Abstract
Study Objectives:
To evaluate the association between sleep duration and neurocognitive function in a representative sample of middle-aged to older Hispanic/Latino adults in the US. We tested the hypothesis that sleep duration has a nonlinear, inverted U-shaped association with neurocognitive function.
Methods:
We performed a cross-sectional analysis from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) participants ages 45–74 years (n = 8,676). HCHS/SOL is a community-based cohort from four US urban areas sampled using a probability design from 2008–2011. Self-reported sleep duration was calculated as a weighted average of the difference between habitual wake and bedtimes assessed by separate questions for weekdays and weekends. Neurocognitive function was measured with standardized scores for Word (Phonemic) Fluency (WF), Brief-Spanish English Verbal learning test (B-SEVLT), and Digit Symbol Substitution (DSS) tests.
Results:
The mean age was 56.5 years; 55% were women; and 40.4% had less than high school education. Average sleep duration was 7.8 ± 1.7 hours. There was an inverted U-shaped association with sleep duration and WF, B-SEVLT sum, and the DSS, with no association with B-SEVLT delayed-recall. Participants with intermediate sleep duration had the best neurocognitive function, while long sleepers had worse neurocognitive function adjusting for demographic, behavioral, and medical factors, daytime sleepiness, and use of sleep medications.
Conclusions:
Sleep duration had curvilinear inverted U-shaped associations with neurocognitive function, with worse scores among participants with longer sleep duration. These findings may provide a framework to further examine sleep duration in the prevention and treatment of neurocognitive disorders.
Citation:
Ramos AR, Tarraf W, Daviglus M, Davis S, Gallo LC, Mossavar-Rahmani Y, Penedo FJ, Redline S, Rundek T, Sacco RL, Sotres-Alvarez D, Wright CB, Zee PC, González HM. Sleep duration and neurocognitive function in the Hispanic Community Health Study/Study of Latinos. SLEEP 2016;39(10):1843–1851.
Keywords: sleep, Hispanic/Latino, epidemiology, neurocognitive
Significance.
Latinos, the largest US ethnic/racial minority, have a 1.5 increased risk of Alzheimer disease and related dementias (ADRD), when compared to Whites. We performed a cross-sectional evaluation of sleep duration and neurocognitive function in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL), the largest study of Latinos in the U.S. We found curvilinear (inverted U-shaped) associations between sleep duration and neurocognitive function. That is, average (7.8 hours ± 1.7 hours) sleep duration was associated with better neurocognitive scores. Our findings confirm that sleep is critical for proper neurocognitive function and brain health among diverse, middle-aged and older Latinos. Our findings provide the opportunity to follow the HCHS/SOL cohort and better understand midlife sleep with neurocognitive decline and ADRD in future studies.
INTRODUCTION
Longitudinal epidemiological studies demonstrate that short (less than 5–6 h) and long (more than 8–9 h) sleep duration is associated with increased mortality and stroke.1 Less is known about associations between neurocognitive function and sleep duration among middle-aged and older adults, especially those from diverse ethnic/racial backgrounds. Neurocognitive disorders now represent a major clinical and public health problem related to inadequate sleep.2,3 Existing research on sleep duration and neurocognitive function mainly involved older or non-Hispanic white cohorts, which limits generalizability to middle-aged and non-white populations.4–6
A few studies have observed associations between long sleep duration and cognitive function, but these studies are mostly from older cohorts that contain relatively small samples of Hispanic/Latinos and only included measures of global cognition such as the mini-mental examination.4–6 In addition, most studies have not accounted for daytime sleepiness, use of sleep medication, and sleep apnea, which are possible confounders between sleep duration and neurocognitive function.7,8 Hispanic/Latinos have a 1.5 increased risk of dementia when compared to non-Hispanic whites,9 but there is a paucity of studies evaluating sleep duration and neurocognitive function in Hispanic/Latino adults.
Previous studies suggest that Hispanic/Latinos may be at risk for both short and long sleep durations, but the associations may differ across different Hispanic/Latino groups.10–15 For example, in a cross-sectional analysis of NHANES,16 Mexicans, compared to non-Hispanic whites, had lower odds of long sleep, while the “other” Hispanic groups had higher odds of very short sleep duration (< 5 h per night). Mexicans born in Mexico, compared to US-born, were less likely to report short sleep duration. Recent data from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) observed significant differences in sleep duration across Hispanics/Latinos from four urban areas in the USA.17 In HCHS/SOL, individuals of Cuban and Mexican backgrounds had the lowest prevalence of short sleep duration, while short sleep was most prevalent among individuals of Puerto Rican background.17 Neurocognitive disorders are expected to increase with the aging population.2 Therefore, evaluating sleep duration and neurocognitive function may provide a critical understanding of how sleep serves as a modifiable risk factor in at-risk populations.
Sleep duration has a U-shaped association with adverse health outcome7,8,18; therefore, we tested the hypothesis that sleep duration has a nonlinear, inverted U-shaped association with neurocognitive function, in the largest study of Hispanic/ Latino adults in the U.S.
METHODS
Population
We analyzed data from HCHS/SOL, a multisite, community-based prospective cohort study. The study's baseline measurements were collected from 2008 to 2011, and included self-identified Hispanic/Latino adults aged 18–74 years (n = 16,415), with an oversample (59%) of participants ages 45–74 years to facilitate examination of target outcomes (e.g., cardiovascular mortality).
The HCHS/SOL sampling frame included 4 major U.S. cities (Bronx, NY; Chicago, IL; Miami, FL; and San Diego, CA) with known substantial Hispanic/Latino concentrations. The study used a 2-stage area probability sample design that includes clustering, stratification, and probability weighting. Detailed description of the HCHS/SOL aims, and study and sampling design are published elsewhere.19–21
Protocol and Measurements
The baseline examination included 22 modules designed to collect extensive sociodemographic (e.g., economic), behavioral (e.g., diet, sleep), and medical (e.g., medical history, and medication use) information on study participants. Questionnaires were administered in Spanish or English based on the participant's language preference. Respondents also underwent medical assessments, fasting laboratory testing and oral glucose tolerance test. The HCHS/SOL was approved by the institutional review boards at each field center, and all participants gave written consent.
Neurocognitive Outcomes
The neurocognitive tests were administered in the participants' preferred language during face-to-face interviews by trained, bilingual research assistants. Three neurocognitive tests were used in this study: (1) the Brief-Spanish English Verbal Learning Test (B-SEVLT),22,23 (2) the Controlled Oral Word Association (or Word/Phonemic Fluency; WF) Test of the Multilingual Aphasia Examination,19 and (3) the Digit Symbol Sub-test (DSS) of the Wechsler Adult Intelligence Scale-Revised.24
The WF is a test of verbal fluency, the B-SEVLT assesses verbal episodic learning and memory function, while the DSS is a test of psychomotor speed and working memory.19
The order of B-SEVLT administration was fixed across 3 learning trials in which participants were asked to recall 15 common words (List A). After the third trial, a 15-item distractor list (List B) was introduced in which participants were asked to repeat aloud each word. Immediately following the interference trial, a delayed free-recall trial for List A occurred.
The WF test has been previously described in detail. Briefly, study participants were instructed to orally generate as many unique words beginning with a specified letter (F and A) as possible within 60 seconds.
The DSS test require participants to rapidly copy symbols encoded to numbers (1–9) onto blank spaces below numbers printed on scoring sheets within 90 seconds. The outcomes were: (1) the summed total number of items correctly recalled across the 3 learning trials (B-SEVLT-sum), (2) the memory or delayed recall trial (B-SEVLT-recall), (3) the sum of correctly generated words beginning with the letters F and A (WF), and (4) the total correct score of the DSS.19
We standardized all neurocognitive scores (i.e. z-score transformed) to facilitate interpretation and comparison of scores across tests. The neurocognitive assessments were chosen based on published validity studies and availability in Spanish and English. Detailed description of the HCHS/SOL neurocognitive tests are published elsewhere.19
Primary Exposure: Sleep Duration
The following questions were used to determine sleep duration in our target population: What time do you usually go to bed? and What time do you usually wake up? Average sleep duration was computed as the weighted average of weekday and weekend sleep (5/7 weekday + 2/7 weekend).17 We also categorized sleep duration into quartiles based on the HCHS/ SOL target population into 3 groups: short duration (lowest quartile), intermediate (interquartile range, [25th to 75th percentile] and long sleep duration [highest quartile]). We used these cutoffs based on the distribution of sleep duration and previous literature on sleep duration and adverse health outcomes.17,25
Covariates
We included sex and continuous age (years). We also controlled for sociodemographic, health behavior, health conditions, mental health indicators, and sleep covariates. Sociodemo-graphic indicators included education in 3 categories (less than high school, high school or equivalent degree, more than high school), income (≤ $20,000, $20,001–50,000, ≥ $50,001, not reported), occupation (non-skilled, service, professional, other), Hispanic background (Cuban, Dominican, Mexican, Puerto Rican, Central American, South American), and language preference (Spanish, English). Health behavior factors included a 4-category indicator of body mass index (BMI; < 18.5; 18.5 to < 25; 25 to < 30; and 30 +), and self-reported smoking status (not current smoker; current smoker). As previously described, impaired glucose tolerance and diabetes mellitus were based on the definition of the American Diabetes Association using fasting glucose, oral glucose tolerance testing, hemoglobin A1C levels and use of hypoglycemic medications. Hypertension was defined as a systolic blood pressure (BP) ≥ 140 and/or diastolic BP ≥ 90 mm Hg, respectively, or antihypertensive medication use. The analyses also controlled for depressive symptoms score (Center for Epidemiological Studies Depression Scale; CES-D10), anxiety score (State-Trait Anxiety Inventory-10-item),26,27 and self-reported prevalent stroke/TIA. Finally, given the HCHS/SOL multi-center design, the final models also controlled for potential field center effects.
Sleep Covariates
Sleep history and symptoms were assessed using items from the Sleep Heart Health Study Sleep Habits Questionnaire and the Epworth Sleepiness Scale (ESS).17,28,29 The sleep habits questionnaire ascertained symptoms of snoring, breathing pauses in sleep, insomnia, and restless legs symptoms. We also accounted for self-reported use of sleep medications with responses ranging from no-use per week to use more than 5 days per week.
Study Subpopulation
The focus of this study was on respondents' ages 45–74 years agreeing to participate in the neurocognitive module of HCHSSOL (n = 9,623). We excluded 227 participants who did not report a specific Latino background (n = 9,396). Furthermore, we only included participants with complete data on the model covariates and the sleep duration variable. Among respondents satisfying criteria for inclusion, 4.7% had missing values on our model covariates (n = 8,950), and an additional 3.1% had missing values on sleep duration (n = 8,676). A detailed accounting of the sample exclusion criteria and missingness patterns is included in the supplemental material (Table S1). To ensure that the impact of missing covariates does not bias our reported results, we conducted bivariate sensitivity analyses to compare the association between sleep duration and neurocognitive scores in (1) the sample of participants not excluding respondents with missing values on the covariates and (2) the subsample of respondents excluding those with incomplete covariate data. This sensitivity check revealed that the results were largely qualitatively unchanged—an indication of the robustness of our reported findings. Respondents with incomplete data on sleep duration were slightly more likely to be male, Puerto Rican, and to originate from the Chicago and Miami sites of the study. Respondents with missing sleep duration values were also more likely to be smokers, more likely to self-report a stroke/TIA, and to have slightly higher average anxiety scores. To accommodate HCHS/SOL's complex sample design, all analyses were performed using survey procedures that account for clustering, stratification, and unequal probability weighting in the Stata software package version 13.1. Methods appropriate for the analyses of subpopulations were applied to generate our parameters' estimates. We used a Taylor Series Linearization approach to variance estimation to obtain sample design adjusted standard errors.
Analytic Approach
First, we generated descriptive statistics to characterize the overall study population and by sleep duration intervals. Second, we fit linear survey regression models to test the relationships between sleep duration and its quadratic order (squared sleep duration) and each of the 4 neurocognitive tests. We fit 3 models for each neurocognitive test. In the first model (unadjusted) we tested the curvilinear (inverted U-shaped) association between sleep duration and neurocognitive scores. We also considered other functional forms including higher order polynomials (e.g., cubic). The second model adjusted the baseline model to account for age, sex, and education. The third model further adjusted for sociodemographic, health behavior, health conditions, and sleep covariates. In additional models, we examined the interactions for several pre-specified sets of analyses; i.e., between sleep duration and sex and sleep duration and age groups (45–54; 55–64; 65–74), to test for differential effects of age and sex on neurocognitive function. We also used U-shaped specific tests to verify both the appropriateness of the second derivative and the range of the inflection point data.30 To facilitate the interpretation of our results, we calculated and plotted the adjusted average neurocognitive scores and their 95% confidence bounds over the range of reported sleep duration.
Sensitivity Analyses
We repeated the primary analysis, excluding participants with self-reported histories of stroke and transient ischemic attacks using all modeling steps detailed above. The findings from these analyses (reported in Table S3 and Figure S1 in the supplemental material) indicated that our reported results and conclusions were robust to this sample re-specification. Additionally, we considered a categorical operationalization of sleep duration using 5 percentile groupings (1 = < 5th; 2 = 5th to < 25th; 3 = 25th to < 75th; 4 = 75th to < 95th; and 5 = 95th +). Analyses were repeated in accordance with the steps detailed above. The result of these analyses are described below and presented in Table S4 in the supplemental material. Additionally, the estimated marginal effects and their 95% confidence intervals are plotted in Figure S2 in the supplemental material to facilitate interpretation. Overall the findings pointed to similar curvilinear associations between sleep duration and cognitive scores with lower scores particularly apparent at higher sleep duration centiles (> 75th percent).
RESULTS
Weighted descriptive statistics for the overall target population and for the three sleep duration groups are presented in Table 1. The overall range of self-reported sleep duration was between 3 and 13.5 h (IQR = 7–8.7 h). The average sleep duration was 7.85 h (SD 1.73 h). The mean age was 57 years, and more than half were females. Mexican background was the largest Hispanic/Latino group represented in our target population, followed by Cuban and Puerto Rican backgrounds. Forty percent had less than high school education (40.4%), nearly half (46%) had a household income ≤ $20,000, and close to three-fifths had a service or non-skilled occupation. Spanish was the language of preference (86%).
Table 1.
Weighted sociodemographic, behavioral and health characteristics of middle-aged and older (45 to 74 years) Hispanics/Latino adults, HCHS/SOL 2008–2011 (n = 8,676).
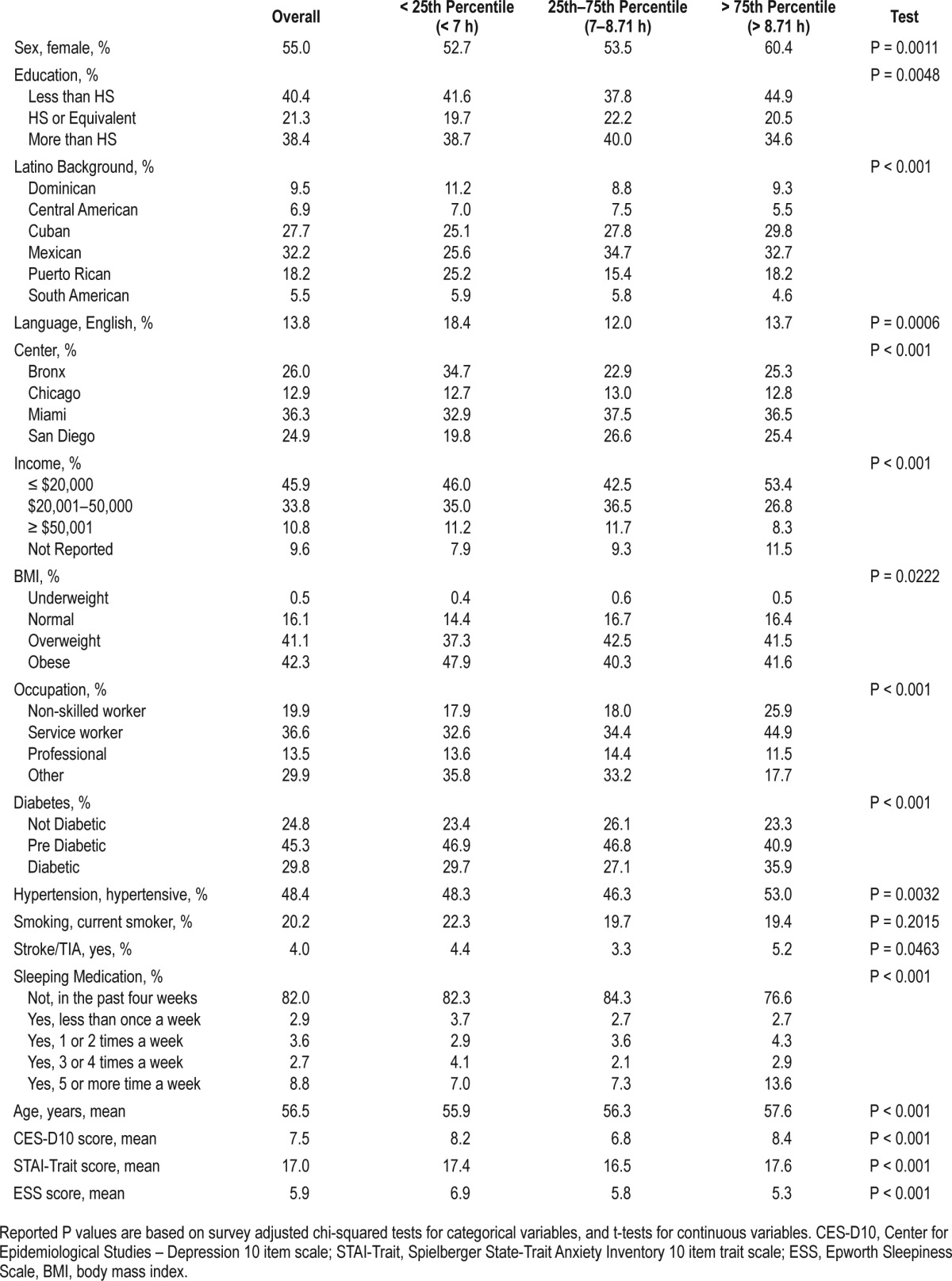
Compared to those with sleep duration within the IQR (7–8.7 h), those in the lowest (< 7 h; short sleep) and highest sleep quartiles (> 8.7 h; long sleep) were more likely to have lower education, lower incomes and to use English as their preferred language. Those with long sleep durations had an increased prevalence of diabetes mellitus and hypertension. Those with short sleep had increased prevalence of obesity and higher sleepiness scores; while those with long sleep reported using more sleep medications.
The regression analyses demonstrated (Table 2) a curvilinear (inverted U-shaped) association between sleep duration and neurocognitive function evidenced across all 4 cognitive tests.
Table 2.
Association between sleep duration and neurocognitive function in middle-aged and older (45 to 74 years) Hispanics/Latino adults, HCHS/SOL 2008–2011.
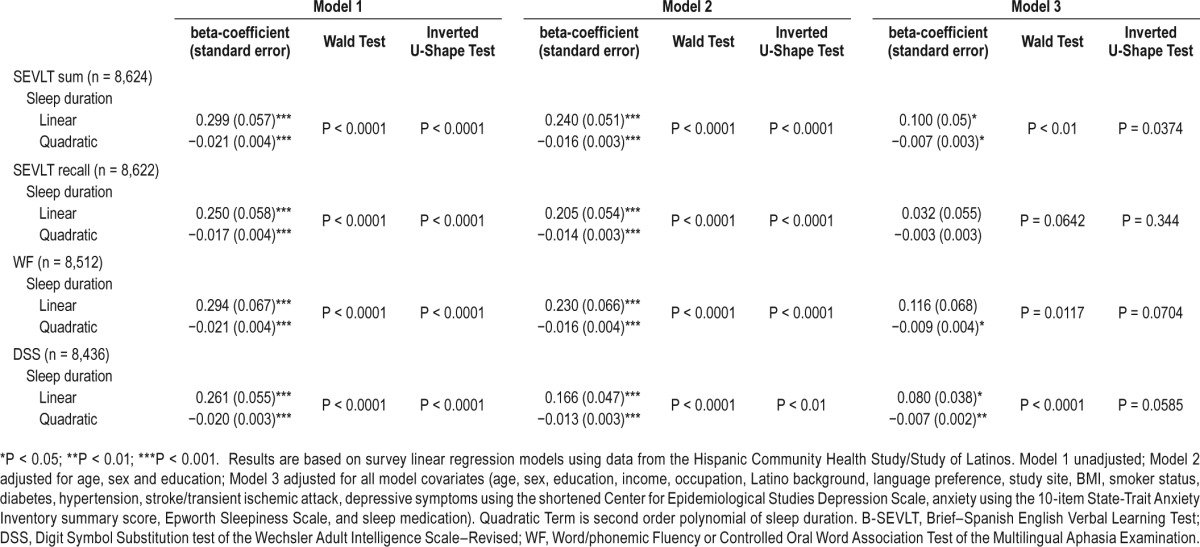
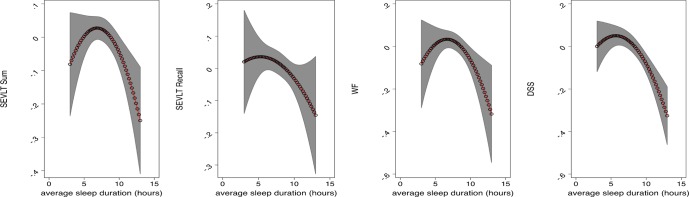
These associations remained after adjusting for age, sex, and education. With the exception of the B-SEVLT-Recall test (delayed memory), the quadratic term of sleep duration and neurocognitive function remained significant after adjustments for sociodemographic, health behavior, medical conditions, sleepiness, and sleep medication covariates. The inflection points for sleep duration in relation to neurocognitive scores that were derived from the adjusted models were B-SEVLTSum (6.8 h; SE = 0.7), B-SEVLT-Recall (5.3 h; SE = 3.2), WF (6.6 h; SE = 0.8), and DSS (5.7 h; SE = 0.9). Table 3 includes the estimated inflection points for the U-shaped curves, for all tests and models, and their SE and 95% CIs. Figure 1 plots the estimated average covariate-adjusted cognitive scores over the sleep duration continuum and their 95% confidence bounds.
Table 3.
Inflection point estimates and their 95% confidence intervals for sleep duration (h) and neurocognitive in middle-aged and older (45 to 74) Hispanics/Latino adults, HCHS/SOL 2008–2011.
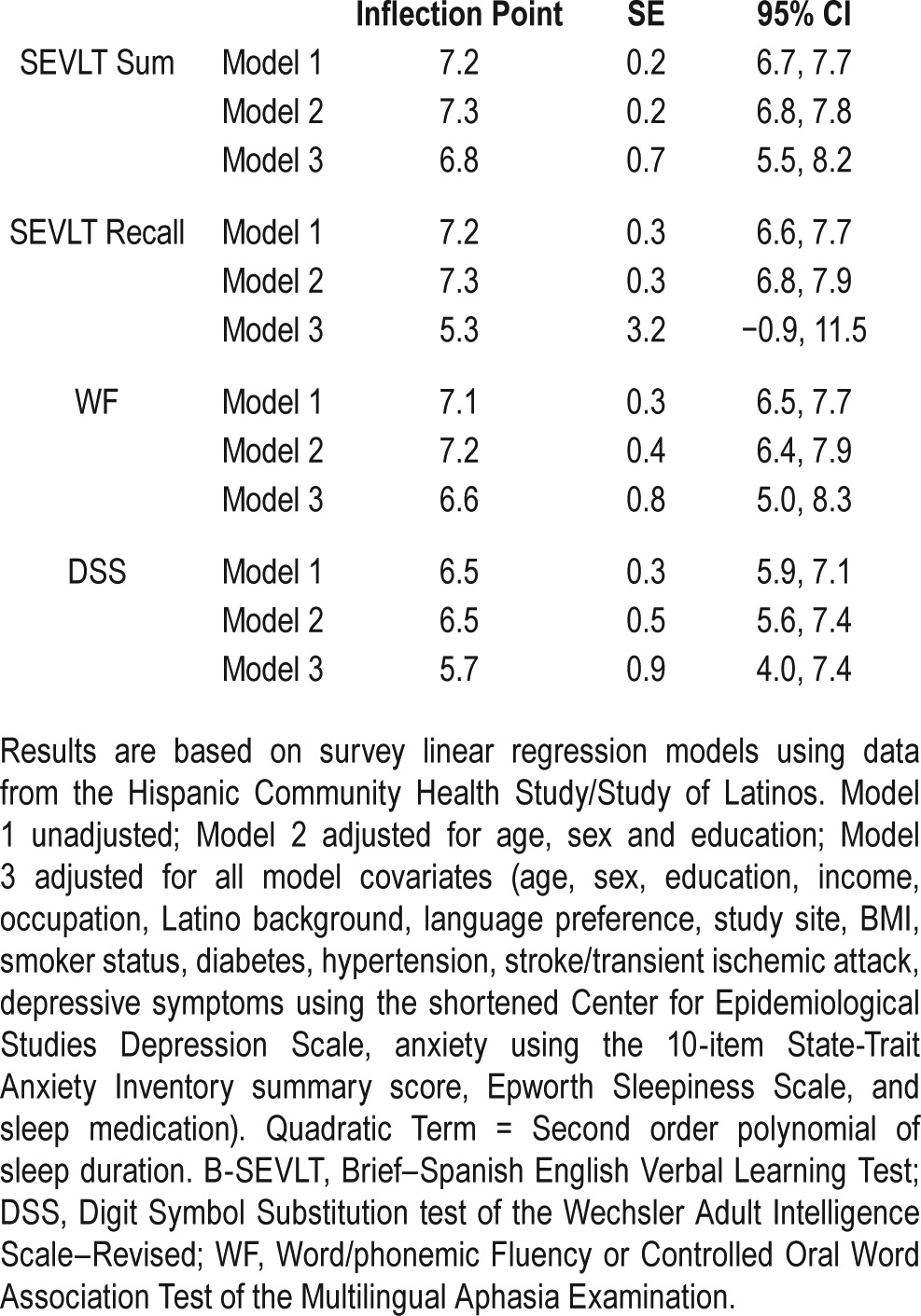
Figure 1.
Standardized neurocognitive score adjusted-mean by sleep duration of middle-aged and older (45 to 74 years) Hispanics/Latino adults, HCHS/ SOL 2008–2011. Estimates and their 95% confidence bounds are derived from survey linear regression models using data from the Hispanic Community Health Study/Study of Latinos. The models were adjusted for age, sex, education, income, occupation, Latino background, language preference, study site, BMI, smoker status, diabetes, hypertension, stroke/transient ischemic attack, depressive symptoms using the shortened Center for Epidemiological Studies Depression Scale, anxiety using the 10-item State-Trait Anxiety Inventory summary score, Epworth sleepiness scale, and sleep medication. The x-axis is the weighted average for weekday and weekend sleep duration in hours. The y-axis represents standardized neurocognitive test score in units of standard deviation. B-SEVLT, Brief–Spanish English Verbal Learning Test; DSS, Digit Symbol Substitution test of the Wechsler Adult Intelligence Scale– Revised; WF, Word/phonemic Fluency or Controlled Oral Word Association Test of the Multilingual Aphasia Examination.
After full adjustment for our covariates, participants with shorter sleep durations had similar neurocognitive scores than participants with intermediate sleep durations, while participants with longer sleep duration had worse neurocognitive scores across all tests (Figure 1, Figure S2 in the supplemental material). Incremental models were constructed to determine the role of our model covariates in explaining the curvilinear association between sleep duration and B-SEVLT-recall (verbal memory). We fit 5 additional models that sequentially controlled for sociodemographic, mental health, self-reported medical conditions, health behavior factors, sleepiness, and sleep medication covariates. The statistical association between sleep duration and B-SEVLT-recall was largely explained by the sociodemographic controls including Hispanic/ Latino background group, language, income, employment, and study center. These results are presented in Table S2 in the supplemental material.
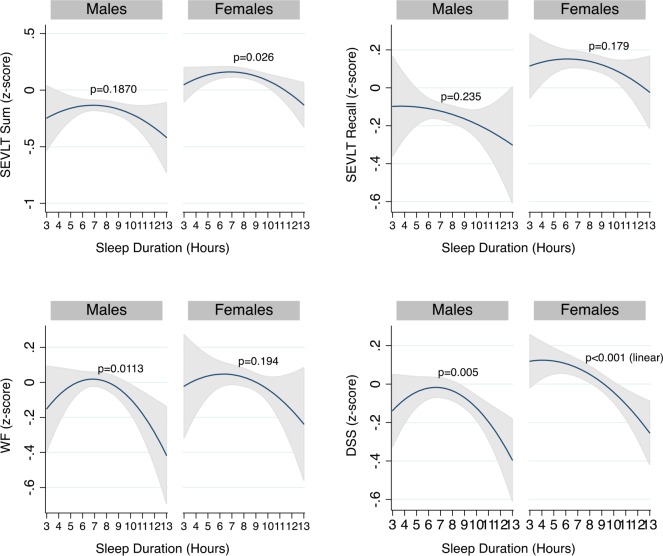
We observed an interaction between sleep duration and sex. Stratified analysis indicated that the association between sleep duration and neurocognitive function differed between males and females (Figure 2). The stratified analyses showed a curvilinear association between sleep duration and SEVLT sum among women (P = 0.026) but not men (P = 0.187). As with the overall analyses, we found that in fully adjusted models the association between sleep duration and SEVLT recall was not significant, explained through controlling for our model covariates, for both women and men. Finally, we found curvilinear associations between sleep duration and both the word fluency and the digit symbol substitution tests among men (P = 0.0113) but not among women (P = 0.005) (Figure 2). Finally, we found no consistent interaction effects between age and sleep duration with neurocognitive function (data not shown). Analyses of the subsample of participants without reported stroke/TIA (reported in Table S3 and Figure S1 in the supplemental material) were quantitatively and qualitatively similar to the overall sample, suggesting that our reported results and conclusions were robust to this sample re-specification. Finally, in Table S4 in the supplemental material, we present the results of the survey linear regression models testing the association between the percentiles of sleep duration and our neurocognitive tests of interest. Figure S2 in the supplemental material shows the estimates and their 95% confidence intervals derived from survey linear regression models across percentiles of sleep duration. The 5th percentile was ≤ 5.5 h of sleep; the 5 th to < 25th percentile was from 5.51 to 7.0 h; the 25th to < 75th percentile was from 7.1 to 8.7 h; the 75th to < 95th percentile was from 8.8 to 10.1 h; and the 95th percentile was > 10.1 h of reported sleep duration. Overall these findings pointed to similar curvilinear associations between sleep duration and cognitive scores with lower NC scores particularly apparent at higher durations of sleep (> 75th percentile).
Figure 2.
Sex differences in estimated average standardized neurocognitive scores over sleep duration in middle-aged and older (45 to 74 years) Hispanics/Latino adults, HCHS/SOL 2008–2011. Average NC score estimates and their 95% confidence bounds and sleep duration (linear and quadratic) stratified by sex in survey linear regression models using data from the Hispanic Community Health Study/Study of Latinos. The models were adjusted for age, education, income, occupation, Latino background, language preference, study site, BMI, smoker status, diabetes, hypertension, stroke/transient ischemic attack, depressive symptoms using the shortened Center for Epidemiological Studies Depression Scale, anxiety using the 10-item State-Trait Anxiety Inventory summary score, Epworth sleepiness scale, and sleep medication. The x-axis is the weighted average for weekday and weekend sleep duration in hours. The y-axis represents standardized neurocognitive test score in units of standard deviation. The P values reported in the graphs are based on survey adjusted Wald-tests that the linear and quadratic coefficients for sleep duration are jointly equal to zero. For the DSS, among females, only the linear term (P < 0.001) reached statistical significance. B-SEVLT, Brief–Spanish English Verbal Learning Test; DSS, Digit Symbol Substitution test of the Wechsler Adult Intelligence Scale–Revised; WF, Word/phonemic Fluency or Controlled Oral Word Association Test of the Multilingual Aphasia Examination.
DISCUSSION
In this large cohort of middle-aged and older Hispanic/Latino adults, the highest neurocognitive performances were observed among participants with intermediate sleep duration.31,32 In our study, the associations between neurocognitive function and sleep duration formed a consistent inverted U-shaped function across neurocognitive tests. The observed associations were not explained by the covariates considered in our study, with the exception of delayed memory, which was explained by sociodemographic factors.
Shorter sleep duration has been associated with increased deposition of brain amyloid33 and poor neurocognitive function34; however, the association was weak to null in our sample. Conversely, studies in young to middle-aged adults have shown associations between short sleep and worse executive function, working memory and attention.25 In contrast, HCHS/ SOL participants with longer sleep durations had markedly lower neurocognitive scores, consistent with evidence from cross-sectional and longitudinal studies suggesting that long sleep predicts impaired neurocognitive function and dementia in older adults.4,6–8
In our study, the association between sleep duration and neurocognitive function was seen across all cognitive tests, except for delayed memory. In a cohort of older men, disturbed sleep was associated primarily with decline in executive function, but not with global cognition.4 Similarly, the Whitehall II study described worse cognitive function (except for memory), in participants who changed their sleep duration, from average to short or average to long sleep, after 5 years of follow-up.35
The observed associations between sleep duration and neurocognitive function were seen in middle-aged and older adults across different neurocognitive tests. These contrast to other studies where self-reported sleep was evaluated in older adults with measures of global cognition (i.e., mini-mental score), which may be insensitive to cognitive variability.2,6
We observed an interaction between sleep duration and sex. Stratified analysis indicated that the association between sleep duration and cognitive function differed between males and females. Other studies have identified sex differences in sleep duration and neurocognitive function such that females with long sleep (> 9 h per night) had cognitive impairment at one year of follow-up; while short sleep (< 5 h per night) predicted cognitive decline in males.36 Our findings suggest that sleep duration may differentially affect neurocognitive function in men and women.
There is a paucity of studies examining a mechanism by which long sleep may cause adverse health consequences or neurocognitive dysfunction.37 Some research suggests that sleep fragmentation may be the critical determinant of neurocognitive function in sleep and aging.25 Long sleep duration is also associated with cerebrovascular disease, increased brain white-matter hyperintensities and stroke; factors linked to worse neurocognitive outcomes.38–40 It is plausible that cerebrovascular damage associated with long sleep, affects white matter tracts and inter-connections between cortical areas and subcortical structures, resulting in neurocognitive dysfunction.41,42
This work represents an important advance in understanding sleep duration and neurocognitive function in a representative sample of middle-aged and older Hispanic/Latino adults from diverse backgrounds. The strengths of our study are the use of a large, diverse sample of Hispanic/Latino backgrounds, with multiple neurocognitive tests, strict quality-control procedures and use of standardized measurements and central scoring for both neurocognitive tests and sleep measures.
The limitations of this study include the use of self-reported sleep duration. In our study, the questions used for sleep duration may reflect time in bed, rather than sleep duration and we may have overestimated sleep by including sleep onset latency and wake after sleep onset.17
Different from previous studies,7,8 we controlled for important sleep confounders such as daytime sleepiness, use of sleep medications and excluded participants with sleep apnea by home sleep testing. However, we did not adjust for shift-work, daytime napping, and number of household members. In addition, we did not assess the participant's sleep schedules over time, which could have been informative. The cross-sectional nature of the analysis does not allow inference of causality. We cannot exclude possible influences of reverse causality due to preexisting disease or other confounders. As such these associations require future considerations.
Additionally, language, a surrogate for acculturation, did not change the associations between sleep duration and neurocognitive function in our sample. However, further investigations of acculturation measures (nativity, years of residence, and language preference) as modifiers of the relationship between sleep duration and neurocognitive performance are necessary. We did not define cases of cognitive impairment or dementia; however, given the cohort's younger mean age (57 years), dementia cases would likely be few. As this cohort ages, our findings provide an opportunity to longitudinally evaluate and act upon the factors associated with sleep-related neurocognitive dysfunction and brain health in midlife and older Hispanics/Latinos.19
In summary, we found that intermediate sleep duration was associated with higher neurocognitive function in the largest study of middle-aged and older Hispanics/Latinos of diverse backgrounds to date. We observed curvilinear inverted U-shaped associations between sleep duration and neurocognitive function, with worse scores among participants with longer sleep durations. Our findings may provide a framework by which sleep and neurocognitive dysfunction could be examined in longitudinally and treatment studies for the prevention of neurocognitive disorders.
DISCLOSURE STATEMENT
This was not an industry supported study. The Hispanic Community Health Study/Study of Latinos was carried out as a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (N01- HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following Institutes/Centers/Offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, NIH Institution-Office of Dietary Supplements. The project described was also supported by Grant Number 1KL2TR000461 (Dr. Ramos), Miami Clinical and Translational Science Institute, from the National Center for Advancing Translational Sciences and the National Institute on Minority Health and Health Disparities. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The project is also supported by grant number AG48642 (Dr. González and Tarraf). Dr. Redline has received research support from Jazz. Dr. Zee has received research support from Jazz and has consulted for Merck, Philips, and Vanda. The other authors have indicated no financial conflicts of interest. Statistical Analysis was performed by Wassim Tarraf, PhD.
ACKNOWLEDGMENTS
The authors thank the staff and participants of HCHS/
SOL for their important contributions. Investigators website: http://www.cscc.unc.edu/hchs
REFERENCES
- 1.Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32:1484–92. doi: 10.1093/eurheartj/ehr007. [DOI] [PubMed] [Google Scholar]
- 2.Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. The Lancet. Neurology. 2014;13:1017–28. doi: 10.1016/S1474-4422(14)70172-3. [DOI] [PubMed] [Google Scholar]
- 3.Lucey BP, Holtzman DM. How amyloid, sleep and memory connect. Nat Neurosci. 2015;18:933–4. doi: 10.1038/nn.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS sleep study. Sleep. 2011;34:1347–56. doi: 10.5665/SLEEP.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackwell T, Yaffe K, Laffan A, et al. Associations of objectively and subjectively measured sleep quality with subsequent cognitive decline in older community-dwelling men: the MrOS sleep study. Sleep. 2014;37:655–63. doi: 10.5665/sleep.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramos AR, Dong C, Elkind MS, et al. Association between sleep duration and the Mini-Mental Score: the Northern Manhattan Study. J Clin Sleep Med. 2013;9:669–73. doi: 10.5664/jcsm.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benito-Leon J, Bermejo-Pareja F, Vega S, Louis ED. Total daily sleep duration and the risk of dementia: a prospective population-based study. Eur J Neurol. 2009;16:990–7. doi: 10.1111/j.1468-1331.2009.02618.x. [DOI] [PubMed] [Google Scholar]
- 8.Benito-Leon J, Louis ED, Villarejo-Galende A, Romero JP, Bermejo-Pareja F. Long sleep duration in elders without dementia increases risk of dementia mortality (NEDICES) Neurology. 2014;83:1530–7. doi: 10.1212/WNL.0000000000000915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alzheimer's Association. 2014 Alzheimer's disease facts and figures. Alzheimer Dement. 2014;10:e47–92. doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based survey. Sleep. 2007;30:1096–103. doi: 10.1093/sleep/30.9.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hale L, Rivero-Fuentes E. Negative acculturation in sleep duration among Mexican immigrants and Mexican Americans. J Immigr Minor Health. 2011;13:402–7. doi: 10.1007/s10903-009-9284-1. [DOI] [PubMed] [Google Scholar]
- 12.Hale L, Troxel WM, Kravitz HM, Hall MH, Matthews KA. Acculturation and sleep among a multiethnic sample of women: the Study of Women's Health Across the Nation (SWAN) Sleep. 2014;37:309–17. doi: 10.5665/sleep.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stamatakis KA, Kaplan GA, Roberts RE. Short sleep duration across income, education, and race/ethnic groups: population prevalence and growing disparities during 34 years of follow-up. Ann Epidemiol. 2007;17:948–55. doi: 10.1016/j.annepidem.2007.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suarez E, Fang SC, Bliwise D, Yaggi HK, Araujo A. Disentangling racial/ethnic and socioeconomic differences in self-reported sleep measures: the Boston Area Community Health Survey. Sleep Health. 2015;1:90–7. doi: 10.1016/j.sleh.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Krueger PM, Friedman EM. Sleep duration in the United States: a cross-sectional population-based study. Am J Epidemiol. 2009;169:1052–63. doi: 10.1093/aje/kwp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whinnery J, Jackson N, Rattanaumpawan P, Grandner MA. Short and long sleep duration associated with race/ethnicity, sociodemographics, and socioeconomic position. Sleep. 2014;37:601–11. doi: 10.5665/sleep.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel SR, Sotres-Alvarez D, Castaneda SF, et al. Social and health correlates of sleep duration in a US Hispanic population: results from the Hispanic Community Health Study/Study of Latinos. Sleep. 2015;38:1515–22. doi: 10.5665/sleep.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grandner MA, Chakravorty S, Perlis ML, Oliver L, Gurubhagavatula I. Habitual sleep duration associated with self-reported and objectively determined cardiometabolic risk factors. Sleep Med. 2014;15:42–50. doi: 10.1016/j.sleep.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez HM, Tarraf W, Gouskova N, et al. Neurocognitive function among middle-aged and older Hispanic/Latinos: results from the Hispanic Community Health Study/Study of Latinos. Arch Clin Neuropsychol. 2015;30:68–77. doi: 10.1093/arclin/acu066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavange LM, Kalsbeek WD, Sorlie PD, et al. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:642–9. doi: 10.1016/j.annepidem.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorlie PD, Aviles-Santa LM, Wassertheil-Smoller S, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:629–41. doi: 10.1016/j.annepidem.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez HM, Mungas D, Haan MN. A verbal learning and memory test for English- and Spanish-speaking older Mexican-American adults. Clin Neuropsychol. 2002;16:439–51. doi: 10.1076/clin.16.4.439.13908. [DOI] [PubMed] [Google Scholar]
- 23.González HM, Mungas D, Reed BR, Marshall S, Haan MN. A new verbal learning and memory test for English- and Spanish-speaking older people. J Int Neuropsychol Soc. 2001;7:544–55. doi: 10.1017/s1355617701755026. [DOI] [PubMed] [Google Scholar]
- 24.Wechsler D. San Antonio, TX: The Psychological Corp.; 1981. WAIS-R Manual. [Google Scholar]
- 25.Scullin MK, Bliwise DL. Sleep, cognition, and normal aging: integrating a half century of multidisciplinary research. Perspect Psychol Sci. 2015;10:97–137. doi: 10.1177/1745691614556680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 27.Spielberger CD. Palo Alto, CA: Consulting Psychologists Press; 1983. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- 28.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 29.Redline S, Sotres-Alvarez D, Loredo J, et al. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med. 2014;189:335–44. doi: 10.1164/rccm.201309-1735OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lind JT, Mehlum H. With or without u? The appropriate test for a u-shaped relationship. Oxf Bull Econ Stat. 2010;72:109–18. [Google Scholar]
- 31.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843–4. doi: 10.5665/sleep.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. J Clin Sleep Med. 2015;11:591–2. doi: 10.5664/jcsm.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spira AP, Gamaldo AA, An Y, et al. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70:1537–43. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goel N, Rao H, Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2009;29:320–39. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrie JE, Shipley MJ, Akbaraly TN, Marmot MG, Kivimaki M, Singh-Manoux A. Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep. 2011;34:565–73. doi: 10.1093/sleep/34.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Potvin O, Lorrain D, Forget H, et al. Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep. 2012;35:491–9. doi: 10.5665/sleep.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grandner MA, Drummond SP. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Med Rev. 2007;11:341–60. doi: 10.1016/j.smrv.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leng Y, Cappuccio FP, Wainwright NW, et al. Sleep duration and risk of fatal and nonfatal stroke: a prospective study and meta-analysis. Neurology. 2015;84:1072–9. doi: 10.1212/WNL.0000000000001371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramos AR, Jin Z, Rundek T, et al. Relation between long sleep and left ventricular mass from a multi-ethnic elderly cohort. Am J Cardiol. 2013;112:599–603. doi: 10.1016/j.amjcard.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramos AR, Dong C, Rundek T, et al. Sleep duration is associated with white matter hyperintensity volume in older adults: the Northern Manhattan Study. J Sleep Res. 2014;23:524–30. doi: 10.1111/jsr.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain. 2005;128:2034–41. doi: 10.1093/brain/awh553. [DOI] [PubMed] [Google Scholar]
- 42.Wright CB, Festa JR, Paik MC, et al. White matter hyperintensities and subclinical infarction: associations with psychomotor speed and cognitive flexibility. Stroke. 2008;39:800–5. doi: 10.1161/STROKEAHA.107.484147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.