Abstract
Study Objectives:
To examine the association between periodic limb movements in sleep (PLMS) and change in selected aspects of cognition in community-dwelling older men.
Methods:
We studied 2,636 older men without dementia who underwent in-home polysomnography with measurement of the periodic limb movement index (PLMI) and periodic limb movement arousal index (PLMAI) using piezoelectric sensors. Random-effects models and logistic regression were used to examine the association between PLMI, PLMAI, and 3- to 4-y change in cognition.
Results:
After multivariable adjustment, men with a high PLMI had greater decline on the Trail Making Test – Part B (P trend = 0.02); those with a PLMI ≥ 30 were 48% more likely (odds ratio = 1.48, 95% confidence interval = 1.05–2.07) to experience the development of significant cognitive impairment (≥ 1 SD above mean change). Further adjustment for sleep efficiency, nocturnal hypoxemia, or dopaminergic medication use and analysis among men without Parkinson disease (n = 2,607) showed similar findings. No significant association was found for PLMAI or for Modified Mini-Mental State Examination scores.
Conclusions:
Among older men without dementia, higher PLMS frequency was associated with greater decline in cognition, particularly in executive function.
Citation:
Leng Y, Blackwell T, Stone KL, Hoang TD, Redline S, Yaffe K. Periodic limb movements in sleep are associated with greater cognitive decline in older men without dementia. SLEEP 2016;39(10):1807–1810.
Keywords: cognition, cognitive decline, periodic limb movements, PLMS, polysomnography
Significance.
Periodic limb movements in sleep (PLMS) were independently associated with subsequent cognitive decline, particularly decline in executive function, in non-demented elderly men. This is the first study to report such an association, and requires confirmation by further studies and exploration of biological mechanisms. Identification of PLMS in the elderly might help to predict future cognitive decline.
INTRODUCTION
Periodic limb movements in sleep (PLMS) are recurring limb movements caused by repetitive muscular contractions in the legs during sleep. It has a reported prevalence of 45% among elderly adults,1 and has been associated with sleep fragmentation and risk of cardiovascular diseases in older men.2,3 The exact mechanism of PLMS is unclear, but impaired dopaminergic transmission is frequently suggested.4–6 Notably, a high prevalence of PLMS has been reported among patients with Parkinson disease (PD) and Lewy body dementia,5,6 further supporting a potentially close link between PLMS and dopaminergic hypoactivity. In addition, higher PLMS has been correlated with impaired executive function in the setting of PD.7 Meanwhile, no study has prospectively studied the relationship between PLMS and change in cognition in community-dwelling older adults. Examination of this relationship will help in the understanding of the effects of PLMS on cognition and, more importantly, contribute to the ongoing debate8 over the clinical significance of PLMS in the general population.
METHODS
We studied participants in an ancillary study to the Osteoporotic Fractures in Men Study (MrOS), the MrOS Sleep Study.9,10 Briefly, from 2000 to 2002, the MrOS baseline examination enrolled 5,994 community-dwelling men 65 y or older at six clinical centers in the United States. In order to participate, men needed to be able to walk without assistance and must not have had a bilateral hip replacement. Of these participants, 3,135 were recruited for a comprehensive sleep assessment between 2003 and 2005. Details of the progression of participants throughout the study have been described.11 After excluding men with significant cognitive impairment at the sleep visit (Modified Mini-Mental State examination (3MS) score < 80 or taking a medication for dementia), the current analysis included 2,636 men who had polysomnography data from Sleep Visit 1 and cognitive function measured at Sleep Visit 1 (2003–2005), Sleep Visit 2 (2005–2006), and Sleep Visit 3 (2007–2009). All men provided written informed consent, and the study was approved by the Institutional Review Board at each site.
In-home sleep studies were completed using unattended polysomography (Safiro, Compumedics, Inc., Melbourne, Australia), following a standardized protocol as described previously.2 Bilateral tibialis leg movements were measured with piezoelectric sensors. PLMS were scored to be consistent with American Academy of Sleep Medicine (AASM) guidelines active at the time of scoring.12 Individual leg movements were scored if there was a clear amplitude increase from baseline and the duration was no less and no more than 0.5 and 5.0 seconds, respectively. To be considered periodic, a minimum of four movements needed to occur in succession no less and no more than 5 and 90 sec apart, respectively. Leg movements following respiratory events were excluded unless part of a movement cluster ≥ 4 with ≥ 2 movements independent of respiratory events. The periodic limb movement index (PLMI) was the total number of periodic leg movements per hour of sleep. The periodic limb movements arousal index (PLMAI) was the number of movements associated with arousals within 3 sec of movement termination. Previously, our group have found high levels of agreement (r = 0.81) between piezoelectric sensors and scoring rules used in this study and electromyographic leg sensors using 2013 AASM scoring rules.13 Sleep efficiency was defined as the percent of time spent asleep from sleep onset to sleep offset. Nocturnal hypoxemia was defined as the percent of time during overnight sleep in which arterial oxygen saturation was below 90%.
Two tests of cognitive function were administered at clinic visits by trained staff: the Trail Making Test – Part B (Trails B) and the 3MS. The Trails B is a timed test of processing speed that measures attention, sequencing, visual scanning, and executive function.14 Participants are given 300 sec to complete the test, and higher scores represent worse cognitive function. The 3MS is a global measurement of cognition, with components for orientation, concentration, language, praxis, and immediate and delayed memory. Scores range from 0 to 100, with higher scores representing better cognitive function.15 Development of clinically significant cognitive decline was defined as having a change in test value ≥ 1 SD worse than the mean of the change value from the Sleep Visit 1 to Sleep Visit 3.16 In addition, all participants completed questionnaires at the time of the Sleep Visit 1, which included items about demographics, education, medical history, physical activity, smoking, and alcohol use. In-clinic measurements of medication use,17 physical activity,18 and body mass index were performed.
The PLMS parameters were categorized as: PLMI, < 5, 5 to < 30, ≥ 30; PLMAI, < 1, 1 to < 5, ≥ 5. Characteristics of participants were compared by categories of PLMS. Random-effects models, as used in similar analysis,11 were utilized to study the association between PLMS and changes in cognition from the baseline to the follow-up visits. Covariates (fixed effects) were selected for inclusion in a multivariable model by examining both the univariate association of the covariate and the PLMS parameters and the association to the changes in 3MS and Trails B in random-effects models. Models were first adjusted by age and site. Then those covariates associated to both a PLMS parameter and an outcome at P < 0.10 were kept in all multivariable models, which included race, education, physical activity level, history of diabetes mellitus, history of hypertension, and history of coronary heart disease. The continuous cognitive scores were transformed to meet model requirements (log transformation for Trails B, cube transformation for 3MS) and back-transformed for display of results. Logistic regression was used to examine the association of PLMS with clinically significant cognitive decline. Secondary analyses were performed, with further adjustment for sleep efficiency, nocturnal hypoxemia, and dopaminergic medication to determine if the associations were independent of these factors. Finally, given the potential link between PLMS and PD, we repeated the analyses among those without a history of PD at baseline. All significance levels reported were two-sided and all analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC).
RESULTS
Of the 2,636 participants, PLMI < 5 in 29.4% 5 to 30 in 25.9%, and ≥ 30 in 44.7% had (39.7%, 33.1%, and 27.2% had a PLMAI of < 1, 1 to 5 and ≥ 5, respectively). The participants were primarily Caucasian (92%) with an average age of 76 ± 5 y at the initial assessment. Those with higher PLMS frequency were older, more likely to be Caucasians, and had a history of coronary heart disease and lower sleep efficiency. The baseline mean Trails B score was 119.4, 112.9, and 116.9 sec, and the mean 3MS score was 93.6, 94.0, and 93.5 points, for men with a PLMI < 5, 5 to < 30, and ≥ 30, respectively. On average, the Trails B test time increased by 9.0 sec, and the 3MS score decreased by 1.2 points over a follow-up of 3.4 y. Detailed descriptive statistics are not presented in this short note, but characteristics of similar study samples can be found in previous publications.2,3,11
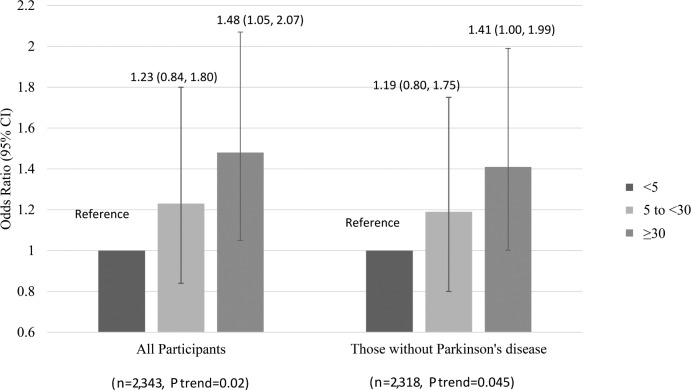
After multivariable adjustment, the rate of increase in Trails B test time (decline in function) was significantly greater for those with a PLMI ≥ 30, whereas the rate of decrease in 3MS scores was smaller, but not significant, for those with a PLMI ≥ 30. The adjusted increase in Trails B test completion time over 3.4 y were 3.5, 6.3, and 8.1 sec for PLMI category of < 5, 5 to 30, and ≥ 30, respectively (P for trend = 0.02); adjusted decline in 3MS score for PLMI categories were 1.3, 0.9, and 1.0 points over 3 y, respectively (P for trend = 0.23). PLMAI was not associated with either of the cognitive outcomes. Figure 1 shows multivariable-adjusted odds ratios (OR) for clinically significant decline in Trails B performance by PLMI categories. The number (%) of men with clinically significant decline in Trails B for those with a PLMI of < 5, 5 to < 30, and ≥ 30 were 59 (8.9%), 63 (10.6%), and 126 (12.8%), respectively. Those in the highest category of PLMI were 48% more likely to experience development of clinically significant cognitive impairment compared to those in the lowest category (OR = 1.48, 95% CI = 1.05–2.07). The associations remained after further adjustment for sleep efficiency (1.47, 1.05–2.06), nocturnal hypoxemia (1.47, 1.05–2.07), or dopaminergic use (1.48, 1.06–2.08). Similar associations were observed after excluding participants with PD (n = 29). Among these 29 PD patients, 17 (58.6%) were on dopaminergic use.
Figure 1.
Multivariable-adjusted* association of periodic limb movements index and clinically significant cognitive impairment as measured by the Trail Making Test – Part B (completion time increased by 58 sec), odds ratio, and 95% confidence interval (CI) (n = 2,343). *Models adjusted by age, site, race (white vs. nonwhite), education level, physical activity level, history of diabetes mellitus, history of hypertension, history of coronary heart disease.
DISCUSSION
Among 2,636 older men without dementia, increasing PLMS frequency was associated with declining cognitive function, particularly on executive function. The worsening on Trails B for men with a PLMI ≥ 30 more than doubled compared to those with a PLMI < 5. The association was independent of sleep efficiency, nocturnal hypoxemia, and dopaminergic use, and remained after excluding men with PD. To our knowledge, this is the first study to show an association between PLMS and cognitive decline in the general population.
Our finding on the association between high PLMI and declining executive function is complemented by prior research, which indicated a correlation between high PLMS and lower executive function in PD patients.7 Although PLMS has been studied mostly in the setting of PD and is frequently associated with dopamine deficiency,6,19 the association observed in the current study remained after adjusting for dopaminergic use and after exclusion of participants with a history of PD. Elevated PLMS could be an early marker of cognitive impairment even in older men free of PD or other apparent dopamine-related disorders. There are a few potential mechanisms. PLMS has been associated with increased sympathetic activity, elevated blood pressure and incident cardiovascular diseases,3,20 which all contribute to vascular-related cognitive impairment, as featured by impairment in executive function.21–23 Moreover, dopamine deficit, as a frequently suggested cause of PLMS,4 also plays a key role in neurodegeneration and affects particularly the prefrontal cortex area, which regulates executive function.24,25 Previous research suggests that executive function is a better predictor of functional decline and mortality in older women26; thus, the current finding on PLMS and decline in executive function might have important clinical implications. Finally, PLMS could be a marker of sleep fragmentation,2 and there is increasing recognition on the role of sleep disturbances in cognitive impairment.27Although previous studies have reported more sleep disruptions associated with PLMS with arousal,2,8 we did not find any association between PLMAI and cognition. The neuropathological effects of PLMS with and without an arousal should be further examined.
The study strengths are its prospective design, participants who were not selected based on PLMS or cognitive function, objective measure of sleep parameters, and consideration of a range of possible confounders. Limitations of the study include limited generalizability because the cohort involves primarily older Caucasian men. Use of piezoelectric sensors for movement detection is not the current standard for PLMI detection, although our methods correlate highly with published methods. In addition, although adjustment for multiple potential confounders did not attenuate the association, the possibility of residual confounding cannot be ruled out. We observed moderate cognitive decline over 3 y, whereas larger differences in the rate of cognitive decline might be observed with longer follow-up. Finally, we did not observe a similar pattern of change for 3MS scores, which could be due to chance, measurement properties, or because PLMS was indeed more closely related to decline in executive function.
In summary, this study shows for the first time that PLMS might be an independent predictor of cognitive decline, particularly decline in executive function, among community-dwelling elderly men. Further studies are needed to explore a link between PLMS and cognitive function and help understand biological mechanisms.
DISCLOSURE STATEMENT
This was not an industry supported study. The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. The National Heart, Lung, and Blood Institute (NHLBI) provides funding for the MrOS Sleep ancillary study “Outcomes of Sleep Disorders in Older Men” under the following grant numbers: R01 HL071194, R01 HL070848, R01 HL070847, R01 HL070842, R01 HL070841, R01 HL070837, R01 HL070838, and R01 HL070839. Dr Yaffe received funding from K24 grant AG031155. The funding sources had no role in the design and conduction of the study; collection, management, analysis, and interpretation of the data; the decision to submit the manuscript for publication; preparation or approval of the manuscript. Terri Blackwell, Dr. Yaffe, Dr. Redline, and Dr. Stone have received grant support from the NIH grant and supporting agencies as listed above. Dr. Redline's institution receives grant support from NIH and PCORU and has received grant funding from Jazz Pharmaceuticals. The other authors have indicated no financial conflicts of interest. Analysis was performed at California Pacific Medical Center, Research Institute.
ACKNOWLEDGMENTS
Investigators in the Outcomes of Sleep Disorders in Older Men study (MrOS Sleep):
Coordinating Center (California Pacific Medical Center Research Institute and University of California, San Francisco): K.L. Stone (Principal Investigator), D.C. Bauer (co-Investigator), S.R. Cummings (co-Investigator), N. Goldschlager (co-Investigator), P. Varosy (co-Investigator), K. Yaffe (co-Investigator), P.M. Cawthon (co-Investigator), R. Fullman (Project Director), R. Benard, T. Blackwell, L. Concepcion, J. Diehl, S. Ewing, C. Fox, M. Jaime-Chavez, E. Kwan, S. Litwack, W. Liu, L.Y. Lui, J. Schneider, R. Scott, D. Tanaka, J. Ziarno; Administrative Center (Oregon Health – Sciences University): E. Orwoll (Principal Investigator), K. Phipps (co-Investigator), L. Marshall (co-Investigator), J. Babich Blank (Project Director), L. Lambert, B. Chan, D. Neevel; University of Alabama, Birmingham: C.E. Lewis (Principal Investigator), J. Shikany (co-Investigator), P. Johnson (Project Director), C. Oden, S. House, N. Webb, K. Hardy, S. Felder, J. Wilkoff, J. King, T. Johnsey, M. Young, J. Smith, C. Sassaman, C. Collier, C. Atkins; University of Minnesota: K. Ensrud (Principal Investigator), H. Fink (co-Investigator), D. King (Program Manager), N. Michaels (Asst. Program Manager), N. Nelson (Clinic Coordinator), C. Bird, D. Blanks, F. Imker-Witte, K. Moen, M. Paudel, M. Slindee; Stanford University: M. Stefanick (Principal Investigator), A. Hoffman (co-Investigator), K. Kent, B. Malig, S. Wong; University of Pittsburgh: J. Cauley (Principal Investigator), J. Zmuda (co-Investigator), M. Danielson (Study Administrator), L. Harper (Project Director), L. Buck (Clinic Coordinator), M. Nasim, D. Cusick, M. Gorecki, N. Watson, C. Bashada, C. Newman; University of California, San Diego: E. Barrett-Connor (Principal Investigator), S. Ancoli-Israel (co-Investigator), T. Dam (co-Investigator), ML Carrion-Petersen (Project Director), P. Miller, N. Kamantigue; Case Western Reserve University/Brigham and Women's Hospital: S. Redline (Principal Investigator), S. Surovec (Project Administrator), N. Scott (Chief Polysomnologist), N. Johnson (Programmer Analyst), J. Arnold (Polysomnologist), R. Nawabit (Polysomnologist), J. Romaniuk (Polysomnologist), S. Seicean (Polysomnologist).
ABBREVIATIONS
- 3MS
Modified Mini-Mental State examination
- AASM
American Academy of Sleep Medicine
- BMI
Body Mass Index
- CI
Confidence Interval
- MrOS
The Osteoporotic Fractures in Men
- OR
Odds Ratio
- PD
Parkinson disease
- PLMI
Periodic Limb Movements Index
- PLMS
Periodic Limb Movements in sleep
- PSG
Polysomnography
- SD
Standard Deviation
- Trails B
Trail Making Test – Part B
REFERENCES
- 1.Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Periodic limb movements in sleep in community-dwelling elderly. Sleep. 1991;14:496–500. doi: 10.1093/sleep/14.6.496. [DOI] [PubMed] [Google Scholar]
- 2.Claman DM, Ewing SK, Redline S, et al. Periodic leg movements are associated with reduced sleep quality in older men: the MrOS Sleep Study. J Clin Sleep Med. 2013;9:1109–17. doi: 10.5664/jcsm.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koo BB, Blackwell T, Ancoli-Israel S, et al. Association of incident cardiovascular disease with periodic limb movements during sleep in older men: outcomes of sleep disorders in older men (MrOS) study. Circulation. 2011;124:1223–31. doi: 10.1161/CIRCULATIONAHA.111.038968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hening WA, Allen RP, Earley CJ, Picchietti DL, Silber MH. An update on the dopaminergic treatment of restless legs syndrome and periodic limb movement disorder. Sleep. 2004;27:560–83. doi: 10.1093/sleep/27.3.560. [DOI] [PubMed] [Google Scholar]
- 5.Hibi S, Yamaguchi Y, Umeda-Kameyama Y, et al. The high frequency of periodic limb movements in patients with Lewy body dementia. J Psychiatr Res. 2012;46:1590–4. doi: 10.1016/j.jpsychires.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Wetter TC, Collado-Seidel V, Pollmacher T, Yassouridis A, Trenkwalder C. Sleep and periodic leg movement patterns in drug-free patients with Parkinson's disease and multiple system atrophy. Sleep. 2000;23:361–7. [PubMed] [Google Scholar]
- 7.Scullin MK, Fairley JA, Trotti LM, Goldstein FC, Factor SA, Bliwise DL. Sleep correlates of trait executive function and memory in Parkinson's disease. J Parkinsons Dis. 2015;5:49–54. doi: 10.3233/JPD-140475. [DOI] [PubMed] [Google Scholar]
- 8.Hornyak M, Feige B, Riemann D, Voderholzer U. Periodic leg movements in sleep and periodic limb movement disorder: prevalence, clinical significance and treatment. Sleep Med Rev. 2006;10:169–77. doi: 10.1016/j.smrv.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26:557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Blackwell T, Yaffe K, Laffan A, et al. Associations between sleep-disordered breathing, nocturnal hypoxemia, and subsequent cognitive decline in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2015;63:453–61. doi: 10.1111/jgs.13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Association TATFotASD. Recording and scoring leg movements. Sleep. 1993;16:748–59. [PubMed] [Google Scholar]
- 13.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 14.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–6. [Google Scholar]
- 15.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–8. [PubMed] [Google Scholar]
- 16.Yaffe K, Lindquist K, Vittinghoff E, et al. The effect of maintaining cognition on risk of disability and death. J Am Geriatr Soc. 2010;58:889–94. doi: 10.1111/j.1532-5415.2010.02818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–11. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 18.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 19.Bliwise DL, Trotti LM, Yesavage JA, Rye DB. Periodic leg movements in sleep in elderly patients with Parkinsonism and Alzheimer's disease. Eur J Neurol. 2012;19:918–23. doi: 10.1111/j.1468-1331.2012.03673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koo BB, Sillau S, Dean DA, 2nd, Lutsey PL, Redline S. Periodic limb movements during sleep and prevalent hypertension in the multi-ethnic study of atherosclerosis. Hypertension. 2015;65:70–7. doi: 10.1161/HYPERTENSIONAHA.114.04193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vicario A, Martinez CD, Baretto D, Diaz Casale A, Nicolosi L. Hypertension and cognitive decline: impact on executive function. J Clin Hypertens. 2005;7:598–604. doi: 10.1111/j.1524-6175.2005.04498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishtala A, Preis SR, Beiser A, et al. Midlife cardiovascular risk impacts executive function: Framingham offspring study. Alzheimer Dis Assoc Disord. 2014;28:16–22. doi: 10.1097/WAD.0b013e3182a715bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Brien JT, Thomas A. Vascular dementia. Lancet. 2015;386:1698–706. doi: 10.1016/S0140-6736(15)00463-8. [DOI] [PubMed] [Google Scholar]
- 24.Dubois B, Pillon B. Cognitive deficits in Parkinson's disease. J Neurol. 1997;244:2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- 25.Puig MV, Rose J, Schmidt R, Freund N. Dopamine modulation of learning and memory in the prefrontal cortex: insights from studies in primates, rodents, and birds. Front Neural Circuits. 2014;8:93. doi: 10.3389/fncir.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson JK, Lui LY, Yaffe K. Executive function, more than global cognition, predicts functional decline and mortality in elderly women. J Gerontol A Biol Sci Med Sci. 2007;62:1134–41. doi: 10.1093/gerona/62.10.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13:1017–28. doi: 10.1016/S1474-4422(14)70172-3. [DOI] [PubMed] [Google Scholar]