Abstract
Study Objectives:
To examine scalp and source power topography in sleep arousals disorders (SADs) using high-density EEG (hdEEG).
Methods:
Fifteen adult subjects with sleep arousal disorders (SADs) and 15 age- and gender-matched good sleeping healthy controls were recorded in a sleep laboratory setting using a 256 channel EEG system.
Results:
Scalp EEG analysis of all night NREM sleep revealed a localized decrease in slow wave activity (SWA) power (1–4 Hz) over centro-parietal regions relative to the rest of the brain in SADs compared to good sleeping healthy controls. Source modelling analysis of 5-minute segments taken from N3 during the first half of the night revealed that the local decrease in SWA power was prominent at the level of the cingulate, motor, and sensori-motor associative cortices. Similar patterns were also evident during REM sleep and wake. These differences in local sleep were present in the absence of any detectable clinical or electrophysiological sign of arousal.
Conclusions:
Overall, results suggest the presence of local sleep differences in the brain of SADs patients during nights without clinical episodes. The persistence of similar topographical changes in local EEG power during REM sleep and wakefulness points to trait-like functional changes that cross the boundaries of NREM sleep. The regions identified by source imaging are consistent with the current neurophysiological understanding of SADs as a disorder caused by local arousals in motor and cingulate cortices. Persistent localized changes in neuronal excitability may predispose affected subjects to clinical episodes.
Citation:
Castelnovo A, Riedner BA, Smith RF, Tononi G, Boly M, Benca RM. Scalp and source power topography in sleepwalking and sleep terrors: a high-density EEG study. SLEEP 2016;39(10):1815–1825.
Keywords: sleep arousal disorders, somnambulism, parasomnias, local sleep, night terrors
Significance.
The current study shows the existence of local sleep differences in patients affected by sleep arousals disorders, a group of NREM sleep parasomnias including sleepwalking and night terrors. Differences consisted of a relative reduction in slow wave activity across the sleep/wake cycle in motor, limbic, and sensori-motor associative cortices.
INTRODUCTION
It is now clear that sleep and waking states are not always whole-brain phenomena regulated strictly at the global level. In fact, along a continuum of brain states, features of NREM sleep, REM sleep and wake can coexist. Marine mammals and birds for instance, can sleep with one brain hemisphere while the other one is awake, and in rodents and humans sleep and wakefulness can co-occur even within the same cortical area in different cortical columns or layers, often after extended wake or during the transition from one behavioral state to another.1–13
In some more extreme cases, however, the abnormal admixture of sleep and wakefulness results in pathological conditions known as parasomnias.14 Specifically, the disruption of the mechanisms underlying the transition between NREM sleep and wake has been proposed to account for the so-called “disorders of arousal.”15 Sleep arousal disorders (SADs), which mainly include sleepwalking and night terrors, are the most striking forms of NREM sleep parasomnias and dissociative states in humans.14 Affected patients may exhibit waking behaviors arising abruptly out of NREM sleep, such as sitting up in bed, screaming, crying, or ambulating. While they remain unresponsive to the external environment, their EEG shows typical sleep features, and they may occasionally report dreaming.16,17 SADs are common and potentially dangerous sleep disorders. They have a prevalence of up to 20% during childhood and up to 4% during adulthood.18–20 Patients can cause severe injuries to themselves and others21,22 and suffer from daytime adverse consequences such as excessive daytime sleepiness (EDS) and increased sensitivity to the cognitive impairment caused by sleep deprivation.23–30
The mechanisms underlying SADs are poorly understood. Behavioral episodes are associated with the emergence of wake-like activation in motor and limbic regions, and with a paradoxical increase in measures of sleep intensity including slow waves over a fronto-parietal network.29,31–34 It remains unclear, however, whether abnormal local patterns of brain activity persist outside of clinical episodes. Scalp sleep EEG studies have described increased sleep fragmentation and several slow wave abnormalities, including decreased slow wave activity during the first sleep cycle and abnormal slow waves build up over the course of the night.15,35–53 However, these studies were limited to the analysis of only one central lead or a small number of electrodes, and thus could not determine whether sleep alterations were local or global. Here we addressed this question by performing high-density EEG (hdEEG) recordings with 256 channels in a group of adult patients with sleepwalking and/or night terrors.
METHODS
Fifteen adult subjects with a diagnosis of SADs and an equal number of sex- and age-matched healthy controls were included for analysis. Patients were identified retrospectively by examining all subjects meeting the diagnostic criteria for SADs according to ICSD-354 who had undergone overnight polysomnography (PSG) testing with combined hdEEG (hdPSG) at the Wisconsin Sleep Laboratory between December 2008 and May 2015. Data were extracted from electronic medical records with a waiver of informed consent and authorization. Matched control participants were drawn from two other studies conducted at the University of Wisconsin-Madison sleep laboratory, both with similar procedures for recording sleep hdPSG as the patient group. All controls participants provided written consent before the study. All study procedures were reviewed and approved by the University of Wisconsin Health Sciences Institutional Review Board.
NREM Parasomnia Patients
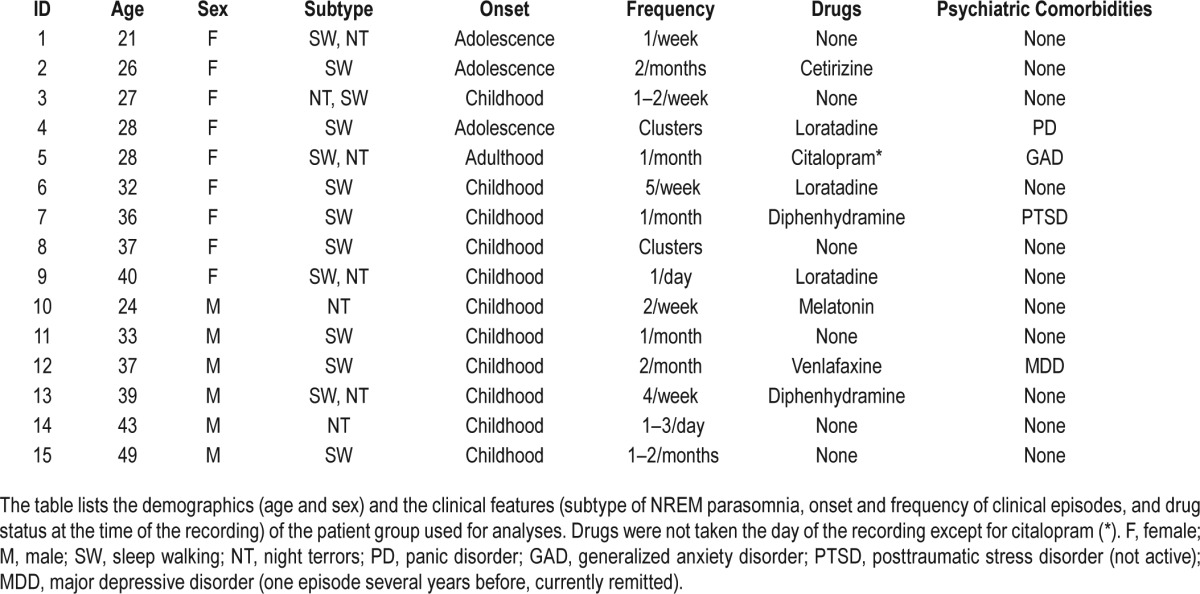
Each patient was evaluated by a physician board-certified in Sleep Medicine and referred to the sleep laboratory for polysomnographic evaluation with extended EEG monitoring because of a clinical history of parasomnia behavior. Good quality recordings were available for 15 patients not currently taking sedative-hypnotic medications. Table 1 presents a summary of the clinical characteristics and the drug status at the time of the recording for all the patients included in the analyses. The group included 2 subjects with final diagnoses of night terrors only, 5 with both night terrors and sleepwalking, and 8 with sleepwalking only. Four of the patients had at least one partial event (e.g. sitting upright and yelling) during the time of the recording. All patients reported relatively frequent clinical episodes (at least one episode per month). SAD onset ranged from early childhood to adulthood. Patients were screened for the presence of apneas and periodic leg movements during sleep. None of the selected patients had an apnea-hypopnea index (AHI) > 5 events/h or a periodic limb movement arousal index during sleep (PLMSAI) ≥ 10/h of sleep. Four patients had a psychiatric diagnosis: one with panic disorder not pharmacologically treated, one with major depressive disorder (single episode, remitted) in treatment with venlafaxine 75 mg per day, one with generalized anxiety disorder treated with citalopram 20 mg per day, and one with posttraumatic stress disorder (currently remitted, and not under treatment). All patients completed the Epworth Sleepiness Scale (ESS)55 on the day of the recording.
Table 1.
Demographics and clinical features for the patient population.
Healthy Controls
Age and sex-matched control participants were drawn from a pool of subjects selected for being good sleepers, who participated as controls in a study on sleep homeostasis in depression (n = 3),56 or in a study on the effects of meditation (n = 14).57 Both studies were also performed at the Wisconsin Laboratory. Exclusion criteria were (1) any current or past neuropsychiatric condition; (2) use of any psychotropic medication or medication that could affect sleep; and (3) evidence of any sleep disorder. All subjects had an initial phone screening and a thorough in person screening visit to determine eligibility. They were asked to complete a sleep diary for at least one week and refrain from hypnotics or drugs that would affect sleep for at least two weeks. Subjects were screened for the presence of psychiatric disorders using a non-patient Structured Clinical Interview for DSM-5 SCID,58 the Profile of Mood States,59 the Montgomery Asberg Depression Rating Scale (MADRS)60 (for the sleep homeostasis study only), or with a medical and psychiatric screening interview and several questionnaires including the Quick Inventory of Depressive Symptoms61 and the Symptom Checklist-90-Revised62 (for the meditation study only). Additionally, each participant completed validated sleep rating scales, including the Stanford Sleepiness Scale63 (both studies), and the ESS (meditation study only). Controls were screened for the presence of apneas and periodic leg movements during sleep. As was the case with the SADs group, none of the selected controls had an AHI > 5 events per hour or a PLMSAI > 10. None of the controls suffered from any psychiatric or sleep disorder or was taking any psychotropic medication at the time of the recording.
General Protocol
Sleep Recordings
All participants underwent an overnight in-laboratory hdEEG recording collected with vertex referencing (256 channels; Electrical Geodesics Inc., Eugene, OR). Lights out was within one hour of the participants most consistently reported bedtime. Three of the parasomnia patients were allowed to sleep ad libitum in the sleep laboratory because they also reported daytime sleepiness and had multiple sleep latency testing (MSLT) the following day; MSLT results were normal for all subjects (mean sleep latencies 11.4 min, 16.2 min, and no sleep, respectively), and no REM sleep periods were observed at sleep onset in any of the three patients. The rest of the patients and all controls were awakened at approximately 06:00–07:00 according to clinical procedures for the laboratory. Sleep staging was performed by registered polysomnographic technicians according to standard criteria using Alice Sleepware (Philips Respironics, Murrysville, PA) based on 30-s epochs for 6 EEG channels at approximate 10–20 locations derived from the hdEEG array with bipolar re-referencing (F3/M2, F4/M1, C3/M2, C4/ M1, O1/M2, O2/M1), EOG, and submental EMG.64 Sleep-disordered breathing and periodic limb movement disorder were ruled out with concurrent PSG. All staging and scoring was reviewed by a board-certified sleep physician (RB).
EEG Scalp Analysis
SADs clinical manifestations occur during stage 3 (N3) in around 80% of cases, and during stage 2 (N2) in the remaining 20% of cases.65 A first analysis was therefore conducted on combined N2N3. A follow-up analysis focused on N2 and N3 separately, as well as on transitional stage N1, REM sleep, and wakefulness, in order to verify potential differences in local power extending outside NREM sleep.
All EEG signals were collected at 500 Hz and high-pass filtered at 0.1 Hz. Prior to spectral analysis, each signal was down-sampled to 200 Hz, band-pass filtered (2-way least-squares FIR, 1–40 Hz) in MATLAB (The MathWorks Inc., Natick, MA), re-referenced to the average of the scalp voltage for all 256 channels, and divided into consecutive 6-s epochs. Semiautomatic artifact rejection procedures were utilized to remove channels and epochs with high frequency noise or interrupted contact with the scalp, as done in other recent studies.66–68 Specifically, thresholds were automatically calculated for low (1–4 Hz) and high (20–40 Hz) frequency ranges at the 99th percentile for each channel. Spectral power in these ranges across all 6-s NREM epochs for each channel was plotted and visually inspected. Channels with artifacts affecting a majority of the recording at the visual inspection were removed. Additional spectral-based and topographic procedures were used to remove individual channels with distinctly greater power relative to neighboring channels. The removed channels were interpolated using spherical interpolation. Overall, ≥ 70% of the data recordings and > 200 of the channels for each participant were retained after the cleaning procedure.
Spectral analysis was performed using all clean 6-s epochs within NREM sleep (Welch averaged modified periodogram with a Hamming window). To increase the signal-to-noise ratio, scalp analyses were restricted to inside channels, yielding 173 channels overlaying the scalp, excluding channels from the face and the neck. For topographic analysis, average spectral density was computed for 6 frequency ranges (slow wave activity or delta: 1–4 Hz; theta: 4–8 Hz; alpha: 8–12 Hz; sigma: 12–16 Hz; beta: 15–25 Hz; and low gamma: 25–40 Hz), consistent with previous studies.66,67,70,71 Topographic maps of both absolute average referenced data and subject-normalized data (z-score across channels) were examined.
Wake and REM sleep were analyzed using a similar procedure. As one subject did not have enough wake before sleep onset, only 14 patients and 14 matched controls were retained for analyses. Bad channels and bad epochs were visually identified, rejected, and replaced with data interpolated from nearby channels using spherical interpolation in MATLAB. Additional bad channels and epochs were identified using the same spectral and threshold methods described before. Independent Component Analysis (ICA) was performed to remove ocular, electrocardiograph, and remaining muscular artifacts using EEGLAB routines.69 Principal component analysis (PCA) was performed before ICA to reduce the number of components to 128. Only ICA components with specific activity patterns and component maps characteristic of artifactual activity were removed.72 After excluding electrodes located on the neck/face region, the signal was re-referenced to the average of the remaining 173 channels.
EEG Source Modeling
Data for source modeling estimates were obtained by visually selecting 5-min of continuous sleep for each subject, using the same procedure as a recent study on insomnia.68 There were several reasons for the use of short, continuous segments: (1) source imaging was computationally practical; (2) it was possible to visually inspect the entire segment, in order to rule out the inclusion of arousal, muscle or eye movement artifacts; (3) segments were comparable for duration and (4) data could be collected at a similar circadian time for all subjects. Because SWA power abnormalities in SADs have been mostly described within N3 from the first 2–3 hours of sleep,38,41 segments were selected from the first part of the night, and primarily from N3 sleep during the first sleep cycle. However, if insufficient continuous N3 sleep was scored, or when there was no deep sleep during the first sleep cycle, segments with some N2 and/or during the second cycle were selected, with a preference for taking the most continuous N3 epochs. Almost all segments were taken from N3 occurring in the first sleep cycle (except for one patient and 3 controls, for which the segments were taken from the second cycle). For all participants, selected segments were within 3 hours of sleep onset and were at least one minute away from any noticeable arousal.
Distributed source imaging is significantly improved by using as many electrodes covering the head surface as possible.73 Thus, each individual segment was reexamined to identify bad channels, which were subsequently replaced by spherical interpolation to ensure that all 256 channels could be used in the source estimation. Source imaging was performed using GeoSource 2.0 (Electrical Geodesics Inc., Eugene, OR) on the 5-min continuous segments of EEG data described above, as in previous studies.68,71,74 In order to examine the source of power for SWA, the segments described above were further band-pass filtered (2-way least-squares FIR, 1–4 Hz) in MATLAB, before source localization.
Statistical Analysis
Between-group differences in demographic and polysomno-graphic variables, as well as global EEG power spectra, were evaluated with unpaired, 2-tailed t-tests. Scalp topography and source images were analyzed using statistical nonpara-metric mapping with supra-threshold cluster tests to correct for multiple comparisons,76 as done before using an appropriate threshold t-value (t = 2 with 50,000 permutations).57,66,68 Although this test faithfully addresses the problem of multiple comparisons across an image, it should also be noted that we did not attempt to strictly correct for the issue of multiple testing across different comparisons (for example across stage) given the exploratory nature of this study. A mixed model analysis of variance (ANOVA) was used to determine the interaction effect between groups and stages, and between groups and cycles, in the region of interest (ROI) identified by the cluster test analysis. Correlations were assessed using the nonparametric Spearman rank-order correlation. All statistical analyses were performed using MATLAB.
RESULTS
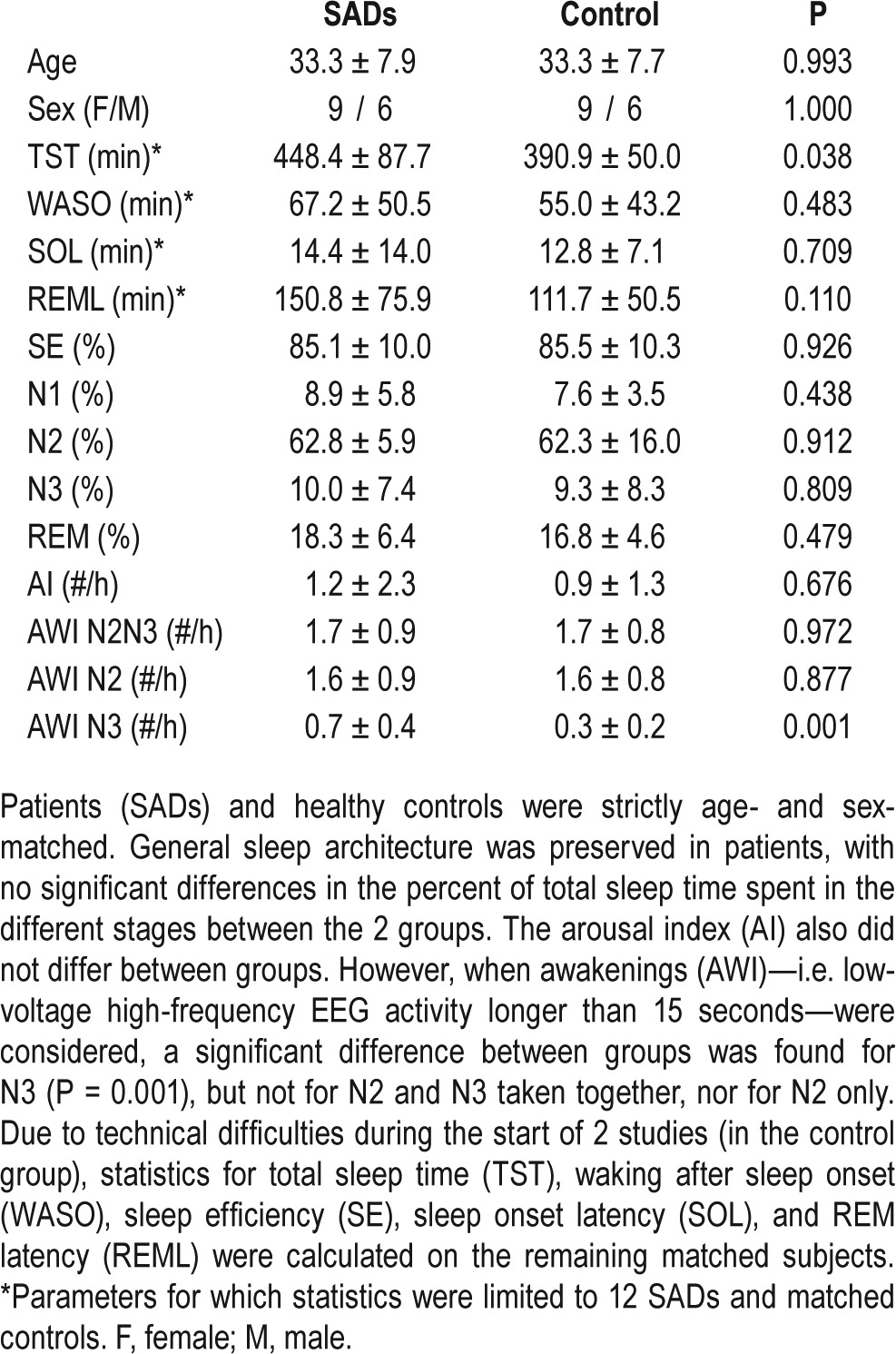
Although the assessment of sleep architecture was not the main focus of this study, we first analyzed group differences in sleep structure. SADs patients had an increased number of awakenings out of N3 (P = 0.0002), in the absence of any significant disruption in general sleep architecture (Table 2). The total sleep time (TST) was greater in the SADs group (P = 0.038), however this difference was primarily driven by 2 outliers in the patient group who were allowed to sleep ad libitum, and was no longer observed when a second analysis was carried removing these 2 subjects (P = 0.142).
Table 2.
Demographics and sleep parameters.
SADs participants demonstrated borderline levels of daytime sleepiness according to ESS scores (mean ± standard deviation = 8.7 ± 4.61), with 6 subjects scoring ≥ 10, whereas all controls for which the scale was available (n = 12) scored within the range of normality (mean ± standard deviation = 4.3 ± 2.63), resulting in a significant difference between groups (P = 0.005) (Figure S1 in the supplemental material). Considering only twelve controls and twelve age-matched matched SADs yielded a similar result (P = 0.004). The difference was even larger when only the subgroup of 8 patients with sleepwalking not associated with night terrors and 8 matched controls were selected (SADs = 10.4 ± 3.3 vs. 3.8 ± 2.7, P = 0.0022). No correlations were found between ESS and the frequency of awakenings out of N3 (r = −0.24, P = 0.35), between the frequency of awakenings out of N3 and the frequency of episodes (r = −0.09, P = 0.74), or between ESS and the frequency of episodes (r = −0.20, P = 0.42).
Global EEG power spectra (power spectral density averaged across all scalp channels) did not differ between SADs patients and controls (Figure S2 in the supplemental material). This was the case for all sleep stages (N2N3, N1, REM sleep, Wake), and persisted when exploratory analyses were conducted in N2 and N3 separately, and in early sleep relative to late sleep (first vs. fourth sleep cycle; data not shown).
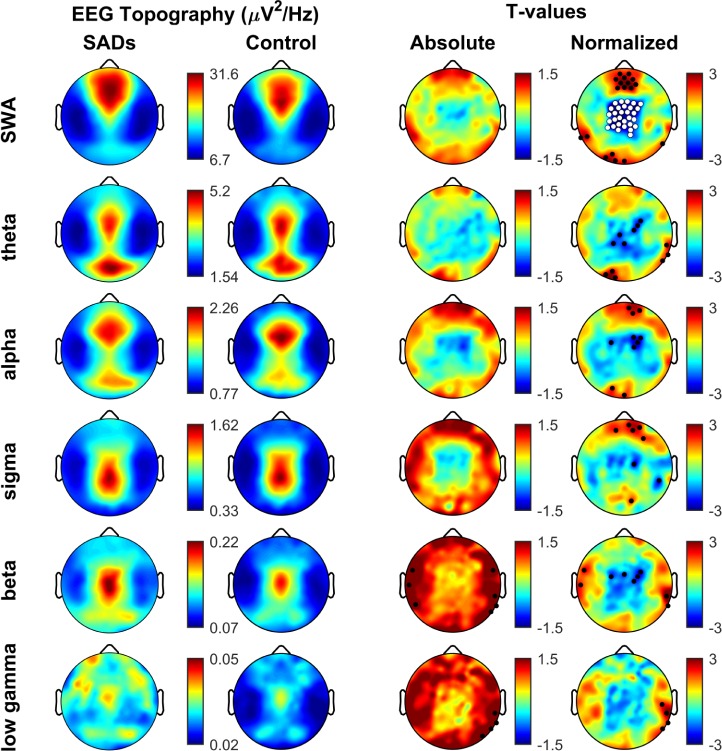
Next, we focused on local EEG changes. While there were no differences between the 2 groups when absolute values were compared (Figure 1), significant changes were apparent in normalized SWA topography across channels. Specifically, during N2N3 SADs patients showed a significant decrease in SWA power in a cluster of electrodes that spanned centro-parietal areas (25 channels, P = 0.0038). A cluster of fronto-central channels showed higher SWA in SAD patients, but this cluster did not survive multi-comparison analyses. Moreover, no other frequency band showed differences between the 2 groups that survived SNPM correction.
Figure 1.
Topographical analysis of N2N3 sleep EEG. Topoplots showing the regional decrease in normalized SWA power in patients (SADs) versus healthy controls. Rows represent frequency bands of interest as indicated: SWA (1–4 Hz), Theta (4–8 Hz), Alpha (8–12 Hz), Sigma (12–15 Hz), Beta (15–25 Hz), Low Gamma (25–40 Hz). First column: average NREM sleep EEG topographies across frequency bands for SADs subjects. Second column: topographical averages for healthy control matches during NREM sleep, scaled the same as SADs subjects. Third column: Map showing the individual electrode t-value (two-tailed, unpaired) maps for the comparison between SADs and control subjects in terms of absolute power. Blue values represent a decrease in absolute EEG power in SADs subjects relative to controls (SADs < control) and red values represent an increase (SADs > control). Fourth column: Same as third column except that each subject was spatially normalized using the z-score across electrodes before creating the t-value comparison. White dots indicate channels that belong to a statistically significant cluster of electrodes (P ≤ 0.05) using statistical nonparametric mapping suprathreshold cluster testing. Black dots indicate individual channels with P < 0.05 (uncorrected).
Similar results were found when N2N3 in the first and the second halves of the night were considered separately (first, 23 channels, P = 0.015; last, 17 channels, P = 0.0366). In order assess whether the effect was stronger during the first cycle as suggested by previous literature,36,38,41 we ran a mixed model ANOVA over a region of interest. The region of interest (ROI) was defined as the cluster of 25 electrodes significantly reduced in SWA power density for SADs relative to controls in N2N3. The effect was equally strong during the first cycle compared to the fourth cycle (F1,14 = 0.01, P = 0.91). The decrease in SWA power also persisted when N2 and N3 were analyzed separately (N2, 30 channels, P = 0.0033; N3, 24 channels, P = 0.0176), with no statistical difference between the 2 stages (F1,14 = 0.96, P = 0.75).
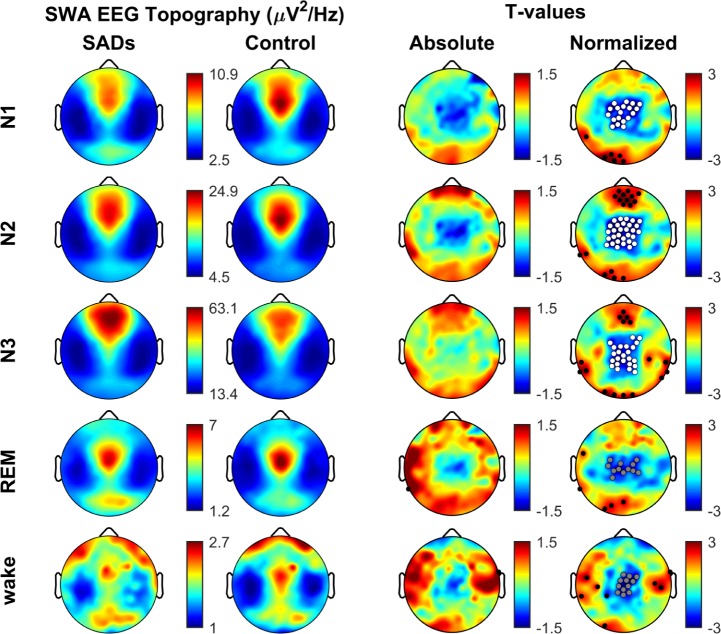
A separate analysis found that a relative decrease in SWA power in a similar centro-parietal area was also evident in N1 (19 channels, 100% of which belong also to the cluster in N2N3, P = 0.0241) (Figure 2). There was an additional decrease in alpha power over the same regions (30 channels, P = 0.0065) (data not shown). A trend for a decline in SWA power in centroparietal areas was also present in SADs patients during REM sleep, although the change did not reach statistical significance (12 channels, 91% of which of which belong also to the cluster in N2N3, P = 0.075, 50000 permutations). Artifact-free wake epochs that occurred while subjects were in bed before sleep onset were also analyzed, and showed again a strong trend toward a decrease in EEG SWA power over the same central areas in SADs patients relative to controls (13 channels, 100% of which belong also to the cluster in N2N3, P = 0.054) (Figure 2).
Figure 2.
SWA topography across the sleep/wake cycle. Same as Figure 1 except only SWA band is shown (1–4 Hz), this time across different phases of the sleep/wake cycle (wake, N1, N2, N3, and REM). Note similarity between sleep/wake phases (fourth column) and consistent differences between groups (clusters of white and gray dots) regardless of the sleep/wake phase. White dots belong to significant clusters (P < 0.05) using SnPM for multi-comparison correction. Gray dots belong to clusters with a trend for significance (P ≤ 0.08). Black dots indicate individual channels with P < 0.05 (uncorrected). Note, wake group comparison was for 14 SADs vs matched controls due to the availability of waking data. See text for details.
No interaction effect between groups and stages was found in a mixed-model ANOVA (F1,14 = 0.17, P = 0.92), suggesting that the effect persisted across the entire sleep/wake cycle. No correlations were found between the average normalized SWA power decrease in N2N3 over the centro-parietal cluster of 25 electrodes (see Figure 1), and clinical measures such as frequency of episodes (number of episodes per month; r = −0.35, P = 0.17), daytime sleepiness (measured by ESS; r = 0.20, P = 0.45) or sleep fragmentation (the number of awakening from N3 per hour − N3 AWI; r = −0.23, P = 0.37).
In order to estimate the cortical areas which might be responsible for these local differences in scalp topography, we selected 5 minutes of the deepest continuous NREM sleep available (stage N3) for all subjects (see Methods). A local decrease in scalp SWA power in centro-parietal regions was still evident in patients compared to controls for these segments (31 channels, P = 0.007). There was an 87% of the channels in the NREM sleep all-night cluster overlapping with the segment data cluster.
Source localization confirmed the presence of a significant reduction in normalized SWA power, which survived the SNPM cluster test (318 voxels, P = 0.0277). The areas that showed the most consistent decrease in power in patients versus controls were the cingulate cortex, the motor and pre-motor/supplementary motor cortices, the associative somato-sensory cortices, the visual associative cortex, and associative parietal areas like the superior parietal lobule and the precuneus (Table 3 and Figure 3). As was the case for the N2 and N3 scalp SWA topography (see Figure 2), there were local areas of apparent increased SWA; however, these areas did not survive multiple comparison correction tests. Voxels with increased SWA in parasomnia patients relative to controls that were significance only at the level of individual t-test appeared in the orbitofrontal cortex (OFC), the ventro-medial prefrontal cortex (VMPFC), the angular cortex (AC), and the primary sensory cortex (S1).
Table 3.
Significant dipoles ranked by averaged t-value.
Figure 3.
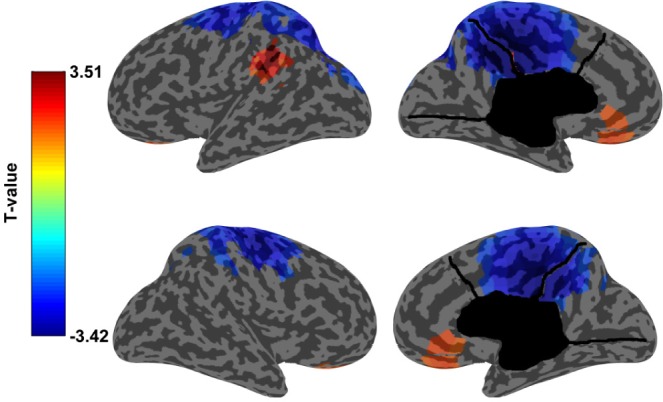
Regional decrease in normalized SWA power. Inflated cortical map showing the results for SWA power source analysis using artifact- and arousal-free 5 minute segments selected from N3. On the top left: lateral surface of the left hemisphere. On the bottom left: lateral surface of the right hemisphere on the bottom left, on the top right: medial surface of the left hemisphere, on the bottom right: medial surface of the right hemisphere. In blue the T-values of the areas that were significantly different (P < 0.05) between SADs and healthy controls using SNPM cluster test. Functional areas that are most significantly different include the cingulate cortex (Brodmann areas: 23, 24, 29, 31), motor and premotor/supplementary motor cortices (Broadmann areas: 4 and 6), primary and associative somatosensory cortices (Broadmann areas: 2, 3 and 5), visual associative cortex (Brodmann areas: 18 and 19) and parietal multi-modal associative cortex (Brodmann area: 7). In red the areas where the increase in SWA power was significantly different (P < 0.05) between SADs and healthy controls at the uncorrected voxel-level.
All scalp analyses were repeated for a subgroup of 11 patients with no psychiatric comorbidities and matched controls and for a subgroup consisting of the 8 patients who only reported sleepwalking. Overall, results for these subgroups were consistent with the described findings for the main group analysis showing a decrease in SWA power during N2N3 (Figure S3 in the supplemental material).
DISCUSSION
This is the first study investigating SADs using hdEEG recordings, which allowed for the analysis of local sleep changes in EEG power topography with high spatial resolution. We examined a group of fifteen subjects diagnosed with sleepwalking and/or night terrors compared to fifteen age- and sex-matched healthy controls. SADs patients showed a reduction of normalized SWA power that was localized to centro-parietal regions, in contrast with preserved global EEG power. While a decrease in SWA power in SADs has been repeatedly reported in the literature, the topography of this decrease had not yet been characterized, due to the low spatial resolution of standard low-density EEG techniques and the focus on a single lead. Specifically, Gaudreau et al. studied SWA power in a group of fifteen sleepwalkers using two leads (C3/A2, O2/A1) and only kept the central lead for analysis.38 Similarly, Guilleminault et al. studied SWA power in a group of twelve sleepwalkers, using four leads (Fz/A1-A2, C2/A2, C4/A1, O1/A1), and restricted the analysis to C4; they found a reduction in SWA power over central leads, which reached significance only during the first cycle.41 They subsequently confirmed this result for a group of ten sleepwalkers, but again limited the analysis to C4.40 Another study in a mixed sample of patients with sleepwalking (three), night terrors (six), or both (two) recorded over two central leads (C3/A2 and C4/A1), did not find any significant differences in any frequency bins for the all night absolute SWA power between patients and controls.36 However, when the distribution of SWA power was considered over time, they again found an abnormal slow wave build-up, with a significant decrease in SWA power during the first cycle in patients versus controls. Of note, our main result was obtained after data normalization across channels to reduce the inter-subject variability, a procedure not applicable in studies using a small number of channels. Moreover, the significant centro-parietal cluster of reduced SWA power observed in SADs in the present study did not overlap with C3/C4, which had been chosen for analysis in previous studies.
Given the increased number of arousals between the two groups in N3, it cannot be excluded that the observed effects are the consequence of sleep disruption. However, power topography examination (Figure 1) revealed noticeable differences compared to previous findings in other sleep disorders, like OSA (obstructive sleep apneas),66 insomnia,68 and depression.77 A study on seven MDD patients with hypersomnia (HYS), seven MDD without HYS and seven healthy controls revealed a decrease in absolute SWA activity over a parieto-occipital cluster in depressed patients with hypersomnia.77 A similar parieto-occipital decrease in power was found for a group of nine asymptomatic OSA.66 These clusters were located more posteriorly than the one described for SAD in normalized SWA power. In OSA patients, the reduction in normalized SWA power was evident across several frequency bands; in contrast to our present findings in SADs, it was also not associated with any observable frontal increase in SWA power. A more recent study on eight chronic insomnia subjects revealed an increase in absolute power in high frequencies (> 16 Hz) during NREM sleep, and a localized absolute increase in alpha power over primary sensory areas during N3 sleep.68
We did not find any correlations between the regional change in SWA power and the clinical features considered (ESS, N3-AWI, frequency of episodes). This negative finding should be treated with caution, since the frequency of parasomnia episodes was based on the patients' clinical report, without the aid of standardized scales, which could be biased by significant amnestic factors. Moreover, since sub-threshold local activations can occur in the absence of any clinical manifestation,31 overt behavioral events might not be a relevant feature to correlate with topographical changes in sleep EEG power. On the other hand, our results are in line with Lopez et al.,27 who, in a study of 30 SADs patients with a diagnosis of sleepwalking or night terrors, did not find any correlation between subjective or objective measures of excessive daytime sleepiness and clinical or polysomnographic parameters. Given the group difference we observed in sleepiness between SADs and controls in our study and that local changes in power were evident throughout the sleep/wake cycle, it is interesting to speculate whether EDS may represent a waking manifestation of blurred state boundaries. A recent neuropsychological investigation of executive functions in SADs showed that daytime consequences of sleepwalking are not limited to EDS, as it includes cognitive impairments in the form of disrupted inhibitory control following sleep deprivation.30 Fatigue during wake could be related to increased frontal lobe susceptibility to sleep-deprivation. Regardless, these results provide further evidence for a possible dissociation between local EEG changes and clinical SADs episodes, which occur exclusively during N2N3 sleep, and especially during N3 and the first 1–3 hours of sleep.15
Source localization analysis suggested that the main areas showing the local decrease in SWA were the cingulate cortex, motor cortex, and to a lesser extent sensory, sensory-motor associative areas and the precuneus. This latter cortical area probably plays a central role in a wide spectrum of highly integrated tasks, including visuo-spatial imagery, episodic memory retrieval and self-processing operations, namely first-person perspective taking and an experience of agency,78 in line with conscious experiences sometimes reported by adult patients with SADs.23 Furthermore, the areas highlighted by source localization largely overlap with the ones found to be activated during sleepwalking episodes.29,31–34 Bassetti et al., in a SPECT case study found that the highest increases of regional cerebral blood flow during sleepwalking, compared with quiet stage N3 sleep, were located in the anterior cerebellum and in the posterior cingulate cortex.29 By contrast, large areas of frontal and parietal association cortices remained deactivated, compared to data obtained from 24 normal volunteers during wakefulness. Subsequently, three stereo-EEG case studies showed the simultaneous coexistence of sleep-like patterns over fronto-parietal associative networks, and wake-like patterns characterized by low-voltage fast activity over the motor and the cingulate cortex.31–33 Janusko et al., in a low resolution (23 channels) source imaging EEG study using eLORETA found an increase in brain activation during the 4-second period preceding the onset of sleepwalking, with greater current density within the beta 3 frequency range (24–30 Hz) in Brod-mann areas 33 and 24 (pregenual and ventral anterior cingu-late cortex),34 although they did not compare patients with age matched controls. It has been hypothesized that local arousal in the motor and cingulate cortices is at the root of the motor behaviors peculiar to SADs.32 This might represent an exaggeration of the normal tendency of these local networks to exhibit a lower arousal threshold, which may have evolved by natural selection to increase the chance of survival by favoring a prompt motor response in case of dangers.6 Of note, a TMS study performed on eight sleepwalkers during waking revealed increased excitability of the motor cortex compared to healthy controls,79 again consistent with the hypothesis that, in SADs, specific motor-limbic thalamocortical circuits may show an increased propensity for local activation.
Although the finding of a local increase in SWA power did not survive multiple comparison correction, and thus should be considered extremely cautiously, its specific localization makes it worthy of notice. The OFC, VMPFC, and AG are cross-modal hubs where converging multisensory information is combined and integrated. Specifically, the OFC and the VMPFC have been proposed as critical structures in a neural systems representation of the affective value of reinforcers and in decision making,80,81 while the AG has been implicated in reorienting attention to relevant information, integrating multi-sensory information to comprehend and give sense to events, and manipulating mental representations.82 During behavioral episodes, patients are largely unresponsive to their external environment and act inappropriately to circumstances, suggesting that these higher order regions may be sleeping deeper than other brain regions, and thus, do not fully awake during episodes. Moreover, during sleepwalking episodes, patients are not awakened by nociceptive stimulation. An increase in sleep at the level of the primary sensory cortex and in the prefrontal cortex, two regions considered central in pain processing, could potentially explain this phenomenon,82,83 although this hypothesis remains highly speculative.
Overall, the observed changes in SWA power suggest a local dysregulation of sleep/wake processes in SADs even outside full-blown clinical episodes.
Limitations and Future Directions
While the total number of subjects considered had adequate statistical power for patient/control comparisons, the heterogeneity of SADs subtypes and their frequent overlap19 limited the possibility of several interesting intra-group comparisons and stratification analyses. Furthermore, while groups were age- and sex-matched, the sample size here did not allow for appropriate evaluation of sex differences. On the other hand, sleepwalking and night terrors are thought to share a similar etiopathogenetic background,19 supporting our choice to group both patient populations in our analyses.
We restricted the analysis of local sleep EEG power in SADs to a sample of adult subjects. This was done to reduce potential biases due to differences in brain maturation and EEG net sizes. Larger studies are required to confirm the presence of local changes in sleep EEG power in children with SADs. These studies would be of particular interest considering the higher presence of these disorders prior to teenage.
Patients with parasomnias often show increased anxiety levels, susceptibility to stress and coexistent sleep disorders,18,84,85 although our study was not intended to address these relationships. We minimized comorbidities by not including data from patients taking sedative-hypnotic medications or with above threshold apnea-hypopnea or periodic limb movement indices. Still, as our sample included four patients with psychiatric disorders, in contrast with none among controls, a separate analysis was conducted without these patients to control for the bias related to psychiatric disorders and medication, with similar results. Although this approach can help determine what is specific to SAD, it could also be argued that our patient group was not representative of the SAD community. A larger sample size study would therefore be needed to fully assess the sensitivity and specificity of local sleep dysregulation in SAD. Moreover, given the interest in using parasomnia diagnoses in criminal cases,86 it is important to point out that evidence of local sleep dysregulation as yet could not establish causality of a remote event and is therefore unlikely to be useful in forensic cases.
Another potential caveat includes the fact that we did not have controlled experimental conditions during the acquisition of waking EEG. This was due to the fact that the present study retrospectively analyzed EEG datasets acquired for clinical purposes. Wakefulness data were extracted from quiet periods in between the end of electrode net setup and the start of the overnight recording. The time required for technical settings and calibrations, however, provided sufficient stable periods of quiet waking (more than 5 minutes per subject).
Unfortunately, the Paris Arousal Disorders scale87 was not assessed in our subjects, as it was only recently published and was therefore not a part of routine clinical practice during data collection. Moreover, as we did not use identical questionnaires or assessments, SADs and control subjects could not be directly compared on relevant measures such as depression, anxiety, sleepiness, pain, executive functions, or reward-related questionnaires. Recent work has also pointed to a specific link between SAD and pain conditions, in particular migraine and headache.88 In our sample only two patients had a clinically relevant pain (migraine and neck pain), but we did not perform group profiling on acute and chronic pain. We also did not rate subjects using the Frontal Lobe Epilepsy and Parasomnias Scale,89 although we did not include patients who had suspicion of epilepsy. Given the potential for overlap between parasomnias and epilepsy, a future comparison between such patients would be warranted.
While this study allowed for investigating stable features of SAD sleep EEG relative to normal controls, future studies examining clinical episodes, surrounding periods of wakefulness and sleep, and critical sleep-wake transitions are essential to further understand the mechanisms of SADs. hdEEG has clear potential in these regard, because of its advantages in the temporal domain, both in terms of resolution and ability to sample uninterrupted for long periods of time, and its improved spatial resolution compared to low-density EEG.
CONCLUSIONS
Using hdEEG we found that patients with sleepwalking and/or night terrors showed a decrease in normalized EEG power that was especially prominent in the SWA range (1–4 Hz) and was source localized mainly to cingulate and motor regions. These results are consistent with the current neurophysiological understanding of NREM sleep parasomnias as disorders characterized by episodic local arousals in motor and limbic cortices. Notably, this local decrease in SWA in centro-parietal regions was present in SADs across sleep cycles and across different NREM sleep stages. A strong trend in the same direction was also present in REM sleep and wake, suggesting that persistent local changes in neuronal excitability may predispose affected subjects to clinical episodes.
DISCLOSURE STATEMENT
This was not an industry supported study. This research was funded in part by the NIH Mental Health Grant P20MH077967, and the NIH National Center for Complementary and Alternative Medicine Grant P01AT004952. Dr. Benca has served as a consultant for Merck and Janssen and receives grant support from Merck. Dr. Tononi is a consultant to the Allen Institute and has been a symposium speaker for Philips Healthcare and for Sanofi. Dr. Tononi was also endowed the David P. White Chair in Sleep Medicine and receives grant support from Philips Healthcare. Dr. Riedner receives partial salary support from the Merck and Philips Healthcare Grants held by Drs. Benca and Tononi and, along with Dr. Tononi, is listed on several joint patent applications between the Wisconsin Alumni Research Foundation and Philips Healthcare related to sleep technology. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the contributions of the following individuals who assisted in subject recruitment and data collection of control subjects: Eric Landsness, Michael J. Peterson, David Bachhuber, Daniela Dentico, Corinna Zennig, and Antoine Lutz and all the sleep technicians and staff at the Wisconsin Sleep Center for all their dedication and hard work. We also thank Armand Mensen, Lampros Perogamvros, Stephanie Jones, and most of all, Chiara Cirelli, for helpful discussions on the manuscript, and Luke Goodpaster for the supervision in sleep scoring.
REFERENCES
- 1.Pigarev IN, Nothdurft HC, Kastner S. Evidence for asynchronous development of sleep in cortical areas. Neuroreport. 1997;8:2557–60. doi: 10.1097/00001756-199707280-00027. [DOI] [PubMed] [Google Scholar]
- 2.Vyazovskiy VV, Olcese U, Hanlon EC, Nir Y, Cirelli C, Tononi G. Local sleep in awake rats. Nature. 2011;472:443–7. doi: 10.1038/nature10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rector DM, Topchiy IA, Carter KM, Rojas MJ. Local functional state differences between rat cortical columns. Brain Res. 2005;1047:45–55. doi: 10.1016/j.brainres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Rattenborg NC, Amlaner CJ, Lima SL. Unilateral eye closure and interhemispheric EEG asymmetry during sleep in the pigeon (Columba livia) Brain Behav Evol. 2001;58:323–32. doi: 10.1159/000057573. [DOI] [PubMed] [Google Scholar]
- 5.Peter-Derex L, Magnin M, Bastuji H. Heterogeneity of arousals in human sleep: a stereo-electroencephalographic study. NeuroImage. 2015;123:229–44. doi: 10.1016/j.neuroimage.2015.07.057. [DOI] [PubMed] [Google Scholar]
- 6.Nobili L, Ferrara M, Moroni F, et al. Dissociated wake-like and sleep-like electro-cortical activity during sleep. NeuroImage. 2011;58:612–9. doi: 10.1016/j.neuroimage.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 7.Mukhametov LM, Supin AY, Polyakova IG. Interhemispheric asymmetry of the electroencephalographic sleep patterns in dolphins. Brain Res. 1977;134:581–4. doi: 10.1016/0006-8993(77)90835-6. [DOI] [PubMed] [Google Scholar]
- 8.Magnin M, Rey M, Bastuji H, Guillemant P, Mauguiere F, Garcia-Larrea L. Thalamic deactivation at sleep onset precedes that of the cerebral cortex in humans. Proc Natl Acad Sci U S A. 2010;107:3829–33. doi: 10.1073/pnas.0909710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lesku JA, Vyssotski AL, Martinez-Gonzalez D, Wilzeck C, Rattenborg NC. Local sleep homeostasis in the avian brain: convergence of sleep function in mammals and birds? Proc Biol Sci. 2011;278:2419–28. doi: 10.1098/rspb.2010.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hung CS, Sarasso S, Ferrarelli F, et al. Local experience-dependent changes in the wake EEG after prolonged wakefulness. Sleep. 2013;36:59–72. doi: 10.5665/sleep.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernardi G, Siclari F, Yu X, et al. Neural and behavioral correlates of extended training during sleep deprivation in humans: evidence for local, task-specific effects. J Neurosci. 2015;35:4487–500. doi: 10.1523/JNEUROSCI.4567-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarasso S, Proserpio P, Pigorini A, et al. Hippocampal sleep spindles preceding neocortical sleep onset in humans. NeuroImage. 2014;86:425–32. doi: 10.1016/j.neuroimage.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 13.Funk CM, Honjoh S, Rodriguez AV, Cirelli C, Tononi G. Local slow waves in superficial layers of primary cortical areas during REM Sleep. Curr Biol. 2016;8(26):396–403. doi: 10.1016/j.cub.2015.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahowald MW, Schenck CH. Insights from studying human sleep disorders. Nature. 2005;437:1279–85. doi: 10.1038/nature04287. [DOI] [PubMed] [Google Scholar]
- 15.Broughton RJ. Sleep disorders: disorders of arousal? Enuresis, somnambulism, and nightmares occur in confusional states of arousal, not in “dreaming sleep”. Science. 1968;159:1070–8. doi: 10.1126/science.159.3819.1070. [DOI] [PubMed] [Google Scholar]
- 16.Zadra A, Pilon M. Non-rapid eye movement parasomnias: diagnostic methods. Sleep Med Clin. 2011;6:447–58. [Google Scholar]
- 17.Zadra A, Desautels A, Petit D, Montplaisir J. Somnambulism: clinical aspects and pathophysiological hypotheses. Lancet Neurol. 2013;12:285–94. doi: 10.1016/S1474-4422(12)70322-8. [DOI] [PubMed] [Google Scholar]
- 18.Petit D, Touchette E, Tremblay RE, Boivin M, Montplaisir J. Dyssomnias and parasomnias in early childhood. Pediatrics. 2007;119:e1016–25. doi: 10.1542/peds.2006-2132. [DOI] [PubMed] [Google Scholar]
- 19.Petit D, Pennestri MH, Paquet J, et al. Childhood Sleepwalking and sleep terrors: a longitudinal study of prevalence and familial aggregation. JAMA Pediatr. 2015;169:653–8. doi: 10.1001/jamapediatrics.2015.127. [DOI] [PubMed] [Google Scholar]
- 20.Ohayon MM, Guilleminault C, Priest RG. Night terrors, sleepwalking, and confusional arousals in the general population: their frequency and relationship to other sleep and mental disorders. J Clin Pscychiatry. 1999;60:268–76. doi: 10.4088/jcp.v60n0413. [DOI] [PubMed] [Google Scholar]
- 21.Siclari F, Khatami R, Urbaniok F, et al. Violence in sleep. Brain. 2010;133:3494–509. doi: 10.1093/brain/awq296. [DOI] [PubMed] [Google Scholar]
- 22.Ingravallo F, Poli F, Gilmore EV, et al. Sleep-related violence and sexual behavior in sleep: a systematic review of medical-legal case reports. J Clin Sleep Med. 2014;10:927–35. doi: 10.5664/jcsm.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oudiette D, Leu S, Pottier M, Buzare MA, Brion A, Arnulf I. Dreamlike mentations during sleepwalking and sleep terrors in adults. Sleep. 2009;32:1621–7. doi: 10.1093/sleep/32.12.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohayon MM, Mahowald MW, Dauvilliers Y, Krystal AD, Leger D. Prevalence and comorbidity of nocturnal wandering in the U.S. adult general population. Neurology. 2012;78:1583–9. doi: 10.1212/WNL.0b013e3182563be5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montplaisir J, Petit D, Pilon M, Mongrain V, Zadra A. Does sleepwalking impair daytime vigilance? J Clin Sleep Med. 2011;7:219. [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez R, Jaussent I, Scholz S, Bayard S, Montplaisir J, Dauvilliers Y. Functional impairment in adult sleepwalkers: a case-control study. Sleep. 2013;36:345–51. doi: 10.5665/sleep.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez R, Jaussent I, Dauvilliers Y. Objective daytime sleepiness in patients with somnambulism or sleep terrors. Neurology. 2014;83:2070–6. doi: 10.1212/WNL.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 28.Desautels A, Zadra A, Labelle MA, Dauvilliers Y, Petit D, Montplaisir J. Daytime somnolence in adult sleepwalkers. Sleep Med. 2013;14:1187–91. doi: 10.1016/j.sleep.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 29.Bassetti C, Vella S, Donati F, Wielepp P, Weder B. SPECT during sleepwalking. Lancet. 2000;356:484–5. doi: 10.1016/S0140-6736(00)02561-7. [DOI] [PubMed] [Google Scholar]
- 30.Labelle MA, Dang-Vu TT, Petit D, Desautels A, Montplaisir J, Zadra A. Sleep deprivation impairs inhibitory control during wakefulness in adult sleepwalkers. J Sleep Res. 2015;24:658–65. doi: 10.1111/jsr.12315. [DOI] [PubMed] [Google Scholar]
- 31.Terzaghi M, Sartori I, Tassi L, et al. Dissociated local arousal states underlying essential clinical features of non-rapid eye movement arousal parasomnia: an intracerebral stereo-electroencephalographic study. J Sleep Res. 2012;21:502–6. doi: 10.1111/j.1365-2869.2012.01003.x. [DOI] [PubMed] [Google Scholar]
- 32.Terzaghi M, Sartori I, Tassi L, et al. Evidence of dissociated arousal states during NREM parasomnia from an intracerebral neurophysiological study. Sleep. 2009;32:409–12. doi: 10.1093/sleep/32.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarasso S, Pigorini A, Proserpio P, Gibbs SA, Massimini M, Nobili L. Fluid boundaries between wake and sleep: experimental evidence from Stereo-EEG recordings. Arch Ital Biol. 2014;152:169–77. doi: 10.12871/0002982920142311. [DOI] [PubMed] [Google Scholar]
- 34.Januszko P, Niemcewicz S, Gajda T, et al. Sleepwalking episodes are preceded by arousal-related activation in the cingulate motor area: EEG current density imaging. Clin Neurophysiol. 2016;127:530–6. doi: 10.1016/j.clinph.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 35.Blatt I, Peled R, Gadoth N, Lavie P. The value of sleep recording in evaluating somnambulism in young adults. Electroencephalogr Clin Neurophysiol. 1991;78:407–12. doi: 10.1016/0013-4694(91)90058-c. [DOI] [PubMed] [Google Scholar]
- 36.Espa F, Ondze B, Deglise P, Billiard M, Besset A. Sleep architecture, slow wave activity, and sleep spindles in adult patients with sleepwalking and sleep terrors. Clin Neurophysiol. 2000;111:929–39. doi: 10.1016/s1388-2457(00)00249-2. [DOI] [PubMed] [Google Scholar]
- 37.Fisher C, Kahn E, Edwards A, Davis DM. A psychophysiological study of nightmares and night terrors: i. physiological aspects of the stage 4 night terror. J Nerv Ment Dis. 1973;157:75–98. doi: 10.1097/00005053-197308000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Gaudreau H, Joncas S, Zadra A, Montplaisir J. Dynamics of slow-wave activity during the NREM sleep of sleepwalkers and control subjects. Sleep. 2000;23:755–60. [PubMed] [Google Scholar]
- 39.Guilleminault C, Kirisoglu C, Bao G, Arias V, Chan A, Li KK. Adult chronic sleepwalking and its treatment based on polysomnography. Brain. 2005;128:1062–9. doi: 10.1093/brain/awh481. [DOI] [PubMed] [Google Scholar]
- 40.Guilleminault C, Kirisoglu C, da Rosa AC, Lopes C, Chan A. Sleepwalking, a disorder of NREM sleep instability. Sleep Med. 2006;7:163–70. doi: 10.1016/j.sleep.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Guilleminault C, Poyares D, Abat F, Palombini L. Sleep and wakefulness in somnambulism: a spectral analysis study. J Psychosom Res. 2001;51:411–6. doi: 10.1016/s0022-3999(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 42.Jaar O, Pilon M, Carrier J, Montplaisir J, Zadra A. Analysis of slow-wave activity and slow-wave oscillations prior to somnambulism. Sleep. 2010;33:1511–6. doi: 10.1093/sleep/33.11.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacobson A, Kales A. Somnambulism: all-night EEG and related studies. Res Publ Assoc Res Nerv Ment Dis. 1967;45:424–55. [PubMed] [Google Scholar]
- 44.Joncas S, Zadra A, Paquet J, Montplaisir J. The value of sleep deprivation as a diagnostic tool in adult sleepwalkers. Neurology. 2002;58:936–40. doi: 10.1212/wnl.58.6.936. [DOI] [PubMed] [Google Scholar]
- 45.Kales A, Jacobson A, Paulson MJ, Kales JD, Walter RD. Somnambulism: psychophysiological correlates. I. All-night EEG studies. Arch Gen Psychiatry. 1966;14:586–94. doi: 10.1001/archpsyc.1966.01730120026004. [DOI] [PubMed] [Google Scholar]
- 46.Perrault R, Carrier J, Desautels A, Montplaisir J, Zadra A. Electroencephalographic slow waves prior to sleepwalking episodes. Sleep Med. 2014;15:1468–72. doi: 10.1016/j.sleep.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 47.Pilon M, Montplaisir J, Zadra A. Precipitating factors of somnambulism Impact of sleep deprivation and forced arousals. Neurology. 2008;70:2284–90. doi: 10.1212/01.wnl.0000304082.49839.86. [DOI] [PubMed] [Google Scholar]
- 48.Pilon M, Zadra A, Joncas S, Rompré S, Montplaisir J. Hypersynchronous delta activity and somnambulism: effects of 38 hours of sleep deprivation. Sleep. 2003;26:A316–7. (Abstract Suppl) [Google Scholar]
- 49.Pilon M, Zadra A, Joncas S, Rompré S, Montplaisir J. Hypersynchronous delta activity and somnambulism: differences between frontal and central leads. Sleep. 2003;26:A318. (Abstract Suppl) [Google Scholar]
- 50.Schenck CH, Pareja JA, Patterson AL, Mahowald MW. Analysis of polysomnographic events surrounding 252 slow-wave sleep arousals in thirty-eight adults with injurious sleepwalking and sleep terrors. J Clin Neurophysiol. 1998;15:159–66. doi: 10.1097/00004691-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 51.Zadra A, Pilon M, Joncas S, Rompré S, Montplaisir J. Analysis of postarousal EEG activity during somnambulistic episodes. J Sleep Res. 2004;13:279–84. doi: 10.1111/j.1365-2869.2004.00404.x. [DOI] [PubMed] [Google Scholar]
- 52.Zadra A, Pilon M, Montplaisir J. Polysomnographic diagnosis of sleepwalking: effects of sleep deprivation. Ann Neurol. 2008;63:513–9. doi: 10.1002/ana.21339. [DOI] [PubMed] [Google Scholar]
- 53.Zucconi M, Oldani A, Ferini-Strambi L, Smirne S. Arousal fluctuations in non-rapid eye movement parasomnias: the role of cyclic alternating pattern as a measure of sleep instability. J Clin Neurophysiol. 1995;12:147–54. [PubMed] [Google Scholar]
- 54.American Academy of Sleep Medicine. International classification of sleep disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 55.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 56.Landsness EC, Goldstein MR, Peterson MJ, Tononi G, Benca RM. Antidepressant effects of selective slow wave sleep deprivation in major depression: a high-density EEG investigation. J Psychiatr Res. 2011;45:1019–26. doi: 10.1016/j.jpsychires.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferrarelli F, Smith R, Dentico D, et al. Experienced mindfulness meditators exhibit higher parietal-occipital EEG gamma activity during NREM sleep. PloS One. 2013;8:e73417. doi: 10.1371/journal.pone.0073417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.First M, Spitzer R, Williams J, Gibbon M. Washington, DC: American Psychiatric Association; 1995. Structured clinical interview for DSM-IV-non-patient edition (SCID-NP, Version 1.0) [Google Scholar]
- 59.McNair D, Lorr M, Droppleman L. Profile of mood states (POMS) 1989 [Google Scholar]
- 60.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 61.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 62.Derogatis LR, Unger R. Symptom checklist-90-revised. Corsini Encyclopedia of Psychology. 2010 [Google Scholar]
- 63.Hoddes E, Zarcone V, Dement W. Development and use of Stanford Sleepiness scale (SSS) Psychophysiology. 1973;10:431–6. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- 64.Iber C, Ancoli-Israel S, Chesson A, Quan SF American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 65.Jacobson A, Kales A, Lehmann D, Zweizig JR. Somnambulism: all-night electroencephalographic studies. Science. 1965;148:975–7. doi: 10.1126/science.148.3672.975. [DOI] [PubMed] [Google Scholar]
- 66.Jones SG, Riedner BA, Smith RF, et al. Regional reductions in sleep electroencephalography power in obstructive sleep apnea: a high-density EEG study. Sleep. 2014;37:399. doi: 10.5665/sleep.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riedner BA, Hulse BK, Murphy MJ, Ferrarelli F, Tononi G. Temporal dynamics of cortical sources underlying spontaneous and peripherally evoked slow waves. Prog Brain Res. 2011;193:201–18. doi: 10.1016/B978-0-444-53839-0.00013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Riedner BA, G M, Plante DT, Rumble ME, Ferrarelli F, Tononi G, Benca RM. Regional patterns of elevated alpha and high-frequency EEG activity during NREM sleep in chronic insomnia. Sleep. 2016:39. doi: 10.5665/sleep.5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 70.Kurth S, Jenni OG, Riedner BA, Tononi G, Carskadon MA, Huber R. Characteristics of sleep slow waves in children and adolescents. Sleep. 2010;33:475–80. doi: 10.1093/sleep/33.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murphy M, Bruno MA, Riedner BA, et al. Propofol anesthesia and sleep: a high-density EEG study. Sleep. 2011;34:283. doi: 10.1093/sleep/34.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jung TP, Makeig S, Humphries C, et al. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37:163–78. [PubMed] [Google Scholar]
- 73.Song J, Davey C, Poulsen C, et al. EEG source localization: sensor density and head surface coverage. J Neurosci Methods. 2015;256:9–21. doi: 10.1016/j.jneumeth.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 74.Murphy M, Riedner BA, Huber R, Massimini M, Ferrarelli F, Tononi G. Source modeling sleep slow waves. Proc Natl Acad Sci U S A. 2009;106:1608–13. doi: 10.1073/pnas.0807933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol. 2002;24:5–12. [PubMed] [Google Scholar]
- 76.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Plante DT, Landsness EC, Peterson MJ, et al. Altered slow wave activity in major depressive disorder with hypersomnia: a high density EEG pilot study. Psychiatr Res. 2012;201:240–4. doi: 10.1016/j.pscychresns.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 79.Oliviero A, Della Marca G, Tonali P, et al. Functional involvement of cerebral cortex in adult sleepwalking. J Neurol. 2007;254:1066–72. doi: 10.1007/s00415-006-0489-0. [DOI] [PubMed] [Google Scholar]
- 80.Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 81.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nature reviews. Neuroscience. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 82.Seghier ML. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist. 2013;19:43–61. doi: 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Treede R-D, Kenshalo DR, Gracely RH, Jones AK. The cortical representation of pain. Pain. 1999;79:105–11. doi: 10.1016/s0304-3959(98)00184-5. [DOI] [PubMed] [Google Scholar]
- 84.Ohayon MM, Priest RG, Zulley J, Smirne S. The place of confusional arousals in sleep and mental disorders: findings in a general population sample of 13,057 subjects. J Nerv Ment Dis. 2000;188:340–8. doi: 10.1097/00005053-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 85.Laberge L, Tremblay RE, Vitaro F, Montplaisir J. Development of parasomnias from childhood to early adolescence. Pediatrics. 2000;106:67–74. doi: 10.1542/peds.106.1.67. [DOI] [PubMed] [Google Scholar]
- 86.Bornemann MA, Mahowald MW, Schenck CH. Parasomnias: clinical features and forensic implications. Chest. 2006;130:605–10. doi: 10.1378/chest.130.2.605. [DOI] [PubMed] [Google Scholar]
- 87.Arnulf I, Zhang B, Uguccioni G, et al. A scale for assessing the severity of arousal disorders. Sleep. 2014;37:127–36. doi: 10.5665/sleep.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lopez R, Jaussent I, Dauvilliers Y. Pain in sleepwalking: a clinical enigma. Sleep. 2015;38:1693–8. doi: 10.5665/sleep.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Manni R, Terzaghi M, Repetto A. The FLEP scale in diagnosing nocturnal frontal lobe epilepsy, NREM and REM parasomnias: data from a tertiary sleep and epilepsy unit. Epilepsia. 2008;49:1581–5. doi: 10.1111/j.1528-1167.2008.01602.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.