Abstract
Study Objectives:
Lost productivity caused by insomnia is a common and costly problem for employers. Although evidence for the efficacy of Internet-based cognitive behavioral therapy for insomnia (iCBT-I) already exists, little is known about its economic effects. This study aims to evaluate the cost-effectiveness and cost-benefit of providing iCBT-I to symptomatic employees from the employer's perspective.
Methods:
School teachers (N = 128) with clinically significant insomnia symptoms and work-related rumination were randomized to guided iCBT-I or a waitlist-control-group, both with access to treatment as usual. Economic data were collected at baseline and 6-mo follow-up. We conducted (1) a cost-effectiveness analysis with treatment response (Reliable Change [decline of 5.01 points] and Insomnia Severity Index < 8 at 6-month follow-up) as the outcome and (2) a cost-benefit analysis. Because both analyses were performed from the employer's perspective, we focused specifically on absenteeism and presenteeism costs. Statistical uncertainty was estimated using bootstrapping.
Results:
Assuming intervention costs of €200 ($245), cost-effectiveness analyses showed that at a willingness-to-pay of €0 for each positive treatment response, there is an 87% probability that the intervention is more cost effective than treatment as usual alone. Cost-benefit analyses led to a net benefit of €418 (95% confidence interval: −593.03 to 1,488.70) ($512) per participant and a return on investment of 208% (95% confidence interval: −296.52 to 744.35). The reduction in costs was mainly driven by the effects of the intervention on presenteeism and to a lesser degree by reduced absenteeism.
Conclusions:
Focusing on sleep improvement using iCBT-I may be a cost-effective strategy in occupational health care.
Clinical Trials Registration:
Title: Online Recovery Training for Better Sleep in Teachers with High Psychological Strain. German Clinical Trial Register (DRKS), URL: https://drks-neu.uniklinik-freiburg.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00004700. Identifier: DRKS00004700.
Commentary:
A commentary on this article appears in this issue on page 1767.
Citation:
Thiart H, Ebert DD, Lehr D, Nobis S, Buntrock C, Berking M, Smit F, Riper H. Internet-based cognitive behavioral therapy for insomnia: a health economic evaluation. SLEEP 2016;39(10):1769–1778.
Keywords: cost-benefit, cost-effectiveness, employer perspective, insomnia, Internet, randomized controlled trial, self-help
Significance.
The intervention that is evaluated in the current study is based on cognitive behavioral therapy (CBT). It has been tailored to the specific situation of employees suffering from both insomnia and work stress. The health economic evaluation shows that the Internet-based CBT intervention significantly reduces insomnia symptoms and costs (due to absenteeism & presenteeism) when compared to nonintervening. Thus, the intervention has a good probability of being cost-effective from an employer's perspective. To our knowledge, the current study is the first economic evaluation of Internet-based CBT for insomnia. Future studies should be conducted with varying types of professions since the present results might only be generalized to teachers and employees with similar working characteristics.
INTRODUCTION
The efficacy of cognitive behavioral therapy for insomnia (CBT-I) has been proven in a large number of studies and is therefore recommended as a first-line treatment for insomnia for various target groups.1–3 The cost-effectiveness of CBT-I, however, is regarded as a largely neglected research topic.4 A recent review5 on health economics of insomnia treatment only includes two studies of behavioral treatment. These studies both report on the cost-effectiveness of face-to-face CBT-I from a health service perspective, which means that they only focused on direct health care costs (e.g., costs for medical drugs or seeing the doctor).6,7 Bonin et al.6 reported a probability of 97% for the cost-effectiveness of brief psychoeducational CBT-I community workshops compared to a waitlist-control group if health policy-makers were willing to pay 150 Great Britain Pound (GBP) ($196) per one-point improvement in the Insomnia Severity Index (ISI). Watanabe and colleagues7 reported brief CBT-I for patients of an outpatient clinic suffering from both insomnia and comorbid depression to be cost-effective at a probability of 95% compared to treatment as usual if decision makers were willing to pay $60,000 for one additional quality-adjusted life year (QALY). These studies indicate a potential cost-effectiveness of CBT-I but they are too few in number to make a robust overall conclusion about the cost-effectiveness of CBT-I. Given the rising costs of prevention and treatment of medical diseases on the one hand, and limited resources on the other hand, health economic evaluations can be an important support tool for various decision makers to make relevant choices between alternative treatment strategies. Overall, more research on the cost-effectiveness of CBT-I based interventions is needed.4,8
A similar knowledge gap can be observed for Internet-based interventions for insomnia (iCBT-I). Internet-based interventions have also been shown to be an effective low-threshold treatment for insomnia.9–12 They have been introduced to both potentially increase the reach of CBT-I and to do so at lower costs than traditional CBT-I.13 However, no evidence is currently available for this assumption. The current study is the first economic evaluation of Internet-based CBT-I. The evaluation was conducted from an employer's perspective because the current CBT-I intervention was an occupational health intervention focusing on employees experiencing work-related insomnia. Furthermore, the indirect costs due to productivity losses at work, e.g., work loss days (absenteeism) and reduced productivity while at work (presenteeism), are primarily relevant to the employer14 and especially substantial with regard to insomnia as shown by a number of epidemiologic studies.15,16
The indirect costs, i.e. production losses, are assumed to outweigh those of direct and indirect medical care costs with an estimated ratio of 1:3.15 For US workers, Kessler et al.16 observed annual costs of $91.7 billion solely due to presenteeism and absenteeism, and presenteeism accounted for two-thirds of these costs. Therefore, we assume that presenteeism will also account for most of the costs and cost reductions in this economic evaluation.
Based on the aforementioned findings researchers have emphasized the need for economic evaluations of insomnia interventions to take the “employer's perspective”.16,17 From this perspective, analyses imply that the employer pays for the costs due to presenteeism/absenteeism and for the intervention costs and benefits from the potential savings due to reduced presenteeism/absenteeism. Reporting the probability to which Internet-based CBT-I may save costs with regard to workplace productivity16,18 could inform employers on how to best spend their limited resources in occupational health care.19
With regard to the form of economic evaluation relevant to an employer, two types of analyses can be distinguished19: first, a cost-benefit analysis providing a return on investment (ROI) by relating costs (e.g., intervention costs) to consequences (e.g., reduced presenteeism and absenteeism), both measured in monetary units, and second, a cost-effectiveness analysis that takes costs and consequences (intervention, presenteeism, and absenteeism) both measured in monetary units and relates them to an effect outcome (e.g., significant improvement of insomnia symptoms) measured in natural units.
To the best of our knowledge no published cost-benefit or cost-effectiveness analyses from an employer's perspective exist for either face-to-face or Internet-based CBT for employees with insomnia. However, there are a number of published economic evaluations of occupational health interventions for other conditions than insomnia such as depression that are conducted from the employer's perspective:
A large systematic review20 (n = 51 studies) on the cost-benefit of face-to-face workplace health promotion programs to employers comprised evidence on ROIs of various interventions and indications, ranging from obesity reduction, smoking cessation, and productivity enhancement programs. The authors found a positive overall ROI of 138% ($1.38 for every dollar invested). However, results were mixed because ROI heavily varied across studies depending on methodology, scope of the program, sample size, and study quality. Better study quality was found in randomized controlled trials (RCTs) correlated with smaller ROIs: The RCTs included (n = 12) exhibited a negative ROI, −0.22 ± 2.41.
With regard to Internet-based occupational health interventions, the first economic evaluation in this area was recently published by Geraedts and collegues.21 They targeted employees with depressive symptoms. Their cost-effectiveness analysis showed that the intervention had a probability of 95% of being cost-effective if the employer would be willing to pay 3,500 euro for an additional clinically meaningful change in depressive symptoms. The authors did not recommend the intervention to employers cost-wise because they regarded 3,500 euro as being too high for the employer to pay and because cost-benefit analyses showed only a moderate probability of positive financial return (66%).
The health economic evaluation of the iCBT-I intervention, which we present here, was conducted parallel to an RCT on the efficacy of Internet-based CBT-I.22 As shown in a previously published article on that trial, the intervention significantly reduced insomnia symptoms among employees (large effect, with Cohen's d = 1.45, 95% confidence interval [CI]: 1.06 – 1.84), with 42.2% of participants in the intervention group being symptom-free at 6-month follow-up (vs. 6.3% in the control group).23 The current evaluation investigates the probability of Internet-based CBT to be cost-effective and cost-beneficial from the employer's perspective with a focus on productivity loss in terms of absenteeism and presenteeism among employees.
METHODS
Design
The current health economic evaluation followed guidelines from the International Society for Pharmacoeconomics and Outcomes Research task force report on good research practices for cost-effectiveness analysis alongside clinical trials (ISPOR RCT-CEA) and the recommendations of the Consolidated Health Economic Evaluation Reporting Standard (CHEERS).24
The study design is described in detail in the study protocol.22 In brief, the study was designed as an evaluation of efficacy with a health-economic evaluation alongside a randomized trial with two parallel groups. Randomization was performed using an automated web-based program (randomisation.eu). The study was approved by the Ethics Committee of the Philipps University of Marburg (Nr.: 2013-01K) and registered as DRKS00004700 in the German Clinical Trial Register (DRKS).
Procedures
Adult schoolteachers were recruited via mailings to schools from March to September 2013. Outcomes were assessed at baseline and at 2- and 6-mo follow-up. In total, 128 currently employed schoolteachers with clinically significant insomnia symptoms (Insomnia Severity Index [ISI] > 14) and elevated work-related rumination (Irritation scale, subscale “Cognitive Irritation” > 14) were randomly allocated to either the guided GET.ON Recovery intervention group (IG) or a waitlist control group (CG) in a ratio of 1:1, both with unrestricted access to treatment as usual. Exclusion criteria were: (1) currently receiving psychological help for insomnia and (2) showing suicidal ideation, for which item 9 from the Beck Depression Inventory II (BDI II)25 was used.
Interventions
The Internet-based CBT-I intervention (GET.ON Recovery22,23) has been specifically tailored for stressed and sleepless employees. It mainly uses CBT-I methods such as sleep restriction, stimulus control, sleep hygiene, and cognitive interventions,26 supplemented by techniques effective in reducing work stress and fostering mental detachment from work-related problems derived from behavioral activation,27 metacognitive therapy,28 gratitude research,29 and research on boundary management.30 The intervention has been shown to be effective in a guided self-help23 and pure self-help31 format. It has also been shown that the intervention's effects on insomnia severity were mediated by both a reduction in perseverative cognitions and sleep effort. Additionally, an increase in number of recovery activities per week was found to be associated with lower perseverative cognitions, which in turn led to a reduction in insomnia severity.31 The intervention consisted of six 1-w modules. The participants in the intervention group received email feedback on every completed module by trained clinical psychologists (e-coaches) following a manual to ensure a standardized procedure of coaching. E-coaches also provided email reminders if participants did not complete a module within 1 w. E-coaches were advised that the total amount of support a subject should receive was to be around 3 h total, with a maximum of 30 min per session, so that the guidance could be kept at a minimal level and comparability of the participants was maximized. A clinical psychologist supervised the e-coaches.
Individuals in the control group eventually did receive the intervention (without coaching) after the last assessment at 6-mo follow-up for ethical reasons.
All study participants had unrestricted access to treatment as usual. Treatment as usual for elevated insomnia symptoms usually indicates visits to the general practitioner (GP) followed by more intensive interventions such as CBT and sleep medication if insomnia symptoms persevere or worsen.
Measures
Clinical Outcome
The primary clinical outcome for the cost-effectiveness analysis was the number of participants with a positive treatment response at 6-mo follow-up using the ISI.32 Participants were labeled as “having a positive treatment response” according to Jacobson and Truax33, by combining two criteria: a sufficiently large change in the widely used Reliable Change Index (RCI) and ISI being below a certain cutoff score. Therefore, treatment response was defined if the ISI score: (1) decreased by 5.01 points on the ISI and (2) fell below the cutoff score of 8 in the ISI, which classifies a participant as being symptom-free.34 The seven items of the ISI are answered on a five-point Likert scale with the total score ranging from 0 to 28. The ISI is a commonly used instrument in CBT-I research and has been validated as a Web-based measure.35 At screening, the cutoff score of ≥ 15 was used to indicate clinical insomnia.32 Participants with a score < 15 were excluded. In our sample, internal consistency was α = 0.91.
Costs
Costs were calculated in euros (€) for the reference year 2013. Main results were translated into US dollars using the Organization for Economic Co-operation and Development's (OECD) Purchasing Power Parities (PPP) of 2013.36 PPPs are the rates of currency conversion eliminating the differences in price levels between countries and therefore correcting for the purchasing power of different currencies.
Absenteeism and Presenteeism
Cost data were measured with the Trimbos/iMTA questionnaire for costs associated with psychiatric illness (TiC-P37), a retrospective (covering the previous three months) self-report questionnaire. The TiC-P is a widely used, feasible, and reliable instrument for collecting data on health care utilization and productivity losses in patients with mild to moderate mental health conditions.38 Participants were instructed to specify all productivity losses.
Costs due to absenteeism were based on the human capital approach.39 In order to measure absenteeism, participants were asked how many days they had been absent from work during the past 3 mo (work loss days). To measure costs, work loss days were then multiplied to the participant's average gross daily wage based on their self-reported monthly salary.
Production losses due to presenteeism were measured by asking participants to report how many days during the past 3 mo they went to work even though they were bothered by their health problems. The number of days was then multiplied by a self-reported inefficiency score, which ranged between 0 and 1 (where 0 means as efficient as when in good health and 1 means totally inefficient) to obtain workday equivalents lost to presenteeism. This method is called the Osterhaus method.40 Subsequently, based on self-reported monthly salary, their gross wages per hour were calculated and were used to calculate the costs that occurred due to presenteeism.37
Intervention Costs
Intervention costs were estimated to be €200 based on the following subcosts. The costs of providing the training, website hosting, maintenance, and technical support per person were obtained by interviewing health care providers about a potential market price for the intervention when implemented in occupational health care. These estimates ranged from €49 to €103. The more conservative estimation of €103 was used and added to the costs of the coaches, which were estimated from the average monthly salary of a full-time psychologist (€65 for 3 h41). This estimate was based on the completion of all six modules by the participants, with the coaches investing 30 minutes per one feedback per person, resulting in 6 × 30 min per participant. Although not all participants complete all intervention modules in Internet-based guided self-help interventions and thus true costs may differ between participants when implementing the intervention in practice, we used the highest possible costs, namely 3 h of coaching per participant. Also, a value-added tax (VAT) of 19% of €168 was added (€30). It was assumed that all participants did the intervention at home, not at work. Therefore, opportunity costs due to the time employees spent working on the intervention were not included.
To summarize, this leads to a total approximate estimate of €200 ($245) that an employer would need to pay per participant for this intervention.
Analytic Plan
Means of presenteeism and absenteeism costs are reported in order to conclude if presenteeism accounts for the larger sum as expected. In order to provide the probability for a positive financial return the employer could expect, cost-benefit analyses were conducted comparing the costs the employer would have to pay (intervention costs) with the benefits he gets (reduced presenteeism and absenteeism). In order to gather evidence on the probability for cost-effectiveness of Internet-based CBT for insomnia at different willingness-to-pay ceilings, we conducted cost-effectiveness analyses relating all costs (intervention, presenteeism, and absenteeism) to the clinical outcome of a significant improvement of insomnia symptoms at 6-mo follow-up.
Statistical Analysis
The current health economic evaluation was conducted alongside a RCT that focused on the efficacy of this intervention and powered accordingly. A sample size of N = 128 was needed to detect a mean clinical difference between the IG and CG of d = 0.50 or larger in the primary outcome (ISI) at posttest. Cost data are usually heavily skewed to the right with large variance requiring very large sample sizes. Therefore, due to ethical and feasibility reasons, the study was not powered to statistically test differences in health economic outcomes. As a result, as it is common in health economic evaluations alongside clinical trials, a probabilistic decision-making approach for making health-economic inferences is used.42 This procedure takes the uncertainty about all measured parameters into account43,44 and aims at informing decision makers on probabilities rather than statistical significance.
Analysis of Costs
Costs were assessed at baseline (T1) and 6-mo follow-up (T3). Missing cost data (monthly wage, weekly working hours, presenteeism days, absenteeism days) at T3 were imputed using a Markov Chain Monte Carlo multivariate imputation algorithm (missing data module in IBM SPSS 20) with 100 estimations per missing value.45 Baseline costs of presenteeism and absenteeism, wage, working hours, age, and sex were used as predictors. At the 6-mo follow-up, participants were asked to only report on the previous 3 mo. Therefore, we had to estimate cumulative costs for each participant during the 6-mo follow-up period. This was done by calculating the area under the curve (AUC) of linearly interpolated 1-mo costs46:
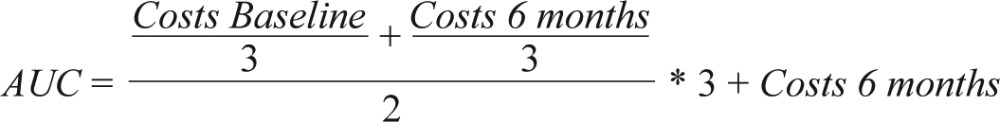 |
Subsequently, we added the respective intervention costs to the IG.
Cost-Benefit Analyses
In the present study, it was assumed that the employer would be the one who pays for all the included costs and the one who benefits from all the savings. Three metrics of cost-benefit analyses were used to report on the comparison between costs and benefits for the employer.19 Benefits in this case indicate the productivity benefits due to reduced presenteeism and absenteeism and can be derived by subtracting absenteeism and presenteeism costs from the CG from those from the IG. Costs are the intervention costs that the employer would have to pay for the online training per employee (estimated at €200). The most frequently used measures of cost-benefit relations are: (1) the net benefits (NB = benefits – costs), the money gained after costs are recovered; (2) the benefit-cost ratio (BCR = benefits / costs), the money returned for one monetary unit invested; and (3) the ROI (ROI = [benefits – costs] / costs × 100), the percentage of profit per monetary unit invested.19 All three measures are reported.
To quantify statistical uncertainty, bootstrapped 95% CIs19,47 were estimated around these measures, with 2,500 replications. Positive financial returns are indicated by the following criteria: NB > 0, BCR > 1, and ROI > 0%.19,48 In addition to this, the probability of financial return was estimated by reporting the proportion of positive bootstrapped financial return estimates.
Analysis of Cost-Effectiveness
All analyses were based on the intention-to-treat principle. All 128 participants completed the ISI at baseline. Missing data from the ISI at follow-up (7.2%) were imputed using a Markov Chain Monte Carlo multivariate imputation algorithm (missing data module in IBM SPSS 20) with 100 estimations per missing value.45
For the cost-effectiveness analyses, costs (presenteeism + absenteeism + intervention costs) and effects (number of participants with treatment response) were calculated for the 6-mo period and then costs and outcomes were related to each other. The incremental cost-effectiveness ratio (ICER) was calculated according to the following formula:
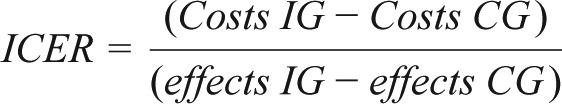 |
To adjust the analyses of cost and effect differences for potential confounders (e.g., baseline depression, age, sex, and marital status), seemingly unrelated regression was used. Baseline depression and marital status were shown to be confounders of the outcome. Since cost data are usually skewed to the right,42 the nonparametric bias-corrected and accelerated bootstrapping method with 2,500 replications was used to handle uncertainty in the ICER. Bootstrap analyses were done using STATA. In ICERs, negative ratios generally can mean reduced costs and positive effects but can also indicate increased costs and negative effects. Therefore, figures are used (the so-called cost-effectiveness planes) in which incremental effects (difference between both groups) are displayed on the x-axis, and incremental costs (difference between both groups) are plotted on the y-axis. If a cost/effect pair (ICER) is located in the northeast quadrant (NE-Q), the intervention is more effective but also more costly as compared to the control condition. In the southeast quadrant (SE-Q) the intervention is estimated to be associated with lower costs, while simultaneously achieving better health effects than the control condition (best possible outcome). ICERs in the northwest quadrant (NW-Q) indicate that the intervention is associated with higher costs and worse outcomes than the control condition. Finally, ICERs in the southwest Quadrant (SW-Q) point to an intervention being less effective but also less costly than the control condition. In the case of the SW-Q and the NE-Q, the amount of money a decision maker is willing to pay for one additional positive outcome is crucial for whether a new intervention is adopted.
The willingness to pay is displayed in the cost-effectiveness acceptability curve, which demonstrates the probability that the intervention is cost-effective compared to treatment as usual-only, given varying willingness to pay ceilings for the intervention by potential decision makers, in this case employers.
Sensitivity Analyses
Two sensitivity analyses of intervention costs were conducted in order to assess the robustness of the findings. In the main analysis, we used intervention costs of €200. However, there exists uncertainty concerning these costs, as prices may differ once the intervention is integrated into occupational health care. Therefore, all analyses were repeated assuming two additional conditions of 50% (€100) and 150% (€300) intervention costs.
RESULTS
Baseline Characteristics
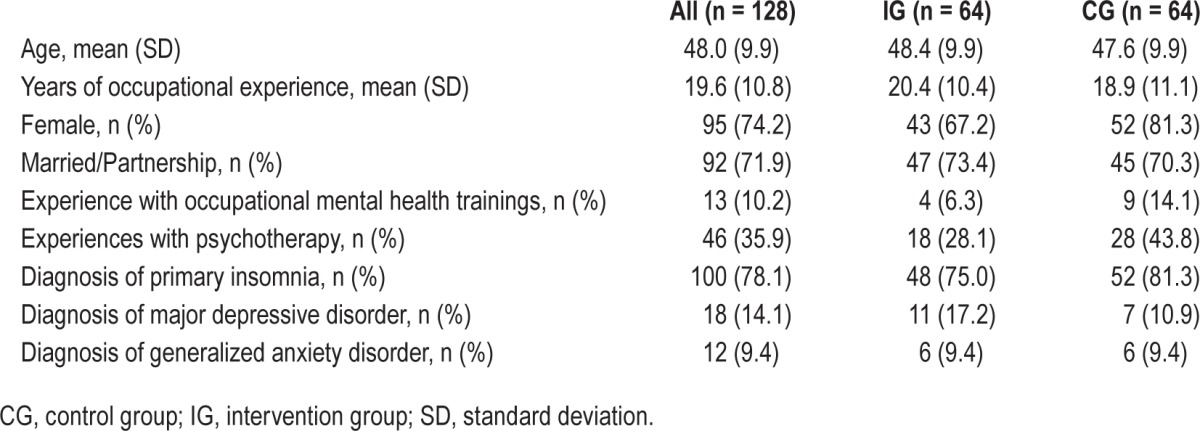
Table 1 summarizes baseline characteristics; details are described in a previous article.32
Table 1.
Demographic characteristics: means/counts, standard deviations/percentages at baseline.
Study Dropouts
Baseline data were available for all participants. The study attrition rate was low; 7.2% did not fill out the 6-mo follow-up questionnaires (n = 5 [7.8%] for the IG and n = 10 [15.6%] in the CG).
Intervention Use and Costs
All of the participants (n = 64) in the IG completed the first three out of six modules of the intervention. Two subjects dropped out after the third module, and one dropped out after the fourth module. Reasons reported were “lack of motivation” and “lack of time.” Overall, 61 participants (95.3%) completed all six sessions.
In Table 2, the mean presenteeism, absenteeism, and intervention costs are presented for the 6-mo follow-up assessment. Total costs are reported for both groups and for the differences between these groups. As Table 2 shows, presenteeism costs accounted for the highest costs in both groups.
Table 2.
Hourly wage, absenteeism, presenteeism, and related costs (in euros) categorized by condition at 6-mo follow-up.
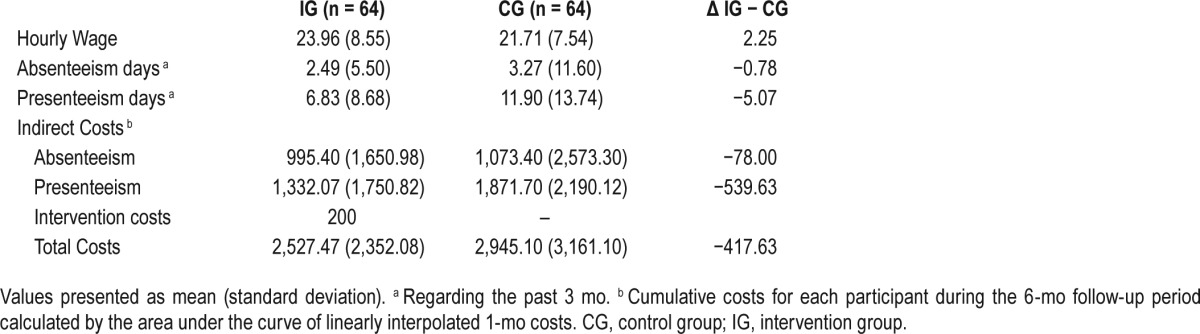
The mean difference in indirect costs at the 6-mo follow-up was €618 per person in favor of the intervention group ([Presenteeism CG + Absenteeism CG] – [Presenteeism IG + Absenteeism IG]). In the intervention group, each participant produced costs of €200 (intervention costs), resulting in average savings in the first 6 mo of €418 ($512) in comparison to the control group.
Cost-Benefit Analyses
Cost-benefit analyses and related sensitivity analyses are displayed in Table 3.
Table 3.
Cost-benefit analyses (main and sensitivity).
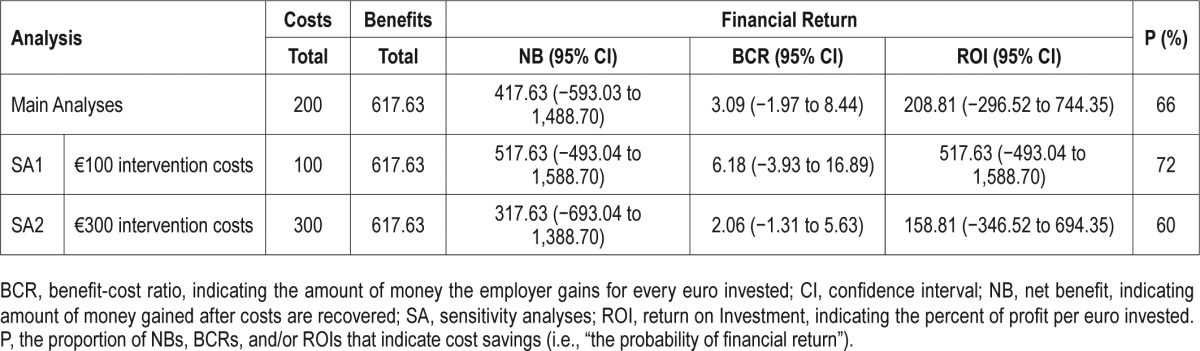
With intervention costs at €200 ($245) and per-person costs at €2,527 ($2,091) in the IG and €2,945 ($3,602) in the CG, the net benefit was €418 ($512) per participant at 6 mo, the benefit cost ratio (BCR [benefit/costs]) was 3.1 (95% CI: −1.97 to 8.44). This indicates that the employer gains €3.1 ($3.7) for every euro (US dollar) invested. This results in an ROI ([benefits – costs] / [costs × 100]) of 208.81% (95% CI: −296.51 to 744.35). Since NB > 0, BCR > 1, and ROI > 0%, financial returns are considered positive. However, CIs are wide and contain 0; thus, cost-benefit lacks statistical significance at a 95% level. The probability of a positive financial return is 66%.
Cost-Effectiveness Analysis
On average, the IG improved by 9.3 (SD = 5.0) points on the ISI, and the CG improved by 2.6 (SD = 4.4) points. There were more participants in the IG (51 [79.7%]) with reliable improvement at posttreatment in insomnia severity than in the CG (18 [28.1%]). In the IG, 27 participants were symptom-free by the 6-mo follow-up as indicated by a score of < 8 in the ISI.41 In the CG, 4 participants were symptom-free. Thus, 42.2% of participants were responders in the IG and 6.3% in the CG.
Cost-effectiveness analyses are displayed in Table 4. The ICER refers to the difference of mean indirect costs between the two groups divided by the difference in positive effects i.e., reliable ISI improvement and symptom-free subjects (C1 − C0) / (E1 − E0). It is shown that (1) the improvement in reliable ISI was greater in the IG than the CG and resulted in increased positive treatment responses (42.2% versus 6.3%), and (2) the intervention group had less indirect cost (€2,527 versus €2,945). The unadjusted point estimate of the ICER resulted in savings of −€1,162 (95% CI: −4,041 to 1,690) (−$1,421) for every participant with a positive treatment response after 6 mo. Using the 2,500 bootstrapped replicates of the ICER that were adjusted for baseline depression and marital status, the ICER was estimated at −€1,512 (95% CI: −4,493 to 1,128) (−$1,849). Figure 1 shows the cost-effectiveness plane, where each dot (n = 2,500) stands for one bootstrap replication of the ICER. The majority of the dots fall in the SE-Q, indicating an 87% probability that the GET.ON Recovery intervention produces greater health effects at lower costs compared to the control condition.
Table 4.
Cost-effectiveness analyses.
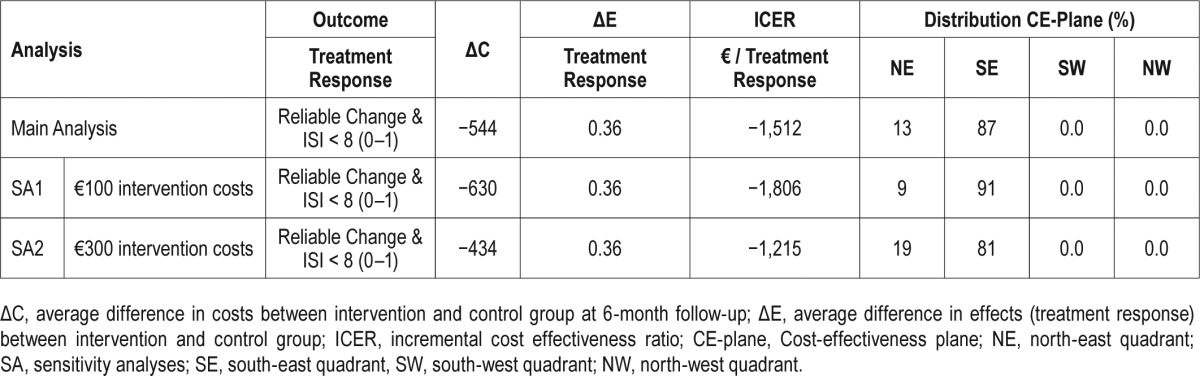
Figure 1.
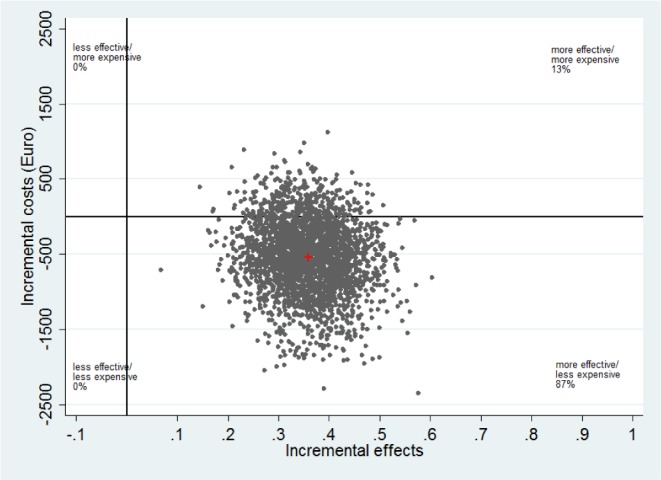
Scatterplot showing the mean differences in costs and effect outcome (positive treatment response) data using 2,500 bootstrap replications.
The cost-effectiveness acceptability curve in Figure 2 shows that if the employer is willing to pay zero for one positive treatment response, there is an 87% chance that GET.ON Recovery is more cost-effective than treatment as usual alone. The 95% probability threshold is reached at €761 ($931) for one positive treatment response.
Figure 2.
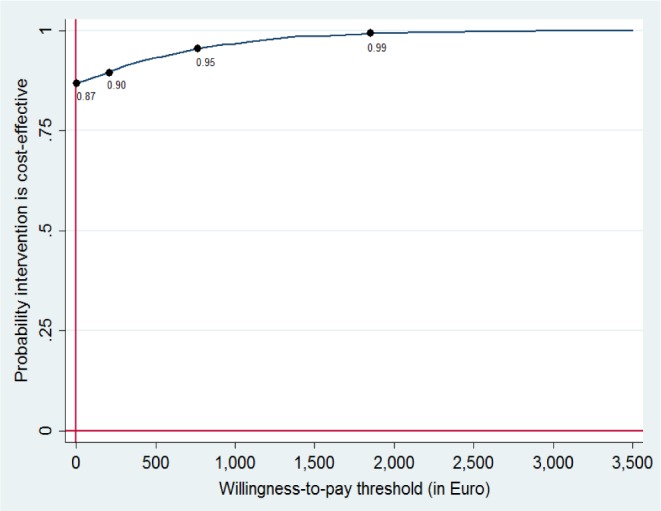
Cost-effectiveness acceptability curve for one positive treatment response.
Sensitivity Analyses
As Tables 3 and 4 show, sensitivity analyses led to similar results. With lower treatment costs (SA1), a positive financial return became more likely in the cost-benefit analyses (72%) and the ICER increased to −€1,806 ($2,209) per additional treatment response. Even with increased intervention costs of €300, the intervention has an 81% probability that it produces greater health effects at lower costs compared to the control condition with the 95% probability threshold reached at a willingness-topay of €1,115 ($1,364) per treatment response.
DISCUSSION
This economic evaluation performed both cost-effectiveness and cost-benefit analyses on Internet-based CBT-I compared to treatment as usual alone in employees with insomnia and work-related rumination from an employer's perspective. As expected, presenteeism was the major cost driver and costs due to presenteeism and absenteeism decreased relatively more in the intervention group as compared to the control condition during the 6-mo follow-up period.
The cost-benefit analyses showed a positive financial return with a net benefit of €418 ($512) and a benefit-cost ratio of 3.09 (95% CI: −1.97 to 8.44). This indicates that the employer would get €3.1 ($3.7) back for every €1 invested. However, CIs were wide and included zero; therefore, our results lacked statistical significance on a 95% level. We only found a moderate probability of 66% for a positive financial return.
With regard to the cost-benefit analyses, this work is the first to investigate CBT for insomnia. However, our results are comparable to Internet-based occupational health for depression (P = 63%)21 and score above the average ROI (−0.22 (SD = 2.41) found for RCTs (n = 12) in a large review20 (2014, n = 51 studies) on the cost-benefit of general workplace health promotion.
With regard to the cost-effectiveness analyses, the probability of the intervention being cost-effective was 87% at a potential willingness-to-pay of zero. If a payer is willing to pay €243, €761, or €1,794 for each treatment response, the probability increases to 90%, 95%, and 99% respectively. Sensitivity analyses confirmed the robustness of these results. Even with intervention costs of €300, the probability of cost-effectiveness is > 80%.
A probability of 87% at a willingness to pay of zero can be generally perceived as a good result because it exceeds the 50% probability cutoff and compares favorably to economic evaluations of CBT-I: Bonin et al.6 found the intervention to have a probability of only 7% of being cost effective. Watanabe et al.7 also reported a probability of less than 10% for a willingness to pay of zero for one additional QALY. A potential explanation for our relatively good results may be that our study focused on non-medical indirect costs, which are considered the major cost-drivers, whereas Bonin et al.6 and Watanabe et al.7 only focused on direct health care costs from a health service perspective. Other explanations include differences with regard to the intervention and the costs associated with it, as well as different outcome measures and another study population. For example, Bonin et al.6 evaluated brief community workshops for the general population (universal prevention) while the present study evaluated Internet-based CBT-I for employees with elevated symptom severity (indicated prevention). Despite these differences, both studies concluded that CBT-I is likely to be cost-effective given the anticipated costs decision makers would be willing to pay.
Since the present Internet-based CBT-I intervention was targeted at employees, its cost-effectiveness may also generally be compared to that found in occupational health interventions for other conditions such as depression.21 Geraedts et al. found a 95% probability of their Internet-based occupational health intervention for depressed employees to be cost-effective at a willingness-to-pay of around three times higher (€3,500) than the one found in this study (€761); therefore, they do not recommend their intervention to employers in terms of cost savings.
Comparisons with the afore-mentioned studies should generally be interpreted with caution as our evaluation differs with regard to target group, methodology, perspective, and/ or setting. Moreover, the willingness-to-pay for occupational health interventions and the acceptability of a certain cost-effectiveness probability threshold varies among payers19; therefore, cost-effectiveness needs to be judged by individual payers and cannot be obtained using the presented cost-acceptability curve or comparisons to other studies.43,44
Limitations of our study include the lack of a longer follow-up period. Although six months is a longer period than in all previous studies on the cost-effectiveness of CBT-I, simulation of costs developing over a much longer period of time may be more useful to show employers the potential of costs to be reduced. With a longer time horizon (i.e., 12 or 24 mo) benefits might accumulate. Thus, our results have a chance of being too conservative, also given its focus on only presenteeism and absenteeism. Further work-related costs were not included. Given that insomnia is associated with 7.2% of all costly workplace accidents and errors and 23.7% of all the costs of these incidents,49 the inclusion of costs other than presenteeism and absenteeism may lead to higher overall costs and a higher potential for cost savings. Limitations further include the fact that the sample size may have been too small to detect statistical significance with appropriate power in the cost-benefit analyses. In economic evaluations that are conducted parallel to a clinical trial, power issues constitute a problem because cost variables often have a higher variance and generally require greater sample sizes than clinical evaluations.44 Hence, a probabilistic decision making approach was used.43,44 In addition, the current evaluation was done from the employer's perspective; further studies should also include calculations of cost-utility from the societal perspective. Another limitation is that participants in this trial were predominantly female teachers, which may limit the generalizability for male teachers. Furthermore, our results may only be generalized to professions with similar characteristics, such as flexible working hours, loose boundaries between work and private life, and work-home interference.50
Internet-based interventions for mental disorders have often been introduced as potential cost-saving alternatives to face-to-face individual or group therapy, but evidence for this assumption needs to be extended.51 Our study helps shed light into this by being the first to show the potential cost-effectiveness of an Internet-based CBT-I intervention from the employer's perspective.
This evaluation shows two main points regarding sleep improvement through Internet-based CBT-I. First, this intervention may potentially be a cost-effective strategy in occupational health care, and second, the cost-savings due to reduced presenteeism at work outweigh those of absenteeism. This is important information for employers willing to primarily target a reduction in sick leave by this kind of intervention. The results of this economic evaluation justify replication of its results preferably with larger sample sizes with sufficient statistical power and as well with professionals other than teachers targeted over longer periods of time. The results of these studies could inform employers about the benefits of CBT-I based occupational health interventions for their employees.
DISCLOSURE STATEMENT
This was not an industry supported study. The European Union funded this study (EU EFRE: ZW6-80119999, CCI 2007DE161PR001). Hanne Thiart, David Daniel Ebert, Dirk Lehr, Stephanie Nobis, and Matthias Berking are stakeholders of the “Institute for Online Health Trainings” which aims to transfer scientific knowledge related to the current research into routine health care. The other authors have indicated no financial conflicts of interest. The current study was performed at Leuphana University of Lueneburg. Hanne Thiart had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
ACKNOWLEDGMENTS
Author contributions: Matthias Berking, David Daniel Ebert, and Dirk Lehr obtained the funding for this study; all authors contributed to the design of the study; Hanne Thiart performed the outcome analyses, led the writing efforts for the first draft of the manuscript, and integrated co-author comments and edits; Stephanie Nobis helped with the analyses; Hanne Thiart, Heleen Riper, Dirk Lehr, Stephanie Nobis, Filip Smit, and David Daniel Ebert supervised the writing process; and all authors contributed to further writing of the manuscript and approved the final manuscript.
ABBREVIATIONS
- AUC
area under the curve
- BCR
benefit cost ratio
- CBT-I
cognitive behavioral therapy for insomnia
- CG
control group
- CHEERS
Consolidated Health Economic Evaluation Reporting Standard
- DRKS
Deutsches Register Klinischer Studien [German Clinical Trial Register]
- ICER
incremental cost effect ratio
- IG
intervention group
- ISI
Insomnia Severity Index
- ITT
intention to treat
- NB
net benefit
- NE-Q
north-east quadrant
- NW-Q
north-west quadrant
- PPP
Purchasing Power Parities
- QALY
quality adjusted life years
- RCI
Reliable Change Index
- ROI
return on investment
- SE-Q
south-east quadrant
- SW-W
south-west quadrant
- TAU
treatment-as-usual
- TiC-P
Trimbos/iMTA questionnaire for costs associated with psychiatric illness
REFERENCES
- 1.Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151:1172–80. doi: 10.1176/ajp.151.8.1172. [DOI] [PubMed] [Google Scholar]
- 2.Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:191–204. doi: 10.7326/M14-2841. [DOI] [PubMed] [Google Scholar]
- 3.Geiger-Brown JM, Rogers VE, Liu W, Ludeman EM, Downton KD, Diaz-Abad M. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2014;23C:54–67. doi: 10.1016/j.smrv.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Botteman M. Health economics of insomnia therapy: implications for policy. Sleep Med. 2009;10:22–5. doi: 10.1016/j.sleep.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Wickwire EM, Shaya FT, Scharf SM. Health economics of insomnia treatments: the return on investment for a good night's sleep. Sleep Med Rev. 2016;30:72–82. doi: 10.1016/j.smrv.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Bonin EM, Beecham J, Swift N, Raikundalia S, Brown JS. Psycho-educational CBT-Insomnia workshops in the community. A cost-effectiveness analysis alongside a randomised controlled trial. Behav Res Ther. 2014;55:40–7. doi: 10.1016/j.brat.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe N, Furukawa TA, Shimodera S, et al. Cost-effectiveness of cognitive behavioral therapy for insomnia comorbid with depression: analysis of a randomized controlled trial. Psychiatry Clin Neurosci. 2014;69:335–43. doi: 10.1111/pcn.12237. [DOI] [PubMed] [Google Scholar]
- 8.van der Zweerde T, Lancee J, Slottje P, et al. Cost-effectiveness of i-Sleep, a guided online CBT intervention, for patients with insomnia in general practice: protocol of a pragmatic randomized controlled trial. BMC Psychiatry. 2016;16:85. doi: 10.1186/s12888-016-0783-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng SK, Dizon J. Computerised cognitive behavioural therapy for insomnia: a systematic review and meta-analysis. Psychother Psychosom. 2012;81:206–16. doi: 10.1159/000335379. [DOI] [PubMed] [Google Scholar]
- 10.Ho FY, Chung KF, Yeung WF, et al. Self-help cognitive-behavioral therapy for insomnia: a meta-analysis of randomized controlled trials. Sleep Med Rev. 2015;19:17–28. doi: 10.1016/j.smrv.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Lancee J, van Straten A, Morina N, Kaldo V, Kamphuis JH. Guided online or face-to-face cognitive behavioral treatment for insomnia? A randomized wait-list controlled trial. Sleep. 2016;39:183–91. doi: 10.5665/sleep.5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zachariae R, Lyby MS, Ritterband LM, O'Toole MS. Efficacy of internet-delivered cognitive-behavioral therapy for insomnia - a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2016;30:1–10. doi: 10.1016/j.smrv.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Mack LJ, Rybarczyk BD. Behavioral treatment of insomnia: a proposal for a stepped-care approach to promote public health. Nat Sci Sleep. 2011;26(3):87–99. doi: 10.2147/NSS.S12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godet-Cayré V, Pelletier-Fleury N, Le Vaillant M, et al. Insomnia and absenteeism at work. Who pays the cost? Sleep. 2006;29:179–84. doi: 10.1093/sleep/29.2.179. [DOI] [PubMed] [Google Scholar]
- 15.Daley M, Morin CM, LeBlanc M, Grégoire JP, Savard J. The economic burden of insomnia: direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep. 2009;32:55–64. [PMC free article] [PubMed] [Google Scholar]
- 16.Kessler RC, Berglund PA, Coulouvrat C, et al. Insomnia and the performance of US workers: results from the America insomnia survey. Sleep. 2011;34:1161–71. doi: 10.5665/SLEEP.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sivertsen B, Lallukka T, Salo P. The economic burden of insomnia at the workplace. An opportunity and time for intervention? Sleep. 2011;34:1151–2. doi: 10.5665/SLEEP.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Donnell MP. What is the ROI for workplace health promotion? It really does depend, and that's the point. Am J Health Promot. 2015;29:5–7. doi: 10.4278/ajhp.29.3.v. [DOI] [PubMed] [Google Scholar]
- 19.van Dongen JM, van Wier MF, Tompa E, et al. Trial-based economic evaluations in occupational health: principles, methods, and recommendations. J Occup Environ Med. 2014;56:563–72. doi: 10.1097/JOM.0000000000000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baxter S, Sanderson K, Venn AJ, et al. The relationship between return on investment and quality of study methodology in workplace health promotion programs. Am J Health Promot. 2014;28:347–63. doi: 10.4278/ajhp.130731-LIT-395. [DOI] [PubMed] [Google Scholar]
- 21.Geraedts AS, van Dongen JM, Kleiboer AM, et al. Economic evaluation of a Web-based guided self-help intervention for employees with depressive symptoms: results of a randomized controlled trial. J Occup Environ Med. 2015;57:666. doi: 10.1097/JOM.0000000000000423. [DOI] [PubMed] [Google Scholar]
- 22.Thiart H, Lehr D, Ebert DD, Sieland B, Berking M, Riper H. Log in and breathe out: efficacy and cost-effectiveness of an online sleep training for teachers affected by work-related strain - study protocol for a randomized controlled trial. Trials. 2013;14:169. doi: 10.1186/1745-6215-14-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thiart H, Lehr D, Ebert DD, Berking M, Riper H. Log in and breathe out: Internet-based recovery training for sleepless employees with work-related strain -results of a randomized controlled trial. Scand J Work Environ Health. 2015;41:164–74. doi: 10.5271/sjweh.3478. [DOI] [PubMed] [Google Scholar]
- 24.Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—Explanation and Elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16:231–50. doi: 10.1016/j.jval.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Hautzinger M, Keller F, Kühner C. Frankfurt am Main: Harcourt Test Services; 2006. BDI-II. Beck Depressions-Inventar Revision [Beck Depression Inventory Revision] [Google Scholar]
- 26.Siebern AT, Manber R. Insomnia and its effective non-pharmacologic treatment. Med Clin North Am. 2010;94:581–91. doi: 10.1016/j.mcna.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Cuijpers P, van Straten A, Warmerdam L. Behavioral activation treatments of depression: a meta-analysis. Clin Psychol Rev. 2007;27:318–26. doi: 10.1016/j.cpr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Wells A. New York, NY: Guilford Press; 2009. Metacognitive therapy for anxiety and depression. [Google Scholar]
- 29.Emmons RA, McCullough ME. Counting blessings versus burdens: an experimental investigation of gratitude and subjective well-being in daily life. J Pers Soc Psychol. 2003;84:377–89. doi: 10.1037//0022-3514.84.2.377. [DOI] [PubMed] [Google Scholar]
- 30.Kreiner GE, Hollensbe EC, Sheep ML. Balancing borders and bridges: negotiating the work-home interface via boundary work tactics. Acad Manage J. 2009;52:704–30. [Google Scholar]
- 31.Ebert DD, Berking M, Thiart H, et al. Restoring depleted resources: efficacy and mechanisms of change of an internet-based unguided recovery training for better sleep and psychological detachment from work. Health Psychol. 2015;34:1240–51. doi: 10.1037/hea0000277. [DOI] [PubMed] [Google Scholar]
- 32.Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–8. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobson N, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research”. J Condult Clin Psychol. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 34.Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 35.Thorndike FP, Ritterband LM, Saylor DK, Magee JC, Gonder-Frederick LA, Morin CM. Validation of the insomnia severity index as a web-based measure. Behav Sleep Med. 2011;9:216–23. doi: 10.1080/15402002.2011.606766. [DOI] [PubMed] [Google Scholar]
- 36.OECD Purchasing Power Parities. [Accessed January 14, 2016]. Available at https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm#indicator-chart.
- 37.Hakkaart-van Roijen L. Rotterdam: Institute for MedicalTechnology Assessment; 2002. Manual Trimbos/iMTA questionnaire for costs associated with psychiatric illness (in Dutch) [Google Scholar]
- 38.Bouwmans C, De Jong K, Timman R, et al. Feasibility, reliability and validity of a questionnaire on healthcare consumption and productivity loss in patients with a psychiatric disorder (tic-p) BMC Health Serv Res. 2013;13:217. doi: 10.1186/1472-6963-13-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drummond MF, Sculpher MJ, Torrance GW, O'Brien BSG. Methods for the economic evaluation of health care programmes. 3rd ed. New York, NY: Oxford University Press; 2005. [Google Scholar]
- 40.Osterhaus JT, Gutterman DL, Plachetka JR. Healthcare resource and lost labor costs of migraine headache in the US. Pharmacoeconomics. 1992;2:67–76. doi: 10.2165/00019053-199202010-00008. [DOI] [PubMed] [Google Scholar]
- 41.Berufsverband deutscher Psychologinnen und Psychologen (o.J.): Durchschnittliche Jahresgehälter von Psychologen [average wages of psychologists] [Accessed April 30, 2015]. Available at http://www.bdpverband.org/beruf/gehalt.shtml.
- 42.van Hout BA, Al MJ, Gordon GS, Rutten FFH. Costs, effects and C/E-ratios alongside a clinical trial. Health Econ. 1994;3:309–19. doi: 10.1002/hec.4730030505. [DOI] [PubMed] [Google Scholar]
- 43.Briggs AH, Gray AM. Methods in health service research: handling uncertainty in economic evaluations of healthcare interventions. Br Med J. 1999;319:635–8. doi: 10.1136/bmj.319.7210.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Briggs A. Economic evaluation and clinical trials: size matters. The need for greater power in cost analyses poses an ethical dilemma. BMJ. 2000;321:1362–3. doi: 10.1136/bmj.321.7273.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7:147–77. [PubMed] [Google Scholar]
- 46.Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–5. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic evaluations in clinical trials. New York, NY: Oxford University Press; 2007. [Google Scholar]
- 48.Leigh JP. Expanding research on the economics of occupational health. Scand J Work Environ Health. 2006;32:1–4. doi: 10.5271/sjweh.969. [DOI] [PubMed] [Google Scholar]
- 49.Shahly V, Berglund PA, Coulouvrat C, et al. The associations of insomnia with costly workplace accidents and errors: results from the America Insomnia Survey. Arch Gen Psychiatry. 2012;69:1054–63. doi: 10.1001/archgenpsychiatry.2011.2188. [DOI] [PubMed] [Google Scholar]
- 50.Lehr D. Belastung und Beanspruchung im Lehrerberuf: Gesundheitliche Situation und Evidenz für Risikofaktoren [Stress and strain in teachers: Health situation and evidence for risk factors] In: Terhart E, Bennewitz H, Rothland M, editors. Handbuch der Forschung zum Lehrerberuf [Manual of research on the teaching profession] Münster: Waxmann; 2011. pp. 757–73. [Google Scholar]
- 51.Donker T, Blankers M, Hedman E, Ljótsson B, Petrie K, Christensen H. Economic evaluations of Internet interventions for mental health: a systematic review. Psychol Med. 2015;45:3357–76. doi: 10.1017/S0033291715001427. [DOI] [PubMed] [Google Scholar]


