Abstract
Background/Objective
Post-transplant diabetes mellitus (PTDM) is both common and associated with poor outcomes after kidney transplantation. Our objective was to examine relationships of uremia-associated inflammation and adiponectin with PTDM.
Methods
Nondiabetic kidney transplant patients were enrolled with donor controls. Inflammatory cytokines and adiponectin were measured before and after transplantation. Adipose tissue was obtained for gene expression analysis. Glucose transport was quantified in vitro in C2C12 cells following cytokine exposure. The patients were monitored up to 12 months for PTDM.
Results
We studied 36 controls and 32 transplant patients, of whom 11 (35%) developed PTDM. Compared to controls, plasma TNFα, IL-6, MCP-1, and CRP levels were higher in transplant patients (p < 0.01). In multivariable analysis, TNFα plasma levels before transplantation were associated with development of PTDM (OR = 2.03, p = 0.04). Visceral adipose tissue TNFα mRNA expression was higher in transplant patients than controls (fold change 1.33; p < 0.05). TNFα mRNA expression was also higher in patients who developed PTDM than in those who did not (fold change 1.42; p = 0.05), and adiponectin mRNA expression was lower (fold change 0.48; p < 0.05). The studies on the C2C12 cells demonstrated an increase in glucose uptake following exposure to adiponectin and no significant change after exposure to TNFα alone. Concomitant TNFα and adiponectin exposure blunted adiponectin-induced glucose uptake (11% reduction; p < 0.001).
Conclusion
Our in vitro and clinical observations suggest that TNFα could contribute to PTDM through an effect on adiponectin. Our study proposes that inflammation is involved in glucose regulation after kidney transplantation.
Key Words: Cytokines, Diabetes, Adiponectin, Kidney transplantation
Introduction
Post-transplant diabetes mellitus (PTDM) is a serious complication of organ transplantation. The incidence of PTDM varies between 15 and 50% of kidney transplants, and PTDM is diagnosed most frequently during the first 12 months after transplantation [1,2,3]. PTDM is an independent risk factor for cardiovascular events with a 60% increase in post-transplantation myocardial infarction [4]. PTDM is also associated with cerebrovascular accidents, aortic or lower-extremity arterial disease, higher rates of renal allograft loss, and mortality [2]. Therefore, PTDM is common and associated with graft loss as well as patient morbidity and mortality.
PTDM shares multiple risk factors with type 2 diabetes, but some characteristics intrinsic to transplanted patients increase the risk of PTDM. Insulin levels and insulin resistance (IR) are altered after kidney transplantation, which may play a role in the development of PTDM. Specifically, insulin clearance is restored after kidney transplantation in the functioning organ. Also, insulin secretion is reduced by calcineurin inhibitors, which are toxic to beta cells and limit insulin secretion [5,6]. Finally, IR worsens after kidney transplantation [7]. Therefore, PTDM is further complicated by the combination of IR, which demands higher insulin secretion for glucose control, and the limited capacity of insulin production by the beta cells. Increased insulin clearance also reduces insulin levels and leads to hyperglycemia. The issue of the contribution of IR as compared to impaired insulin secretion as the seminal event that leads to PTDM remains unresolved [8].
Patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD) are exposed to a milieu of hormones, cytokines, and waste products that interact with the different organ systems. In patients with CKD, IR becomes more pronounced as the kidney function deteriorates [9,10]. The development of IR in kidney disease is driven at least in part by uremic solutes [11] and chronic inflammation [12]. Despite marked reductions in levels of inflammatory cytokines and uremic toxins after kidney transplantation, kidney transplant patients develop diabetes after kidney transplantation at much higher rates than do dialysis patients [3,13].
The role of uremic inflammation and glucose regulation after kidney transplantation has not been clarified. Therefore, we conducted a series of studies including tissue studies from ESRD patients, with post-transplant follow-up, to quantify cytokine production and in vitro experiments in order to examine effects of cytokines on glucose transport. Our hypothesis is that inflammatory cytokines in uremic patients are associated with development of PTDM in this prospective cohort study. Subsequently, due to our findings, we set out to determine if adipose tissue of uremic patients has an increased expression of inflammatory cytokines. Furthermore, we tested in an in vitro model if the cytokines that are differentially expressed in adipose tissue of ESRD patients could affect glucose transport.
Subjects and Methods
General Design and Patient Recruitment
We designed a prospective cohort study of kidney transplant recipients and kidney donor controls whose surgery was performed at Thomas Jefferson University Hospital (TJUH). Criteria for inclusion included having kidney transplantation or donation at TJUH and a plan for follow-up with the TJUH transplant team. Exclusion criteria included multiorgan transplants, functional pancreas transplants, or diabetes before transplantation. Nondiabetic status was verified by fasting glucose measurement at the time of transplantation, as HbA1c was not consistently available for all the participants in their medical record. The patients were observed for 12 months following transplantation and classified as having PTDM if they required treatment for diabetes with drugs or lifestyle modifications, or if they developed laboratory values consistent with diabetes by the ADA criteria [1] at least 3 months after transplant (once steroids had been withdrawn for 1 month).
Plasma as well as adipose tissue samples were obtained at the time of transplantation or during donation. Demographic data and baseline characteristics were obtained from medical records. Plasma samples were also obtained from transplant recipients 3-6 months after transplantation at a clinical appointment once creatinine levels and immunosuppressive therapy had remained stable for at least 1 month (patients had unchanged calcineurin inhibitor dose with goal trough tacrolimus levels of 5-7 ng/ml). The transplant immunosuppression protocol withdrew steroids after the second month following transplantation unless the participants had panel reactive antibody titers >20% or had lost a prior transplant secondary to acute rejection. The immunosuppression regimen of TJUH includes twice-daily tacrolimus to achieve a trough level of 5-7 ng/ml 3 months after transplantation, as well as mycophenolic acid 1,000 mg twice daily. All patients were maintained on tacrolimus. Only 2 participants did not receive mycophenolic acid, and one of them was maintained on azathioprine. Regarding steroid treatment, 18 of 32 ESRD participants received 5 mg of prednisone for maintenance immunosuppression at the time of the follow-up visit.
Procedures
Approximately 500 mg of visceral fat and 10 ml of blood were obtained from ESRD participants and controls while undergoing surgery. Only the transplant recipients had a second blood sample drawn 3-6 months after transplantation. The blood samples were prepared for measurements of plasma adipokines including adiponectin, adiponectin high-molecular-weight fraction, IL-6, IL-8, MCP-1, CRP, and TNFα by ELISA as previously described [14]. The visceral adipose tissue samples were prepared for mRNA analysis of adipokines including adiponectin, CRP, IL-6, IL-8, MCP-1, TNFα, and adiponectin receptors by real-time PCR as previously described [14]. Gene expression was performed using a custom RT2 Profiler PCR array (Qiagen, Valencia, Calif., USA) per the manufacturer's protocol. The data were analyzed using software provided by the manufacturer (http://www.qiagen.com/us/products/genes%20and%20pathways/data-analysis-center-overview-page/). The final expression value was normalized to β2-microglobulin and β-actin. Data are presented as the average ratios in tissue of target mRNA to the reference genes in arbitrary units.
In vitro Experiments
C2C12 myoblasts were obtained from the American Type Culture Collection (Manassas, Va., USA). Cells were cultured and differentiated as previously described [14]. Differentiated myotubes were exposed to different concentrations of TNFα (0, 1, or 5 ng/ml; PeproTech, Rocky Hill, N.J., USA) with or without globular adiponectin (0, 1, and 2 µg/ml; PeproTech) for 48 h. A glucose uptake assay was performed using 2-NBDG (2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-D-glucose; Invitrogen, Carlsbad, Calif., USA). After exposure to TNFα and adiponectin, the myoblasts were incubated with prewarmed 2-NBDG solution (final concentration of 200 µM) for 15 min at 37°C. The cells were then analyzed by flow cytometry using a GFP signal detector with an excitation wavelength of 488 nm and an emission wavelength of 530 nm (to detect 2-NBDG). Data analysis was performed using FlowJo 8.8 software.
Statistical Methods
Continuous data are summarized by medians and the 1st and 3rd quartiles. Categorical data are summarized by frequencies and percentages. Unadjusted comparisons of the distributions of variables between study groups were made using the ranks-based Kruskal-Wallis or Wilcoxon tests when data were continuous, or Fisher's exact test when data were categorical. Where possible, pairwise comparisons were made between controls, ESRD patients with no PTDM, and PTDM patients, with p values adjusted for multiple comparison type I error rate inflation by permutation resampling of Fisher's exact test or by the Dwass-Steel-Critchlow-Fligner method of adjusting for multiple Wilcoxon tests [15]. We compared control baseline adipokine data to ESRD patient baseline and follow-up data. Logistic regression and a best subset selection procedure were used to assist in the development of a parsimonious model of the odds of PTDM among ESRD patients. Given the small number of PTDM events (n = 11), the maximum number of predictors for the final subset model was fixed, in advance, at two. Variables considered for selection included age, gender, BMI, race, cytomegalovirus antibody status, type of transplantation (preemptive vs. other), type of donor (living donor vs. other), prior transplant, prednisone at follow-up, and baseline levels of adipokines. Several of the adipokines were highly skewed and were natural logarithm transformed prior to modeling. The significance level for all tests was set, in advance, at 0.05. All statistical analyses were conducted using SAS 9.3.4 (SAS Institute, Cary, N.C., USA).
Results
Participants' Characteristics
Our final cohort included 32 ESRD patients that received a transplant and 36 controls. Of the 32 nondiabetic ESRD participants, 11 (34%) developed PTDM. PTDM was diagnosed up to 6 months after transplantation in 7 out of the 11 patients, and 4 patients were diagnosed between 6 months and 1 year after transplantation. Table 1 summarizes the patients' and control group's characteristics at the time of kidney transplantation. The fasting blood sugars in ESRD patients were all <100 mg/dl. The baseline characteristics were not significantly different between ESRD patients that developed PTDM and patients that did not (table 1). It is noteworthy, however, that the upper quartile of age was 9 years higher in the PTDM group and our nonparametric testing procedure was not sensitive to that disparity in these data. The transplant patients' characteristics at follow-up and 12 months after transplantation are summarized in online supplementary table 1 (see www.karger.com/doi/10.1159/000446294 for all online suppl. material). There was a similar increase in weight and change in BMI in the patients that developed PTDM and the patients that did not. There were also no statistically significant differences in estimated glomerular filtration rate, time to follow-up, percentage of individuals on steroids, or percentage of patients that suffered acute rejection at any of the follow-up time points between the PTDM and non-PTDM groups.
Table 1.
Baseline characteristics summarized by medians [1st, 3rd quartiles] or frequencies (percentages)
| Demographic variable | Donor controls | pa | ESRD, no DM at transplantation |
pb | |
|---|---|---|---|---|---|
| (n = 36) | no PTDM (n = 21) | PTDM (n = 11) | |||
| Age, years | 43.5 [35, 53] | 0.35 | 46 [38, 53] | 46 [40, 62] | 0.56 |
| Female | 24 (67) | 0.02 | 7 (33) | 3 (27) | 1.00 |
| Race | 0.49 | 1.00 | |||
| African-American | 4 (11) | 2 (10) | 3 (27) | ||
| Caucasian | 31 (86) | 16 (76) | 8 (73) | ||
| Other | 1 (3) | 3 (14) | 0 (0) | ||
| BMI | 25 9 [22.2,28 6] | 0.41 | 27 9 [23.8,31 7] | 27 [25.1,33 5] | 1.00 |
| Weight, lb | 165 [142, 187] | 0.11 | 184 [164, 220] | 180 [175, 220] | 0.92 |
| Albumin, g/dl | 4.4 [4.3, 4.6] | 0.53 | 4.4 [4.2, 4.6] | 4.4 [4.2, 4.5] | 0.96 |
| Fasting glucose, mg/dl | 86 [78, 92] | 0.17 | 86 [84, 93] | 92 [82, 97] | 0.64 |
| Plasma creatinine, mg/dl | 0.8 [0.7, 0.9] | <0.01 | 7.7 [5.5, 9.8] | 7.6 [6.8, 9.6] | 1.00 |
| Prior transplantation | 2 (10) | 3 (27) | 0.31 | ||
| HCV antibody positive | 0 (0) | 0.47 | 1 (5) | 0 (0) | 1.00 |
| CMV antibody positive | 15 (42) | 0.16 | 6 (29) | 7 (64) | 0.30 |
| Type of transplant donor | 0.19 | ||||
| Deceased | 5 (24) | 2 (18) | |||
| Living - related | 11 (52) | 3 (27) | |||
| Living - unrelated | 5 (24) | 6 (55) | |||
| Type of renal replacement | 0.44 | ||||
| Hemodialysis | 10 (48) | 7 (64) | |||
| Peritoneal dialysis | 4 (19) | 0 (0) | |||
| Preemptive | 7 (33) | 4 (36) | |||
| Cause of CKD | 0.07 | ||||
| Hypertension | 0 (0) | 2 (18) | |||
| Glomerulonephritis | 10 (48) | 5 (45) | |||
| Cystic/polycystic | 7 (33) | 0 (0) | |||
| Lithium toxicity | 0 (0) | 1 (9) | |||
| Obstruction | 0 (0) | 1 (9) | |||
| Reflux nephropathy | 1 (5) | 0 (0) | |||
| Systemic lupus erythematosus | 2 (10) | 0 (0) | |||
| Unknown | 1 (5) | 2 (18) | |||
Two degrees offreedomtest for any differences between groups.
No PTDM versus PTDM.
Plasma Adipokines
Adipokine plasma levels before transplantation (baseline) and 3-6 months after transplantation (follow-up) are summarized in tables 2 and 3. At baseline (table 2), ESRD patients had higher IL-6, TNFα, CRP, and MCP-1 levels than controls (p < 0.01). No differences in baseline levels of cytokines or adiponectin were found between those ESRD patients that developed PTDM and the ones who did not develop PTDM in univariable analysis.
Table 2.
Baseline cytokine levels summarized by medians [1st, 3rd quartiles]
| Variable | Donor controls | pa | ESRD, no DM at transplantation |
pb | |
|---|---|---|---|---|---|
| no PTDM | PTDM | ||||
| IL-6, pg/ml | 2.6 [2.1,3.4] | <0.01 | 4.7 [3.3,7.2] | 3.9 [2.7,8.8] | 0.78 |
| IL-8, pg/ml | 10.6 [8.5,14.6] | 0.87 | 12.3 [8.0,16.5] | 10 8 [8.4,13 1] | 0.93 |
| Adiponectin, µg/ml | 8.7 [5.4,13 1] | 0.19 | 12 4 [9.4,17 0] | 9.7 [5.5,15.9] | 0.73 |
| HMW adiponectin, µg/ml | 4.12 [2.57,7.21] | 0.35 | 6.69 [3.61,8.76] | 5.15 [2.57,5.66] | 0.46 |
| Adiponectin ratio, %c | 51 3 [47.3,59 2] | 0.62 | 58 9 [42.0,69 0] | 46.7 [30 8,62.9] | 0.87 |
| TNFα, pg/ml | 9.4 [8.3,10.4] | <0.01 | 15.2 [12 4,20.1] | 17 8 [13.3,30 8] | 0.36 |
| CRP, mg/dl | 1.2 [0.5,2.4] | <0.01 | 4.7 [1.5,6.1] | 2.1 [0.8,4.0] | 0.29 |
| MCP-1, pg/ml | 293 [207, 443] | <0.01 | 406 [332, 491] | 447 [350, 625] | 0.72 |
HMW = High-molecular-weight.
Two degrees of freedom test for any differences between groups.
No PTDM versus PTDM.
HMW adiponectin/total adiponectin × 100.
Table 3.
Follow-up cytokine levels summarized by medians [1st, 3rd quartiles]
| Variable | Donor baseline | pa | ESRD, no DM at transplant |
pb | |
|---|---|---|---|---|---|
| no PTDM follow-up | PTDM follow-up | ||||
| IL-6, pg/ml | 2.6 [2.1,3.4] | 0.02 | 4.5 [2.7,7.4] | 4.7 [2.3,6.4] | 0.83 |
| IL-8, pg/ml | 10.6 [8.5,14.6] | 0.44 | 8.6 [6.1,15.3] | 12 2 [9.6,13 0] | 0.44 |
| Adiponectin, µg/ml | 8.7 [5.4,13 1] | 0.98 | 8.9 [6.0,12 4] | 9.6 [4.7,13.4] | 1.00 |
| HMW adiponectin, µg/ml | 4.12 [2.57,7.21] | 0.32 | 3.86 [1.80,5.15] | 3.09 [2.06,4.63] | 0.94 |
| Adiponectin ratio, %c | 51 3 [47.3,59 2] | <0.01 | 39 3 [37.1,46 2] | 38.5 [30 8,46.8] | 0.90 |
| TNFα, pg/ml | 9.4 [8.3,10.4] | 0.03 | 12.0 [10 8,13.2] | 11 4 [9.9,12 7] | 0.92 |
| CRP, mg/dl | 1.2 [0.5,2.4] | 0.10 | 1.7 [1.1,3.4] | 2.3 [0.8,4.4] | 0.97 |
| MCP-1, pg/ml | 293 [207, 443] | <0.01 | 446 [386, 650] | 436 [353, 628] | 0.98 |
HMW = High-molecular-weight.
Two degrees of freedom test for any differences between groups.
No PTDM versus PTDM.
HMW adiponectin/total adiponectin × 100.
Three to 6 months following transplantation (table 3), plasma cytokine levels were compared between the controls and transplant recipients with restored renal function (mean creatinine at follow-up 1.42 mg/dl). Despite improvement in renal function, IL-6, TNFα, and MCP-1 levels were significantly higher after transplantation when compared to controls at the time of donation (p < 0.05). There were no statistically significant differences in unadjusted post-transplant mean levels of inflammatory cytokines and adiponectin between those who developed PTDM and those who did not at follow-up.
Logistic regression analyses were performed to determine those baseline variables (clinical parameters and cytokines levels) that were most predictive of PTDM. The best two-predictor logistic regression model of the odds of PTDM among nondiabetic ESRD patients included age and baseline log TNFα. TNFα tended to be higher at baseline among those ESRD patients that developed PTDM than among patients who did not, although the difference did not reach statistical significance in unadjusted analysis (online suppl. fig. 1, p = 0.36). However, adjustment for the confounding attributable to the advanced age disparity between the groups revealed that for each 25% increase in TNFα the odds of developing PTDM were significantly increased (2-fold increase; ORTNFα = 2.03, p = 0.04; ORage = 1.02, p = 0.07). These results suggest that elevated TNFα prior to transplantation could be a predictor of subsequent PTDM.
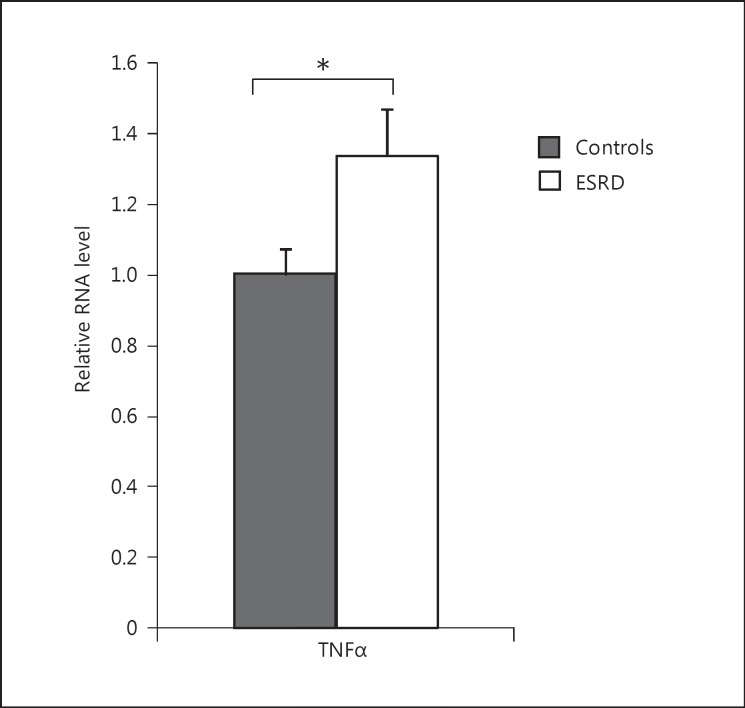
Fig. 1.
Visceral fat cytokine expression in ESRD participants versus controls. mRNA expression of TNFα was assessed in visceral adipose tissue of ESRD participants and compared with controls with normal kidney function. TNFα mRNA fold change: 1.33; * p < 0.05.
Plasma TNFα levels before transplantation were similar in patients with preemptive kidney transplants and patients that were on dialysis (19.3 and 15.95 pg/ml, respectively; p = 0.99), and in patients that had a prior kidney transplant and patients that did not (20.1 and 16.1 pg/ml, respectively; p = 0.22). TNFα levels at follow-up did not differ if the patients had their first post-transplant follow-up level measured early versus late (9.9, 11.2, and 12.7 pg/ml for patients whose level was measured at ≤90, 91-150, and >150 days, respectively; p > 0.05 for the comparison between ≤90 days and >150 days).
Of those patients that developed PTDM, 55% were on prednisone at the time of the follow-up visit; similarly, 57% of those patients that did not develop PTDM were on prednisone at follow-up.
Adipose Tissue mRNA Data
Samples of visceral adipose tissue obtained at the time of surgery were investigated to determine the expression of inflammatory cytokines (fig. 1). Compared to controls, mRNA expression of TNFα was significantly higher in visceral adipose tissue of ESRD patients (1.33-fold change; p < 0.05). mRNA expression of IL-6, IL-8, CRP, and MCP-1 in visceral adipose tissue of ESRD participants was no different than in controls (data not shown). We have previously reported that there is an increase in mRNA expression of adiponectin and adiponectin receptor 1 in ESRD patients compared to controls [16].
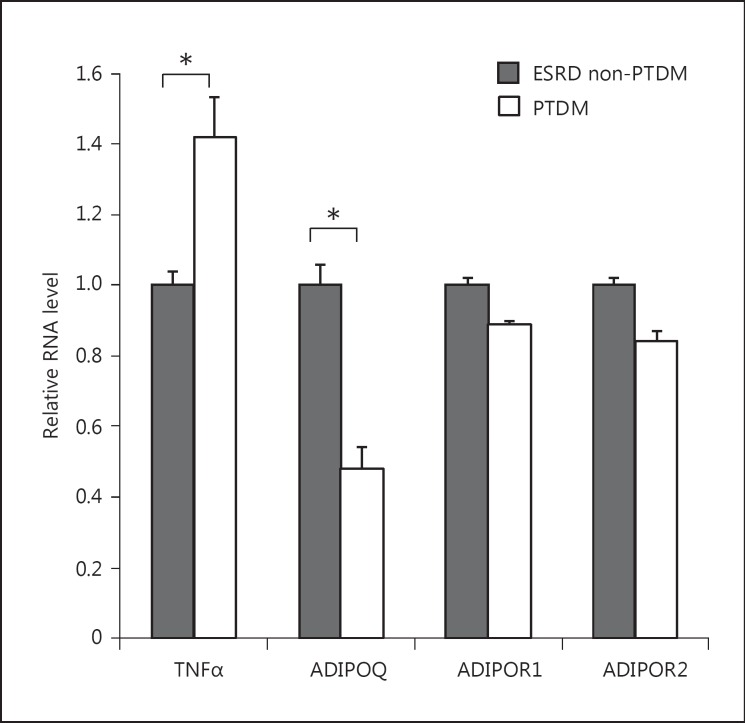
We then examined the expression of cytokines and adiponectin receptors in visceral fat of ESRD participants that developed PTDM compared to those who did not (fig. 2). Patients who developed PTDM had increased mRNA expression of TNFα (1.42-fold change; p = 0.05) and lower adiponectin mRNA expression (0.48-fold change; p < 0.05) compared to those patients who did not. Adiponectin receptor 1 and adiponectin receptor 2 mRNA expression was similar in participants who developed PTDM and nondiabetic patients (fold changes: 0.89 and 0.86, p = 0.33 and p = 0.49, respectively). There were no differences in expression of other inflammatory cytokines (data not shown).
Fig. 2.
Visceral fat mRNA expression of cytokines in ESRD participants. TNFα, adiponectin (ADIPOQ), adiponectin receptor 1 (ADIPOR1), and adiponectin receptor 2 (ADIPOR2) mRNA expression from visceral adipose tissue was assessed in ESRD participants that developed PTDM versus ESRD patients that did not. TNFα mRNA fold change: 1.42, ADIPOQ mRNA fold change: 0.48, * p < 0.05; ADIPOR1 mRNA fold change: 0.89, p = 0.33; ADIPOR2 mRNA fold change: 0.86, p = 0.49.
In vitro Study with C2C12 Cells
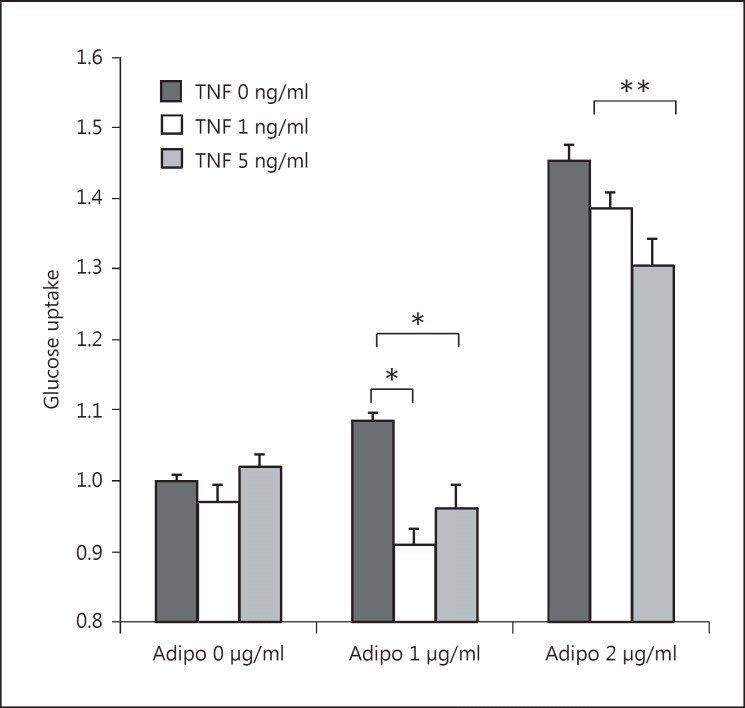
To further explore a possible role of TNFα in PTDM, we conducted a series of in vitro experiments utilizing the myoblast cell line C2C12. We exposed the cells to increasing concentrations of globular adiponectin and TNFα and studied cell glucose uptake by 2-NBDG (green fluorescent 2-deoxyglucose). 2-NBDG is taken up by cells but cannot be catabolized like glucose by hexokinase; hence, fluorescence intensity is a marker of glucose uptake [17,18]. As shown in figure 3, when cells were exposed to adiponectin only, there was a dose-dependent increase in glucose transport (10% increase with adiponectin 1 μg/ml and 45% increase with adiponectin 2 μg/ml; both p < 0.001). Alternatively, myoblast exposure to both adiponectin and TNFα resulted in a reduction of adiponectin-mediated glucose transport at both low- and high-dose adiponectin exposure (12% decrease with adiponectin 1 μg/ml-TNFα 1 ng/ml, p < 0.001; 10% decrease with adiponectin 1 μg/ml-TNFα 5 ng/ml, p < 0.01; 9% decrease with adiponectin 2 μg/ml-TNFα 1 ng/ml, p < 0.15; 11% decrease with adiponectin 2 μg/ml-TNFα 5 ng/ml, p < 0.05). In the absence of adiponectin (adiponectin 0 μg/ml), there was no change in glucose transport when myoblast cells were exposed to increasing concentrations of TNFα (p = 0.25 for TNFα 1 ng/ml and p = 0.32 for TNFα 5 ng/ml). The results of these in vitro experiments demonstrate that TNFα impairs adiponectin-mediated glucose uptake.
Fig. 3.
Glucose uptake by 2-NBDG in C2C12 cells. C2C12 cells were treated with ascending doses of globular adiponectin (0, 1, and 2 µg/ml) and TNFα (0, 1, and 5 ng/ml) for 48 h. * p < 0.01, ** p < 0.05.
Discussion
In this clinical study of ESRD patients that underwent kidney transplantation, we found a significant association between pre-transplant circulating TNFα levels and development of PTDM. We also demonstrated that TNFα mRNA levels in visceral adipose tissue of ESRD patients are higher than those of controls. Moreover, those patients that subsequently developed PTDM had higher TNFα and lower adiponectin mRNA expression in visceral adipose tissue than ESRD participants who did not. Data from our in vitro studies on a myoblast cell line demonstrate that TNFα blunts adiponectin-mediated glucose transport in muscle cells. Therefore, our study supports the concept that there is an interplay between TNFα and adiponectin in ESRD that impairs glucose transport and may facilitate the development of PTDM.
PTDM and diabetes present many similar risk factors [2,19,20,21,22,23], but immunosuppressive medications and infections are specific to organ transplantation [24,25,26,27]. Our study proposes that inflammation, in particular pre-transplant TNFα levels, also plays a significant role in the pathogenesis of PTDM.
The relationship between chronic inflammation and glucose abnormalities in post-transplant patients is unknown, but we demonstrated that inflammation, specifically TNFα levels, is associated with PTDM in CKD patients. On the other hand, there is a well-established association of chronic inflammation with IR and diabetes among individuals with normal kidney function [28,29,30]. Inflammatory cytokines facilitate the development of IR and diabetes through activation of cellular pathways such as NF-κB and JNK or activation of the NLRP3 inflammasome [31,32]. Increased production of TNFα by adipose tissue is believed to partially explain the relationship between inflammation and IR/diabetes with normal kidney function [28]. In humans, TNFα has been shown to impair insulin-mediated glucose transport by directly altering insulin signaling [33]. Specifically, TNFα is known to induce peripheral IR by inactivation of IRS-1 and subsequent inhibition of AKT phosphorylation and glucose transporter exocytosis. To our knowledge, this is the first report that links pre-transplant inflammation with glucose abnormalities after kidney transplantation. Moreover, our in vitro study suggests a novel mechanism by which TNFα can interfere with glucose metabolism.
In contrast to TNFα, the anti-inflammatory cytokine adiponectin enhances glucose uptake and insulin sensitivity [34]. In kidney transplant patients, adiponectin is inversely associated with PTDM despite higher circulating plasma levels compared with controls [35,36]. With our in vitro study, we wanted to determine if there was a relationship of TNFα and adiponectin to glucose transport. In a muscle cell line model, we demonstrated that TNFα exposure blunts adiponectin-mediated glucose transport. Our data replicate those of other studies showing similar effects of adiponectin on glucose transport [34,37], but they also expand the prior knowledge by demonstrating that the adiponectin response is dampened by concomitant TNFα exposure. Our clinical study demonstrates that TNFα plasma levels before transplantation are associated with the development of PTDM, although we were not able to show an association between adiponectin and PTDM, most likely due to the limited sample size. In sum, we hypothesize based on our in vitro and clinical studies that TNFα in ESRD may contribute to subsequent development of PTDM by blunting adiponectin-mediated glucose transport, a mechanism that has not been previously described.
Adiponectin is derived from adipocytes and is considered to be an antidiabetic cytokine. Low circulating levels of adiponectin in human obesity are thought to partially explain the relationship between obesity and IR, metabolic syndrome, or type 2 diabetes [38]. However, in patients with loss of renal function, adiponectin plasma levels are markedly higher than in patients with normal renal function. In previous studies of uremic patients, we found an increased production of adiponectin by adipose tissue and higher circulating levels of adiponectin compared to controls [14]. We also determined that uremia induces adiponectin resistance at the post-receptor level [16]. In our current study, the in vitro experiments demonstrated that TNFα blunts adiponectin-derived glucose transport in myocytes. These findings suggest that TNFα confers adiponectin resistance, thus providing a plausible novel mechanism for the development of PTDM.
Our findings also indicate that uremic patients have a higher expression of TNFα mRNA in visceral adipose tissue than controls; thus, they are consistent with the findings of Roubicek et al. [39]. These investigators reported higher inflammatory cell infiltration in adipose tissue and an increase in TNFα mRNA in visceral adipose tissue in ESRD patients compared to controls. Prior data have linked the dialysis process, clotted grafts, poor dentition, chronic infections, and comorbid conditions with the chronic inflammatory state seen in ESRD patients [40]. These data suggest that adipose tissue inflammation contributes to the chronic inflammatory state seen in CKD and ESRD patients.
Moreover, data from our study indicated that uremic patients who developed PTDM also had a higher expression of TNFα mRNA and lower adiponectin mRNA expression in adipose tissue than uremic patients who did not develop PTDM. These data are in concordance with the observation made by Bayés et al. [41] that lower plasma adiponectin levels are associated with PTDM. Our study was not powered to detect differences in plasma adiponectin levels between PTDM and non-PTDM groups. The lower production of adiponectin by adipose tissue in patients that developed PTDM compared with patients that did not develop PTDM may be a more sensitive marker of impairment of adiponectin production than are plasma levels. It is plausible that patients who develop PTDM not only manifest adiponectin resistance secondary to their kidney disease and inflammation but also have a limited capacity to produce adiponectin. Therefore, the tissue alterations in adipokine metabolism appear to be more pronounced in patients who subsequently develop PTDM.
Our study has some limitations. The sample size was small, which may have limited its statistical power to detect associations between cytokines and PTDM. It was not possible to conduct an OGTT (oral glucose tolerance test) on participants prior to transplantation; therefore, it is possible that some diabetics could have been mislabeled as nondiabetics. HbA1c is not an acceptable alternative to detect diabetes in ESRD. There are many confounding factors that affect the accuracy of HbA1c measurements in ESRD [42], including shortened red blood cell survival, frequent blood loss, erythropoietin treatment, acidosis [43], and increased oxidative stress [44]. In all participants, we corroborated a medical history and medical records with fasting blood glucose measurements prior to transplantation. Because no subject enrolled in the study had a fasting serum glucose level >100 mg/dl, we believe mislabeling of nondiabetic patients due to the lack of an OGTT was unlikely. We did not carry out any assessment of IR at the time of transplantation, since static methods of assessing IR are widely variable and not reliable in ESRD subjects [45]. The incidence of PTDM in our study is slightly higher than in other studies [1,25,46] but probably reflects our particular population in the context of increased incidence of metabolic syndrome, ethnicity, and race stratification [47]. In our adipose tissue studies, we were unable to distinguish whether macrophages or adipose cells are the main contributors to TNFα production. We decided to focus our study on visceral fat as it has been demonstrated to be metabolically more active than subcutaneous fat, and further studies should be done to determine if similar changes are also encountered in subcutaneous fat. Although our in vitro studies suggest that TNFα mediates adiponectin resistance, we did not delineate the exact mechanism.
In summary, TNFα appears to be associated with development of PTDM. Adipose tissue from ESRD patients has an increased expression of TNFα mRNA, suggesting enhanced TNFα production. Our in vitro studies show that TNFα can impair adiponectin-mediated glucose transport. Therefore, a hypothetical mechanism for PTDM is that TNFα induces adiponectin resistance and concurrently there is a reduced capacity to produce adiponectin by adipose tissue. Our study proposes that inflammation is involved in glucose regulation independently of insulin after kidney transplantation.
Statement of Ethics
The Institutional Review Board at TJU approved the study protocol, and written informed consent was obtained from each participant (control No. 10G.261).
Disclosure Statement
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge the transplant coordinators Karen O'Neill, Linda Wright, Cherryl Parcon, Bethany Hosier, Jean Berte, Vanessa Vuong, Christina Petyo, and Kim Phillips for their help in the recruitment of participants. This work was supported in part by an NIH grant (T32GM008562 to M.P.M.C.) and an ADA grant (7-12-JF-41 to M.P.M.C.).
References
- 1.Davidson J, Wilkinson A, Dantal J, Dotta F, Haller H, Hernandez D, Kasiske BL, Kiberd B, Krentz A, Legendre C, Marchetti P, Markell M, van der Woude FJ, Wheeler DC, International Expert Panel New-onset diabetes after transplantation: 2003 International Consensus Guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation. 2003;75((suppl)):SS3–SS24. doi: 10.1097/01.TP.0000069952.49242.3E. [DOI] [PubMed] [Google Scholar]
- 2.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3:178–185. doi: 10.1034/j.1600-6143.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 3.Woodward RS, Schnitzler MA, Baty J, Lowell JA, Lopez-Rocafort L, Haider S, Woodworth TG, Brennan DC. Incidence and cost of new onset diabetes mellitus among US wait-listed and transplanted renal allograft recipients. Am J Transplant. 2003;3:590–598. doi: 10.1034/j.1600-6143.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 4.Lentine KL, Brennan DC, Schnitzler MA. Incidence and predictors of myocardial infarction after kidney transplantation. J Am Soc Nephrol. 2005;16:496–506. doi: 10.1681/ASN.2004070580. [DOI] [PubMed] [Google Scholar]
- 5.Heit JJ, Apelqvist AA, Gu X, Winslow MM, Neilson JR, Crabtree GR, Kim SK. Calcineurin/NFAT signalling regulates pancreatic β-cell growth and function. Nature. 2006;443:345–349. doi: 10.1038/nature05097. [DOI] [PubMed] [Google Scholar]
- 6.Nam JH, Mun JI, Kim SI, Kang SW, Choi KH, Park K, Ahn CW, Cha BS, Song YD, Lim SK, Kim KR, Lee HC, Huh KB. β-Cell dysfunction rather than insulin resistance is the main contributing factor for the development of postrenal transplantation diabetes mellitus. Transplantation. 2001;71:1417–1423. doi: 10.1097/00007890-200105270-00011. [DOI] [PubMed] [Google Scholar]
- 7.Hornum M, Jørgensen KA, Hansen JM, Nielsen FT, Christensen KB, Mathiesen ER, Feldt-Rasmussen B. New-onset diabetes mellitus after kidney transplantation in Denmark. Clin J Am Soc Nephrol. 2010;5:709–716. doi: 10.2215/CJN.05360709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reese PP, Bloom RD. Transplant-associated hyperglycemia: shedding light on the mechanisms. Clin J Am Soc Nephrol. 2010;5:560–562. doi: 10.2215/CJN.01430210. [DOI] [PubMed] [Google Scholar]
- 9.DeFronzo RA, Alvestrand A, Smith D, Hendler R, Hendler E, Wahren J. Insulin resistance in uremia. J Clin Invest. 1981;67:563–568. doi: 10.1172/JCI110067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi S, Maesato K, Moriya H, Ohtake T, Ikeda T. Insulin resistance in patients with chronic kidney disease. Am J Kidney Dis. 2005;45:275–280. doi: 10.1053/j.ajkd.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 11.Koppe L, Pelletier CC, Alix PM, Kalbacher E, Fouque D, Soulage CO, Guebre-Egziabher F. Insulin resistance in chronic kidney disease: new lessons from experimental models. Nephrol Dial Transplant. 2014;29:1666–1674. doi: 10.1093/ndt/gft435. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee D, Recio-Mayoral A, Chitalia N, Kaski JC. Insulin resistance, inflammation, and vascular disease in nondiabetic predialysis chronic kidney disease patients. Clin Cardiol. 2011;34:360–365. doi: 10.1002/clc.20901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tien KJ, Lin ZZ, Chio CC, Wang JJ, Chu CC, Sun YM, Kan WC, Chien CC. Epidemiology and mortality of new-onset diabetes after dialysis: Taiwan National Cohort Study. Diabetes Care. 2013;36:3027–3032. doi: 10.2337/dc12-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez Cantarin MP, Waldman SA, Doria C, Frank AM, Maley WR, Ramirez CB, Keith SW, Falkner B. The adipose tissue production of adiponectin is increased in end-stage renal disease. Kidney Int. 2013;83:487–494. doi: 10.1038/ki.2012.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Critchlow DE, Fligner MA. On distribution-free multiple comparisons in the one-way analysis of variance. Commun Stat Theory Methods. 1991;20:127–139. [Google Scholar]
- 16.Martinez Cantarin MP, Keith SW, Waldman SA, Falkner B. Adiponectin receptor and adiponectin signaling in human tissue among patients with end-stage renal disease. Nephrol Dial Transplant. 2014;29:2268–2277. doi: 10.1093/ndt/gfu249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada K, Saito M, Matsuoka H, Inagaki N. A real-time method of imaging glucose uptake in single, living mammalian cells. Nat Protoc. 2007;2:753–762. doi: 10.1038/nprot.2007.76. [DOI] [PubMed] [Google Scholar]
- 18.Zou C, Wang Y, Shen Z. 2-NBDG as a fluorescent indicator for direct glucose uptake measurement. J Biochem Biophys Methods. 2005;64:207–215. doi: 10.1016/j.jbbm.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Glucose tolerance and mortality comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Europe. Lancet. 1999;354:617–621. [PubMed] [Google Scholar]
- 20.Bayer ND, Cochetti PT, Anil Kumar MS, Teal V, Huan Y, Doria C, Bloom RD, Rosas SE. Association of metabolic syndrome with development of new-onset diabetes after transplantation. Transplantation. 2010;90:861–866. doi: 10.1097/TP.0b013e3181f1543c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosio FG, Kudva Y, van der Velde M, Larson TS, Textor SC, Griffin MD, Stegall MD. New onset hyperglycemia and diabetes are associated with increased cardiovascular risk after kidney transplantation. Kidney Int. 2005;67:2415–2421. doi: 10.1111/j.1523-1755.2005.00349.x. [DOI] [PubMed] [Google Scholar]
- 22.Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care. 1999;22:920–924. doi: 10.2337/diacare.22.6.920. [DOI] [PubMed] [Google Scholar]
- 23.Saydah SH, Loria CM, Eberhardt MS, Brancati FL. Subclinical states of glucose intolerance and risk of death in the US. Diabetes Care. 2001;24:447–453. doi: 10.2337/diacare.24.3.447. [DOI] [PubMed] [Google Scholar]
- 24.Vincenti F, Friman S, Scheuermann E, Rostaing L, Jenssen T, Campistol JM, Uchida K, Pescovitz MD, Marchetti P, Tuncer M, Citterio F, Wiecek A, Chadban S, El-Shahawy M, Budde K, Goto N, DIRECT (Diabetes Incidence after Renal Transplantation Neoral C Monitoring versus Tacrolimus) Investigators: Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant. 2007;7:1506–1514. doi: 10.1111/j.1600-6143.2007.01749.x. [DOI] [PubMed] [Google Scholar]
- 25.Montori VM, Basu A, Erwin PJ, Velosa JA, Gabriel SE, Kudva YC. Posttransplantation diabetes: a systematic review of the literature. Diabetes Care. 2002;25:583–592. doi: 10.2337/diacare.25.3.583. [DOI] [PubMed] [Google Scholar]
- 26.Baid S, Cosimi AB, Farrell ML, Schoenfeld DA, Feng S, Chung RT, Tolkoff-Rubin N, Pascual M. Posttransplant diabetes mellitus in liver transplant recipients: risk factors, temporal relationship with hepatitis C virus allograft hepatitis, and impact on mortality. Transplantation. 2001;72:1066–1072. doi: 10.1097/00007890-200109270-00015. [DOI] [PubMed] [Google Scholar]
- 27.Bloom RD, Rao V, Weng F, Grossman RA, Cohen D, Mange KC. Association of hepatitis C with posttransplant diabetes in renal transplant patients on tacrolimus. J Am Soc Nephrol. 2002;13:1374–1380. doi: 10.1097/01.asn.0000012382.97168.e0. [DOI] [PubMed] [Google Scholar]
- 28.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 29.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 30.Spranger J, Kroke A, Möhlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 31.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes. 2005;54:2939–2945. doi: 10.2337/diabetes.54.10.2939. [DOI] [PubMed] [Google Scholar]
- 34.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 35.Bayés B, Granada ML, Pastor MC, Lauzurica R, Salinas I, Sanmartí A, Espinal A, Serra A, Navarro M, Bonal J, Romero R. Obesity, adiponectin and inflammation as predictors of new-onset diabetes mellitus after kidney transplantation. Am J Transplant. 2007;7:416–422. doi: 10.1111/j.1600-6143.2006.01646.x. [DOI] [PubMed] [Google Scholar]
- 36.Hjelmesæth J, Flyvbjerg A, Jenssen T, Frystyk J, Ueland T, Hagen M, Hartmann A. Hypoadiponectinemia is associated with insulin resistance and glucose intolerance after renal transplantation: impact of immunosuppressive and antihypertensive drug therapy. Clin J Am Soc Nephrol. 2006;1:575–582. doi: 10.2215/CJN.01471005. [DOI] [PubMed] [Google Scholar]
- 37.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 38.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roubicek T, Bartlova M, Krajickova J, Haluzikova D, Mraz M, Lacinova Z, Kudla M, Teplan V, Haluzik M. Increased production of proinflammatory cytokines in adipose tissue of patients with end-stage renal disease. Nutrition. 2009;25:762–768. doi: 10.1016/j.nut.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 40.Stenvinkel P. Inflammation in end-stage renal failure: could it be treated? Nephrol Dial Transplant. 2002;17((suppl 8)):33–38. doi: 10.1093/ndt/17.suppl_8.33. discussion 40. [DOI] [PubMed] [Google Scholar]
- 41.Bayés B, Lauzurica R, Granada ML, Serra A, Bonet J, Fontseré N, Salinas I, Romero R. Adiponectin and risk of new-onset diabetes mellitus after kidney transplantation. Transplantation. 2004;78:26–30. doi: 10.1097/01.tp.0000132561.48217.b1. [DOI] [PubMed] [Google Scholar]
- 42.Sharif A, Baboolal K. Diagnostic application of the A1c assay in renal disease. J Am Soc Nephrol. 2010;21:383–385. doi: 10.1681/ASN.2010010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Marchi S, Cecchin E, Basile A, Donadon W, Lippi U, Quaia P, Tesio F. More on the increase of hemoglobin A1 in chronic renal failure: the role of acidosis. Nephron. 1983;35:49–53. doi: 10.1159/000183044. [DOI] [PubMed] [Google Scholar]
- 44.Selvaraj N, Bobby Z, Sridhar MG. Increased glycation of hemoglobin in chronic renal failure: [corrected] potential role of oxidative stress. Arch Med Res. 2008;39:277–284. doi: 10.1016/j.arcmed.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Pham H, Utzschneider KM, de Boer IH. Measurement of insulin resistance in chronic kidney disease. Curr Opin Nephrol Hypertens. 2011;20:640–646. doi: 10.1097/MNH.0b013e32834b23c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hagen M, Hjelmesæth J, Jenssen T, Mørkrid L, Hartmann A. A 6-year prospective study on new onset diabetes mellitus, insulin release and insulin sensitivity in renal transplant recipients. Nephrol Dial Transplant. 2003;18:2154–2159. doi: 10.1093/ndt/gfg338. [DOI] [PubMed] [Google Scholar]
- 47.Luan FL, Stuckey LJ, Ojo AO. Abnormal glucose metabolism and metabolic syndrome in non-diabetic kidney transplant recipients early after transplantation. Transplantation. 2010;89:1034–1039. doi: 10.1097/TP.0b013e3181d05a90. [DOI] [PMC free article] [PubMed] [Google Scholar]