Abstract
Background
Depression has been linked to disruption in the cerebral levels of specific neurotransmitters. L-tyrosine is a precursor of more than one of the neurotransmitters affected by depression. Even though setbacks of monoamines precursors include high doses and low efficiency, many studies have suggested using L-tyrosine as antidepressant.
Purpose
The purpose of this study was to explore the possible antidepressant effect of L-tyrosine loaded in a nanoparticle-designed formula, using behavioral tests in acute and chronic mild stress (CMS) models of depression in rats.
Methods
Animals from both models received L-tyrosine-loaded nanoparticles (5 or 10 mg/kg), L-tyrosine solution (10 mg/kg), fluoxetine (10 mg/kg) or placebo daily for 21 days. Rats from the acute stress model of depression were subjected to open field and forced swim tests (FSTs). For the CMS model, sucrose preference test was carried out. Additionally, 3 profiles of the nanoparticles formula were tested in vitro. High dissolution rate and entrapment efficiency were obtained from the in vitro tests. Moreover, L-tyrosine-loaded nanoparticles 10 mg/kg and fluoxetine 10 mg/kg significantly decreased the immobility time in the FST, concomitant with restoration of the basal levels of locomotor activity, distance travelled and rearing counts. Also, an increase of the sucrose consumption was recorded in the sucrose preference test after treatment with L-tyrosine-loaded nanoparticles 10 mg/kg and fluoxetine 10 mg/kg.
Results
The positive results after treatment with L-tyrosine-loaded nanoparticles, through behavioral tests, are probably attributed to restorating the basal levels of the cerebral noradrenaline.
Conclusion
The effects of L-tyrosine administration on the cerebral levels of tyrosine hydroxylase and corticotropin-releasing factor should be further investigated.
Key Words: Depression, Open field test, Loco-motor activity, Forced swim test, Sucrose preference test
Introduction
Depression, as a mood disorder, is considered a serious problem to human health because of its relatively high prevalence associated with a significant disability [1].
A number of theories were studied to identify the etiology of depression, including genes and circadian rhythms [2]; however, the monoamine hypothesis was the most influential and widely studied one. This hypothesis suggests that disturbances in the cerebral level of noradrenaline (NA), dopamine or serotonin play a key role in depression [3].
Even though reports on the effectiveness of monoamine precursors for the management of moderate to severe depression cases remain uncritical [4,5,6], several studies have suggested serotonin precursors (tryptophan, 5-hydroxytryptophan) and catecholamines precursors (phenylalanine, tyrosine) as a possible way to manage depression [7,8].
L-tyrosine is a precursor of adrenaline, dopamine and NA, where it may have an impact on depression. Two clinical studies on depressed patients and healthy volunteers have shown that treatment with L-tyrosine has a positive role in depression management, mediated by NA and dopamine levels [8,9].
Rauch and Lieberman [10] and Lieberman et al. [11] have also reported that treatment of stressed rats with L-tyrosine reversed the depressive behavior induced by cold exposure or hyperthermia.
Nanoparticles and Brain Targeting
Using nanoparticles formula may help us deliver the anti-depressant drugs to the brain more efficiently. Positive results from the forced swim test (FST) and tail suspension test on mice treated with minocycline-loaded nanoparticles have suggested nanoparticles as an effective tool for brain targeting [12].
Nanoparticles mostly range in size between 10 and 1,000 nm, where the drug is coated inside or attached on to nanoparticle surface [13]. Polymeric nanoparticle is widely used to load drugs, regarding their controlled-release properties, size in subcellular range and safety [14]. Polymeric nanoparticles are hypothesized to cross the BBB by endocytosis and transcytosis, after binding to specific receptors, or by diffusion of the treatment to endothelial cells [15].
Our work aims at investigating the possible antidepressant effect of L-tyrosine loaded in polymeric nanoparticles, compared to L-tyrosine solution in 2 animal models of depression, using behavioral test batteries.
Methods
Animal Models
Eighty-eight male Wistar rats aged 90 days, weighing 160 ± 25 g, were purchased from the animal house at the Faculty of Science, University of Aleppo. The animals were acclimatized for a period of 1 week before start of the experiment. Rats had free access to food and water and maintained under a standard laboratory condition of temperature, humidity and 12 h of light/dark cycle. Some of housing and feeding conditions were changed as a part of the chronic mild stress (CMS) regimen details as shown in table 1. All experimental procedures were performed during the light phase of the cycle.
Table 1.
List of daily stressors applied on rats during the CMS experiment
| Day | Treatment |
|---|---|
| 1 | Cage soiled, 9 h |
| 2 | Food deprivation, 18 h |
| 3 | Inclination of the cage 45°, 3 h |
| 4 | Water deprivation, 18 h |
| 5 | Noise background, 6 h |
| 6 | Switch the cage mates, 4 h |
| 7 | Continous light, 27 h |
Prior to commencement of the experiment, animals were divided into acute stress model (40 rats) and mild chronic stress (48 rats) model.
The acute stress animals were further sub-divided into 5 groups (comprising of 8 animals each) according to the received treatment: control, L-tyrosine solution 10 mg/kg, L-tyrosine-loaded nanoparticles 5 mg/kg, L-tyrosine-loaded nanoparticles 10 mg/kg and fluoxetine 10 mg/kg.
The CMS animals were also sub-divided into 6 groups (with 8 animals in each group) corresponding to the treatment received: unstressed control, stressed control (both of these groups received placebo), L-tyrosine solution 10 mg/kg, L-tyrosine-loaded nanoparticles 5 mg/kg, L-tyrosine-loaded nanoparticles 10 mg/kg and fluoxetine 10 mg/kg.
Animals from both models were intraperitoneally injected with the treatment medication for 21 days.
The experimental protocol used in this study is consistent with the guidelines of care and use of animals in research [16]. Approval No. 3216 was obtained from the research committee at the University of Aleppo in order to achieve this experiment.
Chemicals and Reagents
The suspension of L-tyrosine-loaded polymeric nanoparticles was prepared using our modified version of nano-precipitation by solvent displacement technique [17], considering the different degrees of L-tyrosine solubility at both neutral and acidic pH conditions [18,19]. Changing the pH degree from acidity to neutral during preparation allowed us to precipitate L-tyrosine into the nanoparticles.
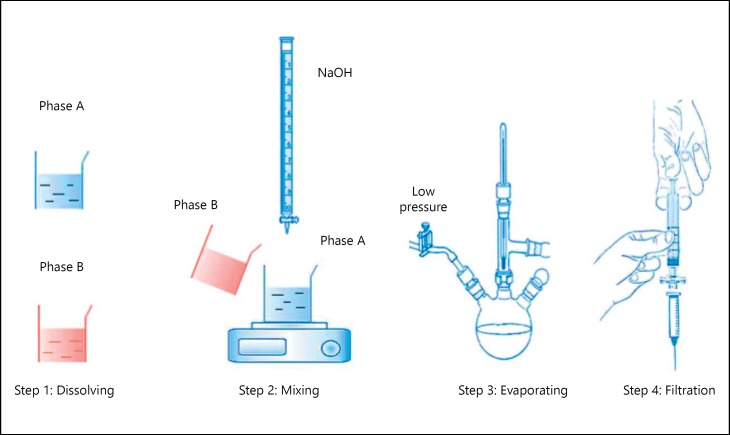
The preparation process (fig. 1) started by dissolving L-tyrosine (Merck, Germany), HCl 1.2 N (Prolabo, EEC), polycaprolactone (Sigma-Aldrich Chemicals, France) and acetone (SureChem Products Ltd., England) all together as phase A. Phase B included polysorbate 20 (BioChemica AppliChem GmbH, Germany), poloxamer 407 (Sigma-Aldrich Chemicals, France) and phosphate buffered saline (Prolabo, EEC).
Fig. 1.
Steps of the preparation of L-tyrosine-loaded nanoparticles formula. Phase A: L-tyrosine, HCL 1.2N, Polycaprolactone, and acetone. Phase B: Polysorbate 20, poloxamer 407, and phosphate buffered saline.
After dissolving all materials, we simultaneously injected phase B and NaOH (Prolabo, EEC) slowly within phase A, accompanied with mixing. Finally, acetone was extracted from the formula by evaporation on 37°C under low pressure. Both L-tyrosine solution 0.4 mg/ml and sucrose solution 1% w/v were prepared by dissolving L-tyrosine (Merck, Germany) and sucrose powder (Cooper, France) in distilled water. Prozac® (fluoxetine hydrochloride) was purchased from Aleppo, Syria.
Placebo solution for control group of the acute stress model, the stressed control group of the CMS model and non-stressed control group of the CMS model was prepared by using all excipients of the nanoparticles formula and following the same method of preparation.
After preparation of all solutions and suspensions, they were sterilized daily, using 0.22 μm nitrocellulose membrane filters under laminar flow (Laminar Flow Clean Bench I Labtech, India).
In Vitro Tests
The average size of L-tyrosine-loaded nanoparticles was measured immediately after preparation and 1 month later. Measurements were taken at 25°C using (Nano-ZS, Malvern Instruments, Malvern, UK). This test is helpful to assess the stability and aggregation of nanoparticles dispersions.
Another characteristic of L-tyrosine-loaded nanoparticles called ‘entrapment efficiency’ was detected after preparation by using (Jasco V-650 Spectrophotometer, Japan). The entrapment efficiency of L-tyrosine in the polymeric nanoparticles was estimated by measuring the concentration of the free drug (unentrapped) content within the supernatant liquid, represented by the absorption at ultraviolet wavelength 282.5 nm. We prepared the supernatant liquid by centrifugation the formula at 15,000 rpm for 2 h at 4°C using (CT ISRE, himac, Hitachi, Japan).
Finally, the dissolution rate of L-tyrosine-loaded nanoparticle formula was estimated in vitro after 24 and 48 h according to de Campos et al. [20] protocol, using a wavelength of 282.5 nm. Measuring the in vitro dissolution rate of the optimal formula is necessary, as it provides us with an approximate estimation of L-tyrosine releasing profile in vivo.
Behavioral Tests
Locomotor Activity and Open Field Tests
The initial activity of a rat placed in a novel surrounding, like the case of locomotor activity test, has been used as an indicator of its emotional state during both acute and chronic stress exposure [21]. Also, horizontal (travelled distance) and vertical (rearing) activities in the open field reflect the excitability and exploration behavior of the animal [22].
For the antidepressant assessment in the acute model, total activity, travelled distance and rearing counts were measured on days 2, 8, 15 and 22 of the experiment using Linton apparatus (Palgrave, Diss Norfolk, UK).
These tests were performed for 30 min, half an hour after administration of the treatment. Inside the measurement chamber, movement of the animal interrupts a beam of photocell light at which a count is recorded.
Forced Swim Test
FST is characterized by considerable reliability for depression assessment in the experimental animals [23]. In our study, we used FST as described by Porsolt et al. [24] with some modifications. The first swimming pre-test was applied for 15 min, 24 h prior to the start of behavioral tests, as it facilitates the development of depressive behavior in animals and increases the sensitivity to detect this behavior [25]. The second swimming test of 5 min was performed half an hour after treatment on days 1, 7, 14 and 21 of the experiment. Each rat was placed individually in a transparent cylinder (height: 50 cm, diameter: 30 cm) filled with 30 cm water and temperature of 23 ± 2°C, such that rat could not touch the bottom or skip out of the cylinder. After a period of struggling, the rat became immobile and moving only as required to keep its head out of the water. The immobility time was measured in blinded manner, using a camera (Sony CCD-TRV90) provided with a recorder.
Sucrose Preference Test
One of the main symptoms of depression is hedonia, defined as loss of interest or pleasure in the daily life activities [26]. Throughout this test, we measured sucrose solution in take because it is a strong representative of hedonia, commonly used and recognized in literatures of CMS [27].
In our experiment, the CMS system includes cage soiled, inclination of the cage, changes in the light-dark cycle, periods of food or water deprivation, noise background and switch of the cage mates [28]. We have applied daily stressors for a period, which varies depending on its type. Stressors mentioned in table 1 are repeated weekly during the CMS experiment.
In order to start the sucrose preference test, rats were first subjected to consume sucrose solution 1% w/v for 1 h, 4 days before the commencement of the CMS experiment, as a basic test of sucrose preference. The non-stressed control group was used for comparison with other stressed groups as an indicator of CMS model success. Stressed animals were deprived of food and water for 14 h; then they were represented to sucrose solution 1% w/v for 1 h. Sucrose consumption was measured by comparing the weight of the sucrose solution bottle before and after the test. We measured the consumption of sucrose once a week for 2 weeks of CMS before the treatment and once a week during 3 weeks of the daily treatment, where CMS procedures were continued during this period.
In addition to the above-mentioned tests, any changes in the features of the rat outputs, including urine and stool colour or stool consistency were recorded as possible side or toxic effects of the nanoparticle formula.
Statistical Analysis of Results
Results are expressed as (mean ± SD) of the individual values, obtained from each group. Statistical comparisons were performed using a statistical program Prism 5.0 for analysis of variance (ANOVA). One-way ANOVA, followed by Tukey test, was used to analyze the data. Values were considered significant if p value was ≤0.05.
Results
In Vitro Tests
The average size of L-tyrosine-loaded nanoparticles was 141.8 nm immediately after preparation and then slightly decreased up to 131.8 nm after 1 month (table 2).
Table 2.
Average size, entrapment efficiency and dissolution rates of the L-tyrosine-loaded nanoparticles formula
| Duration | Average size, nm | Entrapment efficiency, % | Dissolution rate, % |
|---|---|---|---|
| Immediately | 141.8±1.967 | 87.4479 | – |
| After 1 month | 131.8±1.25 | 66.9347 | – |
| After 24 h | – | – | 66.54 |
| After 48 h | – | – | 88.65 |
Average size represented as mean ± SD.
The entrapment efficiency of L-tyrosine inside the polymeric nanoparticles was also estimated, and 87.4479 and 66.9347% of L-tyrosine quantity were successfully entrapped immediately and 1 month after preparation, respectively (table 2).
The entrapment efficiency of L-tyrosine was calculated by the equation:
100 × (loading concentration - supernatant concentration)/loading concentration
The dissolution rate test on L-tyrosine-loaded nanoparticles showed that up to 66.54 and 88.65% of the loaded doses were released after 24 and 48 h of the test commencement, respectively, indicating to a prolonged release profile of L-tyrosine-loaded nanoparticles.
Behavioral Tests
Locomotor Activity and Open Field Tests
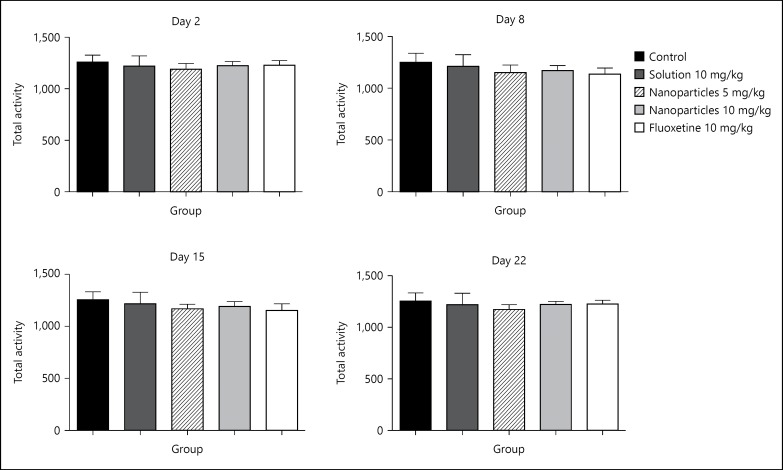
Total locomotor activity has shown no clear changes on day 2 of the experiment, but a decrease was recorded on day 8 in all groups. However, on days 15 and 22 of the experiment, there was an increase of the total activity in the groups of L-tyrosine-loaded nanoparticles 10 mg/kg and fluoxetine 10 mg/kg. This increase almost restored the locomotor activity basal levels (fig. 2).
Fig. 2.
Treatment effect on the total activity on days 2, 8, 15 and 22. Total activity was measured for 30 min, half hour after treatment on days 2, 8, 15, 22. Data are presented as mean ± SD, with 8 rats per group (one-way ANOVA followed by Tukey test). Total degree of freedom = 39. p < 0.05: no symbol, * p < 0.05, ** p < 0.01, *** p < 0.001.
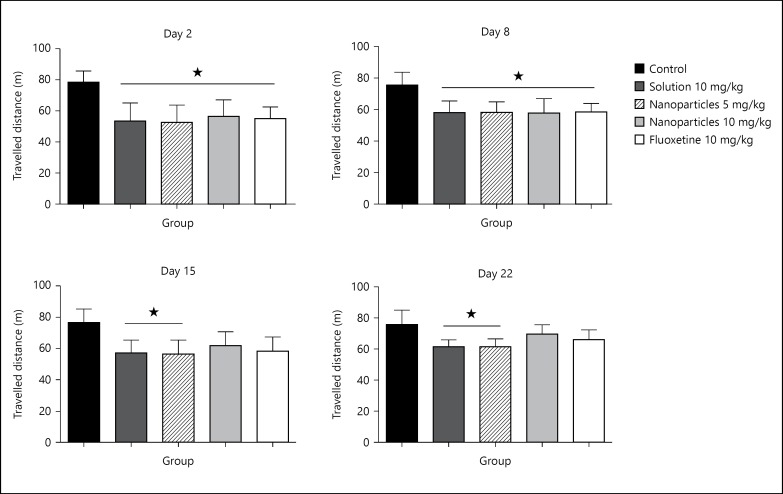
Figure 3 depicts the travelled distance during 30 min in the open field test. Significant decrease (p > 0.05) came on day 2 of the experiment in all groups, compared to control. Fifteen and 22 days of treatment with L-tyrosine-loaded nanoparticles 10 mg/kg and fluoxetine 10 mg/kg resulted in gradual restoration of the travelled distance.
Fig. 3.
Treatment effect on the travelled distance on days 2, 8, 15 and 22. Travelled distance was measured for 30 min, half hour after treatment on days 2, 8, 15, 22. Data are presented as mean ± SD, with 8 rats per group (one-way ANOVA followed by Tukey test). Total degree of freedom = 39. p < 0.05: no symbol, * p < 0.05, ** p < 0.01, *** p < 0.001.
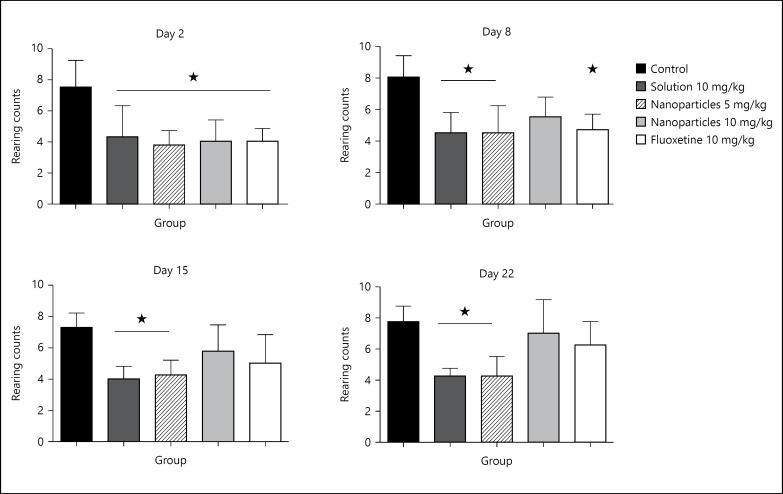
Similar results were obtained by measuring the rearing counts, where general decease was recorded on day 2 of the experiment (p > 0.05), in comparison with control. Restoration of the rearing counts came on days 8 and 15 of treatment with L-tyrosine-loaded nanoparticles 10 mg/kg and fluoxetine 10 mg/kg, respectively (fig. 4).
Fig. 4.
Treatment effect on the rearing counts on days 2, 8, 15 and 22. Rearing counts were measured for 30 min, half hour after treatment on days 2, 8, 15 and 22. Data are presented as mean ± SD, with 8 rats per group (one-way ANOVA followed by Tukey test). Total degree of freedom = 39. p < 0.05: no symbol, * p < 0.05, ** p < 0.01, *** p < 0.001.
Forced Swim Test
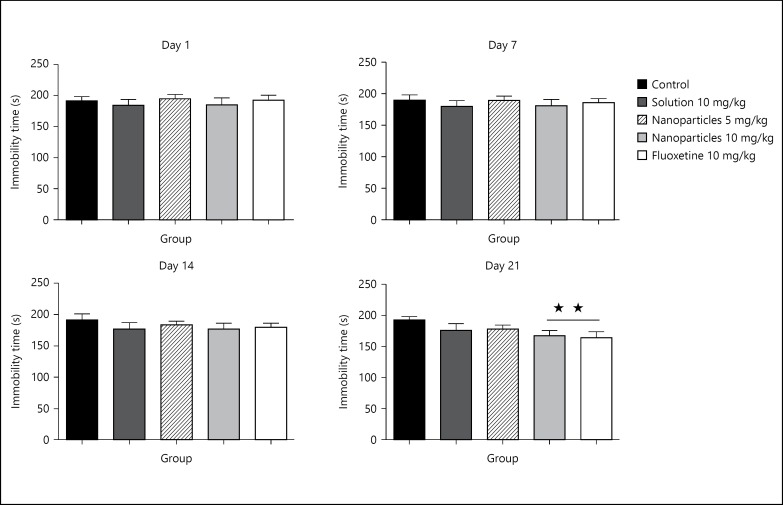
There was a trend to reduction in all treated groups on day 14 of the experiment. The significant effect on the immobility time came after 21 days of treatment at groups of L-tyrosine-loaded nanoparticles 10 mg/kg and fluoxetine 10 mg/kg as compared to control (p > 0.01; fig. 5).
Fig. 5.
Effect of treatment on the immobility time on days 1, 7, 14 and 21. The immobility time was measured for 30 min, half hour after treatment on days 1, 7, 14 and 21. Data are presented as mean ± SD, with 8 rats per group (one-way ANOVA followed by Tukey test). Total degree of freedom = 39. p < 0.05: no symbol, * p < 0.05, ** p < 0.01, *** p < 0.001.
Sucrose Preference Test
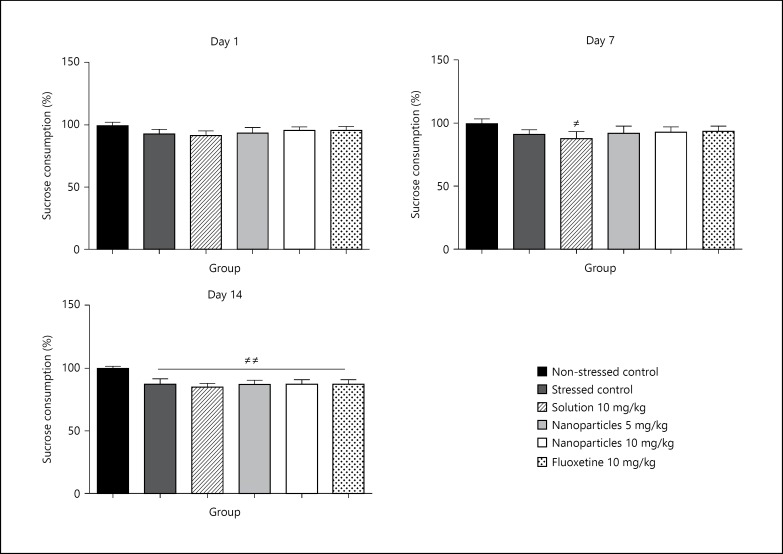
No significant difference in sucrose consumption was observed among groups by the beginning of CMS procedure. However, after 2 weeks of CMS, there was a significant reduction (p < 0.01) in sucrose consumption in all groups of CMS compared to the non-stressed control group as shown in figure 6.
Fig. 6.
Effect of CMS on the sucrose consumption on days 1, 7 and 14 before starting treatment with the corresponding drugs (non-stressed control, stressed control, L-tyrosine solution 10 mg/kg, L-tyrosine nanoparticles 5 mg/kg, L-tyrosine nanoparticles 10 mg/kg, fluoxetine 10 mg/kg). Sucrose consumption was measured for 1 h, half hour after treatment. Data are presented as mean ± SD with, 8 rats each group (one-way ANOVA followed by Tukey test). Total degree of freedom = 47. # The significant difference between non-stressed control and rest of groups. p < 0.05: no symbol, # p < 0.05, ## p < 0.01, ### p < 0.001.
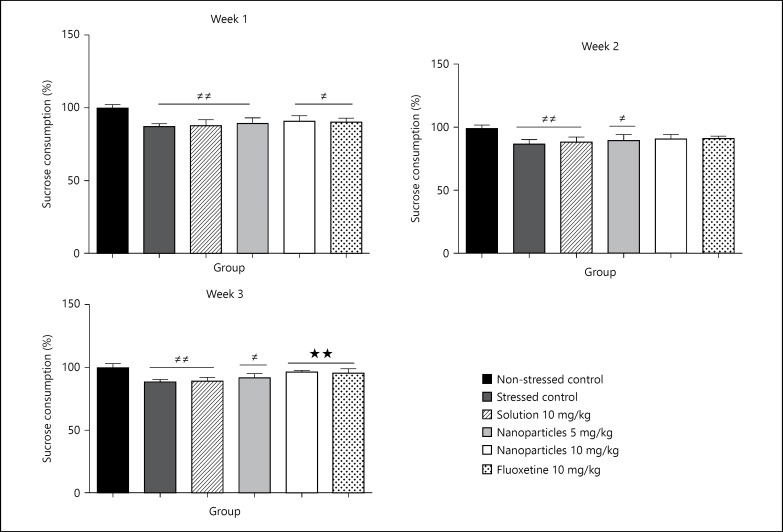
After 2 weeks of treatment, the sucrose consumption increased in all stressed groups except the stressed control. However, 3 weeks of treatment resulted in a significant effect on the sucrose consumption at groups of L-tyrosine-loaded nanoparticles 10 mg/kg and fluoxetine 10 mg/kg compared to stressed control (p > 0.01; fig. 7).
Fig. 7.
Effect of treatment on the sucrose consumption 1, 2 and 3 weeks after starting treatment with the corresponding drugs (non-stressed control, stressed control, L-tyrosine solution 10 mg/kg, L-tyrosine nanoparticles 5 mg/kg, L-tyrosine nanoparticles 10 mg/kg, fluoxetine 10 mg/kg). Sucrose consumption was measured for 1 h, half hour after treatment. Data are presented as mean ± SD, with 8 rats each group (one-way ANOVA followed by Tukey test). Total degree of freedom = 47. # The significant difference between non-stressed control and rest of groups. * The significant difference between stressed control and rest of groups except non-stressed control. p < 0.05: no symbol, *, # p < 0.05, **, ## p < 0.01, ***, ### p < 0.001.
Discussion
At the present time, the effect of some anti-depressant drugs involves changes at the cerebral levels of certain neurotransmitters [29]. As precursor of NA and dopamine, L-tyrosine probably has an impact on depression. Through this study, we tested the anti-depressant effect of L-tyrosine-loaded nanoparticles in 2 animal models of depression using different behavioral tests.
In the acute model of depression, FST is characterized by strong validity but concomitant locomotor activity test is mandatory to avoid false results [30]. Open field test also represents a reflection of anxiety and depression-like behaviors in rodents [31].
In parallel, sucrose preference test is considered the hedonic criterion widely used so far in the CMS model of depression [32].
Our findings from the in vitro tests include average size below 200 nm and entrapment efficiency (87.4479% immediately after preparation), together with prolonged dissolution rates of L-tyrosine-loaded nanoparticles (66.54 and 88.65% doses after 24 and 48 h of the test, respectively). All these in vitro results demonstrate high efficiency and low toxicity of our formula [33]. Using poloxamer 407 in the nanoparticles formula to get prolonged release properties is useful to avoid any fluctuations of the in vivo L-tyrosine levels [34].
However, according to Misra et al. [35], physiochemical factors such as surface area and crystal structure of nanoparticle can also affect the in vivo performance of nanoparticle formula, making it more difficult to predict the dissolution rate inside the body. Gatoo et al. [36] suggested that physicochemical characteristics of nanoparticles can also influence the toxic or the undesirable manifestations of the treatment.
Our nanoparticle formula has shown a good degree of tolerance and safety with intraperitoneal injection of treatments. No changes were observed in the animal outputs during the experiment, added to that the chemicals used to prepare the nanoparticles formula are widely tested and used so far. Johnston and Miller [37] reported that poloxamer 407 irritancy and toxicity levels are comparable to saline.
Polycaprolactone is also well known to have biodegradable and biocompatible characteristics [38].
Nanoparticle method is of advantage to overcome the possible toxicity related to the required high doses to reach the therapeutic threshold. With the help of nanoparticles formula, we decreased to great extent the L-tyrosine dose compared to earlier studies [10,11]. Treatment with L-tyrosine-loaded nanoparticles 10 mg/kg had significant effect in both animal models, unlike the earlier study, where depressed patients have shown no clinical improvement with 100 mg/kg of L-tyrosine solution [39].
In vivo tests, locomotor activity decreased after 8 days, and then gradually restored on days 15 and 22 of the acute model experiment. In the same model, 15 and 22 days of treatment with L-tyrosine-loaded nanoparticles 10 mg/kg or fluoxetine 10 mg/kg also reversed the reduction of travelled distance and rearing counts, which happened on day 2 of the experiment.
Additionally, L-tyrosine-loaded nanoparticles 10 mg/kg or fluoxetine 10 mg/kg significantly reduced (p < 0.01) the immobility time during the FST in the acute model.
These findings are in line with reports from number of experiments on the effect of acute stress and L-tyrosine treatment on motor activity and NA turnover rhythm [10,40]. L-tyrosine has shown the ability to reverse the reduced levels of motor activity and cerebral NA, induced by stress exposure [41]. According to Płaźnik et al. [42], intra-accumbens injection of NA or dopamine increased the rat rearing and locomotor activity in the open filed test. However, another study that used NA microinjection has shown no increase in the exploratory activity [43]. This contrast of results is probably attributed to the site of NA injection or the type of rearing activity recorded.
In the CMS experiment, 3 weeks of treatment with L-tyrosine-loaded nanoparticles 10 mg/kg or fluoxetine 10 mg/kg significantly increased (p < 0.01) the sucrose consumption compared to the stressed control.
Our results agree with another study about the effect of CMS stress through sucrose preference test [28]. Comparing the anti-depressant effect of L-tyrosine-loaded nanoparticles with NA reuptake inhibitors shows that both treatments induce an increase of the sucrose consumption [32]. Using different protocol of CMS, Willner et al. [32] recorded the significant effect of desmethylimipramine on the sucrose consumption after 2 weeks, whereas 3 weeks of treatment with L-tyrosine-loaded nanoparticles 10 mg/kg were needed in our study to get a similar effect.
More detailed studies about the impact of acute stress and CMS have shown an activation of corticotropin-releasing factor (CRF), following stress regimen [44,45]. CRF is neuropeptide expressed both outside and inside the brain, where possibly it has a role in the stress response [46]. Moreover, Jedema et al. [47] reported that the intraventricular injection of CRF has increased NA firing inside the brain.
Taken together, Dunn et al. [48] have suggested a feed-forward loop including NA and CRF, which may play a key role in the pathology of depression. According to this loop, NA neurons in the brain stem stimulate CRF neurons during stress. In turn, CRF projections, from neurons of different regions in the brain activate NA neurons in the locus coeruleus, which represents the main brain region of NA production. In rats, intravenous injection of CRF resulted in no change of locomotor activity levels, with decrease in rearing counts [49]. These results were dependent on the CRF dose and test conditions.
Noteworthy, the rats administrated CRF chronically showing an activation of tyrosine hydroxylase (TH), which is the rate-limiting enzyme of NA biosynthesis, in the locus coeruleus [50]. Under normal situations, high levels of TH hydroxylase enzyme are saturated with tyrosine [41].
Activation of TH induced by CRF during stress [50] is shown to be inversed after chronic treatment with different types of antidepressants, including NA reuptake inhibitors and selective serotonin reuptake inhibitors [51]. Additionally, TH mRNA and protein levels were also found to be decreased after this chronic treatment [51] suggesting that NA turn over in the locus coeruleus also decreased.
Reports on the effect of chronic antidepressant treatment have involved a decrease in the levels of CRF or one of its receptors (CRF-R1) in different regions of rat brain [52,53]. This means that both CRF and its receptor could be possible targets for anti-depressant treatments. Results from clinical experiments on CRF antagonists in depressed patients are still not critical, with the expectation to be helpful at least for patients with high CRF levels during stress [54,55].
Treatment with L-tyrosine-loaded nanoparticles in our study, and L-tyrosine solution in previous experiments [41] have shown positive results. This is considered as additional evidence that L-tyrosine is involved in the stress response, probably by changing NA cerebral levels according to the CRF-NA loop. Different levels of TH reach saturation with tyrosine during stress and after treatment may have also changed according to NA levels.
Our results from both stress models suggest L-tyrosine-loaded nanoparticles as an effective formula for depression treatment. However, this suggestion should be supported by calibrations of cerebral L-tyrosine and monoamine concentrations. Also, the effects of treatment with L-tyrosine-loaded nanoparticles on the cerebral levels of TH and CRF should be investigated.
Authorship Contributions
A. Alabsi, MA in Neuroscience; and A.C. Khoudary, MA in Neuroscience, University of Alexandria and University of Bordeaux II; W. Abdelwahed, PhD in Pharmaceutics and Pharmaceutical Technology, University of Claude Bernard Lyon I share equal effort contribution in this paper.
Disclosure Statement
All authors have seen and agreed with the contents of this manuscript and certified that the submission is not under review in any other publication. There is no potential conflict of interest or competing interest. This study did not receive any form of sponsorship or funding.
Acknowledgment
This work was funded by the Faculty of Pharmacy, University of Aleppo. Thanks to the laboratory of Neuroscience at the Faculty of Pharmacy, Aleppo University, and to the Numeric Campus of Francophonie for their worthy assistance.
References
- 1.Moussavi S, Chatterji S, Verdes E, et al. Depression, chronic diseases, and decrements in health: results from the world health surveys. Lancet. 2007;370:851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 2.Carlson NR. Physiology of Behavior. ed 11. Boston: Pearson; 2013. [Google Scholar]
- 3.Nestler EJ, Barrot M, DiLeone RJ, et al. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 4.Beckmann H, Kasper S. [Serotonin precursors as antidepressive agents: a review] Fortschr Neurol Psychiatr. 1983;51:176–182. doi: 10.1055/s-2007-1002225. [DOI] [PubMed] [Google Scholar]
- 5.Gelenberg AJ, Wojcik JD, Growdon JH, et al. Tyrosine for the treatment of depression. Am J Psychiatry. 1980;137:622–623. doi: 10.1176/ajp.137.5.622. [DOI] [PubMed] [Google Scholar]
- 6.Meyers S. Use of neurotransmitter precursors for treatment of depression. Altern Med Rev. 2000;5:64–71. [PubMed] [Google Scholar]
- 7.Ouakki S, Mrabet F, Hessni A, Mesfioui A, et al. Conversion of L-tryptophan into melatonin is the possible action pathway involved in the effect of L-tryptophan on antidepressant-related behavior in female rats: analysis of the influence of treatment duration. J Behav Brain Sci. 2013;3:362–372. [Google Scholar]
- 8.McLean A, Rubinsztein JS, Robbins TW, et al. The effects of tyrosine depletion in normal healthy volunteers: implications for unipolar depression. Psychopharmacology (Berl) 2004;171:286–297. doi: 10.1007/s00213-003-1586-8. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg IK. L-tyrosine in depression. Lancet. 1980;2:364–365. doi: 10.1016/s0140-6736(80)90356-6. [DOI] [PubMed] [Google Scholar]
- 10.Rauch TM, Lieberman HR. Tyrosine pretreatment reverses hypothermia-induced behavioral depression. Brain Res Bull. 1990;24:147–150. doi: 10.1016/0361-9230(90)90299-f. [DOI] [PubMed] [Google Scholar]
- 11.Lieberman HR, Georgelis JH, Maher TJ, et al. Tyrosine prevents effects of hyperthermia on behavior and increases norepinephrine. Physiol Behav. 2005;84:33–38. doi: 10.1016/j.physbeh.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 12.Nagpal K, Singh SK, Mishra D. Evaluation of safety and efficacy of brain targeted chitosan nanoparticles of minocycline. Int J Biol Macromol. 2013;59:20–28. doi: 10.1016/j.ijbiomac.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Mohanraj VJ, Chen Y. Nanoparticles - a review. Trop J Pharm Res. 2006;5:561–573. [Google Scholar]
- 14.Pinto Reis C, Neufeld RJ, Ribeiro AJ, et al. Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomedicine. 2006;2:8–21. doi: 10.1016/j.nano.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Kreuter J. Drug delivery to the central nervous system by polymeric nanoparticles: what do we know? Adv Drug Deliv Rev. 2014;71:2–14. doi: 10.1016/j.addr.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Parodi A. Guide for the Ethical Evaluation of Experiments using Laboratory Animals. ed 1. Boulogne-Billancourt: Gircor; [Google Scholar]
- 17.Fessi H, Puisieux F, Devissaguet JP, et al. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int J Pharm. 1989;55:R1–R4. [Google Scholar]
- 18.Hitchcock DI. The solubility of tyrosine in acid and in alkali. J Gen Physiol. 1924;6:747–757. doi: 10.1085/jgp.6.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Y, Evans LB, Chen CC. Phase partitioning of biomolecules - solubilities of amino-acids. Biotechnol Prog. 1989;5:111–118. [Google Scholar]
- 20.de Campos VE, Teixeira CA, da Veiga VF, et al. L-tyrosine-loaded nanoparticles increase the antitumoral activity of direct electric current in a metastatic melanoma cell model. Int J Nanomedicine. 2010;5:961–971. doi: 10.2147/IJN.S13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz RJ, Roth KA, Carroll BJ. Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neurosci Biobehav Rev. 1981;5:247–251. doi: 10.1016/0149-7634(81)90005-1. [DOI] [PubMed] [Google Scholar]
- 22.Klenerová V, Sída P, Krejcí I, et al. Effects of two types of restraint stress on spontaneous behavior of Sprague-Dawley and Lewis rats. J Physiol Pharmacol. 2007;58:83–94. [PubMed] [Google Scholar]
- 23.Castagné V, Moser P, Roux S, et al. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci. 2011 doi: 10.1002/0471142301.ns0810as55. chapter 8:unit 8.10A. [DOI] [PubMed] [Google Scholar]
- 24.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 25.Borsini F, Lecci A, Sessarego A, et al. Discovery of antidepressant activity by forced swimming test may depend on pre-exposure of rats to a stressful situation. Psychopharmacology (Berl) 1989;97:183–188. doi: 10.1007/BF00442247. [DOI] [PubMed] [Google Scholar]
- 26.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 27.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 28.Grønli J, Bramham C, Murison R, et al. Chronic mild stress inhibits BDNF protein expression and CREB activation in the dentate gyrus but not in the hippocampus proper. Pharmacol Biochem Behav. 2006;85:842–849. doi: 10.1016/j.pbb.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 29.Leonard BE. Stress, norepinephrine and depression. J Psychiatry Neurosci. 2001;26((suppl)):S11–S16. [PMC free article] [PubMed] [Google Scholar]
- 30.Slattery DA, Cryan JF. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc. 2012;7:1009–1014. doi: 10.1038/nprot.2012.044. [DOI] [PubMed] [Google Scholar]
- 31.Chen LJ, Shen BQ, Liu DD, et al. The effects of early-life predator stress on anxiety- and depression-like behaviors of adult rats. Neural Plast. 2014;2014:163908. doi: 10.1155/2014/163908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willner P, Towell A, Sampson D, et al. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 33.Masserini M. Nanoparticles for brain drug delivery. ISRN Biochem. 2013;2013:238428. doi: 10.1155/2013/238428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ricci EJ, Lunardi LO, Nanclares DM, et al. Sustained release of lidocaine from poloxamer 407 gels. Int J Pharm. 2005;288:235–244. doi: 10.1016/j.ijpharm.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 35.Misra SK, Dybowska A, Berhanu D, et al. The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. Sci Total Environ. 2012;438:225–232. doi: 10.1016/j.scitotenv.2012.08.066. [DOI] [PubMed] [Google Scholar]
- 36.Gatoo MA, Naseem S, Arfat MY, et al. Physicochemical properties of nanomaterials: implication in associated toxic manifestations. Biomed Res Int. 2014;2014:498420. doi: 10.1155/2014/498420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnston TP, Miller SC. Toxicological evaluation of poloxamer vehicles for intramuscular use. J Parenter Sci Technol. 1985;39:83–89. [PubMed] [Google Scholar]
- 38.Sinha VR, Bansal K, Kaushik R, et al. Poly-epsilon-caprolactone microspheres and nanospheres: an overview. Int J Pharm. 2004;278:1–23. doi: 10.1016/j.ijpharm.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 39.Gelenberg AJ, Wojcik JD, Falk WE, et al. Tyrosine for depression: a double-blind trial. J Affect Disord. 1990;19:125–132. doi: 10.1016/0165-0327(90)90017-3. [DOI] [PubMed] [Google Scholar]
- 40.Lehnert H, Reinstein DK, Strowbridge BW, et al. Neurochemical and behavioral consequences of acute, uncontrollable stress: effects of dietary tyrosine. Brain Res. 1984;303:215–223. doi: 10.1016/0006-8993(84)91207-1. [DOI] [PubMed] [Google Scholar]
- 41.Young SN. Behavioral effects of dietary neurotransmitter precursors: basic and clinical aspects. Neurosci Biobehav Rev. 1996;20:313–323. doi: 10.1016/0149-7634(95)00022-4. [DOI] [PubMed] [Google Scholar]
- 42.Płaźnik A, Danysz W, Kostowski W. A stimulatory effect of intraaccumbens injections of noradrenaline on the behavior of rats in the forced swim test. Psychopharmacology (Berl) 1985;87:119–123. doi: 10.1007/BF00431791. [DOI] [PubMed] [Google Scholar]
- 43.Pelosi GG, Resstel LL, Soares VP, et al. Anxiolytic-like effect of noradrenaline microinjection into the dorsal periaqueductal gray of rats. Behav Pharmacol. 2009;20:252–259. doi: 10.1097/FBP.0b013e32832c7098. [DOI] [PubMed] [Google Scholar]
- 44.Stout SC, Mortas P, Owens MJ, et al. Increased corticotropin-releasing factor concentrations in the bed nucleus of the stria terminalis of anhedonic rats. Eur J Pharmacol. 2000;401:39–46. doi: 10.1016/s0014-2999(00)00412-x. [DOI] [PubMed] [Google Scholar]
- 45.Shimizu N, Nakane H, Hori T, et al. CRF receptor antagonist attenuates stress-induced noradrenaline release in the medial prefrontal cortex of rats. Brain Res. 1994;654:145–148. doi: 10.1016/0006-8993(94)91580-6. [DOI] [PubMed] [Google Scholar]
- 46.Binder EB, Nemeroff CB. The CRF system, stress, depression and anxiety-insights from human genetic studies. Mol Psychiatry. 2010;15:574–588. doi: 10.1038/mp.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jedema HP, Finlay JM, Sved AF, et al. Chronic cold exposure potentiates CRH-evoked increases in electrophysiologic activity of locus coeruleus neurons. Biol Psychiatry. 2001;49:351–359. doi: 10.1016/s0006-3223(00)01057-x. [DOI] [PubMed] [Google Scholar]
- 48.Dunn AJ, Swiergiel AH, Palamarchouk V. Brain circuits involved in corticotropin-releasing factor-norepinephrine interactions during stress. Ann N Y Acad Sci. 2004;1018:25–34. doi: 10.1196/annals.1296.003. [DOI] [PubMed] [Google Scholar]
- 49.Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- 50.Melia KR, Duman RS. Involvement of corticotropin-releasing factor in chronic stress regulation of the brain noradrenergic system. Proc Natl Acad Sci U S A. 1991;88:8382–8386. doi: 10.1073/pnas.88.19.8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nestler EJ, Mcmahon A, Sabban EL, et al. Chronic antidepressant administration decreases the expression of tyrosine hydroxylase in the rat locus coeruleus. Proc Natl Acad Sci U S A. 1990;87:7522–7526. doi: 10.1073/pnas.87.19.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aubry JM, Pozzoli G, Vale WW. Chronic treatment with the antidepressant amitriptyline decreases CRF-R1 receptor mRNA levels in the rat amygdala. Neurosci Lett. 1999;266:197–200. doi: 10.1016/s0304-3940(99)00295-5. [DOI] [PubMed] [Google Scholar]
- 53.Stout SC, Owens MJ, Nemeroff CB. Regulation of corticotropin-releasing factor neuronal systems and hypothalamic-pituitary-adrenal axis activity by stress and chronic antidepressant treatment. J Pharmacol Exp Ther. 2002;300:1085–1092. doi: 10.1124/jpet.300.3.1085. [DOI] [PubMed] [Google Scholar]
- 54.Paez-Pereda M, Hausch F, Holsboer F. Corticotropin releasing factor receptor antagonists for major depressive disorder. Expert Opin Investig Drugs. 2011;20:519–535. doi: 10.1517/13543784.2011.565330. [DOI] [PubMed] [Google Scholar]
- 55.Valdez GR. Development of CRF1 receptor antagonists as antidepressants and anxiolytics: progress to date. CNS Drugs. 2006;20:887–896. doi: 10.2165/00023210-200620110-00002. [DOI] [PubMed] [Google Scholar]