkel T. J. Activation of the vrg6 promoter of Bordetella pertussis by RisA. J. Bacteriol 187, 1648–1658 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenson T. H., Allen A. G., Al-Meer J. A., Maskell D. & Peppler M. S. Bordetella pertussis risA, but not risS, is required for maximal expression of Bvg-repressed genes. Infect Immun 73, 5995–6004 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungnitz H., West N. P., Walker M. J., Chhatwal G. S. & Guzman C. A. A second two-component regulatory system of Bordetella bronchiseptica required for bacterial resistance to oxidative stress, production of acid phosphatase, and in vivo persistence. Infect Immun 66, 4640–4650 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings C. A., Bootsma H. J., Relman D. A. & Miller J. F. Species- and strain-specific control of a complex, flexible regulon by Bordetella BvgAS. J. Bacteriol 188, 1775–1785 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine R. et al. New virulence-activated and virulence-repressed genes identified by systematic gene inactivation and generation of transcriptional fusions in Bordetella pertussis. J. Bacteriol 182, 5902–5905 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman T. J., Cummings C. A., Liew S. Y., Relman D. A. & Armstrong S. K. Transcriptional profiling of the iron starvation response in Bordetella pertussis provides new insights into siderophore utilization and virulence gene expression. J. Bacteriol 193, 4798–4812 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez Hayes J. et al. Identification of a new protective antigen of Bordetella pertussis. Vaccine 29, 8731–8739 (2011). [DOI] [PubMed] [Google Scholar]
- Vidakovics M. L. et al. Profiling the Bordetella pertussis proteome during iron starvation. J. Proteome Res 6, 2518–2528 (2007). [DOI] [PubMed] [Google Scholar]
- Boinett C. J. et al. Complete Genome Sequence of Bordetella pertussis D420. Genome Announc 3, e00657–15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawa et al. Glutamate Limitation, BvgAS Activation, and (p)ppGpp Regulate the Expression of the Bordetella pertussis Type 3 Secretion System. J. Bacteriol 198, 343–351 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M. M. et al. Growth phase- and nutrient limitation-associated transcript abundance regulation in Bordetella pertussis. Infect. Immun. 74, 5537–5548 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römling U., Galperin M. Y. & Gomelsky M. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev 77, 1–52 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerley B. J. & Miller J. F. Flagellin gene transcription in Bordetella brobchispetica is regulated by the BvgAS virulence control system. J. Bacteriol 175, 3468–3479 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerley B. J., Monack D. M., Falkow S. & Miller J. F. The bvgAS locus negatively controls motility and synthesis of flagella in Bordetella bronchiseptica. J Bacteriol 174, 980–990 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisti F., Ha D. G., O’Toole G. A., Hozbor D. & Fernandez J. Cyclic-di-GMP signalling regulates motility and biofilm formation in Bordetella bronchiseptica. Microbiology 159, 869–879 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veal-Carr W. L. & Stibitz S. Demonstration of differential virulence gene promoter activation in vivo in Bordetella pertussis using RIVET. Mol Microbiol 55, 788–798 (2005). [DOI] [PubMed] [Google Scholar]
- Menozzi F. D. et al. Heparin-inhibitable lectin activity of the filamentous hemagglutinin adhesin of Bordetella pertussis. Infect Immun 62, 769–778 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stibitz S. & Yang M. S. Subcellular localization and immunological detection of proteins encoded by the vir locus of Bordetella pertussis. J Bacteriol 173, 4288–4296 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quandt J. & Hynes M. F. Versatile suicide vectors which allow direct selection for gene replacement in Gram-negative bacteria. Gene 127, 15–21 (1993). [DOI] [PubMed] [Google Scholar]
- Stibitz S., Black W. & Falkow S. The construction of a cloning vector designed for gene replacement in Bordetella pertussis. Gene 50, 133–140 (1986). [DOI] [PubMed] [Google Scholar]
- Stibitz S. Use of conditionally counterselectable suicide vectors for allelic exchange. Methods Enzymol 235, 458–465 (1994). [DOI] [PubMed] [Google Scholar]
- Simon R., Priefer U. & Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Biotechnology 1, 784–791 (1983). [Google Scholar]
- Locht C., Geoffroy M. C. & Renauld G. Common accessory genes for the Bordetella pertussis filamentous hemagglutinin and fimbriae share sequence similarities with the papC and papD gene families. EMBO J 11, 3175–3183 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouillard J. M., Zuker M. & Gulari E. OligoArray 2.0: design of oligonucleotide probes for DNA microarrays using a thermodynamic approach. Nucleic Acids Res 31, 3057–3062 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth G. K., Yang Y. H. & Speed T. Statistical issues in cDNA microarray data analysis. Methods Mol Biol 224, 111–136 (2003). [DOI] [PubMed] [Google Scholar]
- Lonnstedt I. & Speed T. Replicated microarray data. Stat Sinica 12, 808 (2002). [Google Scholar]
- Benjamini Y. & Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met 811, 289–300 (1995). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.