Abstract
Background
Previous studies examining the association of apolipoprotein E (APOE) gene polymorphism with the risk of ischemic stroke (IS) have yielded conflicting results. Therefore, we performed a meta-analysis to investigate the association between APOE ε4 gene polymorphism and risk of IS.
Summary
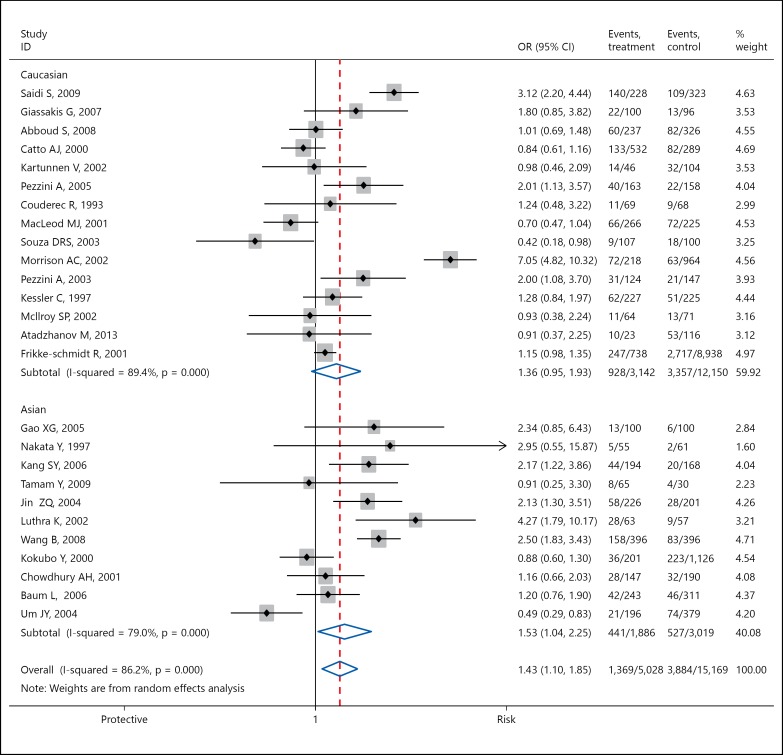
A literature search for genetic association studies published before May 30, 2015, was conducted in the PubMed, EMBASE and Google Scholar databases. The following search terms were used: (apolipoprotein E) or (APOE) and (ε4) and (polymorphism) or (polymorphisms) and (‘ischemic stroke’ or ‘IS’) and (‘cerebral infarction’ or ‘CI’) and (‘genetic polymorphism’ or ‘single nucleotide polymorphisms’ or ‘SNP’). ORs and 95% CIs were used to calculate the strength of association. Begg's funnel plot was used to assess the potential for publication bias. In our meta-analysis, 26 case-control studies involving 6,397 IS cases and 19,053 controls were included. Overall significant association between carrier of ε4 allele and risk of IS was observed (OR 1.43, 95% CI 1.10-1.85, p = 0.007). In the subgroup analysis based on ethnicity, a significant association between Apo ε4 carrier and risk of IS was observed in Asian studies (OR 1.53, 95% CI 1.04-2.25, p = 0.031) whereas borderline significant association between APO ε4 carrier and risk of IS was observed in Caucasian studies (OR 1.36, 95% CI 0.95-1.93, p = 0.093).
Key Messages
Our meta-analysis suggests that APOE ε4 allele is associated with higher risk of IS in Asian population as compared to Caucasian population.
Key Words: Apolipoprotein-E, Association study, Ischemic stroke, Cerebral infarction, Meta-analysis, Gene polymorphism
Introduction
Stroke is the second major leading cause of death and adult disability after ischemic heart disease [1]. Stroke has accounted for nearly 5.7 million deaths globally and 87% of these deaths take place in low and middle income nations [2]. In the last 4 decades, incidence of stroke in South Asian countries has been amplified by more than 100% while this is decreased by 42% in the developed European countries [3,4]. This increase in the incidence of stroke in developing countries could have been influenced by environmental and genetic factors. Ischemic stroke (IS) is a multifactorial, polygenic disease and comprises of 80-85% of overall stroke [5]. Epidemiological and animal studies have robustly recommended genetic influence in the pathogenesis of IS.
Apolipoprotein E (APOE) gene is one of the commonly studied genes in vascular and neurodegenerative diseases, which is located on chromosome 19q13.2 [6]. Its protein products are composed of glycoprotein with 3 common isoforms E2, E3 and E4 encoded by the respective alleles ε2, ε3 and ε4 giving rise to 6 genotypes. Apo-E protein plays an important role in lipid metabolism and transport and is also significantly expressed in the brain. There is substantial evidence of association between Apo ε4 allele and elevated low density lipoprotein cholesterol levels and thereby there is an increase in the risk of cardiovascular disease [7,8,9,10]. It has been shown that elevated level of ApoE in plasma is an important risk factor for stroke. Several studies have shown inconsistent results for the association between APOE gene polymorphism and risk of IS [11]. Factors responsible for inconsistent results include different study designs and inadequate characterization of phenotypes, variation in sample size and lack of proper case-control matching. Thus, we conducted a meta-analysis to investigate the association between APOE gene polymorphism and risk of IS.
Methods
Identification of Relevant Studies
A literature search for genetic association studies published before May 30, 2015, was conducted in the PubMed, EMBASE and Google Scholar databases. The following search terms were used: (apolipoprotein E) or (APOE) and (ε4) and (polymorphism) or (polymorphisms) and (‘ischemic stroke’ or ‘IS’) and (‘cerebral infarction’ or ‘CI’) and (‘genetic polymorphism’ or ‘single nucleotide polymorphisms’ or ‘SNP’). We included studies that were conducted on human subjects and the studies were searched without any limitations on language. We thoroughly reviewed all the references to find out the relevant published studies in the literature.
Inclusion and Exclusion Criteria
The inclusion criteria for the studies were as following: (1) case-control studies exploring the association between the APOE ε4 gene polymorphism and risk of IS, (2) diagnosis of IS according to World Health Organization and (3) studies with enough reported genotypic and allelic data. The exclusion criteria were: (1) study design other than case-control study, (2) publications with overlapping cases and controls from the similar study and (3) no genotypic data available. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline [12].
Data Extraction
Two authors (A.K. and P.K.) separately reviewed each full-text article for eligibility and extracted the data. Any disagreements were resolved by discussion among all the authors.
Quality Assessment
We also checked the methodological quality of each study using a methodological quality assessment scale [13] for the genetic association studies and it was modified by us to increase the relevance of our study. This scale took into account both traditional epidemiological considerations and genetic issues. The scores ranged from 0 (worst) to 16 (best). Details of the scale items are presented in table 1. Two authors (A.K. and P.K.) independently assessed the quality of included studies. Discrepancies over quality scores were resolved by discussion among all the authors and subsequent consensus was reached.
Table 1.
Scale for quality assessment
| Criteria | Score |
|---|---|
| Representativeness of cases | |
| Selected from any population disease registry or multiple center sites | 2 |
| Selected from any cardiology/neurology | 1 |
| Not described | 0 |
| Source of controls | |
| Population or neighbour based | 3 |
| Hospital based | 2 |
| Healthy volunteers with total description | 1 |
| Healthy volunteers without total description | 0.5 |
| Matching of controls | |
| Age and sex match | 2 |
| Smoking, hypertensive, diabetics | 1 |
| Not matched | 0 |
| Ascertainment of IS | |
| Adequate confirmation | 2 |
| Diagnosis of IS by patient medical record | 1 |
| Not described | 0 |
| Ascertainment of controls | |
| Stroke frees status by using appropriate QVSS or CT/MRI | 1 |
| Not described | 0 |
| Genotyping | |
| Genotyping done under blinded conditions | 1 |
| Unblinded or not mentioned | 0 |
| Genotyping method | |
| DNA sequencing/multiplex polymerase chain reaction | 2 |
| Polymerase chain reaction-restriction fragment length polymorphism | 1 |
| Others | 0 |
| HWE | |
| Allelic frequency in accordance HWE | 2 |
| Not HWE but followed statistics to adjust confounding | 1 |
| Not checked | 0 |
| Association assessment | |
| Appropriate statistics and examining confounders and effect modifiers | 1 |
| Inappropriate statistics used inappropriate statistics used | 0 |
| Total score | 16 |
Statistical Analysis
Hardy-Weinberg equilibrium (HWE) using the chi-square test was used to check the distribution of genotypes. Pooled OR and 95% CI were used to test the magnitude of association between the APOE ε4 gene polymorphism and risk of IS. Heterogeneity between studies was checked by using I2 metric [14]. I2 >50% was considered as presence of significant heterogeneity. Fixed effects model was used when I2 <50%, or else random effects model was used. Along with an overall comparison, stratified analysis on the basis of ethnicity and age was used to explore whether differences in association are present between different ethnicities and different age groups. Begg's funnel plot was used to assess the potential for publication bias. All the statistical analysis was performed using STATA version 13.1 software.
Results
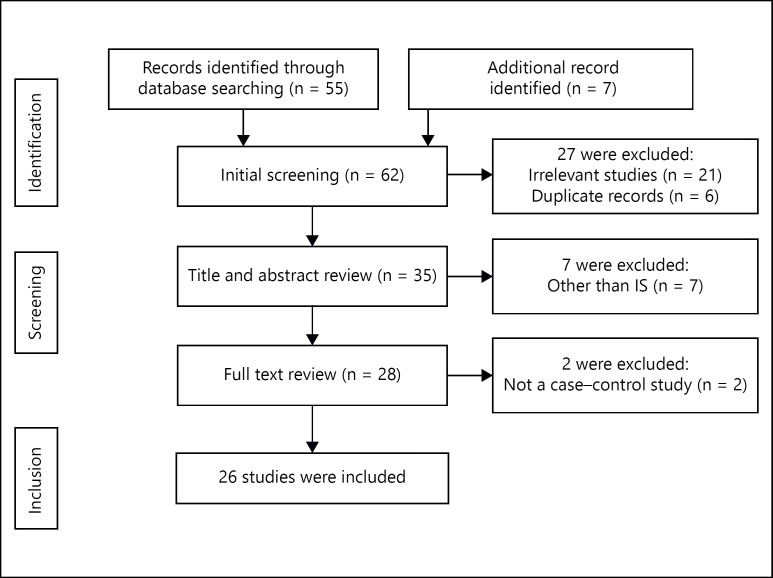
A total of 62 published articles were identified using the pre-specified search strategy. Figure 1 represents a flow chart of included and excluded studies along with their causes for exclusion. Out of 62 retrieved articles, 21 studies were excluded because they were irrelevant to our interests, 6 studies were excluded as they were in duplicate records, 7 studies were excluded due to conducted in other than IS and 2 studies were excluded as they were not of case-control study design. Keeping the inclusion criteria in mind, 26 case-control studies were included in our meta-analysis. Based on ethnicity, studies were carried out in 2 major ethnic populations; 11 studies were conducted in Asian while 15 studies were conducted in Caucasian population. We found the studies published in the literature from year 1993 to 2013. The genotype distribution in controls of 12 studies included in the present meta-analysis was in accordance with HWE. The methodological qualities of most of the studies were found to be moderately high. Out of 26 studies, the source of controls was hospital based in 13 studies, population based in 10 studies and 3 studies did not report their source of controls. A summary of the characteristics and methodological quality of the included studies in the present meta-analysis are mentioned in table 2.
Fig. 1.
Flow diagram of the selection of studies and specific reasons for exclusion from the present meta-analysis.
Table 2.
Characteristic of studies included in the meta-analysis of the association of APOE ε4 gene polymorphism with the risk of ischemic stroke
| No. | First author, year | Origin | Ethnicity | Sample size, n (case/control) | PCR method | Matching criteria | M/F (case/control) | Age (case/control) | HWE | Source of control | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Saidi [17], 2007 | Tunisia | Caucasian | 228/323 | PCR-RFLP | Age-sex | 114/114 117/146 | 61.5±12.1/60.9±12.8 | Yes | PB | 12 |
| 2 | Gao [18], 2006 | Chinese | Asian | 100/100 | PCR-DHPLC | Age-sex | 71/29 71/29 | 60.08±10.77/60.9±10.64 | No | HB | 11 |
| 3 | Giassakis [19], 2007 | Greece | Caucasian | 100/96 | Nested PCR-RFLP | Age-sex | 70/30 66/30 | 60.7±9.8/61.3±9.8 | No | PB | 9 |
| 4 | Nakata [20], 1997 | Japan | Asian | 55/61 | PCR-RFLP | Age-sex | 25/30 30/31 | 66±14/67±8 | Yes | PB | 12 |
| 5 | Abboud [21], 2008 | Beligum | Caucasian | 237/326 | PCR | NA | NA | NA | Yes | PB | 12 |
| 6 | Kang [22], 2006 | Korea | Asian | 194/168 | PCR-sequencing | Age | 116/78 94/74 | 62±9.5/62.3±6.3 | No | HB | 11 |
| 7 | Tamam [23], 2009 | Turkey | Asian | 65/30 | PCR | Age-sex | 44/21 10/20 | 65.5±14.3/61.9±14.7 | No | NA | 6.5 |
| 8 | Catto [24], 2000 | UK | Caucasian | 513/289 | PCR-RFLP | Age-sex | 297/295 150/139 | 73 (64-80)/72.5 (58-79) | Yes | HB | 12 |
| 9 | Karttunen [25], 2002 | Finland | Caucasian | 46/104 | PCR-RFLP | Age-sex | 27/19 59/45 | 46 (15-60)/46 (17-62) | No | PB | 10 |
| 10 | Jin [26], 2004 | China | Asian | 226/201 | PCR-RFLP | Age-sex | 129/97 109/92 | 48.5±3.4/47.1±2.4 | Yes | PB | 11 |
| 11 | Pezzini [27], 2005 | Italy | Caucasian | 163/158 | Multiplex-PCR | Age-sex | 84/79 85/73 | 35±7.5/34±6.1 | No | HB | 11 |
| 12 | Luthra [28], 2002 | India | Asian | 63/57 | PCR-RFLP | NA | NA | 56.4±13.1/39.4±8 | No | HB | 8 |
| 13 | Wang [29], 2009 | China | Asian | 396/396 | PCR-RFLP | Age-sex | 209/187 201/195 | 57.3±8/57.2±8.09 | Yes | HB | 12 |
| 14 | Couderc [30], 1993 | France | Caucasian | 69/68 | PCR-RFLP | Age-sex | 36/33 33/35 | 72.3±11.6/72.1±11.5 | No | NA | 7.5 |
| 15 | MacLeod [31], 2001 | UK | Caucasian | 266/225 | PCR-RFLP | NA | 150/116 94/105 | 65.7±12.2/77±1 | Yes | PB | 9 |
| 16 | Kokubo [32], 2000 | Japan | Asian | 201/1,126 | PCR-RFLP | NA | 187/135 334/792 | 67.9±11/64.3 vs. 10.5 | No | PB | 8 |
| 17 | Souza [33], 2003 | Brazil | Caucasian | 107/100 | PCR-RFLP | Age-sex | NA | 68.8±9.17/69.4±8.29 | No | NA | 6 |
| 18 | Morrison [34], 2002 | US | Caucasian | 218/964 | PCR-RFLP | Age-sex | 113/105 415/549 | 56.6±0.4/53.9±0.1 | Yes | PB | 13 |
| 19 | Pezzini [35], 2004 | Italy | Caucasian | 124/147 | PCR-RFLP | Age-sex | 68/56 80/67 | 34.7±7.3/34.8±6.1 | No | HB | 9 |
| 20 | Kessler [36], 1997 | Germany | Caucasian | 227/225 | PCR-RFLP | Age-sex | 108/119 108/117 | 62.3±14.2/62.6±14 | No | HB | 9 |
| 21 | Mcllroy [37], 2002 | Ireland | Caucasian | 64/71 | PCR-RFLP | Smoking-hypertension | 37/27 14/57 | 73.8±8.1/74.3±7.6 | No | HB | 9 |
| 22 | Chowdhury [38], 2001 | Bangladesh | Asian | 147/190 | PCR-RFLP | NA | 117/30 129/61 | 57.9±11.1/60.3±9.6 | No | HB | 7 |
| 23 | Atadzhanov [39], 2013 | Zambia | Caucasian | 23/116 | TaqMan assay - direct sequencing | Age-sex | NA | NA | Yes | HB | 13 |
| 24 | Baum [40], 2006 | Hong Kong | Asian | 246/336 | PCR-RFLP | Age | 134/112 152/184 | 70.7±12/71.0±5.9 | Yes | HB | 12 |
| 25 | Frikke-Schmidt [41], 2001 | Denmark | Caucasian | 738/8,938 | PCR-RFLP | Age-sex | 457/281 4,022/4,916 | 63.2±0.4/57.2±0.2 | Yes | PB | 11 |
| 26 | Um [42], 2003 | Korea | Asian | 196/379 | PCR-RFLP | NA | NA | NA | Yes | HB | 8 |
PB = Population based; HB = hospital based; PCR-RFLP = polymerase chain reaction-restriction fragment length polymorphism; NA = not applicable.
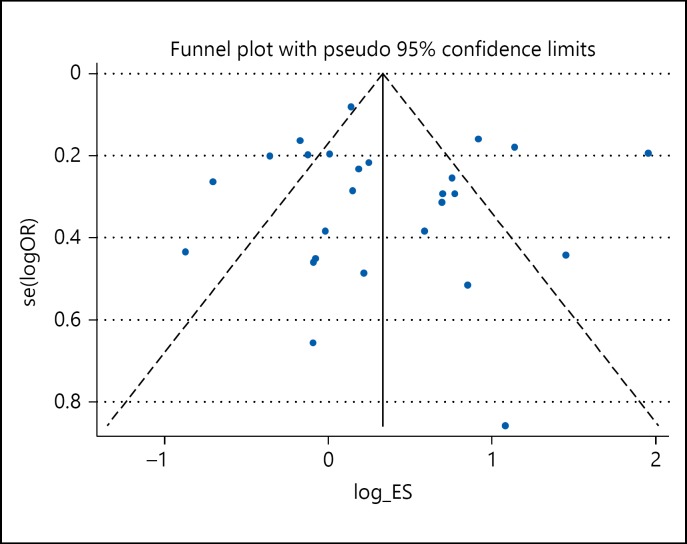
A total of 26 case-control studies involving 6,397 cases and 19,053 controls were included in our meta-analysis. Overall, a significant association between carrier of ε4 allele and risk of IS was observed (OR 1.43, 95% CI 1.10-1.85, p = 0.007). In the subgroup analysis based on ethnicity, significant association between Apo ε4 carrier and risk of IS was observed in 11 Asian studies involving 2,327 IS cases and 2,546 controls (OR 1.53, 95% CI 1.04-2.25, p = 0.031) but borderline significant association was observed in 15 Caucasian studies involving 4,070 IS cases and 15,507 controls (OR 1.36, 95% CI 0.95-1.93, p = 0.093). A significant heterogeneity was observed (I2 = 86.2%; pHet < 0.0001; fig. 2). The shape of the Begg's funnel plot suggests the presence of significant publication bias (fig. 3).
Fig. 2.
Forest plot for the association between APOE ε4 gene polymorphism and IS risk.
Fig. 3.
Begg's funnel plot for investigating publication bias for the included studies.
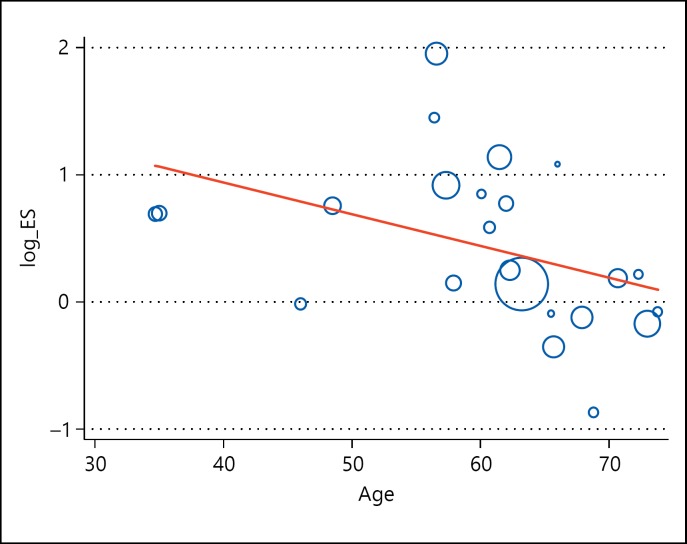
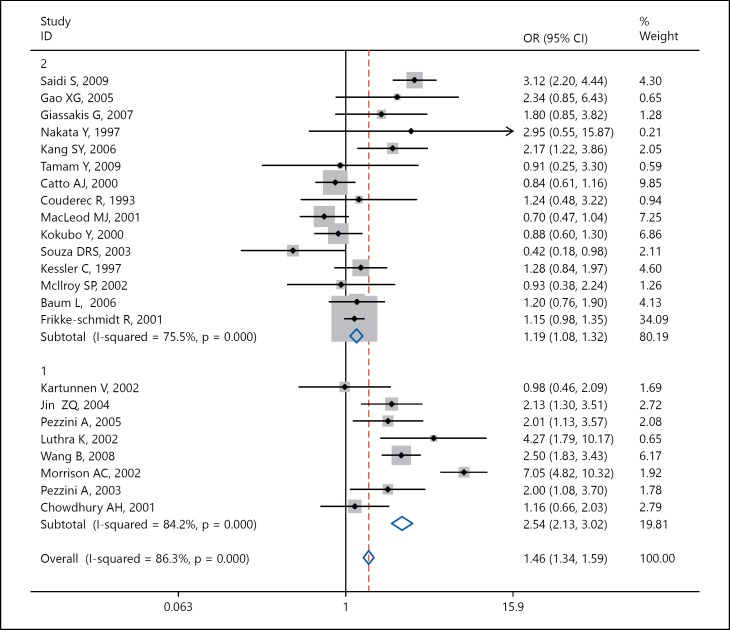
We analyzed the data using meta-regression analysis to explore whether age plays a significant role in the association between Apo-E polymorphism and risk of IS and found that increasing age is associated with decrease in effect size of association of APOE polymorphism with the risk of IS (p = 0.05; fig. 4). Further stratified analysis based on age category grouped as ≤60 and >60 years was done, and we found patients with IS having age ≤60 years (OR 2.54, 95% CI 2.13-3.02) and age >60 years (OR 1.19, 95% CI 1.08-1.32) had a significant association of APO-E ε4 genotype with the risk of IS (fig. 5).
Fig. 4.
Meta-regression plot for age-stratified analysis. Increasing age suggests a decrease in log odds of association of APOE polymorphism with IS (p value = 0.05).
Fig. 5.
Forrest plot of stratified analysis by age for association between APOE ε4 gene polymorphism and IS risk. Stratified analysis based on mean age of the cases in individual study ≤60 and >60 years suggests that age ≤60 years had double OR (OR 2.54) as compared to those who had mean age >60 years (OR 1.19).
Discussion
There is evidence about the involvement of genetic factors for development of IS. In the present meta-analysis, we investigated the association between Apo-ε4 gene polymorphism and risk of IS. Our study results suggest that there is a higher risk of IS in subjects who are carriers of ε4 allele of APOE gene. Our findings are consistent with the previously published meta-analysis [15] involving 4,096 IS cases and 16,117 controls suggesting Apo-E polymorphism contributes to the risk of stroke (OR 1.11, 95% CI 1.01-1.22). A recently published meta-analysis suggested that APO-ε4 allele is associated with increased risk for cerebral infarction in Chinese population [16].
We also conducted a subgroup analysis based on ethnicity and observed that the Apo-ε4 carrier allele is more prone to have the risk of IS in Asian population as compared to the Caucasian population. In the current study, we observed that 14 studies were deviated from HWE and a potential publication bias with significant heterogeneity was found.
We conducted a meta-regression analysis using mean age of cases of individual study as continuous variables and found out that increasing age is associated with decrease in effect size of association of APOE polymorphism with the risk of IS (p = 0.05; fig. 4). Furthermore, we stratified the data on the basis of the age category as ≤60 and >60 years and observed that patients with IS having age ≤60 years had significant association of APOE ε4 genotype with the risk of IS with an OR 2.54 and 95% CI 2.13-3.02 while patient with IS having age >60 years had a significant association with an OR 1.19 and 95% CI 1.08-1.32 (fig. 5).
There were a few limitations in our study. (1) Some studies included in the meta-analysis had small sample size and may have provided inconsistent results due to low statistical power. (2) Stroke risk varies as per specific subtypes of stroke; however, most of the studies included in the meta-analysis have not presented the data as per subtype of stroke; therefore, meta-analysis based on association between APOE polymorphism and subtype of stroke has not been done. (3) The use of different methodologies for genotyping method, selection of controls and matching criteria may have led to heterogeneity. (4) Heterogeneity may also be due to the variations in ethnicity, age and environmental factors. (5) Survival bias may be present in included case-control studies as these studies may not be designed to recruit the critically ill patient at the acute onset.
In spite of the limitations listed above, our findings demonstrate that Apo-ε4 allele is associated with increased risk of IS. Our meta-analysis suggests that IS patients have higher frequency of ε4 allele in Asian population than in Caucasian population. To explore a definitive conclusion, further well designed and large sample size epidemiological studies are needed to be performed in the near future.
Authorship Contribution
A.K. and P.K.: concept, data search, extraction; M.P.: writing of manuscript; S.M.: data entry and drafting of manuscript; A.K.P.: manuscript writing; K.C.: writing and drafting of manuscript.
Disclosure Statement
There is no potential conflict of interest. This study received no funding or sponsorship of any form.
References
- 1.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 2.Reddy KS, Yusuf S. Emerging epidemic of cardiovascular disease in developing countries. Circulation. 1998;97:596–601. doi: 10.1161/01.cir.97.6.596. [DOI] [PubMed] [Google Scholar]
- 3.Abegunde DO, Mathers CD, Adam T, Ortegon M, Strong K. The burden and costs of chronic diseases in low-income and middle-income countries. Lancet. 2007;370:1929–1938. doi: 10.1016/S0140-6736(07)61696-1. [DOI] [PubMed] [Google Scholar]
- 4.Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet Neurol. 2007;6:182–187. doi: 10.1016/S1474-4422(07)70031-5. [DOI] [PubMed] [Google Scholar]
- 5.Della-Morte D, Guadagni F, Palmirotta R, Testa G, Caso V, Paciaroni M, et al. Genetics of ischemic stroke, stroke-related risk factors, stroke precursors and treatments. Pharmacogenomics. 2012;13:595–613. doi: 10.2217/pgs.12.14. [DOI] [PubMed] [Google Scholar]
- 6.Ribalta J, Vallvé JC, Girona J, Masana L. Apolipoprotein and apolipoprotein receptor genes, blood lipids and disease. Curr Opin Clin Nutr Metab Care. 2003;6:177–187. doi: 10.1097/00075197-200303000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 8.Laskowitz DT, Horsburgh K, Roses AD. Apolipoprotein E and the CNS response to injury. J Cereb Blood Flow Metab. 1998;18:465–471. doi: 10.1097/00004647-199805000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Anthopoulos PG, Hamodrakas SJ, Bagos PG. Apolipoprotein E polymorphisms and type 2 diabetes: a meta-analysis of 30 studies including 5423 cases and 8197 controls. Mol Genet Metab. 2010;100:283–291. doi: 10.1016/j.ymgme.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Al-Khedhairy AA. Apolipoprotein E polymorphism in Saudis. Mol Biol Rep. 2004;31:257–260. doi: 10.1007/s11033-005-2713-x. [DOI] [PubMed] [Google Scholar]
- 11.Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Attia J, Thakkinstian A, D'Este C. Meta-analyses of molecular association studies: methodologic lessons for genetic epidemiology. J Clin Epidemiol. 2003;56:297–303. doi: 10.1016/s0895-4356(03)00011-8. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu L, Su L, Chen Q, Liang B, Qin Y, Xie J, et al. Association between the apolipoprotein E gene polymorphism and ischemic stroke in Chinese populations: new data and meta-analysis. Exp Ther Med. 2013;5:853–859. doi: 10.3892/etm.2012.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Q, Wang WJ, Wu L, Liu L, Han LZ. Meta-analysis of APOE ε2/ε3/ε4 polymorphism and cerebral infarction. J Neural Transm (Vienna) 2013;120:1479–1489. doi: 10.1007/s00702-013-1019-8. [DOI] [PubMed] [Google Scholar]
- 17.Saidi S, Slamia LB, Ammou SB, Mahjoub T, Almawi WY. Association of apolipoprotein E gene polymorphism with ischemic stroke involving large-vessel disease and its relation to serum lipid levels. J Stroke Cerebrovasc Dis. 2007;16:160–166. doi: 10.1016/j.jstrokecerebrovasdis.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Gao X, Yang H, ZhiPing T. Association studies of genetic polymorphism, environmental factors and their interaction in ischemic stroke. Neurosci Lett. 2006;398:172–177. doi: 10.1016/j.neulet.2005.12.078. [DOI] [PubMed] [Google Scholar]
- 19.Giassakis G, Veletza S, Papanas N, Heliopoulos I, Piperidou H. Apolipoprotein E and first-ever ischaemic stroke in Greek hospitalized patients. J Int Med Res. 2007;35:127–133. doi: 10.1177/147323000703500114. [DOI] [PubMed] [Google Scholar]
- 20.Nakata Y, Katsuya T, Rakugi H, Takami S, Sato N, Kamide K, et al. Polymorphism of angiotensin converting enzyme, angiotensinogen, and apolipoprotein E genes in a Japanese population with cerebrovascular disease. Am J Hypertens. 1997;10((12 pt 1)):1391–1395. doi: 10.1016/s0895-7061(97)00315-4. [DOI] [PubMed] [Google Scholar]
- 21.Abboud S, Viiri LE, Lütjohann D, Goebeler S, Luoto T, Friedrichs S, et al. Associations of apolipoprotein E gene with ischemic stroke and intracranial atherosclerosis. Eur J Hum Genet. 2008;16:955–960. doi: 10.1038/ejhg.2008.27. [DOI] [PubMed] [Google Scholar]
- 22.Kang SY, Lee WI. Apolipoprotein e polymorphism in ischemic stroke patients with different pathogenetic origins. Korean J Lab Med. 2006;26:210–216. doi: 10.3343/kjlm.2006.26.3.210. [DOI] [PubMed] [Google Scholar]
- 23.Tamam Y, Tasdemir N, Toprak R, Tamam B, Iltumur K. Apolipoprotein E genotype in patients with cerebrovascular diseases and its effect on the disease outcome. Int J Neurosci. 2009;119:919–935. doi: 10.1080/00207450802686350. [DOI] [PubMed] [Google Scholar]
- 24.Catto AJ, McCormack LJ, Mansfield MW, Carter AM, Bamford JM, Robinson P, et al. Apolipoprotein E polymorphism in cerebrovascular disease. Acta Neurol Scand. 2000;101:399–404. doi: 10.1034/j.1600-0404.2000.90308a.x. [DOI] [PubMed] [Google Scholar]
- 25.Karttunen V, Alfthan G, Hiltunen L, Rasi V, Kervinen K, Kesäniemi YA, et al. Risk factors for cryptogenic ischaemic stroke. Eur J Neurol. 2002;9:625–632. doi: 10.1046/j.1468-1331.2002.00464.x. [DOI] [PubMed] [Google Scholar]
- 26.Jin ZQ, Fan YS, Ding J, Chen M, Fan W, Zhang GJ, et al. Association of apolipoprotein E 4 polymorphism with cerebral infarction in Chinese Han population. Acta Pharmacol Sin. 2004;25:352–356. [PubMed] [Google Scholar]
- 27.Pezzini A, Grassi M, Del Zotto E, Archetti S, Spezi R, Vergani V, et al. Cumulative effect of predisposing genotypes and their interaction with modifiable factors on the risk of ischemic stroke in young adults. Stroke. 2005;36:533–539. doi: 10.1161/01.STR.0000155741.31499.c2. [DOI] [PubMed] [Google Scholar]
- 28.Luthra K, Prasad K, Kumar P, Dwivedi M, Pandey RM, Das N. Apolipoprotein E gene polymorphism in cerebrovascular disease: a case-control study. Clin Genet. 2002;62:39–44. doi: 10.1034/j.1399-0004.2002.620105.x. [DOI] [PubMed] [Google Scholar]
- 29.Wang B, Zhao H, Zhou L, Dai X, Wang D, Cao J, et al. Association of genetic variation in apolipoprotein E and low density lipoprotein receptor with ischemic stroke in Northern Han Chinese. J Neurol Sci. 2009;276:118–122. doi: 10.1016/j.jns.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Couderc R, Mahieux F, Bailleul S, Fenelon G, Mary R, Fermanian J. Prevalence of apolipoprotein E phenotypes in ischemic cerebrovascular disease. A case-control study. Stroke. 1993;24:661–664. doi: 10.1161/01.str.24.5.661. [DOI] [PubMed] [Google Scholar]
- 31.MacLeod MJ, de Lange RP, Breen G, Meiklejohn D, Lemmon H, Clair DS. Lack of association between apolipoprotein E genoype and ischaemic stroke in a Scottish population. Eur J Clin Invest. 2001;31:570–573. doi: 10.1046/j.1365-2362.2001.00851.x. [DOI] [PubMed] [Google Scholar]
- 32.Kokubo Y, Chowdhury AH, Date C, Yokoyama T, Sobue H, Tanaka H. Age-dependent association of apolipoprotein E genotypes with stroke subtypes in a Japanese rural population. Stroke. 2000;31:1299–1306. doi: 10.1161/01.str.31.6.1299. [DOI] [PubMed] [Google Scholar]
- 33.Souza DR, Campos BF, Arruda EF, Yamamoto LJ, Trindade DM, Tognola WA. Influence of the polymorphism of apolipoprotein E in cerebral vascular disease. Arq Neuropsiquiatr. 2003;61:7–13. doi: 10.1590/s0004-282x2003000100002. [DOI] [PubMed] [Google Scholar]
- 34.Morrison AC, Ballantyne CM, Bray M, Chambless LE, Sharrett AR, Boerwinkle E. LPL polymorphism predicts stroke risk in men. Genet Epidemiol. 2002;22:233–242. doi: 10.1002/gepi.0191. [DOI] [PubMed] [Google Scholar]
- 35.Pezzini A, Grassi M, Del Zotto E, Bazzoli E, Archetti S, Assanelli D, et al. Synergistic effect of apolipoprotein E polymorphisms and cigarette smoking on risk of ischemic stroke in young adults. Stroke. 2004;35:438–442. doi: 10.1161/01.STR.0000112973.00867.98. [DOI] [PubMed] [Google Scholar]
- 36.Kessler C, Spitzer C, Stauske D, Mende S, Stadlmüller J, Walther R, et al. The apolipoprotein E and beta-fibrinogen G/A-455 gene polymorphisms are associated with ischemic stroke involving large-vessel disease. Arterioscler Thromb Vasc Biol. 1997;17:2880–2884. doi: 10.1161/01.atv.17.11.2880. [DOI] [PubMed] [Google Scholar]
- 37.McIlroy SP, Dynan KB, Lawson JT, Patterson CC, Passmore AP. Moderately elevated plasma homocysteine, methylenetetrahydrofolate reductase genotype, and risk for stroke, vascular dementia, and Alzheimer disease in Northern Ireland. Stroke. 2002;33:2351–2356. doi: 10.1161/01.str.0000032550.90046.38. [DOI] [PubMed] [Google Scholar]
- 38.Chowdhury AH, Yokoyama T, Kokubo Y, Zaman MM, Haque A, Tanaka H. Apolipoprotein E genetic polymorphism and stroke subtypes in a Bangladeshi hospital-based study. J Epidemiol. 2001;11:131–138. doi: 10.2188/jea.11.131. [DOI] [PubMed] [Google Scholar]
- 39.Atadzhanov M, Mwaba MH, Mukomena PN, Lakhi S, Rayaprolu S, Ross OA, et al. Association of the APOE, MTHFR and ACE genes polymorphisms and stroke in Zambian patients. Neurol Int. 2013;5:e20. doi: 10.4081/ni.2013.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baum L, Ng HK, Wong KS, Tomlinson B, Rainer TH, Chen X, et al. Associations of apolipoprotein E exon 4 and lipoprotein lipase S447X polymorphisms with acute ischemic stroke and myocardial infarction. Clin Chem Lab Med. 2006;44:274–281. doi: 10.1515/CCLM.2006.047. [DOI] [PubMed] [Google Scholar]
- 41.Frikke-Schmidt R, Nordestgaard BG, Thudium D, Moes Grønholdt ML, Tybjaerg-Hansen A. APOE genotype predicts AD and other dementia but not ischemic cerebrovascular disease. Neurology. 2001;56:194–200. doi: 10.1212/wnl.56.2.194. [DOI] [PubMed] [Google Scholar]
- 42.Um JY, Moon KS, Lee KM, Cho KH, Heo Y, Moon BS, et al. Polymorphism of angiotensin-converting enzyme, angiotensinogen, and apolipoprotein E genes in Korean patients with cerebral infarction. J Mol Neurosci. 2003;21:23–28. doi: 10.1385/JMN:21:1:23. [DOI] [PubMed] [Google Scholar]