1. Introduction
Migraine and other severe headache disorders, which affect an estimated 16–23% of the U.S. population [40], are a major cause of personal suffering and functional impairment [29; 40]. Patients with chronic headaches often have high levels of psychological distress [8], including depressive symptoms [23; 37], anxiety [25; 41; 54], and somatization [21; 48]. Psychological distress can exacerbate the adverse impact of headache on health-related quality of life (HRQOL) [22], and may reduce efficacy of headache interventions [22]. Interventions targeting both physical and psychological dimensions of pain may produce maximal improvements in HRQOL.
1.1. Can targeted dietary changes modulate physical and psychological pain?
Dietary n-3 and n-6 fatty acids could alter biochemical mechanisms underlying physical and psychological dimensions of pain. Notably, n-3 and n-6 fatty acids are major structural components of neuronal, glial, and immune cell membranes [38], and biosynthetic precursors to several families of bioactive lipid mediators (e.g., eicosanoids, endovanilloids, resolvins)[34]. With several notable exceptions [19; 46], lipid mediators derived from n-6 fatty acids have pronociceptive properties [2; 32; 51], while mediators derived from n-3 fatty acids have antinociceptive actions [27; 31; 39; 53]. n-3 and n-6 fatty acids are also proposed to modulate the risk of developing depression and anxiety [1; 4; 16; 18; 24; 44; 50]. Therefore, in addition to physical pain relief, targeted manipulation of dietary n-3 and n-6 fatty acids may be able to reduce psychological distress and enhance HRQOL.
1.2. The Chronic Daily Headache (CDH) trial
The CDH trial was a randomized, parallel-group, 12-week trial designed to test the biochemical and clinical effects of a diet high in n-3 and low in n-6 fatty acids (the H3-L6 intervention) compared to a diet low in n-6 fatty acids (the L6 intervention) in a population with chronic headaches. In a previous manuscript [34], we reported that the H3-L6 intervention produced statistically significant, clinically relevant improvements in headache frequency and severity (Figure 1) [26; 34; 36]. However, we do not yet know whether this pain relief was accompanied by beneficial effects on psychological dimensions of pain or HRQOL. Nor do we know how the changes in individual n-3 and n-6 fatty acids in circulation relate to headache pain, psychological distress, and HRQOL.
Fig. 1. Pain reduction induced by H3-L6 and L6 dietary interventions.
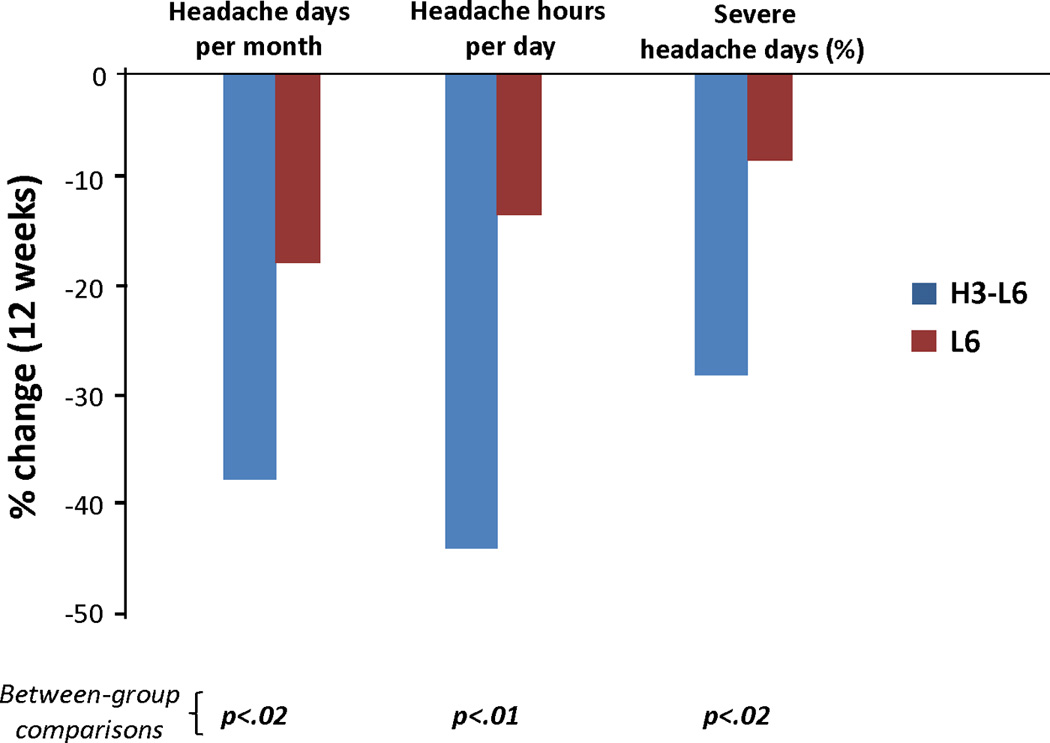
The H3-L6 intervention produced statistically significant, clinically relevant reductions in pain frequency and intensity in a population with Chronic Daily Headache.
The objectives of this paper are: (1) to determine the effect of the interventions on psychological distress and HRQOL, as measured by the Brief Symptom Inventory (BSI-18) and the Medical Outcomes Study Short Forms 12 (SF-12), respectively; (2) to examine the effect of the dietary interventions on the number of cases with substantial physical or mental impairments as defined by cutoff values in BSI-18, SF-12, Headache Impact Test-6 (HIT-6), and the number of Headache Days per month; and (3) examine how changes in n-3 and n-6 fatty acids relate to headache pain, psychological distress, and HRQOL.
2. Material and Methods
2.1 Trial overview
The trial protocol, detailed dietary composition and intervention methods, and the primary pain-related and biochemical outcomes were previously described [26; 34; 35; 47]. Briefly, adult women and men with any primary headache type meeting our CDH criteria—headaches ≥4 hours per day and ≥15 days per month for at least 3 months as well as a headache history of ≥2 years with headaches managed by a physician—were recruited to participate in an outpatient dietary trial. Inclusion and exclusion criteria are shown in Supplementary Table S1. After the nature and possible consequences of the trial were explained, all participants provided written informed consent. During the 4-week pre-intervention run-in phase, participants continued their usual care and habitual diets and recorded headache characteristics and medication use in an online daily headache diary. Upon completion of the run-in phase, participants were randomized to either the H3-L6 or L6 intervention, to be maintained for 12 weeks. Participants were advised to continue seeing their regular headache physician for usual care throughout the trial. The trial was conducted at the University of North Carolina at Chapel Hill (UNC) from April 2009 to November 2011. Trial procedures were approved by the UNC Institutional Review Board. This trial is registered under ClinicalTrials.gov (NCT01157208).
2.2. Randomization and masking
Randomization and masking procedures were previously described [34]. Briefly, participants were randomized by the dietitian using an on-line, uneditable treatment assignment algorithm with a random permuted block design using a number sequence (1:1 allocation ratio). Only the dietitian was unmasked by necessity at randomization in order to assign patients to their group and to administer the interventions. Participants were provided dietary advice and foods in accordance with their assigned intervention, and were masked to the nature and content of the other group’s intervention. All other investigators, staff, and each participant’s personal physician were masked to group assignment for the full duration of the trial.
2.3. Dietary interventions
The H3-L6 intervention was designed to reduce dietary n-6 linoleic acid (LA), and concurrently increase dietary n-3 eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). The L6 intervention was designed to reduce dietary n-6 LA, and to maintain the low n-3 EPA and DHA intakes typical of U.S. diets [26]. The nutrient intakes of the two groups were previously published [26] and are summarized in Supplementary Table S3. Foods meeting nutrient targets were provided to participants sufficient for two meals and two snacks per day. The interventions were designed to be equally credible and equivalent with respect to: (1) macronutrient and caloric intake, (2) the amounts of study foods provided, (3) interactions with the study dietitian and other investigators, and (4) the intensity and breadth of the dietary advice and intervention materials [26]. A registered dietitian administered dietary counseling at randomization and at regular 2-week intervals throughout the 12-week intervention phase.
2.4. Measures of psychological distress and HRQOL
Psychological distress was assessed at baseline and week 12 with the BSI-18, which is a self-reported survey for screening psychological distress and measuring treatment outcomes [7]. The BSI-18 assesses three symptom dimensions—somatization, depression, and anxiety—which together form the global severity index (GSI) of psychological distress. A higher BSI-18 score indicates a greater degree of psychological distress. For this study, we removed the suicide query from the depression dimension and replaced its value with the mean of the remaining depression questions.
HRQOL was assessed at baseline and week 12 with the SF-12 [16] which is a self-reported survey for measuring physical and mental health and function. The SF-12 [19] contains eight subdomains—physical functioning, role physical, bodily pain, general health, vitality, role emotional, mental health, and social functioning—which in turn comprise separate physical and mental health summary measures. Lower SF-12 scores indicate a lower quality of physical and mental health. The SF-12 was found to be reliable, valid, and responsive to change in a population of chronic-back-pain patients, and has been employed in migraine research [19].
In addition to those surveys, both psychological distress and HRQOL were assessed at baseline and week 12 with the HIT-6, which is a self-reported survey for measuring headache-related disability [3; 45]. The HIT-6 encompasses the health dimensions of pain, social functioning, role functioning, vitality, cognitive functioning, and psychological distress. Higher HIT-6 scores indicate a higher level of headache-related disability.
2.5 Cutoff values for cases of substantial physical or mental impairment
Cases of substantial impairment were defined as those subjects with BSI-18 scores > 60, SF-12 physical and mental health summary scores < 40, HIT-6 scores ≥ 56, or ≥ 20 headache days per month. Since the BSI-18 and SF-12 scores are standardized (norm-referenced) with 50 points equal to the mean of the general population, we use a one standard deviation (10 points) difference to determine the cutoff points. This means, for example, that people with a BSI-18 score of 60 or above function at a level lower than 84% of the general population. Also, an SF-12 score of < 40 can be interpreted as moderate to severe disability [42]. For HIT-6, subjects with scores ≥ 56 range from having “substantial impact on life” corresponding to severe pain to having “very severe impact” corresponding to disabling pain [3]. We also classified subjects with 20 or more headache days per month as having substantial pain-related impairment.
2.6. Medication use
Daily headache-related medication use was captured in the online headache diary. Medications were classified into 3 broad categories: acute, preventive, or adjunctive (Supplementary Table S2). Medication change was previously reported as an exploratory outcome measure [34]. Participants in the H3-L6 group significantly reduced their use of acute medications and adjunctive medications compared to baseline. Participants did not significantly alter their use of preventive medications in either group.
2.7. Sample collection and laboratory analysis of n-3 and n-6 fatty acids
Sample preparation and analyses were performed by investigators who were blinded to study protocol and clinical data. Fasting blood was drawn at baseline and after 12-weeks of diet exposure. Plasma fatty acids were analyzed at the National Institute on Aging Brain Physiology and Metabolism Section (Bethesda, MD) by gas chromatography as previously described [47]. Plasma fatty acids are expressed as a percentage of total esterified fatty acids. Composite fatty acid indices (n-6 in HUFA score and EPA+DHA) were calculated as described by Stark [43].
2.8. Data Analysis
All analyses were conducted using Stata version 13 (College Station, Texas) and followed intention-to-treat principle. To estimate the headache-related clinical measures for the 11 participants (six in the L6 group, five in the H3-L6 group) who had missing data at week 12, we used multiple imputation (MI). The MI regression model consisted of independent variables with the other headache-related measures at baseline plus age, gender, and intervention group.
We used a Wilcoxon matched-pairs signed-ranks test to assess differences in pre-to-post intervention values of the BSI-18 and SF-12 subscales within each diet group. To determine whether diet group assignment had an effect on the post-intervention value of each variable, we used an analysis of covariance including the baseline value of each respective variable. To determine whether diet group assignment had an effect on the likelihood of experiencing substantial mental or physical impairments, we used logistic regression models, adjusting for the respective baseline value of each outcome.
For the fatty acids, we employed a combination of non-parametric and parametric approaches (normalizing variables when necessary) using all available data without imputations. The effects of changes in plasma fatty acids on post-intervention clinical outcomes were calculated using regression models adjusted for the baseline values of each outcome and fatty acid.
3. Results
Sixty-seven subjects with CDH were randomized to either the H3-L6 or L6 intervention. Fifty-six participants (28 in each group) completed the 12-week intervention phase, but data from all 67 randomized participants were included in the analysis. Figure 2 shows how the participants were processed throughout the trial.
Fig. 2. CONSORT Trial Profile (Adapted from PAIN publication32).
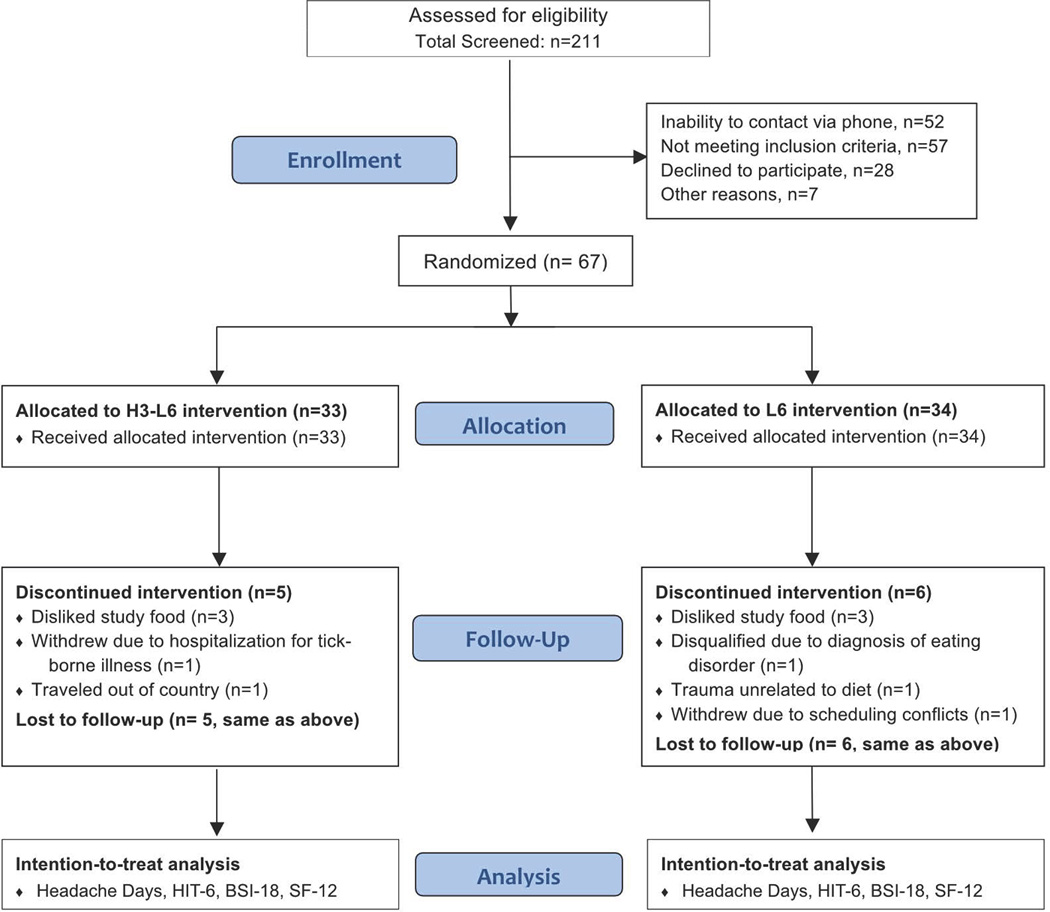
3.1. Baseline demographic and clinical characteristics
The H3-L6 and L6 groups had comparable baseline demographic and clinical characteristics (Table 1) [34]. Participants reported an average of 23 headache days per month and 10 headache hours per day, despite taking an average of 6 different headache-related medications. Mean scores for the HIT-6 at baseline (60.8, SD 4.9) were consistent with severe adverse impact on headache-specific quality of life [3].
Table 1.
Baseline characteristics of 67 patients with chronic headachesa
| H3-L6 diet n = 33 |
L6 diet n = 34 |
|
|---|---|---|
| Age, y, mean (SD) | 41 (13.4) | 42 (11.1) |
| Female, n (%) | 28 (84.8) | 30 (88.2) |
| Education, n (%) | ||
| High school | 2 (6.1) | 2 (6.2) |
| Attended college | 18 (54.5) | 17 (53.1) |
| Master’s degree or higher | 13 (40.6) | 13 (39.4) |
| % Chronic migraineb, n (%) | 26 (78.8) | 24 (70.6) |
| Headache Impact Test (HIT-6), mean (SD) | 61.0 (4.32) | 60.6 (5.56) |
| Headache days per month, mean (SD) | 23.3 (20.9, 25.8) | 23.2 (20.2, 25.8) |
| Headache hours per day, mean (SD) | 10.2 (8.4, 12.3) | 9.8 (8.1, 11.8) |
| Number of different headache-related medications reported, mean (SD) |
6.4 (3.4) | 5.6 (3.3) |
| Number using antidepressantsd, n (%) | 11 (33.3) | 14 (41.2) |
| Two or more antidepressants, n (%) | 3 (9.1) | 2 (5.9) |
| Number using anticonvulsantsd, n (%) | 12 (36.4) | 11 (32.4) |
| Two or more anticonvulsants, n (%) | 3 (9.1) | 2 (5.9) |
| Number using anxiolyticsd, n (%) | 6 (18.2) | 6 (17.6) |
| Two or more anxiolytics, n (%) | 1 (3.0) | 0 (0.0) |
Adapted from PAIN publication13.
Subjects classified as chronic migraine met International Headache Disorders-2 criteria. Subjects classified as Chronic Daily Headache (CDH) with migraine features had some characteristics of migraine (e.g. unilateral, pulsating, severe, sensory sensitivity, or aggravated by physical activity) but did not meet all criteria needed for chronic migraine diagnosis. Subjects classified with CDH without migraine features had no evidence of migraine.
95% confidence interval around the median per binomial-based method in Stata 12.
Headache medication categories are shown in Supplementary Table S3.
3.2 Changes in psychological distress (BSI-18) and health-related quality of life (SF-12)
Table 2 summarizes the effect of the H3-L6 and L6 diets on the BSI-18 and SF-12 scores over the 12-week trial. At baseline, there was no statistically significant difference between the diet groups. Compared to baseline, participants in the H3-L6 group (but not the L6 group) experienced statistically significant reductions in psychological distress and improvements in HRQOL (p-values < 0.05). Compared to the L6 intervention, participants in the H3-L6 intervention group experienced statistically significant reductions in psychological distress (mean difference in BSI-18 scores at week 12 was −6.56; 95% confidence interval (CI): −11.43, −1.69). Participants in the H3-L6 intervention group also experienced statistically significant improvements in HRQOL, as measured by the SF-12 mental health summary (mean difference 6.01; 95% CI: 0.57, 11.45) and the SF-12 physical health summary (mean difference 6.65; 95% CI; 2.14, 11.16). Participants in the H3-L6 group experienced statistically significant improvements in most, but not all, subdomains of the BSI-18 and SF-12 compared to the L6 group. Changes in BSI-18 and SF-12 subdomains can be found in Supplementary Table S4.
Table 2.
Psychological distress and health-related quality of life at baseline and after the 12-week diet interventions, means (95% CI)
| H3-L6 Diet (n=33) | L6 Diet (n=34) | p-values between- group difference1 |
|||
|---|---|---|---|---|---|
| Baseline | 12-weeks | Baseline | 12-weeks | ||
| Physical Health (SF-12)2 | 43.8 (40.6, 47.1) | 50.6 (47.9, 53.2)3 | 43.4 (40.0, 46.7) | 43.9 (40.5, 47.4) | <0.001 |
| Mental Health (SF-12)2 | 45.0 (41.3, 48.6) | 51.0 (47.3, 54.8)3 | 43.7 (40.6, 46.8) | 45.0 (41.4, 48.7) | 0.003 |
| Psychological distress (BSI-18 GSI)4 |
51.5 (47.9, 55.2) | 44.8 (41.2, 48.4)3 | 53.1 (50.4, 55.8) | 51.4 (48.2, 54.5) | 0.003 |
Between-group difference p-values are based on analysis of covariance comparing post-intervention values controlling for baseline value
Higher score = better quality of life.
pre-to post-intervention change p-value <0.05
Higher score = more psychological distress.
Abbreviations: GSI, global severity index
3.3 Changes in the number of people experiencing substantial physical or mental impairment
Figure 3 shows the change in the number of cases of substantial health impairment as defined by cutoff points for BSI-18, SF-12, HIT-6, and headache days per month. Each bar chart represents the percentage of the sample with substantial impairment at baseline and week 12. At baseline, a majority of the subjects experienced substantial to disabling headache-related impairments—70% had greater than or equal to 20 headache days per month and 88% had a HIT-6 score of 56 or more. There were no statistically significant between-group differences at baseline for any of the outcomes. By week 12, subjects in the H3-L6 group had improvements in four of the five indicators shown, with statistically significant between-group differences at the 0.01 level for HIT-6 and headache days per month and at the 0.05 level for the SF-12 physical health measure and the BSI-18.
Fig. 3. Proportion of subjects experiencing substantial physical or mental impairment.
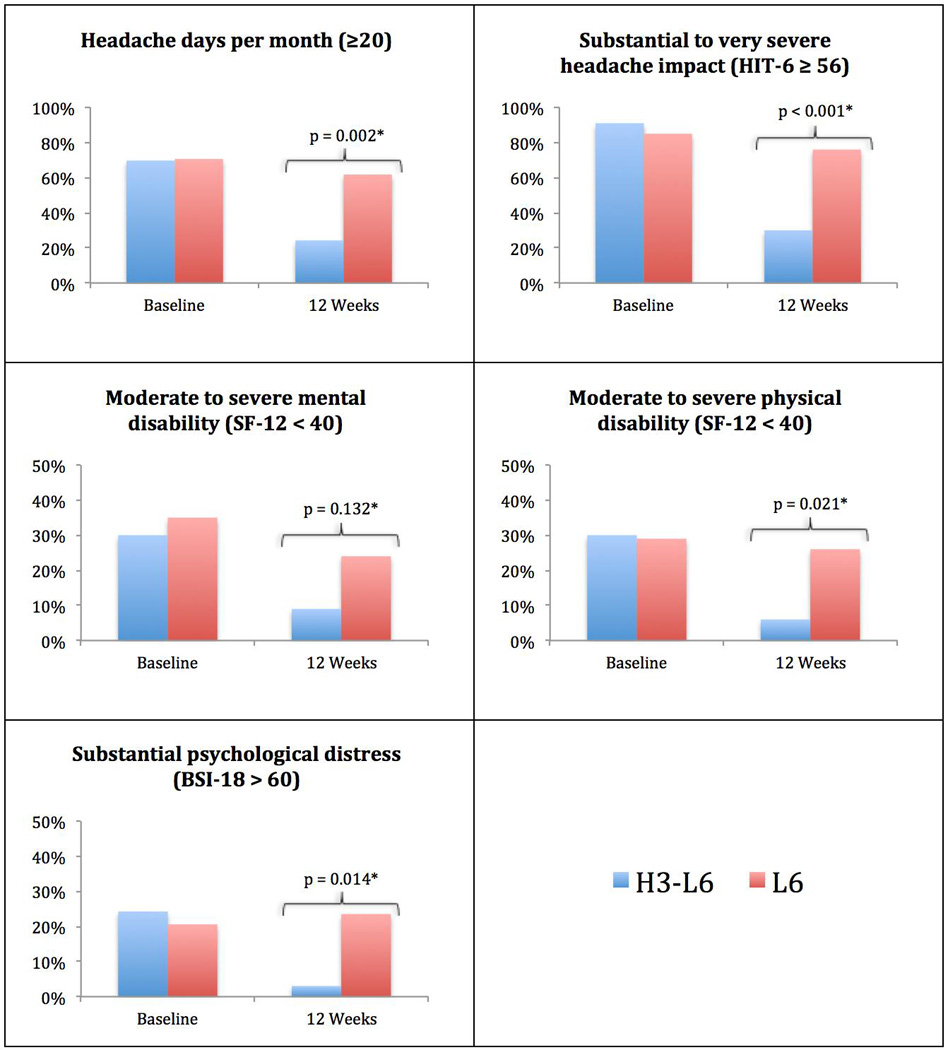
Subjects in the H3-L6 group had statistically significant improvements in HIT-6, headache days per month, SF-12 physical health and BSI-18.
*p-value from logistic regression for effect of diet group on 12-week outcomes, adjusted for baseline values.
3.4 Diet-induced changes in plasma n-3 and n-6 fatty acids
Compared to baseline, both interventions significantly increased plasma n-3 EPA and DHA, and reduced the n-6 in HUFA score (Table 3). Compared to the L6 intervention, the H3-L6 intervention had significantly greater increases in n-3 EPA and DHA and greater reduction in n-6 arachidonic acid (AA) and n-6 in HUFA score. Both interventions produced comparable, statistically significant reductions in plasma n-6 LA.
Table 3.
Plasman-3 and n-6 fatty acids at baseline and after 12-week dietary interventions1
| H3-L6 group2 | L6 group2 | Between- group difference p-value4 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12-weeks | % change |
p value3 |
Baseline | 12-weeks | % change |
p value3 |
||
| Esterified plasma fatty acids (% of total fatty acids) | |||||||||
| n-3 family | |||||||||
| DHA | 2.08(1.68, 2.97) | 4.08(3.27, 5.32) | +112 | <0.001 | 1.94 (1.57, 2.51) | 2.09 (1.83, 2.86) | +15 | 0.003 | <0.001 |
| EPA | 0.47(0.38, 0.61) | 1.92(1.11, 2.33) | +229 | <0.001 | 0.54(0.32, 0.69) | 0.77(0.54, 1.00) | +46 | <0.001 | <0.001 |
| n-6 family | |||||||||
| LA | 22.1(20.6, 24.4) | 18.8(16.4, 20.8) | −16 | <0.001 | 22.7 (21.4, 24.9) | 20.2(19.8, 22.3) | −12 | <0.001 | 0.055 |
| AA | 10.9(9.15, 12.8) | 8.42(7.76, 9.72) | −18 | <0.001 | 10.1 (8.29, 11.6) | 9.00(7.91, 11.0) | −5 | 0.048 | <0.001 |
| Composite indices | |||||||||
| n6 in HUFA score5 |
78.0(75.4, 80.5) | 55.2(50.6, 65.6) | −25 | <0.001 | 77.1(74.6, 81.3) | 73.3(69.5, 77.0) | −4 | <0.001 | <0.001 |
| EPA+DHA6 | 2.66 (2.25, 3.36) | 6.22 (4.88, 7.91) | +123 | <0.001 | 2.54 (2.01,3.04) | 2.9 (2.56, 3.94) | +19 | <0.001 | <0.001 |
Values are medians with inter-quartile ranges in parenthesis.
High n-3, Low n-6 Diet n=27; Low n-6 Diet n=28. There were no differences between diet groups at baseline (p>0.05)
Wilcoxon matched-pairs signed-ranks tests used for within-group comparisons.
ANCOVA for intervention effect (i.e., effect of diet group assignment on post-intervention value of analyte, adjusted for respective baseline value).
The n-6 in HUFA score is equal to the proportion of n-6 fatty acids in total highly unsaturated fatty acids (HUFA). The equation for calculation of the n-6 in HUFA score is 100 × (20:3n-6 + 20:4n-6 + 22:4n-6 + 22:5n-6)/(20:5n-3 + 22:5n-3 + 22:6n-3 + 20:3n-6 + 20:4n-6 + 22:4n-6 + 22:5n-6 + 20:3n-9).
Plasma EPA+DHA is calculated by summing EPA and DHA.
DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid; LA, linoleic acid; AA, arachidonic acid.
3.5 Association between 12-week changes in plasma fatty acids and clinical measures
For each standard deviation increase in plasma DHA, we observed a 16% reduction in the number of headache days per month (p<0.001), a 24% reduction in the number of headache hours per day (p<0.001), and a 44% reduction in the number of severe headache hours per day (p<0.001)(Table 4). Increases in DHA were also related to improvements in the functional dimensions of pain (p=0.005), but were unrelated to psychological dimensions of pain. Increases in EPA were related to decreased pain frequency and severity (p<0.001) and improvements in the functional dimensions of pain (p=0.009), and tended to correlate with reduced psychological distress (p=0.022).
Table 4.
Associations between 12-week changes in plasma fatty acids and physical pain, pain-related quality of life measures and psychological stress (n=55)1
| n-3 family | n-6 family | Composite n-3 and n-6 scores | ||||
|---|---|---|---|---|---|---|
| DHA | EPA | AA | LA | n6 in HUFA score |
EPA+DHA | |
| Pain frequency and intensity2 | ||||||
| Headache days/month | −16% (<0.001) | −14%(<0.001) | −0.2% (0.954) | 15% (0.001) | 13% (<0.001) | −15% (<0.001) |
| Headache hours/day | −24% (<0.001) | −21%(<0.001) | −1.0% (0.908) | 32% (<0.001) | 23% (<0.001) | −23% (<0.001) |
| Severe headache hours/day |
−44% (<0.001) | −34%(<0.001) | 24% (0.027) | 56% (<0.001) | 52% (<0.001) | −41% (<0.001) |
| Functional dimensions of pain3 | ||||||
| Headache Impact on quality of life (HIT-6) 4 |
−0.36 (0.004) | −0.30(0.009) | 0.30 (0.025) | 0.05 (0.711) | 0.30 (0.006) | −0.35 (0.004) |
| Physical Health Composite Score (SF-12)5 |
0.35 (0.005) | 0.33(0.006) | −0.38 (0.004) | −0.07 (0.607) | −0.35 (0.001) | 0.36 (0.003) |
| Psychological dimensions of pain3 | ||||||
| Mental Health Composite Score (SF-12)5 |
0.19 (0.150) | 0.25(0.035) | −0.11 (0.447) | −0.04 (0.753) | −0.11 (0.332) | 0.23 (0.056) |
| Psychological distress (BSI-18)6 |
−0.18 (0.134) | −0.24 (0.022) | 0.19 (0.128) | −0.03 (0.829) | 0.14 (0.190) | −0.21 (0.062) |
Results are beta coefficients and respective p-values. Abbreviations: DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; LA, linoleic acid; AA, arachidonic acid.
Analyzed using Poisson regression and presented as % change in count for each standard deviation change in the respective fatty acid.
Analyzed using linear regressions and presented as standard deviation change in Y for each standard deviation change in X.
Higher score = more headache impact on quality of life.
Higher score = better quality of life.
Higher score = more psychological distress.
Decreases in n-6 LA were strongly related to decreased pain frequency and intensity, but unrelated to functional and psychological dimensions of pain. For each standard deviation decrease in LA, there was a 15% decrease in headache days per month (p=0.001), a 32% decrease in headache hours per day (p<0.001) and a 56% reduction in the number of severe headache hours per day (p<0.001). Decreases in n-6 AA were not related to improvements in physical or psychological dimensions of pain, but did correlate with improvement in physical function. Changes in the n-6 in HUFA and EPA+DHA composite scores were related to decreased pain frequency and intensity (p<0.001) and improvements in the functional dimensions of pain (p=0.006), but were unrelated to changes in the psychological dimensions of pain.
4. Discussion
This population of subjects with CDH had frequent and often severe physical pain, and substantial headache-related impairments in psychological distress and HRQOL. We previously demonstrated that the combination of increasing dietary n-3 fatty acids with concurrent reduction in n-6 LA (the H3-L6 intervention) produced statistically significant, clinically relevant pain reduction, and reduced headache-related disability (HIT-6) [34]. In the present analysis, the H3-L6 intervention was found to significantly reduce psychological distress, improve physical and mental HRQOL, and significantly reduce the proportion of subjects with moderate to severe headache impact and physical impairment. These clinical improvements were accompanied by reduced use of headache-related analgesic and adjunctive medications, suggesting that the beneficial effects of the H3-L6 intervention were not due to changes in psychoactive medication use. These findings demonstrate that the beneficial effects of the H3-L6 intervention extended beyond pain reduction, favorably impacting the quality-of-life and function of this chronic headache population. However, it is not yet clear whether these improvements in psychological distress and HRQOL were secondary to reduction in physical pain, or to more direct mechanism(s) targeting anxiety or psychological resiliency.
4. 1 Dietary n-3 and n-6 fatty acids and physical pain
Physical pain reduction in the H3-L6 group may be due to diet-induced alteration in the balance of pro- and anti-nociceptive lipid-autacoids derived from n-6 and n-3 fatty acids (e.g. endovanilloids, endocannabinoids, eicosanoids, resolvins, protectins). Notably, 2-series prostaglandins derived from n-6 arachidonic acid can elicit migraines in humans [2], and monohydroxy derivatives of n-6 LA have demonstrated pro-nociceptive properties in rodent pain models [32; 33]. By contrast, several families of mediators derived from n-3 EPA and DHA have demonstrated anti-nociceptive actions in rodent pain models [31; 39; 53; 55]. Hence, diet-induced reductions in pro-nociceptive mediators and increases in anti-nociceptive mediators represent a relatively straightforward, plausible mechanism underlying pain relief.
4. 2 Dietary n-3 and n-6 fatty acids and psychological distress
Several, but not all, randomized controlled trials testing n-3 supplement interventions reported substantial reductions of depressive symptoms. Meta-analyses of randomized controlled trials have reported that EPA predominant n-3 supplements reduce depressive symptoms among subjects with clinically significant depressive illnesses [44], [13]. These findings are consistent with epidemiological studies [14] and rodent models [42]. Proposed mechanisms include diet-induced alterations in monoamine neurotransmitter metabolism [17; 45], endocannabinoid signaling [10], immune activation [6; 9], and hypothalamic-pituitary axis activation [5].
Patients with physical pain are at increased risk of developing a first episode of depressive and anxiety disorders [12]. Given the adverse effect of physical pain on psychological health, the finding in the present trial that marked reduction in physical pain in the H3-L6 group was accompanied by reduced psychological distress is perhaps not surprising. However, it is not clear whether the reduction in psychological distress was secondary to relief of physical pain, or to a more direct mechanism affecting psychological aspects of the pain experience. Since the BSI-18 somatization subscale includes somatic symptoms often experienced during migraines (e.g., nausea, tingling), the beneficial effects of the H3-L6 intervention on migraine frequency may have contributed to reductions in BSI-18.
Chronic pain has well-known adverse impacts on social and family relationships, job status, and financial security, which in turn can promote psychological distress [15]. Pain relief may not quickly lead to improvements in these social and financial consequences of chronic pain; therefore, there may be a substantial lag between reductions in physical pain and consequent psychological distress. Notably, the maximal clinical efficacy of some antidepressant medications for treating major depression does not occur for several weeks or longer [11; 49]. Moreover, because participants in the present trial did not have moderate to severe levels of depression at baseline it was unlikely that significant reductions in depressive symptoms would be detected [20]. Hence, the 12-week intervention period in the present trial may not have been of sufficient duration to produce maximal improvements in psychological distress. Future, longer trials, including subjects with moderate to severe depressive symptoms, are needed to establish whether the H3-L6 intervention can produce more marked and sustained psychological improvements.
Reductions in physical pain and psychological distress in the H3-L6 group were accompanied by improvements in physical and mental function, and reductions in the percentages of subjects meeting cutoff values indicative of moderate-to-severe physical and psychological impairment and disability. The robustness of these findings indicates that the intervention had a broad beneficial impact on the lives of participants randomized to the H3-L6 group in this population. If these results are reproducible in other populations with severe headaches, and perhaps other chronic pain populations, this dietary approach would represent a novel therapeutic option for treating pain, common pain-related comorbidities, and functional consequences of chronic pain.
4.3 Mechanistic considerations linking n-3 and n-6 fatty acids to clinical improvements
Physical pain reduction was closely correlated with increases in plasma n-3 EPA and DHA, and reduction in n-6 LA, but was not related to changes in plasma n-6 AA. These findings are consistent with the hypothesis that changes in anti- and pro-nociceptive lipid mediators derived from n-3 EPA and DHA and n-6 LA respectively, could have contributed to the observed anti-nociception. Anti-nociceptive mediators derived from DHA include D-series resolvins [31; 53], neuroprotectins [30; 52], maresins [39], and DHA-epoxides [39]; anti-nociceptive mediators derived from EPA include E-series resolvins [31] and EPA-epoxides [27]. Pro-nociceptive mediators derived from LA include hydroxyoctadecadienoic acids [32; 33] and linoleoylethanolamine [28]. The lack of association between changes in plasma n-6 AA and pain reduction is somewhat surprising since AA is the precursor to prostaglandins that are classically linked to headache pathogenesis. Notably, AA is also the precursor to lipid mediators with anti-nociceptive properties (eg, epoxyeicosatrienoic acids, lipoxins), which might counteract the pro-nociceptive effects of prostaglandins. Increases in plasma n-3 EPA and DHA and reductions in n-6 AA were related to improvement in functional dimensions of pain. These collective biochemical findings suggest that both n-3 and n-6 fatty acids contributed to the beneficial effects of the intervention.
4.4 Limitations
The present trial was relatively small and of short duration. In addition, because targeted dietary fatty acids could not be altered as independent variables, we cannot rule out the possibility that changes in other nutrients could have contributed to the favorable effects of the H3-L6 intervention. Therefore the present trial should be replicated in a larger and longer trial, with nutrients altered as controlled variables.
4.4 Future directions
Human trials are needed to better characterize the effects of the H3-L6 intervention on the many specific lipid autacoids linked to nociception and psychological distress (e.g. prostanoids, endovanilloids, endocannabinoids, maresins), and to investigate the relationship of these biochemical changes to physical and psychological dimensions of the pain experience. Future trials should also capture daily measures of both physical pain and psychological distress in order to investigate the directionality and temporal relationship between these different aspects of pain. Animal studies are needed to characterize diet-induced biochemical alterations in pain signaling pathways and specific peripheral and central brain nervous system regions linked to pain processing and psychological distress.
4.5 Summary & Conclusion
Targeted dietary manipulation of n-3 and n-6 fatty acids reduced physical pain and psychological distress, and improved HRQOL and physical function in a chronic headache population. Future trials are needed to better define the role that targeted dietary alterations could play as a complementary strategy for managing the psychological distress and physical impairments that often accompany chronic pain.
Supplementary Material
Acknowledgments
The authors thank the patients who participated in the trial, and acknowledge the following individuals for their research assistance: Chanee Lynch, Rebecca Coble, and Amit Ringel for research assistance; Marjorie Busby for expertise with study design; Beth Fowler, Carol Carr, Regina McCoy, and Tim McCaskill for design and functionality of the study website; Meg Mangan for 24-hour recall data collection and management; Sharon Majchrzak-Hong for data management support and referencing; and Mark Horowitz for editing and statistical programming. This project was supported by the Mayday Fund (primary source); the UNC Research Fellowship in Complementary and Alternative Medicine (grant T32-AT003378, NCCAM, NIH); the North Carolina Clinical and Translational Sciences Institute (grant UL1RR025747, NCRR, NIH); the UNC Nutrition Obesity Research Center, CHAI Core (grant DK056350, NIDDK, NIH); and the Intramural Programs of the National Institute on Aging and the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health.
The authors alone are responsible for the content and writing of the article.
Footnotes
Conflict of interest statement
The authors declare that they have no competing interests.
References
- 1.Almeida-Santos AF, Gobira PH, Rosa LC, Guimaraes FS, Moreira FA, Aguiar DC. Modulation of anxiety-like behavior by the endocannabinoid 2-arachidonoylglycerol (2-AG) in the dorsolateral periaqueductal gray. Behavioural brain research. 2013;252:10–17. doi: 10.1016/j.bbr.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 2.Antonova M, Wienecke T, Olesen J, Ashina M. Prostaglandin E(2) induces immediate migraine-like attack in migraine patients without aura. Cephalalgia : an international journal of headache. 2012;32(11):822–833. doi: 10.1177/0333102412451360. [DOI] [PubMed] [Google Scholar]
- 3.Bayliss MS, Batenhorst AS. The HIT-6TM A User’s guide. Lincoln RI: QualityMetric Incorporated; [Google Scholar]
- 4.Beydoun MA, Fanelli Kuczmarski MT, Beydoun HA, Hibbeln JR, Evans MK, Zonderman AB. omega-3 fatty acid intakes are inversely related to elevated depressive symptoms among United States women. The Journal of nutrition. 2013;143(11):1743–1752. doi: 10.3945/jn.113.179119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buydens-Branchey L, Branchey M, Hibbeln JR. Higher n-3 fatty acids are associated with more intense fenfluramine-induced ACTH and cortisol responses among cocaine-abusing men. Psychiatry research. 2011;188(3):422–427. doi: 10.1016/j.psychres.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calder PC. n-3 fatty acids, inflammation and immunity: new mechanisms to explain old actions. The Proceedings of the Nutrition Society. 2013;72(3):326–336. doi: 10.1017/S0029665113001031. [DOI] [PubMed] [Google Scholar]
- 7.Derogatis L. BSI-18: Administration, Scoring, and Procedures Manual. Minneapolis, MN: National Computer Systems; 2000. [Google Scholar]
- 8.Eskin M, Akyol A, Celik EY, Gultekin BK. Social problem-solving, perceived stress, depression and life-satisfaction in patients suffering from tension type and migraine headaches. Scandinavian journal of psychology. 2013;54(4):337–343. doi: 10.1111/sjop.12056. [DOI] [PubMed] [Google Scholar]
- 9.Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaetani S, Dipasquale P, Romano A, Righetti L, Cassano T, Piomelli D, Cuomo V. The endocannabinoid system as a target for novel anxiolytic and antidepressant drugs. International review of neurobiology. 2009;85:57–72. doi: 10.1016/S0074-7742(09)85005-8. [DOI] [PubMed] [Google Scholar]
- 11.Georgotas A, McCue RE, Cooper TB, Nagachandran N, Friedhoff A. Factors affecting the delay of antidepressant effect in responders to nortriptyline and phenelzine. Psychiatry research. 1989;28(1):1–9. doi: 10.1016/0165-1781(89)90192-3. [DOI] [PubMed] [Google Scholar]
- 12.Gerrits MM, van Oppen P, van Marwijk HW, Penninx BW, van der Horst HE. Pain and the onset of depressive and anxiety disorders. Pain. 2014;155(1):53–59. doi: 10.1016/j.pain.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Grosso G, Pajak A, Marventano S, Castellano S, Galvano F, Bucolo C, Drago F, Caraci F. Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PloS one. 2014;9(5):e96905. doi: 10.1371/journal.pone.0096905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351(9110):1213-1213. doi: 10.1016/S0140-6736(05)79168-6. [DOI] [PubMed] [Google Scholar]
- 15.Hibbeln JR. Seafood consumption, the DHA content of mothers' milk and prevalence rates of postpartum depression: a cross-national, ecological analysis. Journal of affective disorders. 2002;69(1–3):15–29. doi: 10.1016/s0165-0327(01)00374-3. [DOI] [PubMed] [Google Scholar]
- 16.Hibbeln JR, Davis JM. Considerations regarding neuropsychiatric nutritional requirements for intakes of omega-3 highly unsaturated fatty acids. Prostaglandins, leukotrienes, and essential fatty acids. 2009;81(2–3):179–186. doi: 10.1016/j.plefa.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hibbeln JR, Linnoila M, Umhau JC, Rawlings R, George DT, Salem N. Essential fatty acids predict metabolites of serotonin and dopamine in cerebrospinal fluid among healthy control subjects, and early- and late-onset alcoholics. Biol Psychiat. 1998;44(4):235–242. doi: 10.1016/s0006-3223(98)00141-3. [DOI] [PubMed] [Google Scholar]
- 18.Hibbeln JR, Salem N., Jr Dietary polyunsaturated fatty acids and depression: when cholesterol does not satisfy. The American journal of clinical nutrition. 1995;62(1):1–9. doi: 10.1093/ajcn/62.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Inceoglu B, Jinks SL, Ulu A, Hegedus CM, Georgi K, Schmelzer KR, Wagner K, Jones PD, Morisseau C, Hammock BD. Soluble epoxide hydrolase and epoxyeicosatrienoic acids modulate two distinct analgesic pathways. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(48):18901–18906. doi: 10.1073/pnas.0809765105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: A meta-analysis of data submitted to the food and drug administration. Plos Med. 2008;5(2):260–268. doi: 10.1371/journal.pmed.0050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kucuksen S, Genc E, Yilmaz H, Salli A, Gezer IA, Karahan AY, Salbas E, Cingoz HT, Nas O, Ugurlu H. The prevalence of fibromyalgia and its relation with headache characteristics in episodic migraine. Clinical rheumatology. 2013;32(7):983–990. doi: 10.1007/s10067-013-2218-2. [DOI] [PubMed] [Google Scholar]
- 22.Lanteri-Minet M, Radat F, Chautard MH, Lucas C. Anxiety and depression associated with migraine: influence on migraine subjects' disability and quality of life, and acute migraine management. Pain. 2005;118(3):319–326. doi: 10.1016/j.pain.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Ligthart L, Gerrits MM, Boomsma DI, Penninx BW. Anxiety and depression are associated with migraine and pain in general: an investigation of the interrelationships. The journal of pain : official journal of the American Pain Society. 2013;14(4):363–370. doi: 10.1016/j.jpain.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Liu JJ, Galfalvy HC, Cooper TB, Oquendo MA, Grunebaum MF, Mann JJ, Sublette ME. Omega-3 polyunsaturated fatty acid (PUFA) status in major depressive disorder with comorbid anxiety disorders. The Journal of clinical psychiatry. 2013;74(7):732–738. doi: 10.4088/JCP.12m07970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucchetti G, Peres MF, Lucchetti AL, Mercante JP, Guendler VZ, Zukerman E. Generalized anxiety disorder, subthreshold anxiety and anxiety symptoms in primary headache. Psychiatry and clinical neurosciences. 2013;67(1):41–49. doi: 10.1111/j.1440-1819.2012.02405.x. [DOI] [PubMed] [Google Scholar]
- 26.MacIntosh BA, Ramsden CE, Faurot KR, Zamora D, Mangan M, Hibbeln JR, Mann JD. Low-n-6 and low-n-6 plus high-n-3 diets for use in clinical research. The British journal of nutrition. 2013;110(3):559–568. doi: 10.1017/S0007114512005181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morisseau C, Inceoglu B, Schmelzer K, Tsai HJ, Jinks SL, Hegedus CM, Hammock BD. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. Journal of lipid research. 2010;51(12):3481–3490. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Movahed P, Jonsson BA, Birnir B, Wingstrand JA, Jorgensen TD, Ermund A, Sterner O, Zygmunt PM, Hogestatt ED. Endogenous unsaturated C18 N-acylethanolamines are vanilloid receptor (TRPV1) agonists. The Journal of biological chemistry. 2005;280(46):38496–38504. doi: 10.1074/jbc.M507429200. [DOI] [PubMed] [Google Scholar]
- 29.Munakata J, Hazard E, Serrano D, Klingman D, Rupnow MF, Tierce J, Reed M, Lipton RB. Economic burden of transformed migraine: results from the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2009;49(4):498–508. doi: 10.1111/j.1526-4610.2009.01369.x. [DOI] [PubMed] [Google Scholar]
- 30.Park CK, Lu N, Xu ZZ, Liu T, Serhan CN, Ji RR. Resolving TRPV1- and TNF-alpha-mediated spinal cord synaptic plasticity and inflammatory pain with neuroprotectin D1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(42):15072–15085. doi: 10.1523/JNEUROSCI.2443-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park CK, Xu ZZ, Liu T, Lu N, Serhan CN, Ji RR. Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin D1, D2, and E1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(50):18433–18438. doi: 10.1523/JNEUROSCI.4192-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patwardhan AM, Akopian AN, Ruparel NB, Diogenes A, Weintraub ST, Uhlson C, Murphy RC, Hargreaves KM. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. The Journal of clinical investigation. 2010;120(5):1617–1626. doi: 10.1172/JCI41678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patwardhan AM, Scotland PE, Akopian AN, Hargreaves KM. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(44):18820–18824. doi: 10.1073/pnas.0905415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramsden CE, Faurot KR, Zamora D, Suchindran CM, Macintosh BA, Gaylord S, Ringel A, Hibbeln JR, Feldstein AE, Mori TA, Barden A, Lynch C, Coble R, Mas E, Palsson O, Barrow DA, Mann JD. Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: a randomized trial. Pain. 2013;154(11):2441–2451. doi: 10.1016/j.pain.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramsden CE, Mann JD, Faurot KR, Lynch C, Imam ST, MacIntosh BA, Hibbeln JR, Loewke J, Smith S, Coble R, Suchindran C, Gaylord SA. Low omega-6 vs. low omega-6 plus high omega-3 dietary intervention for chronic daily headache: protocol for a randomized clinical trial. Trials. 2011;12:97. doi: 10.1186/1745-6215-12-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramsden CE, Ringel A, Feldstein AE, Taha AY, MacIntosh BA, Hibbeln JR, Majchrzak-Hong SF, Faurot KR, Rapoport SI, Cheon Y, Chung YM, Berk M, Mann JD. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins, leukotrienes, and essential fatty acids. 2012;87(4–5):135–141. doi: 10.1016/j.plefa.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rist PM, Schurks M, Buring JE, Kurth T. Migraine, headache, and the risk of depression: Prospective cohort study. Cephalalgia : an international journal of headache. 2013;33(12):1017–1025. doi: 10.1177/0333102413483930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sastry PS. Lipids of nervous tissue: composition and metabolism. Progress in lipid research. 1985;24(2):69–176. doi: 10.1016/0163-7827(85)90011-6. [DOI] [PubMed] [Google Scholar]
- 39.Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26(4):1755–1765. doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smitherman TA, Burch R, Sheikh H, Loder E. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53(3):427–436. doi: 10.1111/head.12074. [DOI] [PubMed] [Google Scholar]
- 41.Smitherman TA, Kolivas ED, Bailey JR. Panic disorder and migraine: comorbidity, mechanisms, and clinical implications. Headache. 2013;53(1):23–45. doi: 10.1111/head.12004. [DOI] [PubMed] [Google Scholar]
- 42.Song C, Zhang XY, Manku M. Increased phospholipase A2 activity and inflammatory response but decreased nerve growth factor expression in the olfactory bulbectomized rat model of depression: effects of chronic ethyl-eicosapentaenoate treatment. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(1):14–22. doi: 10.1523/JNEUROSCI.3569-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stark KD. The percentage of n-3 highly unsaturated fatty acids in total HUFA as a biomarker for omega-3 fatty acid status in tissues. Lipids. 2008;43(1):45–53. doi: 10.1007/s11745-007-3128-3. [DOI] [PubMed] [Google Scholar]
- 44.Sublette ME, Ellis SP, Geant AL, Mann JJ. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. The Journal of clinical psychiatry. 2011;72(12):1577–1584. doi: 10.4088/JCP.10m06634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sublette ME, Galfalvy HC, Hibbeln JR, Keilp JG, Malone KM, Oquendo MA, Mann JJ. Polyunsaturated fatty acid associations with dopaminergic indices in major depressive disorder. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2014;17(3):383–391. doi: 10.1017/S1461145713001399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun T, Yu E, Yu L, Luo J, Li H, Fu Z. LipoxinA(4) induced antinociception and decreased expression of NF-kappaB and pro-inflammatory cytokines after chronic dorsal root ganglia compression in rats. European journal of pain. 2012;16(1):18–27. doi: 10.1016/j.ejpain.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Taha AY, Cheon Y, Faurot KF, Macintosh B, Majchrzak-Hong SF, Mann JD, Hibbeln JR, Ringel A, Ramsden CE. Dietary omega-6 fatty acid lowering increases bioavailability of omega-3 polyunsaturated fatty acids in human plasma lipid pools. Prostaglandins, leukotrienes, and essential fatty acids. 2014;90(5):151–157. doi: 10.1016/j.plefa.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tietjen GE, Brandes JL, Peterlin BL, Eloff A, Dafer RM, Stein MR, Drexler E, Martin VT, Hutchinson S, Aurora SK, Recober A, Herial NA, Utley C, White L, Khuder SA. Allodynia in migraine: association with comorbid pain conditions. Headache. 2009;49(9):1333–1344. doi: 10.1111/j.1526-4610.2009.01521.x. [DOI] [PubMed] [Google Scholar]
- 49.Tylee A, Walters P. Onset of action of antidepressants. Bmj. 2007;334(7600):911–912. doi: 10.1136/bmj.39197.619190.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaz JS, Kac G, Nardi AE, Hibbeln JR. Omega-6 fatty acids and greater likelihood of suicide risk and major depression in early pregnancy. Journal of affective disorders. 2014;152–154:76–82. doi: 10.1016/j.jad.2013.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wen H, Ostman J, Bubb KJ, Panayiotou C, Priestley JV, Baker MD, Ahluwalia A. 20-Hydroxyeicosatetraenoic acid (20-HETE) is a novel activator of transient receptor potential vanilloid 1 (TRPV1) channel. The Journal of biological chemistry. 2012;287(17):13868–13876. doi: 10.1074/jbc.M111.334896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu ZZ, Liu XJ, Berta T, Park CK, Lu N, Serhan CN, Ji RR. Neuroprotectin/protectin D1 protects against neuropathic pain in mice after nerve trauma. Annals of neurology. 2013;74(3):490–495. doi: 10.1002/ana.23928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, Serhan CN, Ji RR. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nature medicine. 2010;16(5):592–597. doi: 10.1038/nm.2123. 591p following 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yavuz BG, Aydinlar EI, Dikmen PY, Incesu C. Association between somatic amplification, anxiety, depression, stress and migraine. The journal of headache and pain. 2013;14(1):53. doi: 10.1186/1129-2377-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoo S, Lim JY, Hwang SW. Resolvins: Endogenously-Generated Potent Painkilling Substances and their Therapeutic Perspectives. Current neuropharmacology. 2013;11(6):664–676. doi: 10.2174/1570159X11311060009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


