Abstract
Background
Chronic renal hypoxia influences the progression of chronic kidney disease (CKD). Blood oxygen level-dependent (BOLD) magnetic resonance (MR) is a noninvasive tool for the assessment of renal oxygenation. The impact of beta-blockers on renal hemodynamics and oxygenation is not completely understood. We sought to determine the association between beta-blocker use, renal cortical and medullary oxygenation, and renal blood flow in patients suspected of renal artery stenosis.
Methods
We measured renal cortical and medullary oxygenation using BOLD MR and renal artery blood flow using MR phase contrast techniques in 38 participants suspected of renal artery stenosis.
Results
Chronic beta-blocker therapy was associated with improved renal cortical (p < 0.001) and medullary (p = 0.03) oxygenation, while the use of calcium channel blockers or diuretics showed no association with either cortical or medullary oxygenation. Receipt of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers was associated with reduced medullary oxygenation (p = 0.01). In a multivariable model, chronic receipt of beta-blockers was the only significant predictor of renal tissue oxygenation (β = 8.4, p = 0.008). Beta-blocker therapy was not associated with significant changes in renal artery blood flow, suggesting that improved renal oxygenation may be related to reduced renal oxygen consumption.
Conclusions
In addition to known benefits to reduce cardiovascular mortality in patients with renal disease, beta-blockers may reduce or prevent the progression of renal dysfunction in patients with hypertension, diabetes, and renovascular disease, partly by reducing renal oxygen consumption. These observations may have important implications for the treatment of patients with CKD.
Key Words: Beta-blockers, Renal oxygenation, MR imaging
Introduction
Chronic kidney disease (CKD) has an impact on morbidity and mortality and is associated with hypertension (HTN), diabetes, and vascular disease [1]. In many of these disorders, chronic renal hypoxia is common and may contribute to the progression of renal injury [2]. Beta-blockers are popular medications used to treat HTN and cardiovascular diseases; however, their effectiveness in blood pressure control and the prevention of renal disease progression has been questioned [3,4]. Renin-angiotensin system blockade is considered a first-line therapy for renal protection in patients with CKD and diabetes [4]. Beta-blockers reduce cardiovascular mortality in patients with CKD and systolic heart failure. However, it is unclear whether this is related to vasodilatory or reduced antiarrhythmic properties [5].
Theoretically, beta-blockers may reduce cardiac output and subsequently renal perfusion pressure, thereby exacerbating renal dysfunction. The sympathetic nervous system (SNS) is activated in CKD, which is a known factor in the progression of renal disease [6]. Hypoxia causes SNS activation and may play an important role in the progression of renal dysfunction [7]. The impact of beta-blockers on renal hemodynamics and oxygenation is not completely understood. Accordingly, we sought to determine the influence of beta-blocker therapy on renal tissue oxygenation and renal hemodynamics. To accomplish this, we used magnetic resonance (MR) imaging measures of tissue oxygenation [blood oxygen level-dependent MR (BOLD MR)] and renal artery blood flow in hypertensive patients suspected of renal artery stenosis.
Methods
Study Population
Thirty-eight participants were included based on suspicion of renal artery stenosis after renal Doppler examinations. Subjects were ineligible for participation if they had a contraindication for MR examination including a pacemaker, defibrillator, implanted metal devices, or other electronic devices. Other exclusion criteria were claustrophobia, significant valvular disease, or active acute coronary or peripheral vascular syndromes. After enrollment, the glomerular filtration rate (GFR) was estimated in each participant using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [8], and the serum creatinine concentration was measured from a blood sample obtained within 4 weeks prior to each MR examination. Participants were asked to abstain from taking any diuretics including furosemide for at least 12 h prior to the MR scan. Clinical characteristics including medication histories were obtained from electronic medical records in accordance with the Wake Forest School of Medicine Institutional Review Board policies.
MR Imaging Techniques
Each participant underwent a comprehensive MR imaging examination of both kidneys including BOLD MR, phase contrast techniques, and anatomical imaging of the kidney parenchyma on a 1.5T Siemens Magnetom Avanto scanner (Siemens Medical Solutions USA, Malvern, Pa., USA), as previously described [9]. Electrocardiographic leads and respiratory gating bellows were applied to account for cardiac and respiratory motion, respectively. Blood pressure and heart rate were monitored periodically during the MR examination to ensure hemodynamic stability.
A three-dimensional (3D), segmented, steady-state, free-precession sequence with nonselective radiofrequency excitation was utilized to acquire noncontrasted angiograms of the renal arteries. The fields of view (FOV) ranged from 30 to 40 cm to cover the entire abdomen in the axial position for 3D volume acquisitions. Imaging parameters included a repetition time (TR) of 2.3 ms, an echo time (TE) of 1 ms, a 90° flip angle, a readout bandwidth of 980 Hz per pixel, a 256 × 256 matrix, and a total of approximately 40-50 3D partitions with a slice thickness of 3 mm. A parallel imaging technique, generalized autocalibrating, partially parallel acquisition (GRAPPA) with an acceleration factor of 2 was applied to shorten scan times. Percentage stenosis of each renal artery was visually estimated by a blinded board-certified cardiologist and cardiovascular imager.
After locating the renal arteries, an image series of the vessels in double-oblique, cross-sectional orientation was obtained to ensure a circular lumen throughout the cardiac cycle to minimize partial volume effects during image acquisition. Interleaved, phase-contrast, gradient-echo sequences were used to determine cardiac, cycle-dependent measurements of the vessel area and velocity according to previously published techniques from our laboratory [10]. These sequences were positioned in an oblique plane 2 cm distal to the origin of the renal artery at the aorta. These scans utilized 7-mm thick slices with a 256 × 256 matrix, a 32-cm FOV (yielding voxel sizes of 0.94 × 0.94 × 7 mm for the renal artery), a 40° flip angle, a TR of 11 ms, and a TE of 3.5 ms.
During suspended respiration, three-plane, single-shot, fast spin-echo localizing images of the kidneys were performed, followed by additional scout images oriented parallel to the coronal axis of each kidney. BOLD imaging consisted of a 2D fast spoiled gradient echo sequence with eight echoes (TEs ranged from 2.5 to 30 ms) obtained at each slice location, and a middle kidney coronal image was evaluated for each kidney. BOLD imaging parameters included a slice thickness of 10 mm, a 224 × 160-192 imaging matrix, a FOV of 32-40 cm, a 45° flip angle, and a TR of 140 ms. The FOV was adjusted based on participants' body sizes, and the imaging matrix and TRs were adjusted in participants with diminished breath-holding capabilities. Three regions of interest were manually drawn in the superior, middle, and inferior segments of the kidney for both the cortex and medulla to determine the mean T2* signal intensity of each region. T2* signal intensity is higher in tissues with less deoxyhemoglobin (i.e. higher tissue oxygenation).
Statistical Analyses
All statistical analyses were performed using SAS 9.2 software (SAS Institute, Cary, N.C., USA). Pearson's correlation coefficients were used to determine relationships between cardiovascular medications and renal tissue oxygenation and renal blood flow. A p value of <0.05 was considered to be statistically significant. Subjects were then divided into two groups: those receiving beta-blocker therapy and those not taking beta-blockers. Student's t tests were performed to evaluate the differences in the mean values for oxygenation and blood flow between these two groups. Additional mixed model analyses were performed to assess the effects of beta-blocker therapy and other covariates with a p value of <0.20 on univariate analyses of renal tissue oxygenation.
Statement of Ethics
This study was approved by the Institutional Review Board at Wake Forest School of Medicine. All participants provided written informed consent.
Results
Overall, 97% of the participants were hypertensive, and 42% were diabetic. Sixty-three percent of the participants were women, and 26% were African American. The mean age of the participants was 69 years.
Participant characteristics subdivided by the use of beta-blockers are listed in table 1. There were no significant differences in age, gender, race, or BMI. There were no significant differences in renal size or estimated GFR. A diagnosis of diabetes was more prevalent in patients receiving beta-blockers. Patients chronically receiving beta-blockers were on significantly more antihypertensive medications compared to those not receiving beta-blockers (3.8 ± 0.2 vs. 1.6 ± 0.2, p = 0.001). Forty-four percent (n = 4/9) of the participants in the no-beta-blocker group and 41% (n = 12/29) of the participants in the beta-blocker group had ≥50% stenosis of at least one renal artery.
Table 1.
Patient characteristics subdivided by receipt of beta-blocker therapy
| Patient characteristics | Beta-blocker therapy (n = 29) | No beta-blocker (n = 9) | p value |
|---|---|---|---|
| Age, years | 68.3±1.2 | 69.4±1.6 | 0.63 |
| Race | 0.45 | ||
| White | 76 | 67 | |
| African American | 24 | 33 | |
| Male | 41 | 22 | 0.15 |
| BMI | 29.8±0.9 | 29.7±1.6 | 0.93 |
| Mean renal artery stenosis (%) | 25±5.0 | 22±9 | 0.71 |
| Patients with significant renal artery stenosis (≥50%) | 41 | 44 | 0.88 |
| Mean kidney length, cm | 10.5±0.2 | 10.6±0.2 | 0.83 |
| GFR, ml/min/1,73 m2 | 53.1±6.6 | 56.7 ±6.9 | 0.61 |
| Hemoglobin, g/dl | 12.7±0.3 | 11.6±0.5 | 0.07 |
| Diabetes diagnosis | 52 | 11 | 0.002* |
| Mean antihypertensive medications (n) | 3.8±1.3 | 1.6±1 | <0.001* |
| Patients on ACEIs or ARBs | 15 (52) | 5 (56) | 0.78 |
| Patients on mineralocorticoid receptor antagonists | 5 (17) | 0 (0) | 0.06 |
| Patients on thiazide diuretics | 10 (35) | 3 (33) | 0.92 |
| Patients on loop diuretics | 14 (48) | 1 (11) | 0.004* |
| Patients on calcium channel blockers | 18 (62) | 4 (44) | 0.30 |
| Patients on statins | 19 (66) | 7 (78) | 0.33 |
Values are presented as percentages, means ± SE, or n (%).
p < 0.05.
Renal Tissue Oxygenation Is Higher in Patients Receiving Beta-Blocker Therapy
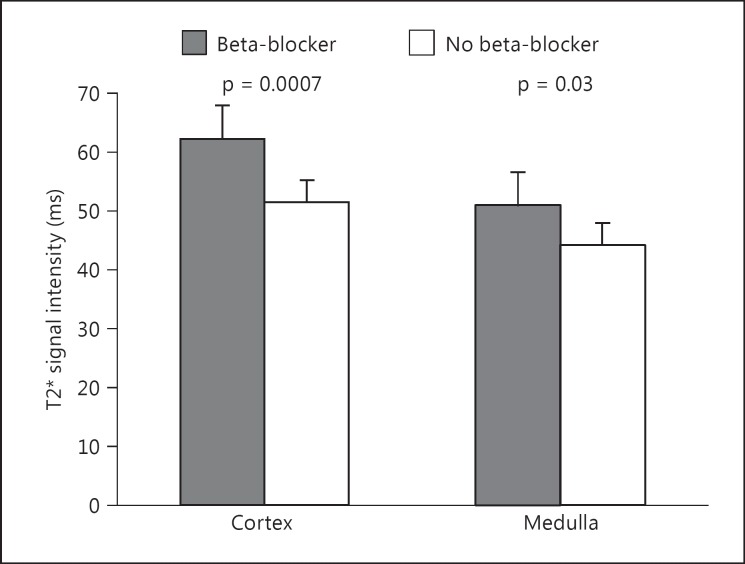
Beta-blocker therapy was significantly associated with improved whole kidney tissue oxygenation (T2*), as assessed by BOLD MR (r = 0.33, p = 0.004). There were also significant positive relationships with beta-blocker therapy and renal cortical (p < 0.001) and renal medullary (p = 0.03) tissue oxygenation (T2*, table 2). Patients receiving beta-blockers had higher mean cortical and medullary renal T2* values compared with those not receiving beta-blockers (fig. 1). The use of angiotensin-converting inhibitors (ACEI) or angiotensin receptor blockers (ARBs) was associated with reduced renal medullary tissue oxygenation (p = 0.01). There were no significant relationships with renal oxygenation and receipt of other antihypertensive medications or statins. We further evaluated the effect of beta-blockers on renal tissue oxygenation after accounting for other factors that were identified as significantly different between those chronically receiving beta-blockers and those not. In a multivariable model including gender, hemoglobin, diagnosis of diabetes, or use of mineralocorticoid receptor antagonists, loop diuretics, or beta-blockers, the use of beta-blockers was the only significant predictor of renal tissue oxygenation (table 3).
Table 2.
Correlations of renal tissue oxygenation (T2* signal intensity) and cardiovascular medications
| Kidney region | Medication | Pearson correlation coefficient (r) | p value |
|---|---|---|---|
| Cortex | ACEI or ARB | −0.15 | 0.28 |
| Thiazide diuretic | 0.12 | 0.40 | |
| Loop diuretic | −0.01 | 0.94 | |
| Beta-blocker | 0.44 | 0.0007* | |
| Calcium channel blocker | 0.16 | 0.24 | |
| Mineralocorticoid receptor antagonist | 0.07 | 0.60 | |
| Statin | −0.06 | 0.68 | |
| Medulla | ACEI or ARB | −0.33 | 0.01* |
| Thiazide diuretic | 0.09 | 0.50 | |
| Loop diuretic | −0.04 | 0.76 | |
| Beta-blocker | 0.29 | 0.03* | |
| Calcium channel blocker | 0.14 | 0.32 | |
| Mineralocorticoid receptor antagonist | 0.09 | 0.51 | |
| Statin | −0.21 | 0.12 | |
p < 0.05.
Fig. 1.
Renal cortical (left bars) and medullary (right bars) oxygenation as measured by BOLD MR is higher in patients chronically receiving beta-blocker therapy compared with those not receiving beta-blockers.
Table 3.
Multivariable model examining the effects of factors predicting renal tissue oxygenation (T2*)
| Variables | Parameter estimate (SE) | p value |
|---|---|---|
| Male | –0.44 (2.62) | 0.86 |
| Hemoglobin | 0.85 (0.60) | 0.16 |
| Diabetes | 0.13 (2.89) | 0.96 |
| Loop diuretic use | –3.40 (2.46) | 0.19 |
| Mineralocorticoid antagonist use | 4.16 (4.29) | 0.34 |
| Beta-blocker use | 8.40 (3.09) | 0.008* |
p < 0.05.
Renal Blood Flow Is Lower in Patients on Beta-Blockers
Using phase contrast techniques in the middle renal artery, blood flow was quantified in each patient. Although not statistically significant, renal artery blood flow was 21% lower in patients receiving beta-blockers compared to those not on beta-blockers (177 ± 14 vs. 224 ± 54 ml/min, p = 0.23). No other cardiovascular medications evaluated exhibited a significant relationship with resting renal artery blood flow.
Discussion
We evaluated the relationships between widely prescribed cardiovascular medications and renal tissue oxygenation and renal blood flow using comprehensive, noninvasive MR techniques in a hypertensive patient population with evidence of renovascular disease. We found that the treatment with beta-blockers is associated with higher renal tissue oxygenation and that this relationship is not related to increased renal artery blood flow. Our findings suggest that use of beta-blockers may be associated with increased oxygenation of the kidney despite a neutral or potentially negative effect on renal artery blood flow.
There were some notable differences in the two patient groups, including a higher prevalence of diabetes in patients receiving beta-blocker therapy. Patients receiving beta-blockers were also more likely to have treatment-resistant HTN given the observation that they were on more than twice as many antihypertensive medications, on average, compared with those not receiving beta-blockers. These patients also received more loop diuretics and mineralocorticoid receptor antagonists, although these differences were not statistically significant. However, there were no significant differences in renal function, as assessed by GFR, renal size, or renal artery stenosis severity between the two groups. Of note, these patients were suspected of severe renal artery stenosis; however, less than half of them had ≥50% stenosis of at least one kidney. Most of these patients were hypertensive, requiring multiple antihypertensive medications, and most of them had some degree of renovascular disease, albeit noncritical.
Despite having a similar GFR and renal blood flow as the no-beta-blocker group, the group receiving beta-blockers had higher T2* signal intensities, suggestive of higher renal oxygenation. This observation was opposite to what we anticipated given the higher rate of diabetes and more severe HTN in that group. The receipt of beta-blockers was significantly associated with higher tissue oxygenation in both the renal cortex and renal medulla independent of renal blood flow.
There has been debate over the safety of beta-blockers in patients with chronic renal disease due to the concern for reduced cardiac output, which could theoretically reduce renal artery blood flow and kidney perfusion [11]. Although we observed lower renal artery blood flow in patients receiving beta-blockers, this was not statistically significant. However, cardiovascular disease is a significant cause of mortality in CKD patients, and beta-blockers reduce mortality in patients who have had a myocardial infarction and in those with chronic systolic heart failure [5,11,12]. Given the high prevalence of coronary artery disease and heart failure in CKD patients, beta-blockers may be particularly beneficial in this patient population. A large systemic review of eight clinical trials found that in CKD patients with heart failure, beta-blockers reduced all-cause mortality (RR 0.72, CI 0.64-0.80) and cardiovascular mortality (RR 0.66, CI 0.49-0.89) compared to placebo [5]. However, data from the United States Renal Data System indicate that only 20% of the chronic dialysis patients with heart failure were receiving beta-blocker therapy [11,13]. In a 10-year study of renal transplant patients, beta-blocker therapy was associated with improved survival (HR 0.61, p = 0.04) compared with not being on a beta-blocker, and this was consistent, regardless of the presence of HTN, diabetes, myocardial infarction or left ventricular dysfunction [14].
Our findings suggest that beta-blocker therapy may be associated with improved renal tissue (cortical and medullary) oxygenation in hypertensive and diabetic patients. Increases in tissue oxygenation, in theory, could result from either increased oxygen delivery or reduced renal oxygen consumption. Since we did not observe an increase in renal artery blood flow, our findings are consistent with the possibility that beta-blockers may reduce renal oxygen consumption. One potential mechanism for this beneficial effect is blockade of multiple beta-adrenergic actions of the SNS, which is known to be activated in CKD [7]. Most of the renal oxygen consumption is normally related to tubular reabsorption and sodium-potassium ATPase activity [15]. Antagonism of β-1 receptors would tend to reduce renin release, which would reduce angiotensin II formation and renal sodium reabsorption. However, if this were the only mechanism leading to increased renal tissue oxygenation, then participants treated with ACEIs or ARBs would also have higher renal oxygenation, which we did not observe. Regardless of the specific mechanism, beta-blocker therapy is associated with improved renal tissue oxygenation, and further studies assessing the specific mechanisms should be performed.
Our study has a few limitations. The number of participants was fairly small. Less than half of the patients evaluated had significant (≥50% stenosis) renal artery stenosis, but there was no difference in the mean stenosis severity or number of patients between the groups with significant stenosis. We do not know what type of beta-blocker the participants were receiving (β-1 vs. nonselective beta-blocker). However, our observations are hypothesis generating and should be investigated in large-scale studies. Several participants in the beta-blocker therapy group were also receiving mineralocorticoid antagonists, while none in the comparison group were, although this difference was not statistically significant. Mineralocorticoids, most notably aldosterone, have proinflammatory and profibrotic effects [16]. In addition to its blood pressure-lowering effects, the mineralocorticoid antagonist spironolactone has been demonstrated to reduce renal tubulointerstitial fibrosis and inflammation [17,18]. Administration of spironolactone to rats undergoing renal ischemia-reperfusion injury either before or after ischemia prevented CKD by inhibiting activation of profibrotic and proinflammatory pathways. However, after controlling for mineralocorticoid use in a multivariable model, the treatment with beta-blockers was the only significant predictor of renal tissue oxygenation.
Our observation that patients receiving beta-blockers have better tissue oxygenation even in the absence of changes in renal blood flow suggests that beta blockade may have important effects to reduce the progression of renal dysfunction beyond their vasodilatory functions. Our findings suggest that beta-blockers may improve renal oxygenation by reducing renal oxygen consumption, although the mechanisms for this effect are unclear. In addition to their known benefits to reduce cardiovascular mortality in patients with renal disease, beta-blockers may reduce or prevent progression of renal dysfunction in patients with HTN, diabetes, and renovascular disease. Unfortunately, there are very few large-scale clinical trials assessing the effects of beta-blockers to prevent CKD. Further studies will be needed to determine the specific mechanisms by which beta-blockers may improve renal tissue oxygenation and reduce the risk of CKD progression.
Disclosure Statement
This study was funded in part by NIH grant R42 AG030248, a Small Business Initiative Grant award to Prova Inc. for which Drs. Hundley and Hamilton are minor stock holders. The other authors have no conflicts of interest to disclose.
Acknowledgments
The authors express their gratitude to the study participants, the MR technologists (Jenny Hagee, Jennifer McGuinn, and Meredith Gammons), and to the study coordinators Crosby Moss and Kim Lane. Research was supported in part by National Institutes of Health (NIH) grants R42AG030248, P30AG21332, R01HL076438, and T32HL091824. M.E.H. has received funding by the American Heart Association (14SDG20490339) and is currently funded by a NIH/National Institutes of Diabetes and Digestive and Kidney Diseases grant (1K08DK099415-01A1).
References
- 1.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17:2034–2047. doi: 10.1681/ASN.2005101085. [DOI] [PubMed] [Google Scholar]
- 2.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17:17–25. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 3.Kalaitzidis R, Bakris G. Should nephrologists use beta-blockers? A perspective. Nephrol Dial Transplant. 2009;24:701–702. doi: 10.1093/ndt/gfn695. [DOI] [PubMed] [Google Scholar]
- 4.Tomiyama H, Yamashina A. Beta-blockers in the management of hypertension and/or chronic kidney disease. Int J Hypertens. 2014;2014:919256. doi: 10.1155/2014/919256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badve SV, Roberts MA, Hawley CM, Cass A, Garg AX, Krum H, Tonkin A, Perkovic V. Effects of beta-adrenergic antagonists in patients with chronic kidney disease: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58:1152–1161. doi: 10.1016/j.jacc.2011.04.041. [DOI] [PubMed] [Google Scholar]
- 6.Zoccali C, Mallamaci F, Parlongo S, Cutrupi S, Benedetto FA, Tripepi G, Bonanno G, Rapisarda F, Fatuzzo P, Seminara G, Cataliotti A, Stancanelli B, Malatino LS. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation. 2002;105:1354–1359. doi: 10.1161/hc1102.105261. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher EC, Bao G, Li R. Renin activity and blood pressure in response to chronic episodic hypoxia. Hypertension. 1999;34:309–314. doi: 10.1161/01.hyp.34.2.309. [DOI] [PubMed] [Google Scholar]
- 8.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall ME, Rocco MV, Morgan TM, Hamilton CA, Edwards MS, Jordan JH, Hurie JB, Hundley WG. Chronic diuretic therapy attenuates renal BOLD magnetic resonance response to an acute furosemide stimulus. J Cardiovasc Magn Reson. 2014;16:17. doi: 10.1186/1532-429X-16-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hundley WG, Li F, Willard JE, Landau C, Lange RA, Meshack BM, Hillis LD, Peshock RM. Magnetic resonance imaging assessment of the severity of mitral regurgitation: comparison of invasive techniques. Circulation. 1995;92:1151–1158. doi: 10.1161/01.cir.92.5.1151. [DOI] [PubMed] [Google Scholar]
- 11.Bakris GL, P Hart, E Ritz. Beta-blockers in the management of chronic kidney disease. Kidney Int. 2006;70:1905–1913. doi: 10.1038/sj.ki.5001835. [DOI] [PubMed] [Google Scholar]
- 12.McCullough PA, Sandberg KR, Borzak S, Hudson MP, Garg M, Manley HJ. Benefits of aspirin and beta-blockade after myocardial infarction in patients with chronic kidney disease. Am Heart J. 2002;144:226–232. doi: 10.1067/mhj.2002.125513. [DOI] [PubMed] [Google Scholar]
- 13.Abbott KC, Trespalacios FC, Agodoa LY, Taylor AJ, Bakris GL. Beta-blocker use in long-term dialysis patients: association with hospitalized heart failure and mortality. Arch Intern Med. 2004;164:2465–2471. doi: 10.1001/archinte.164.22.2465. [DOI] [PubMed] [Google Scholar]
- 14.Aftab W, Varadarajan P, Rasool S, Kore A, Pai RG. Beta and angiotensin blockades are associated with improved 10-year survival in renal transplant recipients. J Am Heart Assoc. 2013;2:e000091. doi: 10.1161/JAHA.112.000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopp UC. Neural Control of Renal Function. San Rafael: Morgan & Claypool Life Sciences; 2011. [PubMed] [Google Scholar]
- 16.Brem AS. Targeting activated mineralocorticoid receptor: Occam's razor revisited. Kidney Int. 2012;82:619–620. doi: 10.1038/ki.2012.157. [DOI] [PubMed] [Google Scholar]
- 17.Barrera-Chimal J, Pérez-Villalva R, Rodríguez-Romo R, Reyna J, Uribe N, Gamba G, Bobadilla NA. Spironolactone prevents chronic kidney disease caused by ischemic acute kidney injury. Kidney Int. 2013;83:93–103. doi: 10.1038/ki.2012.352. [DOI] [PubMed] [Google Scholar]
- 18.Remuzzi G, Cattaneo D, Perico N. The aggravating mechanisms of aldosterone on kidney fibrosis. J Am Soc Nephrol. 2008;19:1459–1462. doi: 10.1681/ASN.2007101079. [DOI] [PubMed] [Google Scholar]