Abstract
Study Objectives:
Narcolepsy with cataplexy (NC) is a chronic neurological disorder thought to result from an altered immune response based on a genetic predisposition coupled with environmental factors. Pandemrix vaccination has been reported to increase the risk of narcolepsy. We aimed at identifying other vaccines associated with the onset of narcolepsy.
Methods:
Case series and retrospective database study.
Results:
We identified four cases of NC following a tick-borne encephalitis (TBE) vaccination with FSME Immun. Additional four cases could be detected in the database of the Paul-Ehrlich-Institut, Federal Institute for Vaccines and Biomedicines in Germany.
Conclusions:
Our findings implicate TBE vaccination as a potential additional environmental factor for the development of NC and add additional evidence for an immunological mechanism in the pathogenesis of the disease.
Citation:
Hidalgo H, Kallweit U, Mathis J, Bassetti CL. Post tick-borne encephalitis virus vaccination narcolepsy with cataplexy. SLEEP 2016;39(10):1811–1814.
Keywords: narcolepsy, cataplexy, hypocretin, tick-borne encephalitis virus vaccination, granzyme, dendritic cells
Significance.
Here we describe for the first time tick born vaccination as potential triggering factor of narcolepsy-cataplexy.
INTRODUCTION
Narcolepsy with cataplexy (NC) is a chronic neurological sleep-wake disorder with a prevalence of 25–50 per 100,000. Symptom onset typically peaks during the second decade of life.
The etiology of NC is still incompletely understood, but increasing evidence underlines that NC may be an immune-mediated disorder affecting genetically predisposed individuals after exposure to environmental factors.1 This immune process eventually results in a selective loss of hypocretin (HCRT) cells in the posterior hypothalamus. Correspondingly, cerebro-spinal fluid (CSF) hypocretin-1 (hcrt-1) levels are low or below detection limit in ∼95% of NC patients. The strong association (> 98%) of NC with the HLA DQB1*0602 haplotype underlines the importance of the DQB1 locus for both genetic risk and protection in the development of NC.2
Different environmental factors have been suggested to be associated with the onset of NC. In early reports on the etiology of NC,3 associations with various infections have been reported at the onset of NC. Elevated anti-Streptococcal antibodies have been observed near disease onset, suggesting that streptococcal infections may act as a trigger for NC.4 Further, H1N1 virus infection in 2010 led to a major increase of NC incidence in China.5 The increase of NC incidence after H1N1 vaccination with Pandemrix in several European countries since 2009, particularly in children and young adults, was a key event and pointed to the vaccination as a possible environmental trigger for disease onset.6 Initially, the AS03 adjuvant in Pandemrix was supposed to be the pivotal trigger for post H1N1 NC.7 However, the ASO3 adjuvant has also been used in Arepanrix, another H1N1 vaccine, without any increase of NC rate.8 A higher amount of polymeric H1N1 virus nucleo-protein (NP) for Pandemrix, probably due to different purification process was detected in Pandemrix when compared to Arepanrix and may have led to higher levels of IgG-antibodies in children with predisposed susceptibility for developing post-H1N1-NC.8 There are no reports on other vaccines associated with the onset of NC.
Here we report four cases of NC onset shortly after tick-borne encephalitis (TBE) vaccine in Germany and Switzerland.
CASE REPORTS
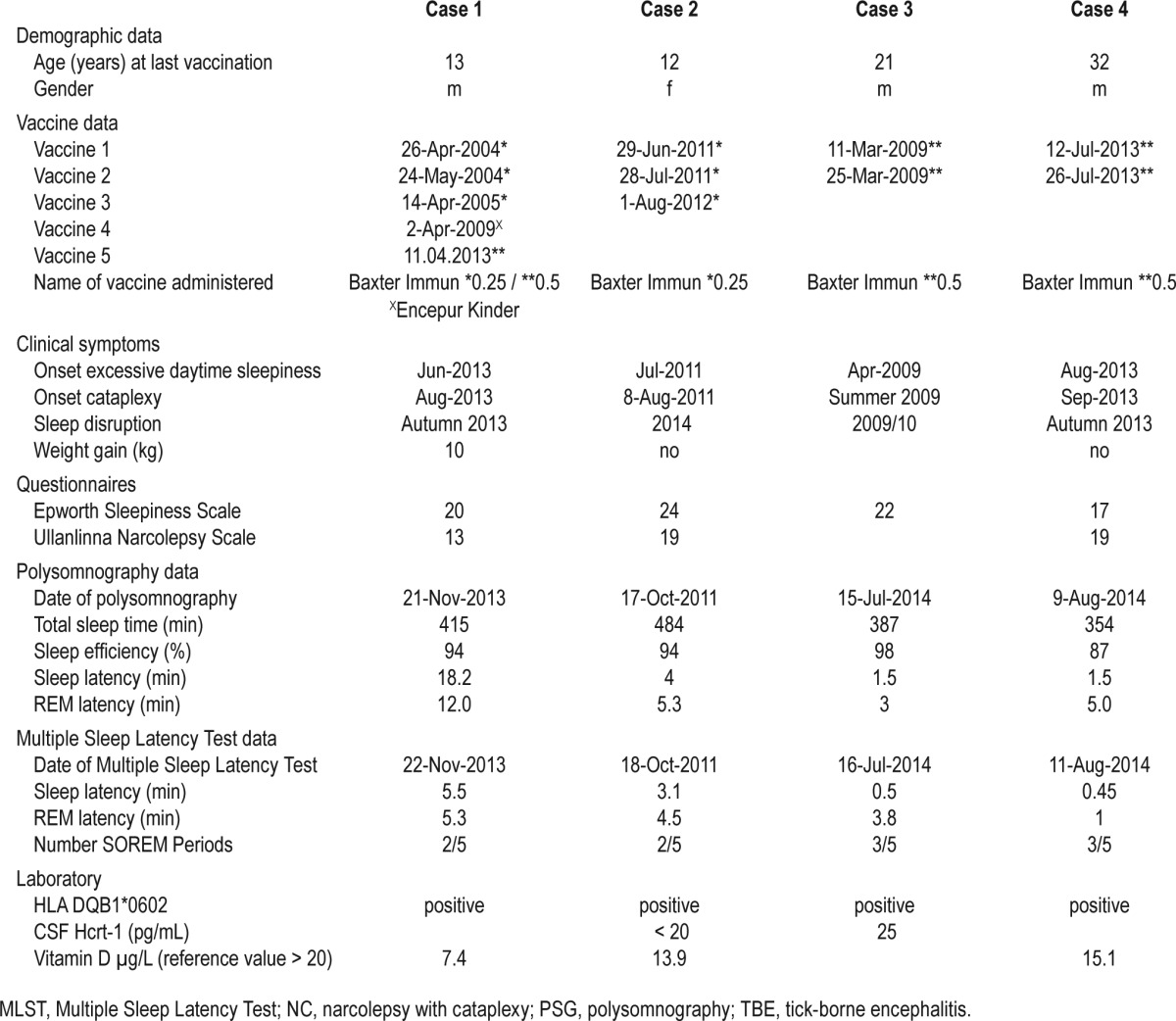
For further data please see Table 1.
Table 1.
Demographic, clinical, sleep laboratory (PSG, MSLT), laboratory (HLA, hypocretin) and vaccine data of four NC patients suffering from NC after TBE vaccination.
General
Clinical, neurological, and psychiatric examinations as well as brain MRI and EEG studies were without pathological findings in all cases. CSF examinations for measuring hypocretin levels were declined by patient 1 and 4.
Patient 1
A 13-year-old boy together with his heterozygotic twin brother and his 15-year-old sister, received on 11 April 2013 the second booster injection against TBE with FSME Immun 0.5 mL (Baxter Germany). This injection followed primary immunizations in 2004 and 2005 with FSME junior Baxter 0.25 mL and a first booster with Encepur Kinder in 2009.
In June 2013 he developed excessive daytime sleepiness (EDS) followed by the onset of cataplexy a few weeks later. He presented a weight gain of 10 kg in 6 months. He was diagnosed for narcolepsy with cataplexy on 20 November 2013 by clinical symptoms and Multiple Sleep Latency Test (MSLT). Laboratory data were unremarkable except an eosinophilia of 6.4% (normal range < 5.5%). Medical history revealed a history of frequent upper airway infections until the age of 2 years in contrast to his twin brother. Subsequently, he complained of disrupted night sleep and hypnagogic hallucinations and described increased perspiration at nighttime and further worsening of cataplexy. In March 2014, Epworth Sleepiness Scale (ESS) score was 20/24 points and Ullanlinna narcolepsy scale 13; laboratory findings revealed a vitamin D deficiency (7.4 ng/mL); ASL titer was negative. There was no family history of narcolepsy or daytime sleepiness. His HLA-DQB1*0602 positive siblings did not present any symptoms of narcolepsy.
Patient 2
A previously healthy 13-year-old girl with an unremarkable personal and family history of neurological or sleep disorders presented with EDS one month after her first TBE vaccine with FSME-Immun 0.25 junior (Baxter) in June 2011. Ten days after the second administration of FSME-Immun 0.25 junior, the first cataplexy occurred in July of 2011. She was diagnosed for NC in October 2011. She is HLA DQB1*0602 positive, and CSF hcrt-1 was below detection limit. Another booster immunization of FSME-Immun 0.25 junior was administered in 2012, but did not lead to any change of symptoms.
Patient 3
A 21-year-old healthy male without family history of NC or other neurologic disorders presented in 2009, one month after TBE-vaccinations on 11 March 2009 and 25 March 2009 with FSME Immun (Baxter)—according to a rapid immunization pattern—with EDS, rapidly followed by sleep paralysis, hypnagogic hallucinations, cataplexy, nightmares, and REM sleep behaviour disorder (RBD). Diagnosis of NC was made in July 2014. He is HLA DQB1 0602 positive; CSF hcrt-1 was 25 pg/mL.
Patient 4
A 32-year-old male reporting an increased sleep need since infancy and a family history of a “sleepy grandfather” presented with EDS and cataplexy of increasing number and severity a few (approximately 2) weeks after receiving TBE-vaccinations with FSME Immun adult (Baxter) on 12 July 2013 and 26 July 2013 following a rapid immunization schedule. Night sleep was disrupted with the patient spending often more than 3 hours awake. He is HLA DQB1 0602 positive. Diagnosis of NC was established in November 2014 based upon clinical symptoms and MSLT.
DISCUSSION
TBE vaccine is a recommended prophylaxis for pathogenic flavivirus tick-borne encephalitis virus infections in endemic regions of Germany, Switzerland, and Austria same as in other European countries and northern Asia.9 Vaccination coverage in endemic regions is rated at 31–37% (grade schoolers: 33– 44%) and 15% (12%) in non-risk regions for Germany and 34% for Switzerland, respectively.10,11 Vaccine is offered in Europe in two preparations: Encepur (Novartis) and FSME Immun (Baxter). Encepur Kinder is approved for children from 1–11 years (FSME Immun Junior: 1–15 years) and Encepur Erwachsene for adolescents and adults ≥ 12 years (FSME Immun Erwachsene: 16–60 years). Of such immunizations, FSME Immun has been prescribed in 60.6% (children: 64.12%) vs. Encepur in 39.4% (35.88%) of cases (data for Germany, mean from period 2011–2015).10 Key differences between vaccines are the presence of human albumin: only in FSME Immun, and in the strain and dose of utilized viruses (1.5 μg Karlsruhe 23 virus strain vs 2.4 μg Neudörfl strain). Both preparations contain formaldehyde inactivated antigens adsorbed to a similar dose of aluminium hydroxide (0.35 mg vs 0.3–0.4 mg). Preparations for children of both vaccines contain half of adult doses and are recommended for children under 12 or 16 years in FSME Immun, respectively.12,13
Conventional schedules for both preparations in Germany and Switzerland involve two injections with a 4-week interval between injections and a booster after one year, which is to be repeated after 3 years. The rapid schedule suggests application on day 0, 14, and 300.12,13 No information is available on the rate of conventional vs. rapid immunisations.
In addition to our 3 German cases, the serious adverse events (SAE) database of the Paul-Ehrlich-Institut-Federal Institute for Vaccines and Biomedicines in Germany, documents additional 4 cases of narcolepsy following FSME Immun vaccination.14 No case of narcolepsy following Encepur vaccine has been described so far. Due to data protection laws, no additional information on these cases is available.
Common features of all our cases is the use of Baxter FSME Immun vaccine and in three patients the deviation of the classic immunization schedule, including one patient receiving double of dose the recommended dose for his age (case 1), and two the accelerated schedule (cases 3, 4).
The time interval between vaccinations and onset of first symptoms is between one and six weeks (cases 2–4) and approximately 11 weeks in case 1 and hence similar to cases with post H1N1 vaccination NC.15 Our patients rapidly evolved a full-blown phenotype of narcolepsy, which also has been described frequently in post H1N1 NC.16
There is no known congruence of ingredients between Pandemrix and TBE vaccines. Aluminium hydroxide (AH) is a frequently used adjuvant in many vaccines. There are no reports of NC after vaccination with other vaccines using AH. Human albumin (HA) in FSME Immun is used as a component to reduce TNF-α, IL-1 β, IL-6, and IL-8 induced side effects.17 HA can potentially cause allergic reactions. In very rare cases, TBE vaccines were associated with the induction of autoimmune diseases such as Guillain Barré Syndrome or multiple sclerosis.18
No case of narcolepsy following TBE virus infection has previously been described.
A special feature of FSME Immun is that it has been found to suppress expression of granzyme B (GrB) by human plasmacytoid dendritic cells (pDC).19 Although the mechanism of this suppression is still unclear, this finding is not observed in other vaccines. Granzymes are serine proteases expressed by several types of immune cells and seem to have immunomodulatory functions such as suppression of T cell expansion at the site of an infection. Granzymes also potentially suppress auto-reactive T cells. Extracellular soluble granzymes are elevated in the circulation of patients with autoimmune diseases.20 The role of granzymes in the development of narcolepsy, however, remains speculative.
This case series is to our knowledge the first report of a potential association between TBE vaccination and NC. Due to coverage of TBE vaccination in only 15% to 30% of the population and the low incidence of narcolepsy, a limited number of NC cases associated with the vaccine can be expected among those presenting with new onset “sporadic” narcolepsy. However, it remains striking that the only reported cases of NC occurred following FSME Immun and not Encepur, even though both vaccines were used quite similarly.
Although there are no common features with the influenza vaccine Pandemrix, which has been shown to induce antibodies to influenza nucleoprotein that cross-react with hypo-cretin receptor 2,21 it is possible that a specific, not yet defined, component in the TBE FSME Immun vaccine may trigger in predisposed individuals, a cross-reactive immune response that may lead to NC. We hypothesize that a stronger immune reaction was induced in our patients by either deviation of dosage or rapid immunization. If true, it may also be possible that rather than specific vaccine ingredients, NC may be triggered in a bystander fashion as a consequence of an enhanced immune response. Collectively, our data adds additional evidence of potential immunological mechanisms in the patho-physiology of NC and calls for further evaluation of alternative cellular immune response mechanisms, possibly involving, e.g., DC and GrB.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Bassetti has consulted for Jazz, UCB Europe, Zambon, and Servier and has received research support from Swiss National Science Foundation, ResMed, Respironics, Vifor Pharma, UCB, Parkinson Schweiz, Tropos Stiftung, and Schweizerische Herzstiftung. Dr. Kallweit has consulted for UCB Germany and Biogen Germany.
ACKNOWLEDGMENTS
The authors thank Wiebke Hellenbrand, MD, Robert-Koch-Institute (RKI), Germany for information on TBE vaccines and epidemiological data and Markus Schmidt, MD, PhD, Bern, Switzerland for his critical review of the manuscript. The work was performed at the Neurocenter Rhine-Lahn, Clinic Katzenelnbogen, Katzenelnbogen, Germany and at the Department of Neurology, Bern University Hospital, Bern, Switzerland.
REFERENCES
- 1.Liblau RS, Vassalli A, Seifinejad A, Tafti M. Hypocretin (orexin) biology and the pathophysiology of narcolepsy with cataplexy. Lancet Neurol. 2015;14:318–28. doi: 10.1016/S1474-4422(14)70218-2. [DOI] [PubMed] [Google Scholar]
- 2.Tafti M, Hor H, Dauvilliers, et al. DQB1 locus alone explains most of the risk and protection in narcolepsy with cataplexy in Europe. Sleep. 2014;37:19–25. doi: 10.5665/sleep.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orellana C, Villemin E, Tafti M, Carlander B, Besset A, Billiard M. Stressful life events in the year preceding the onset of narcolepsy. Sleep. 1994;17:S50–3. doi: 10.1093/sleep/17.suppl_8.s50. [DOI] [PubMed] [Google Scholar]
- 4.Aran A, Lin L, Nevismalova S, et al. Elevated anti-streptococcal antibodies in patients with recent narcolepsy onset. Sleep. 2009;32:979–83. doi: 10.1093/sleep/32.8.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han F, Warby SC, Faraco J, Li J, Dong SX, et al. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Ann Neurol. 2011;70:410–7. doi: 10.1002/ana.22587. [DOI] [PubMed] [Google Scholar]
- 6.Nohynek H, Jokinen J, Partinen M, et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS One. 2012;7:e33536. doi: 10.1371/journal.pone.0033536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaarala O, Vuorela A, Partinen M, et al. Antigenic differences between AS03 Adjuvanted Influenza A (H1N1) pandemic vaccines: implications for Pandemrix-associated narcolepsy Risk. PLoS One. 2014;9:e114361. doi: 10.1371/journal.pone.0114361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacob L, Leib R, Ollila HM, Bonvalet M, Adams CM, Mignot E. Comparison of Pandemrix and Arepanrix, two pH1N1 AS03-adjuvanted vaccines differentially associated with narcolepsy development. Brain Behav Immun. 2015;47:44–57. doi: 10.1016/j.bbi.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Schöndorf I, Beran J, Cizkova D, et al. Tick-borne encephalitis (TBE) vaccination: applying the most suitable vaccination schedule. Vaccine. 2007;25:1470–5. doi: 10.1016/j.vaccine.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 10.Robert Koch Institut: Aktuelle Daten und Informationen zu Infektionskrankheiten und Public Health. FSME: Risikogebiete in Deutschland (Stand: Mai 2015) Epidemiologisches Bulletin. 2015;21:175–90. [Google Scholar]
- 11.Heininger U. Schutz vor FSME - Update 2015. ARS MEDICI. 2014;24:1251–3. [Google Scholar]
- 12.Robert Koch Institut: Aktuelle Daten und Informationen zu Infektionskrankheiten und Public Health. Mitteilung der Ständigen Impfkommission am Robert Koch-Institut (RKI) - Empfehlungen der Ständigen Impfkommission (STIKO) am Robert Koch-Institut/Stand: August 2014. Epidemiologisches Bulletin 25. August 2014 / Nr. 34. [Google Scholar]
- 13.Bundesamt für Gesundheit (BAG), Schweizerische Kommission für Impffragen (SKIF): Zeckenenzephalitis: Empfehlungen zur Impfung gegen Zeckenenzephalitis. Bull BAG. 2006:225–231. Nr. 13. [Google Scholar]
- 14.Germany: Robert-Koch-Institute; http://www.pei.de/DE/arzneimittelsicherheit-vigilanz/pharmakovigilanz/uaw-datenbank/uaw-datenbank-node.html. [Google Scholar]
- 15.Dauvilliers Y, Montplaisir J, Cochen V, et al. Post-H1N1 narcolepsycataplexy. Sleep. 2010;33:1428–30. doi: 10.1093/sleep/33.11.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pizza F, Peltola H, Sarkanen T, Moghadam KK, Plazzi G, Partinen M. Childhood narcolepsy with cataplexy: comparison between post-H1N1 vaccination and sporadic cases. Sleep Med. 2014;15:262–5. doi: 10.1016/j.sleep.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Marth E, Kleinhappl B, Jelovcan S. Stimulation of the immune system by different TBE-virus vaccines. Int J Med Microbiol. 2004;293:139–44. doi: 10.1016/s1433-1128(04)80025-1. [DOI] [PubMed] [Google Scholar]
- 18.Koller A, Doser K, Hartmann K, Fleisch F, Kuhn M. Vermutete neurologische Nebenwirkungen der FSME-Impfung: Erfahrungen der Schweizerischen Arzneimittel-Nebenwirkungs-Zentrale (SANZ) Praxis. 2002;91:159–62. doi: 10.1024/0369-8394.91.5.159. [DOI] [PubMed] [Google Scholar]
- 19.Fabricius D, Nußbaum B, Busch D, et al. Antiviral vaccines license T cell responses by suppressing granzyme B levels in human plasmacytoid dendritic cells. J Immunol. 2013;191:1144–53. doi: 10.4049/jimmunol.1203479. [DOI] [PubMed] [Google Scholar]
- 20.Wensink AC, Hack CE, Bovenschen N. Granzymes regulate proinflammatory cytokine responses. J Immunol. 2015;194:491–7. doi: 10.4049/jimmunol.1401214. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed SS, Volkmuth W, Duca J, et al. Antibodies to influenza nucleoprotein cross-react with human hypocretin receptor 2. Sci Transl Med. 2015;7:294ra105. doi: 10.1126/scitranslmed.aab2354. [DOI] [PubMed] [Google Scholar]


