Abstract
Aims
To examine the effects of 2 weeks of high-NaCl diet on left ventricular (LV) morphology and serum levels of cardiac troponin T (cTnT) in rats with adenine-induced chronic renal failure (ACRF).
Methods
Male Sprague-Dawley rats either received chow containing adenine or were pair-fed an identical diet without adenine [controls (C)]. Approximately 10 weeks after the beginning of the study, the rats were randomized to either remain on a normal NaCl diet (NNa; 0.6%) or to be switched to high-NaCl chow (HNa; 4%) for 2 weeks, after which acute experiments were performed.
Results
Rats with ACRF showed statistically significant increases (p < 0.001) in arterial pressure (AP), LV weight and fibrosis, and serum cTnT levels compared to controls. Two weeks of high-NaCl intake augmented the increases in AP, LV weight and fibrosis, and serum cTnT concentrations only in ACRF rats (p < 0.05 for group × NaCl intake interaction). Compared to group C-NNa, cTnT levels were elevated approximately 6-fold in group ACRF-NNa and 24-fold in group ACRF-HNa. Focal LV injury with cardiomyocyte necrosis, scarring, and fibrinoid necrosis of small arteries were only detected in group ACRF-HNa. There was a strong correlation between the degree of LV fibrosis and serum cTnT levels in ACRF rats (r = 0.81, p < 0.01).
Conclusion
Two weeks of high-NaCl diet in rats with ACRF produces LV injury and aggravates increases in serum cTnT levels, presumably by causing hypertension-induced small artery lesions leading to myocardial ischemia. This model may be suitable for studying pathophysiological mechanisms in chronic renicardiac syndromes.
Key Words: Chronic renal failure, Adenine, Hypertension, Left ventricular hypertrophy, Troponin T, High-sodium diet
Introduction
Chronic kidney disease (CKD) is a major global health problem due to its high prevalence and poor prognosis [1,2]. In patients with a glomerular filtration rate (GFR) less than 45 ml/min/1.73 m2, cardiovascular (CV) disease is the major cause of death [3], and in end-stage renal disease (ESRD), sudden cardiac death (SCD) accounts for approximately 25% of mortality [4,5]. Interestingly, SCD in ESRD patients does not seem to be caused by ischemia and coronary artery disease to the same extent as in the general population [4]. The pathophysiological mechanisms causing SCD in these patients are not clear, although a number of predisposing factors have been suggested, e.g. left ventricular (LV) hypertrophy (LVH) and remodeling, autonomic dysfunction, and electrolyte abnormalities [4,5].
Increased serum levels of cardiac troponins are frequently observed in CKD patients even in the absence of acute coronary syndrome [6,7,8]. In addition, cardiac troponin T (cTnT) is a powerful prognostic marker in patients with ESRD, and elevated levels are associated with increased risk of CV events and mortality [9,10,11]. The mechanism underlying elevated serum levels of cTnT in asymptomatic ESRD patients is not fully understood [3].
In patients with ESRD, comorbidity is high, making it difficult to identify the mechanisms that cause myocardial injury. Hence, we have established a rat model of adenine-induced chronic renal failure (ACRF) which bears close resemblance to the uremic syndrome in patients [12,13,14]. In this well-characterized model, rats develop severe renal failure and GFR values are approximately 10% of those in controls [12,13,14].
The aim of the present study was to investigate LV histology and serum levels of cTnT in rats with established ACRF and to examine the effects of 2 weeks of high-NaCl diet. We hypothesized that high-NaCl diet, by inflicting a hemodynamic challenge on the left ventricle, might aggravate myocardial injury and increase serum concentrations of cTnT.
Methods
General Procedures and Protocols
All experiments were approved by the regional ethics committee in Gothenburg, Sweden. Chemicals were purchased from Sigma (St. Louis, Mo., USA) if not stated otherwise.
Protocol A
Thirty-nine male Sprague-Dawley rats (Harlan, Horst, The Netherlands), approximately 10 weeks old and weighing ∼300 g, were used. Chronic renal failure was induced by feeding the animals with chow containing adenine as previously described [12,13,14]. At the beginning of the study, the animals were provided with standard pelleted rat chow containing adenine (group ACRF, n = 20) or identical chow without adenine [pair-fed controls (C), n = 19]. The chow (R34, Lantmännen, Kimstad, Sweden) contained 0.63% phosphorus, 0.74% calcium, 0.53% potassium, and 0.6% sodium chloride. The concentration of adenine in the chow was 0.5% for the first 3 weeks, followed by 0.3% for 2 weeks and 0.15% thereafter until acute experiments were performed and the study was terminated after 10-12 weeks.
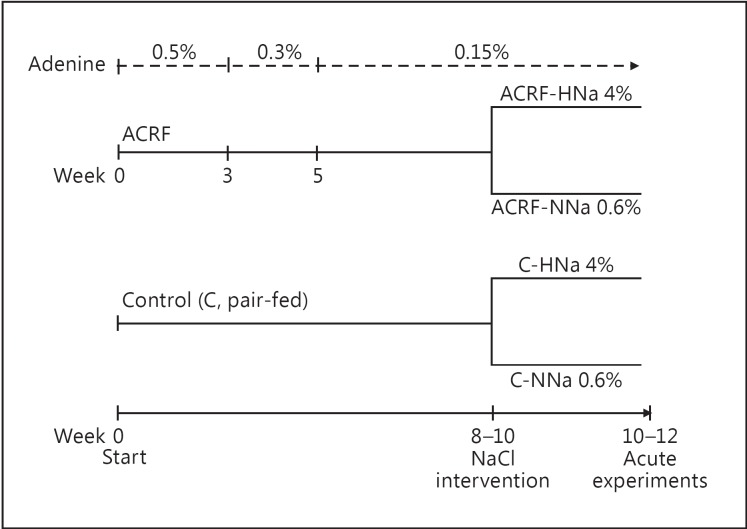
Two weeks prior to the acute experiments, the rats were randomized to either continue with the same chow with a normal NaCl content (NNa; 0.6% NaCl) or switch to an identical chow with a high NaCl content (HNa; 4.0% NaCl). Hence, acute experiments were carried out on four groups of animals: (1) C-NNa (n = 9); (2) C-HNa (n = 10); (3) ACRF-NNa (n = 10), and (4) ACRF-HNa (n = 10). An overview of the feeding procedure for protocols A and B is presented in figure 1. Chow intake was measured throughout the study, and as a consequence of our pair-feeding protocol there were no differences between groups in chow intake. Data on kidney function and renal hemodynamics from acute experiments have previously been published [14].
Fig. 1.
Schematic presentation of the feeding protocol and study groups. Rats with ACRF ate chow supplemented with adenine (0.5-0.15%, upper panel) throughout the study period, and controls were pair-fed a normal diet without adenine. The chow had a normal NaCl content (0.6%) from the beginning of the study until 2 weeks prior to the acute experiments, when the rats were randomized to either remain on normal NaCl chow (NNa; 0.6% NaCl) or be switched to high-NaCl chow (HNa; 4% NaCl).
Protocol B (Plasma Brain Natriuretic Peptide-32 Concentrations)
Sixty-two male Sprague-Dawley rats (Harlan) weighing ∼300 g were used. These rats were subjected to a feeding protocol identical to that for the rats in study A, resulting in four groups: (1) C-NNa (n = 16); (2) C-HNa (n = 16); (3) ACRF-NNa (n = 15), and (4) ACRF-HNa (n = 15). The rats were killed by decapitation 10 weeks after the beginning of the study, and free-flowing trunk blood was collected and tissues were harvested. Heparin plasma was obtained following centrifugation (5,000 rpm for 10 min) and was stored at 80°C until analyzed. From these rats, only data on plasma concentrations of brain natriuretic peptide-32 (BNP-32), the biologically active 32-amino acid polypeptide, are presented.
Plasma BNP-32 concentrations were measured by a commercially available ELISA kit (Abcam, Cambridge, UK) according to the manufacturer's instructions. Samples were analyzed in duplicate and the results were averaged.
Acute Renal Clearance Experiment and Arterial Pressure Measurements (Protocol A)
In brief, the rats were anesthetized with isoflurane (Pharmacia & Upjohn, Stockholm, Sweden) and prepared for renal clearance experiments as described [14]. Isoflurane mixed with air was administered by spontaneous breathing using an isoflurane vaporizer (Univentor-1200; AgnTho's, Lidingö, Sweden). For induction and maintenance of anesthesia, isoflurane concentrations of 5 and 1.5% (vol/vol), respectively, were used. Arterial pressure (AP) and heart rate were recorded continuously via a polyethylene catheter in the femoral artery using the data acquisition program Biopac MP 150 (Biopac Systems, Santa Barbara, Calif., USA). Systolic AP (SAP), diastolic AP (DAP), mean AP (MAP), and pulse pressure (PP) were derived from the data acquisition program. Immediately after catheterization, an arterial blood sample (0.25 ml) was collected for analysis of cTnT and an equivalent volume of 4% bovine serum albumin in isotonic saline was administered. The left kidney was exposed by a flank incision and immobilized in a plastic cup, and the ureter was catheterized (polyethylene-25) for urine collection. The GFR of the left kidney was determined by measuring renal 51Cr-EDTA (51Cr-ethylenediamine tetraacetic acid; Amersham Laboratories, Amersham, UK) clearance. The rats were infused with a total volume of 10 ml/kg/h of isotonic saline throughout.
After a 45-min equilibration period, 2 consecutive 20-min renal clearance periods were performed for analyses of kidney function. The rats were killed by an overdose of pentobarbital sodium after the second clearance period, and the heart and kidneys were immediately excised and weighed. AP decreased significantly in both groups during the equilibration period, most likely due to the AP-lowering effects of isoflurane [15]. Hence, the presented AP data are average values of the initial 5 min of the equilibration period, as these data more accurately reflected AP in the awake state.
LV Histology (Protocol A)
The left ventricle was cut transversally into base, middle, and apical parts. The tissue was immediately immersion fixed in 4% neutrally buffered formaldehyde (Histolab Products AB, Gothenburg, Sweden) for 24 h, after which it was stored in 70% ethanol at 4°C until further processing. Using routine techniques, 3-μm-thick transverse sections were prepared and stained with hematoxylin and eosin, Picrosirius red (analysis of fibrosis), von Kossa (assessment of calcifications), or Miller's elastin. LV calcification was scored semiquantitatively as either present or absent. All assessments were made by an investigator blinded to treatment group.
Morphometric Analyses of LV Fibrosis (Protocol A)
Images of sections stained with Picrosirius red were derived using an Olympus BX60 microscope (camera Olympus DP72) and the imaging software cellSens (Olympus). The imaging software BioPix iQ 2.0 (BioPix, Gothenburg, Sweden) was used to objectively measure general and perivascular fibrosis.
For the analysis of general fibrosis, one section (×10 magnification) from each of the three levels which included the whole LV circumference was used. Ten visual fields per section were randomly selected, and the area percentage of fibrosis was determined. Perivascular areas were excluded from measurements.
For the analysis of perivascular fibrosis, two sections (×10 magnification) from each of the three levels which included the whole LV circumference was used. All arteries that had been cut transversally and had a circular shape were analyzed (an average of 38 ± 13 arteries per rat). The artery area (wall area + lumen area) and fibrotic area immediately surrounding the artery were determined. Assuming a circular shape, the artery outer diameter was calculated from the vessel area. To correct for vessel size, perivascular fibrosis is expressed as fibrotic area per artery outer diameter [16]. The average diameter of the examined arteries was 95 ± 13 µm.
Immunohistochemistry (Protocol A)
The tissue sections were incubated with normal goat serum (Vector Laboratories Inc., Burlingame, Calif., USA) for 20 min before being incubated overnight at 4°C with primary antibody (monoclonal CD68 mouse anti-rat antibody, 1:200; LifeSpan Biosciences Inc., Seattle, Wash., USA). Subsequently, the sections were washed with PBS and treated with biotinylated goat anti-mouse (Vector Laboratories) for 30 min followed by an avidin-biotin peroxidase complex for another hour (Vectastain Elite ABC Kit; Vector Laboratories). Localization of the peroxidase conjugates was achieved using 3,3′-diaminobenzidine tetrahydrochloride as a chromogen.
Analysis of cTnT (Protocol A)
cTnT was measured in serum samples diluted 3.5 times with water using the Elecsys hs-cTnT immunoassay on a fully automated cobas 8000 analyzer (Roche, Germany) with a limit of the blank as determined by the manufacturer of 3 ng/l and a limit of quantitation of 5 ng/l. This assay uses two monoclonal antibodies directed against a cardiac-specific amino acid sequence in the tropomyosin-binding domain of cTnT and does not detect skeletal muscle isoforms of troponin T [17]. The locally determined within-run coefficient of variation using pooled human serum samples and controls with human cTnT was 4.9% at 4.6 ng/l, 2.3% at 9.5 ng/l, and 1.1% at 27.9 ng/l.
Statistical Analysis
Values are means ± SD. Analyses were performed using two-factorial ANOVA. The degree of correlation between variables was analyzed by determining the Pearson correlation coefficient (r). A p value <0.05 was considered statistically significant. The statistical software SPSS 20.0 (SPSS Inc., Chicago, Ill., USA) was used.
Results
AP and Kidney Function
Early during the equilibration period, 2 animals in group ACRF-HNa developed severe hypotension and died, and they were excluded from analysis. The rats with ACRF showed statistically significant increases in SAP, DAP, PP, and MAP versus controls, whereas their heart rate was not significantly altered (table 1). There was a statistically significant between-factor interaction in SAP, DAP, and MAP that was caused by high NaCl intake producing increases in pressure only in ACRF rats. On average, the MAP was elevated by 21 ± 13 mm Hg in ACRF-HNa versus ACRF-NNa rats. High NaCl intake decreased the heart rate in both ACRF and control rats (table 1).
Table 1.
AP and HR in anesthetized rats
| C-NNa | C-HNa | ACRF-NNa | ACRF-HNa | ANOVA effects, p value |
|||
|---|---|---|---|---|---|---|---|
| (n = 9) | (n = 10) | (n = 10) | (n = 8) | adenine | NaCl intake | interaction | |
| SAP, mm Hg | 138±18 | 134±11 | 181±23 | 206±16 | <0.001 | n. s. | <0.05 |
| DAP, mm Hg | 91±12 | 91±8 | 114±8 | 129±11 | <0.001 | <0.05 | <0.05 |
| PP, mm Hg | 48±7 | 43±5 | 68±18 | 77±7 | <0.001 | n. s. | n. s. |
| MAP, mm Hg | 110±14 | 108±9 | 138±12 | 159±13 | <0.001 | <0.05 | <0.01 |
| HR, beats/min | 304±22 | 286±26 | 300±21 | 285±23 | n. s. | <0.05 | n. s. |
Data are derived from clearance experiments on isoflurane-anesthetized animals performed approximately 10-12 weeks after the beginning of the study (see Methods). All animals received chow with a normal (0.6%) NaCl content from the beginning of the study until 2 weeks prior to the clearance experiments, when the animals were randomized to either remain on the same diet (NNa) or be switched to high-NaCl (4%) chow (HNa). Values are means ± SD. Main effects and between-factor interaction from two-factorial ANOVA are presented. HR = Heart rate; n. s. = not significant.
The GFR was markedly reduced (p < 0.001) in ACRF rats (0.04 ± 0.03 and 0.04 ± 0.01 ml/min/100 g body weight in groups ACRF-NNa and ACRF-HNa, respectively) versus controls (0.24 ± 0.06 and 0.31 ± 0.03 ml/min/100 g body weight in groups C-NNa and C-HNa, respectively). Complete data on renal hemodynamics, kidney function, and plasma electrolytes have previously been published [14].
Cardiac Weights, Serum cTnT, and LV Fibrosis
Macroscopically, left ventricles from ACRF rats showed a striking wall thickening. LV and right ventricular weights were significantly elevated in ACRF rats versus controls. There was a statistically significant between-factor interaction in LV weight that was explained by high NaCl intake causing an increase in LV weight specifically in ACRF rats.
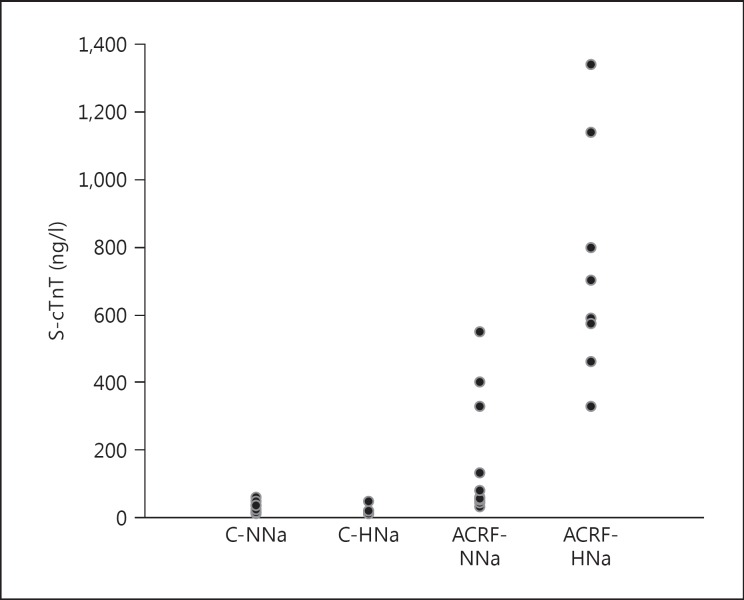
Serum concentrations of cTnT were markedly elevated in ACRF rats versus controls (table 2; fig. 2). In addition, high NaCl intake produced a marked increase in cTnT levels specifically in ACRF rats. Compared to group C-NNa, serum levels of cTnT were elevated approximately 6-fold in group ACRF-NNa and 24-fold in group ACRF-HNa.
Table 2.
Cardiac weights, LV fibrosis, and serum levels of cTnT
| C-NNa | C-HNa | ACRF-NNa | ACRF-HNa | ANOVA effects, p value |
|||
|---|---|---|---|---|---|---|---|
| (n = 9) | (n = 10) | (n = 10) | (n = 8) | adenine | NaCl intake | interaction | |
| Body weight, g | 369±31 | 343±23 | 387±27 | 346±32 | n. s. | <0.01 | n.s. |
| LVW, g/kg BW | 2.33±0.24 | 2.25±0.12 | 3.24±0.35 | 3.54±0.40 | <0.001 | n.s. | <0.05 |
| RVW, g/kg BW | 0.54±0.09 | 0.56±0.09 | 0.66±0.08 | 0.71±0.13 | <0.001 | n.s. | n.s. |
| LV fibrosis, % | 2.9±0.9 | 2.5±0.6 | 3.4±1.4 | 10 7±5.1 | <0.001 | <0.001 | <0.001 |
| LV PV fibrosis, µm2/µm | 76±15 | 50±7 | 83±12 | 90±11 | <0.001 | <0.05 | <0.001 |
| Serum cTnT, ng/l | 30±17 | 16±12 | 172±184 | 740±342 | <0.001 | <0.001 | <0.001 |
Data are derived from acute experiments performed approximately 10–12 weeks after the beginning of the study (see Methods). All animals received chow with a normal (0.6%) NaCl content from the beginning of the study until 2 weeks prior to the acute experiments, when the animals were randomized to either remain on the same diet (NNa) or be switched to high-NaCl (4%) chow (HNa). Values are means ± SD. Main effects and between-factor interaction from two-factorial ANOVA are presented. LVW = LV weight; RVW = right ventricular weight; BW = body weight; PV = perivascular; n. s. = not significant.
Fig. 2.
Serum levels of cTnT (S-cTnT) measured in isoflurane-anesthetized rats 10-12 weeks after the beginning of the study (see Methods). Rats received chow with a normal (0.6%) NaCl content from the beginning of the study until 2 weeks prior to the acute experiments, when the animals were randomized to either remain on the same diet (NNa) or be switched to high-NaCl (4%) chow (HNa). Numeric data and results of the statistical analyses are presented in table 2.
Both general and perivascular LV fibrosis were significantly elevated in ACRF rats versus controls (table 2). There were statistically significant between-factor interactions as a consequence of high NaCl intake producing increases in fibrosis only in ACRF rats.
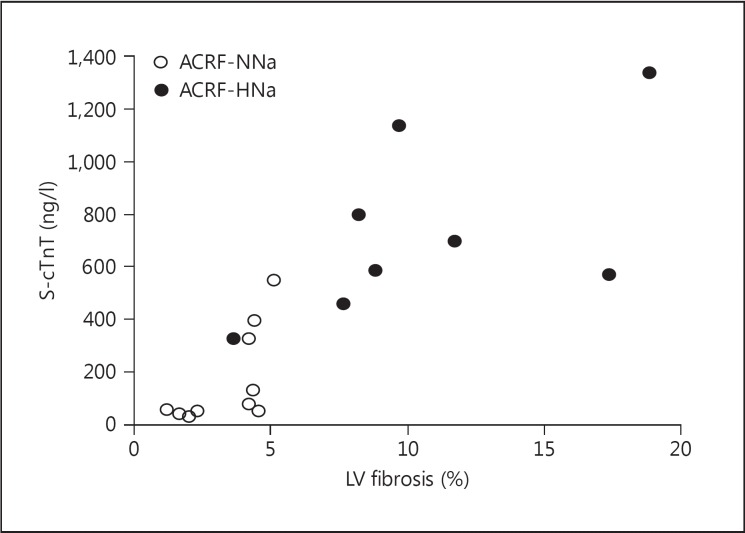
There was a positive correlation between LV general fibrosis and cTnT levels in ACRF rats (r = 0.81, p < 0.01; fig. 3). In addition, there were significant correlations between SAP (r = 0.69, p < 0.01), DAP (r = 0.65, p < 0.01), and PP (r = 0.57, p < 0.05) and cTnT concentrations. There were no statistically significant correlations between perivascular fibrosis, or LV weight, and cTnT levels in ACRF rats (data not shown). The correlation between GFR and cTnT levels in ACRF rats was negative but did not reach statistical significance (r = −0.41, p = 0.09).
Fig. 3.
Scatterplot illustrating the correlation between LV fibrosis and serum levels of cTnT (S-cTnT) in rats with ACRF analyzed 10-12 weeks after the beginning of the study (see Methods). Rats received chow with a normal (0.6%) NaCl content from the beginning of the study until 2 weeks prior to the acute experiments, when the animals were randomized to either remain on the same diet (NNa) or be switched to high- NaCl (4%) chow (HNa). There was a significant correlation between LV fibrosis and cTnT levels (r = 0.81, p < 0.01).
Plasma BNP-32
The plasma concentrations of BNP-32 were 545 ± 116, 1,061 ± 117, 1,034 ± 160, and 1,314 ± 121 pg/ml in groups C-NNa, C-HNa, ACRF-NNa, and ACRF-HNa, respectively. Two-factorial ANOVA revealed statistically significant effects of adenine (p < 0.01) and high-NaCl diet (p < 0.001) on plasma BNP-32 concentrations, but no statistically significant between-factor interactions.
LV Histology
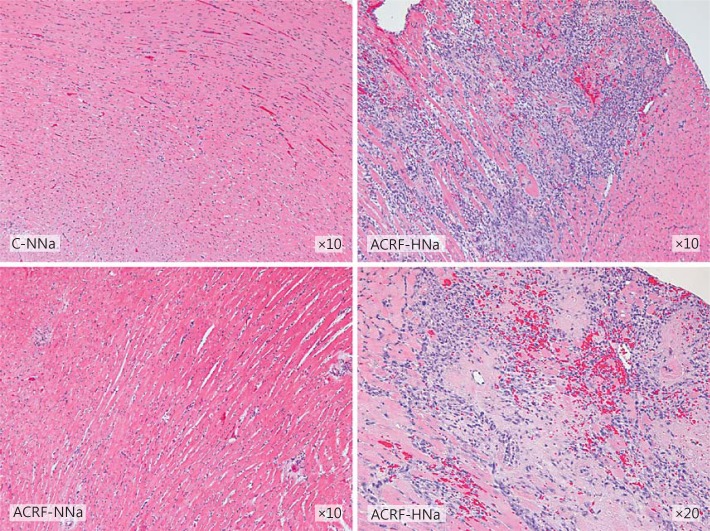
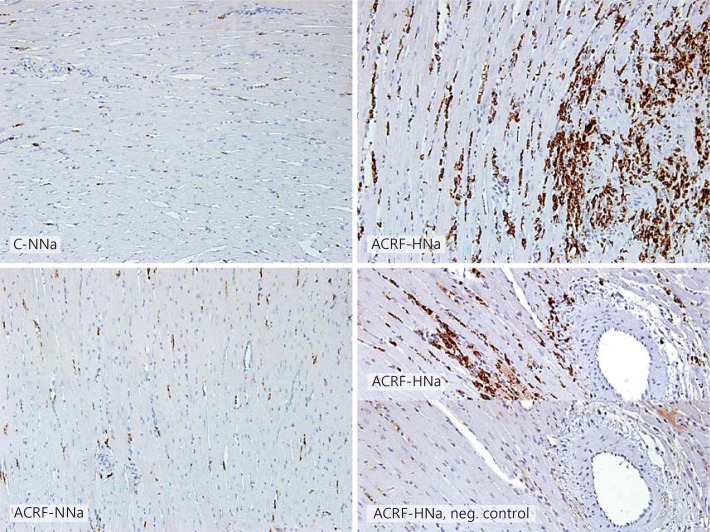
In groups C-HNa and ACRF-NNa, general LV histology did not appear different from the one in C-NNa rats. However, all rats in group ACRF-HNa showed focal areas with pronounced interstitial inflammatory cell infiltration, fibrosis, necrotic cardiomyocytes, and perivascular erythrocytes, indicating microvascular injury (fig. 4). A large proportion of the cells within the inflammatory infiltrate were positive for CD68, indicating that these cells were macrophages/monocytes (fig. 5).
Fig. 4.
LV histology appeared normal in pair-fed controls (C-NNa) and in rats with ACRF on normal (0.6%) NaCl diets (ACRF-NNa). In ACRF rats that had received 2 weeks of high-NaCl (4%) chow (ACRF-HNa), the left ventricle showed focal areas with inflammatory cell infiltration, fibrosis, necrotic cardiomyocytes, and perivascular erythrocytes, indicating hemorrhages. Sections were stained with hematoxylin and eosin.
Fig. 5.
Immunohistochemistry identifying CD68-positive cells (monocytes and macrophages) in the left ventricle of pair-fed controls (C) and rats with ACRF on a normal (0.6%; NNa) or high-NaCl (4%; HNa) diet. A large proportion of the cells within injured areas in the left ventricle of ACRF-HNa rats showed CD68 positivity. In the lower right panel, a negative control, in which no primary antibody was applied, has been included. ×20.
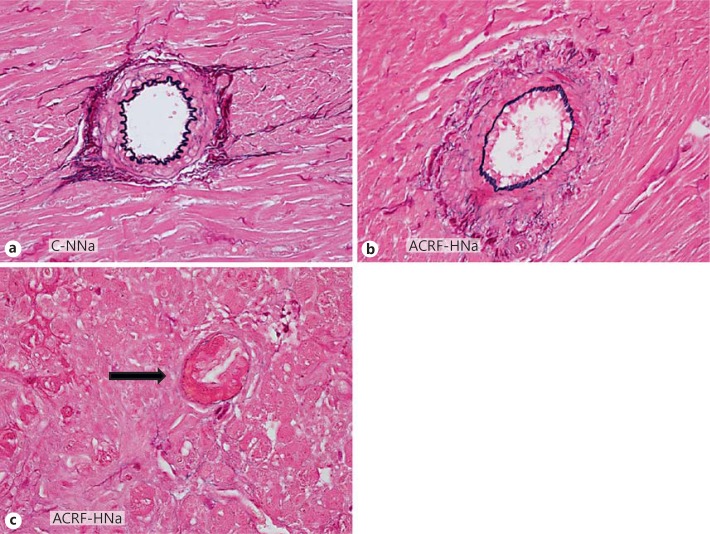
In ACRF rats, arteries within areas of a preserved myocardial architecture showed only modest medial thickening (fig. 6b). However, in group ACRF-HNa small arteries and arterioles within focal areas with scarring and inflammation showed fibrinoid necrosis and severe luminal obstruction (fig. 6c). There were no arterial abnormalities observed in controls with normal renal function (fig. 6a). No myocardial calcifications could be detected on von Kossa-stained sections in any of the groups.
Fig. 6.
LV arteries from pair-fed controls on normal (0.6%) NaCl diet (C-NNa; a) and rats with ACRF subjected to 2 weeks of high-NaCl (4%) diet (ACRF-HNa; b, c). In ACRF-HNa rats, arteries from noninjured areas of the myocardium showed modest alterations characterized by thickening of the medial layer (b). In myocardial areas with severe focal tissue injury, arteries (arrow) demonstrated fibrinoid necrosis with destruction of the internal elastic lamina and pronounced occlusion of the vessel lumen (c). Sections were stained with Miller's elastin. ×60.
Discussion
The main finding in the present study was that 2 weeks of high-NaCl diet in rats with ACRF caused LV injury and augmented increases in serum cTnT levels. Myocardial arteries in ACRF-HNa rats showed fibrinoid necrosis with luminal narrowing, suggesting that LV injury was at least partially caused by severe hypertension and tissue ischemia.
Serum concentrations of cTnT were clearly elevated also in ACRF rats on a normal NaCl diet, although these animals did not demonstrate any obvious histopathological injuries to the left ventricle by light microscopy. This observation resembles findings on CKD patients with a reduced GFR, in whom serum levels of cTnT are frequently elevated in the absence of symptoms [2,7]. Theoretically, this increase in cTnT levels could be caused by either an enhanced release of cTnT from the myocardium or a decreased plasma clearance. However, still little is known about the mechanisms of plasma clearance of cTnT and the role of the kidneys. It is feasible to hypothesize that the observed increase in serum cTnT in ACRF rats was mainly caused by an elevated release from the myocardium secondary to cardiomyocyte injury. First, the rats in group ACRF-NNa were hypertensive and had LVH, and both of these factors have been shown to predict plasma levels of cTnT in patients with CKD independently of GFR [7,18]. Secondly, cTnT is a relatively large molecule (molecular weight 37 kDa), indicating a low rate of filtration in glomeruli. For comparison, the glomerular sieving coefficient for α1-microglobulin, a somewhat smaller protein (31 kDa), is estimated to be only 0.092 in humans [19]. It has been speculated that smaller cTnT fragments might accumulate in CKD patients with reduced GFRs and cause, or contribute to, increased cTnT immunoreactivity [20]. In support of this notion, Cardinaels et al. [21], by using the original Roche antibodies that were applied also in the present study, were able to detect cTnT fragments of 29 and 14-18 kDa in sera from patients with acute myocardial infarction. In contrast, Fahie-Wilson et al. [22] did not detect any low-molecular-mass cTnT fragments in ESRD patients and found that free, intact cTnT was the predominant form in plasma.
In group ACRF-HNa, cTnT levels were elevated 4.3-fold versus ACRF-NNa rats, although there was no significant difference between the groups in GFR, clearly indicating that 2 weeks of high-NaCl diet aggravated myocardial injury and cTnT release in rats with chronic renal failure independently of the GFR. Indeed, LV lesions with necrotic cardiomyocytes and scarring were only detected in ACRF-HNa rats. In addition, there was a strong correlation between the degree of LV general fibrosis and serum levels of cTnT in ACRF rats. In accord with a previous study on this model [12], in which AP was measured continuously by radiotelemetry, ACRF rats developed a marked increase in AP in response to a high-NaCl diet. It is likely that the increase in AP was caused by sodium retention and an expansion of the extracellular fluid volume considering that the GFR values in ACRF rats were only about 15% of those in controls. In support of this notion, plasma concentrations of BNP-32 were elevated in ACRF rats versus controls in the present study, and the highest concentration was observed in group ACRF-HNa. Although LV function was not examined in the present study, we have previously shown that ACRF rats with a normal dietary NaCl intake have increased LV end-diastolic pressure compared to controls [12]. It is feasible to hypothesize that the high-NaCl diet, by increasing the plasma volume, elevated LV end-diastolic pressure even further in group ACRF-HNa in the present study. The small artery lesions in areas of myocardial injury in ACRF-HNa rats were characterized by medial thickening, fibrinoid necrosis, and marked luminal narrowing, abnormalities that are typically seen in malignant hypertension. Hence, we hypothesize that hypertension-induced arterial lesions caused myocardial ischemia and hypoxic injury. In support of this notion, the myocardial injury in ACRF-HNa rats mimics the one described in salt-loaded, malignant hypertensive, double transgenic rats harboring human renin and angiotensinogen genes [23]. However, in contrast to double transgenic rats, hypertension in ACRF animals is characterized by low plasma renin levels and is not angiotensin II dependent [12]. In addition, the marked hypertension in ACRF-HNa animals and increase in LV afterload are expected to enhance tissue oxygen demand, thereby aggravating myocardial hypoxia.
The cardiac structural changes in ACRF rats showed similarities to those previously reported for other rodent CKD models [24]. Interestingly, Amann and coworkers [25] found that subtotally nephrectomized rats developed LVH, intramyocardial arteriolar wall thickening, capillary rarefaction, and expansion of the myocardial interstitium that could not be explained by hypertension. Thus, nonhemodynamic pathophysiological mechanisms may also be involved in the cardiac injury developing in CKD. For instance, we have previously demonstrated elevated plasma levels of aldosterone, a well-known promoter of myocardial fibrosis and remodeling, in ACRF rats with a normal NaCl intake [13].
In conclusion, rats with ACRF developed LVH and elevated serum levels of cTnT. Two weeks of high-NaCl diet aggravated the increases in serum cTnT concentrations and produced LV injury presumably by causing hypertension-induced small artery lesions and myocardial ischemia. We consider this model suitable for investigating pathophysiological mechanisms leading to myocardial injury and elevated serum cTnT levels in chronic renicardiac syndromes. In addition, our results support the notion that a high dietary intake of NaCl may have deleterious effects on LV integrity in patients with ESRD.
Statement of Ethics
This study was performed on rats, and all experiments were approved by the regional ethics committee in Gothenburg, Sweden.
Disclosure Statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Acknowledgements
The technical assistance of Jaana Lundgren, Elisabeth Grimberg, and Lisa Nguy is acknowledged. This study was supported by grants from the Swedish Heart-Lung Foundation, Swedish Federal Government under the LUA/ALF agreement, Göteborg Medical Society, Swedish Medical Society, Swedish Association for Kidney Patients, Swedish Society of Nephrology, Inger Bendix Foundation, Paul Frankenius Foundation, Britt Wennerström's Research Foundation, IngaBritt and Arne Lundbergs Research Foundation, Marianne and Marcus Wallenbergs Foundation, Swedish Cancer Society, and Swedish Pain Foundation (SSF).
References
- 1.Matsushita K, Sang Y, Ballew SH, Astor BC, Hoogeveen RC, Solomon SD, Ballantyne CM, Woodward M, Coresh J. Cardiac and kidney markers for cardiovascular prediction in individuals with chronic kidney disease: the Atherosclerosis Risk in Communities study. Arterioscler Thromb Vasc Biol. 2014;34:1770–1777. doi: 10.1161/ATVBAHA.114.303465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Köttgen A, Levey AS, Levin A. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382:158–169. doi: 10.1016/S0140-6736(13)60439-0. [DOI] [PubMed] [Google Scholar]
- 3.Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. doi: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 4.Green D, Roberts PR, New DI, Kalra PA. Sudden cardiac death in hemodialysis patients: an in-depth review. Am J Kidney Dis. 2011;57:921–929. doi: 10.1053/j.ajkd.2011.02.376. [DOI] [PubMed] [Google Scholar]
- 5.Whitman IR, Feldman HI, Deo R. CKD and sudden cardiac death: epidemiology, mechanisms, and therapeutic approaches. J Am Soc Nephrol. 2012;23:1929–1939. doi: 10.1681/ASN.2012010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freda BJ, Tang WH, van Lente F, Peacock WF, Francis GS. Cardiac troponins in renal insufficiency: review and clinical implications. J Am Coll Cardiol. 2002;40:2065–2071. doi: 10.1016/s0735-1097(02)02608-6. [DOI] [PubMed] [Google Scholar]
- 7.Dubin RF, Li Y, He J, Jaar BG, Kallem R, Lash JP, Makos G, Rosas SE, Soliman EZ, Townsend RR, Yang W, Go AS, Keane M, Defilippi C, Mishra R, Wolf M, Shlipak MG, CRIC Study Investigators Predictors of high sensitivity cardiac troponin T in chronic kidney disease patients: a cross-sectional study in the Chronic Renal Insufficiency Cohort (CRIC) BMC Nephrol. 2013;14:229. doi: 10.1186/1471-2369-14-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjurman C, Petzold M, Venge P, Farbemo J, Fu ML, Hammarsten O. High-sensitive cardiac troponin, NT-proBNP, hFABP and copeptin levels in relation to glomerular filtration rates and a medical record of cardiovascular disease. Clin Biochem. 2015;48:302–307. doi: 10.1016/j.clinbiochem.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 9.Khan NA, Hemmelgarn BR, Tonelli M, Thompson CR, Levin A. Prognostic value of troponin T and I among asymptomatic patients with end-stage renal disease: a meta-analysis. Circulation. 2005;112:3088–3096. doi: 10.1161/CIRCULATIONAHA.105.560128. [DOI] [PubMed] [Google Scholar]
- 10.Mallamaci F, Zoccali C, Parlongo S, Tripepi G, Benedetto FA, Cutrupi S, Bonanno G, Fatuzzo P, Rapisarda F, Seminara G, Stancanelli B, Bellanuova I, Cataliotti A, Malatino LS. Troponin is related to left ventricular mass and predicts all-cause and cardiovascular mortality in hemodialysis patients. Am J Kidney Dis. 2002;40:68–75. doi: 10.1053/ajkd.2002.33914. [DOI] [PubMed] [Google Scholar]
- 11.deFilippi C, Wasserman S, Rosanio S, Tiblier E, Sperger H, Tocchi M, Christenson R, Uretsky B, Smiley M, Gold J, Muniz H, Badalamenti J, Herzog C, Henrich W. Cardiac troponin T and C-reactive protein for predicting prognosis, coronary atherosclerosis, and cardiomyopathy in patients undergoing long-term hemodialysis. JAMA. 2003;290:353–359. doi: 10.1001/jama.290.3.353. [DOI] [PubMed] [Google Scholar]
- 12.Nguy L, Johansson ME, Grimberg E, Lundgren J, Teerlink T, Carlström M, Lundberg JO, Nilsson H, Guron G. Rats with adenine-induced chronic renal failure develop low-renin, salt-sensitive hypertension and increased aortic stiffness. Am J Physiol Regul Integr Comp Physiol. 2013;304:R744–R752. doi: 10.1152/ajpregu.00562.2012. [DOI] [PubMed] [Google Scholar]
- 13.Nguy L, Nilsson H, Lundgren J, Johansson ME, Teerlink T, Scheffer PG, Guron G. Vascular function in rats with adenine-induced chronic renal failure. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1426–R1435. doi: 10.1152/ajpregu.00696.2011. [DOI] [PubMed] [Google Scholar]
- 14.Saeed A, DiBona GF, Grimberg E, Nguy L, Mikkelsen ML, Marcussen N, Guron G. High-NaCl diet impairs dynamic renal blood flow autoregulation in rats with adenine-induced chronic renal failure. Am J Physiol Regul Integr Comp Physiol. 2014;306:R411–R419. doi: 10.1152/ajpregu.00383.2013. [DOI] [PubMed] [Google Scholar]
- 15.Seyde WC, Durieux ME, Longnecker DE. The hemodynamic response to isoflurane is altered in genetically hypertensive (SHR), as compared with normotensive (WKY), rats. Anesthesiology. 1987;66:798–804. doi: 10.1097/00000542-198706000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Shi YX, Chen Y, Zhu YZ, Huang GY, Moore PK, Huang SH, Yao T, Zhu YC. Chronic sodium hydrosulfide treatment decreases medial thickening of intramyocardial coronary arterioles, interstitial fibrosis, and ROS production in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;293:H2093–H2100. doi: 10.1152/ajpheart.00088.2007. [DOI] [PubMed] [Google Scholar]
- 17.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56:254–261. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 18.Mishra RK, Li Y, DeFilippi C, Fischer MJ, Yang W, Keane M, Chen J, He J, Kallem R, Horwitz EJ, Rafey M, Raj DS, Go AS, Shlipak MG, CRIC Study Investigators Association of cardiac troponin T with left ventricular structure and function in CKD. Am J Kidney Dis. 2013;61:701–709. doi: 10.1053/j.ajkd.2012.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norden AG, Lapsley M, Lee PJ, Pusey CD, Scheinman SJ, Tam FW, Thakker RV, Unwin RJ, Wrong O. Glomerular protein sieving and implications for renal failure in Fanconi syndrome. Kidney Int. 2001;60:1885–1892. doi: 10.1046/j.1523-1755.2001.00016.x. [DOI] [PubMed] [Google Scholar]
- 20.Diris JH, Hackeng CM, Kooman JP, Pinto YM, Hermens WT, van Dieijen-Visser MP. Impaired renal clearance explains elevated troponin T fragments in hemodialysis patients. Circulation. 2004;109:23–25. doi: 10.1161/01.CIR.0000109483.45211.8F. [DOI] [PubMed] [Google Scholar]
- 21.Cardinaels EP, Mingels AM, van Rooij T, Collinson PO, Prinzen FW, van Dieijen-Visser MP. Time-dependent degradation pattern of cardiac troponin T following myocardial infarction. Clin Chem. 2013;59:1083–1090. doi: 10.1373/clinchem.2012.200543. [DOI] [PubMed] [Google Scholar]
- 22.Fahie-Wilson MN, Carmichael DJ, Delaney MP, Stevens PE, Hall EM, Lamb EJ. Cardiac troponin T circulates in the free, intact form in patients with kidney failure. Clin Chem. 2006;52:414–420. doi: 10.1373/clinchem.2005.062307. [DOI] [PubMed] [Google Scholar]
- 23.Luft FC, Mervaala E, Müller DN, Gross V, Schmidt F, Park JK, Schmitz C, Lippoldt A, Breu V, Dechend R, Dragun D, Schneider W, Ganten D, Haller H. Hypertension-induced end-organ damage: a new transgenic approach to an old problem. Hypertension. 1999;33:212–218. doi: 10.1161/01.hyp.33.1.212. [DOI] [PubMed] [Google Scholar]
- 24.Bongartz LG, Braam B, Gaillard CA, Cramer MJ, Goldschmeding R, Verhaar MC, Doevendans PA, Joles JA. Target organ cross talk in cardiorenal syndrome: animal models. Am J Physiol Renal Physiol. 2012;303:F1253–F1263. doi: 10.1152/ajprenal.00392.2012. [DOI] [PubMed] [Google Scholar]
- 25.Tornig J, Amann K, Ritz E, Nichols C, Zeier M, Mall G. Arteriolar wall thickening, capillary rarefaction and interstitial fibrosis in the heart of rats with renal failure: the effects of ramipril, nifedipine and moxonidine. J Am Soc Nephrol. 1996;7:667–675. doi: 10.1681/ASN.V75667. [DOI] [PubMed] [Google Scholar]