Abstract
Background
Adjunctive behavioral smoking cessation treatments have the potential to improve outcomes beyond standard care. The present study had two aims: 1) compare standard care (SC) for smoking (four weeks of brief counseling and monitoring) to SC plus prize-based contingency management (CM), involving the chance to earn prizes on days with demonstrated smoking abstinence (carbon monoxide (CO) ≤6ppm); and 2) compare the relative efficacy of two prize reinforcement schedules - one a traditional CM schedule, and the second an early enhanced CM schedule providing greater reinforcement magnitude in the initial week of treatment but equal overall reinforcement.
Methods
Participants (N = 81 nicotine-dependent cigarette smokers) were randomly assigned to one of the three conditions.
Results
Prize CM resulted in significant reductions in cigarette smoking relative to SC. These reductions were not apparent at follow-up. We found no meaningful differences between the traditional and enhanced CM conditions.
Conclusions
Our findings reveal that prize CM leads to significant reductions in smoking during treatment relative to a control intervention, but the benefits did not extend long-term.
Keywords: Prize, contingency management, cigarette, tobacco, treatment efficacy
1. INTRODUCTION
Cigarette smoking results in 1 of every 5 deaths and extraordinary economic costs (Centers for Disease Control and Prevention, 2008). Contingency management (CM) has demonstrated efficacy for treating substance use disorders (Dutra et al., 2008; Prendergast et al., 2006), including smoking (Ledgerwood, 2008 review). However, CM can be costly, with reinforcement exceeding $1,000 (Higgins et al., 2004; Lamb et al., 2004). Petry and colleagues developed a prize reinforcement program lower-cost by design, with demonstrated efficacy with cocaine, opioid, alcohol and poly-substance-dependent patients (e.g., Peirce et al., 2006; Petry et al., 2000; Petry et al., 2012). To date, one small, non-randomized pilot has examined prize CM for smoking among substance abuse patients, with prize CM engendering greater proportions of negative CO tests than standard care (Alessi et al., 2008). The present study examines the efficacy of Prize CM for smoking in a randomized trial.
A second purpose is to evaluate a scheduled increase in reinforcement magnitude early in treatment. Reinforcement magnitude is a parameter that can increase treatment response (Petry et al., 2004) and may thereby increase long-term abstinence (Kenford et al., 1994; Higgins et al., 2006), but this has not yet been examined in a randomized clinical trial of treatment-seeking smokers.
The two aims of the present study are: assess the efficacy of prize-based CM for cigarette smoking; and compare a traditional versus early-treatment enhanced reinforcement schedule.
2. METHODS
2.1 Participants
Participants were nicotine-dependent smokers (N = 81) who responded to advertisements in local newspapers, bulletin boards and at health fairs, and broadcast messages to staff of a large health center and university. Inclusion criteria were: Fagerström Test of Nicotine Dependence (Heatherton et al., 1991) score ≥4; age ≥18; and English literate. Exclusion criteria were: uncontrolled psychiatric disorders (acute suicidality, psychosis); current substance dependence excluding nicotine or caffeine; in recovery for pathological gambling; or already receiving smoking treatment. Recruitment occurred December 2007 to January 2011.
2.2 Procedures
Individuals were screened for eligibility and scheduled for intake if appropriate. During intake, written informed consent and self-report assessments and CO and urine cotinine tests were completed.
After Intake, participants attended the clinic twice daily (separated by 5+ hours) Monday-Friday for 5 weeks. Unexcused absences were considered positive CO readings; excused absences (e.g., family emergency) were without consequences. Follow-up assessments occurred 2- and 6-months after starting treatment, with $20 compensation per follow-up.
2.3 Measures
2.3.1
Demographic data were collected at intake. A brief screen of suicidality, psychosis and substance abuse was adapted using the Structured Clinical Interview for the DSM-IV-TR (First et al., 2002) to assess inclusion/exclusion. Pathological gambling was assessed using the NORC-DSM Screen (Gerstein et al., 1999).
2.3.2
Smoking history included ages first smoked and smoked daily, and cigarettes smoked daily.
2.3.3
Fagerström Test for Nicotine Dependence questionnaire assessed nicotine dependence (Fagerstrom, 1978).
2.3.4
Expired Carbon Monoxide (CO) levels were assessed at intake, twice daily during baseline and treatment, and at follow-ups using an EC50-MP Micro CO monitor (Bedfont). Levels ≤6ppm were considered smoking-negative for reinforcement purposes, consistent with other studies (range: 4ppm–8ppm; Corby et al., 2000; Lamb et al., 2004).
2.3.5
Urinary Cotinine samples were collected at intake, Mondays weeks 2–5, and follow-ups, and tested using the Accutest® NicAlertTM test-strip system (JANT Pharmacal Corporation, Encino, CA). During treatment weeks 2–4, cotinine samples served as a measure of weekend smoking abstinence (≤100ng/mL) to establish CM bonus draws.
2.4. Baseline
All participants received $1 per sample, independent of results, with a $20 bonus for submitting all 10 samples, to motivate compliance. Submitting ≥5 samples was required for randomization, else individuals were discontinued and referred for treatment elsewhere.
The baseline phase allowed for assessment of smoking pre-treatment and time to prepare to quit. On the last baseline visit, participants met with a research therapist to review a smoking cessation self-help quit guide to prepare to quit (U.S. Public Health Service, June 2000). Based on standards of care (Fiore et al., 2008), the materials emphasize motivation, social support and behavioral skills to help reduce smoking.
Random assignment to one of the three treatment conditions and stratified by gender and any CO ≤6 ppm during baseline (none or ≥1) occurred on treatment day 1 (quit date). Statistician-prepared sequentially numbered randomization envelopes concealed group assignments until assigned Randomization to CM conditions and standard treatment occurred at a 2:1 ratio to ensure adequate power to compare the two CM conditions.
2.5. Treatments
2.5.1. Standard care
Standard care (SC; weeks 2–5) involved monitoring CO and cotinine, and brief counseling (Fiore et al., 2008). Participants received $1/sample regardless of test results and a $20 weekly bonus for submitting all samples (maximum $120). Each session, the therapist provided immediate feedback about CO/cotinine test results, briefly (≈5 minutes) discussed recent smoking/abstinence, and praised quit efforts.
2.5.2. Traditional prize CM
In addition to SC, traditional CM (TCM) patients earned chances to win prizes for negative CO and cotinine samples (similar to Petry and Martin, 2002; Petry et al., 2000), with no compensation for compliance with only submitting samples.
On treatment day 1, participants drew for a prize if CO was reduced at least 3ppm from his/her intake level. Subsequently, draws were contingent on CO reading ≤6ppm. Draws from the prize urn increased by 1 (up to 5) each consecutive day both daily CO tests met criterion. CO levels >6 ppm, refusal to submit a sample, or unexcused absences reset draws to one for the next negative sample.
The TCM prize urn contained 250 slips of paper, with typical prize percentages (e.g., Petry and Martin, 2002); 50% (125) did not result in prizes, 44.8% were Small (worth about $1, e.g., snacks, toiletries); 4.8% were Large (worth about $20, e.g., gift certificates, electronics); and 0.4% were Jumbo (worth $100, e.g., DVD players, gift certificates).
To reinforce weekend abstinence, each cotinine sample ≤100 ng/ml on Mondays resulted in five bonus draws in weeks 3–5. Overall, 180 draws and 15 bonus draws were possible for CO- and cotinine-negative tests, respectively.
2.5.3. Early-treatment enhanced prize reinforcement (ECM)
ECM participants received SC, CO and cotinine monitoring and reinforcement criteria, described above. Draws available and reinforcement criteria were identical to TCM, but the chance of receiving reinforcement early in treatment was scheduled to be enhanced by providing guaranteed prizes (100% probability, versus 50% chance in TCM) for negative CO tests during treatment week 1.
During week 1, the ECM urn included 91.2% Small, 8% Large and 0.8% Jumbo prizes. For the remaining three weeks, an urn with 65.8% slips resulting in no prize, and 30% Small, 4% Large and 0.2% Jumbo prizes was used. Draws for cotinine-negative tests were made from the urn with 100% winning slips, for the same overall reinforcement magnitude in both CM conditions.
2.6. Analysis
We employed an intent-to-treat approach, including all participants who entered the treatment phase. Analyses compared combined CM conditions versus SC, and TCM versus ECM. Chi-square and t-tests were used to analyze baseline characteristics.
Outcomes were average CO, longest duration of smoking abstinence (days; LDA), and percent CO tests <4ppm. The CO criterion for reinforcement was ≤6ppm to maximize opportunities for reinforcement, but CO <4ppm was used for analysis to ensure conservative reporting of findings. A day of abstinence was defined as two consecutive CO tests <4ppm. If sessions encompassed a weekend, the patient was considered abstinent for three consecutive days if tests immediately before and after the weekend were negative. Unexcused absences broke the string of abstinence, excused absences did not if preceded and followed by negative samples.
Linear mixed models were used to analyze group differences in CO levels baseline through treatment. Fixed factors included treatment condition, time (50 testing sessions) and the condition X time interaction. Cases were included as random-effects variables.
T-tests were used to assess LDA and attendance. LDA data were normalized using square-root transformation for analysis, with raw data presented in tables to facilitate interpretation. Percent negative CO was the number of CO tests <4ppm divided by number of treatment sessions attended (maximum 40). Percent negative CO data and prize dollars earned could not be transformed to normality so Mann-Whitney U tests were used. Analyses were re-run using a cutoff of ≤6ppm (the reinforcement criterion). Although LDA and percent negative were predictably higher than when we used the more stringent CO <4ppm, overall direction and significance remained the same (available from first author).
Chi-square analysis was used for group differences at follow-ups on 7-day point prevalence (PPA; self-reported no smoking in the past 7 days and cotinine ≤100 ng/ml). Missing data were analyzed as smoking-positive.
3. RESULTS
3.1. Demographics
See Supplemental Figure 1 for participant flow and Table 1 for demographic and baseline data. CM participants had significantly more education than those in SC. Otherwise, differences were nonsignificant (ps >.05). Overall, cigarettes per day ranged from 14.7 to 19.3 (p > .10) and average Fagerström scores revealed “moderate” nicotine dependence.
Table 1.
Demographic, Smoking and Treatment Outcome variables for each treatment condition and for combined contingency management conditions. Mean (standard deviation) longest duration of abstinence, percent negative carbon monoxide (CO) tests, and treatment attendance data for standard versus combined CM conditions, and for traditional CM versus enhanced CM conditions are included as outcome variables. (Note: Longest duration of abstinence was square-root transformed prior to analysis; Test statistics are t-test and Mann-Whitney U test)
| Variable | Standard (n = 17) | Traditional CM (n = 28) | Enhanced CM (n = 36) | Combined CM Conditions (n = 64) | Standard vs. Combined CM t or χ2 |
Traditional vs. Enhanced t or χ2 |
|---|---|---|---|---|---|---|
| Age M(SD) | 44.0(12.1) | 45.8(11.7) | 44.5(12.7) | 45.1(12.2) | t(79) = −.32, p = .75 | t(62) = .40, p = .69 |
|
| ||||||
| Gender N(%) | χ2 (1,N=81) = .08, p = .78 | χ2 (1,N=64) = .07, p = .80 | ||||
| Male | 7(41.2) | 10(35.7) | 14(38.9) | 24(37.5) | ||
| Female | 10(58.8) | 18(64.3) | 22(61.1) | 40(62.5) | ||
|
| ||||||
| Race N(%) | χ2 (2,N=81) = .45, p = | χ2 (2,N=64) = 2.98, p = | ||||
| European American | 5(29.4) | 7(31.8) | 15(41.7) | 22(34.4) | .80 | .23 |
| African American | 12(70.6) | 21(70.0) | 20(55.6) | 41(64.1) | ||
| Other | 0(0) | 0(0) | 1(2.8) | 1(1.6) | ||
|
| ||||||
| Employed N(%) | 16(94.1) | 24(85.7) | 29(80.6) | 53(82.8) | χ2 (1,N=81) = 1.36, p = .24 | χ2 (1,N=64) = .29, p = .59 |
|
| ||||||
| Marital N(%) | χ2 (3,N=81) = 0.90, p = .83 | χ2 (3,N=64) = .88, p = .83 | ||||
| Never Married | 5(29.4) | 10(35.7) | 13(36.1) | 23(35.9) | ||
| Married/Cohab. | 6(35.3) | 6(21.4) | 11(30.6) | 17(25.6) | ||
| Widowed | 1(5.9) | 1(3.6) | 1(2.8) | 2(3.1) | ||
| Seperated/Divorced | 5(29.4) | 11(39.3) | 11(30.6) | 22(34.4) | ||
|
| ||||||
| Education M(SD) | 13.4(1.9) | 14.1(1.8) | 14.6(2.0) | 14.3(1.9) | t(79) = −2.00, p = .05 | t(62) = −1.18, p = .24 |
|
| ||||||
| Income (Median/IQ range) | $22,000 ($22,900) | $17,500 ($23,750) | $23,000 ($29,972) | $18,000 ($28,000) | U = 636.00, p = .24 | U = 487.50, p = .97 |
|
| ||||||
| Age first smoking M(SD) | 16.1(3.5) | 17.7(5.1) | 16.3(3.6) | 16.9(4.3) | t(78) = −.77, p = .44 | t(61) = 1.28, p = .21 |
|
| ||||||
| Number of cigarettes/day M(SD) | 19.3(8.8) | 14.7(9.8) | 16.8(8.0) | 15.9(8.8) | t(77) = 1.40, p = .16 | t(60) = −.92, p = .36 |
|
| ||||||
| Fagerstrom Score M(SD) | 6.4(1.2) | 6.0(1.2) | 6.4(1.2) | 6.2(1.2) | t(77) = .55, p = .59 | t(60) = −1.26, p = .21 |
|
| ||||||
| Treatment Outcomes | ||||||
|
| ||||||
| Longest Duration of Abstinence (days) | 1.6(3.2) | 6.9(8.6) | 3.7(5.7) | 5.1(7.2) | t(79) = −2.21, p < .05 | t(62) = 1.63, p = .11 |
|
| ||||||
| Percent of Negative CO Tests (<4ppm)/Sessions Attended (Median/IQ range) | 2.5(13.6) | 42.4(79.6) | 29.1(52.3) | 36.1(55.3) | U = 774.00, p < .01 | U = 433.00, p = .33 |
|
| ||||||
| Percent of Baseline Sessions Attended | 93.5(9.3) | 85.7(15.5) | 86.7(18.5) | 86.3(17.1) | t(79) = 1.68, p = .10 | t(65) = −.22, p = .83 |
|
| ||||||
| Percent of Treatment Sessions Attended | 74.7(31.0) | 69.8(24.4) | 68.5(30.7) | 69.1(27.9) | t(79) = .72, p = .48 | t(62) = −.18, p = .86 |
|
| ||||||
| Total Prize Dollar amount (Median/IQ Range) | N/A | $121.00(197.55) | $120.56(257.09) | $120.56(216.51) | N/A | U = 507.5, p = .81 |
|
| ||||||
| Prize Dollar Amount Week 1 (Median/IQ Range) | N/A | $21.00(46.00) | $23.50(54.00) | $23.00(48.00) | N/A | U = 595.5, p =.37 |
3.2. Standard Care versus CM
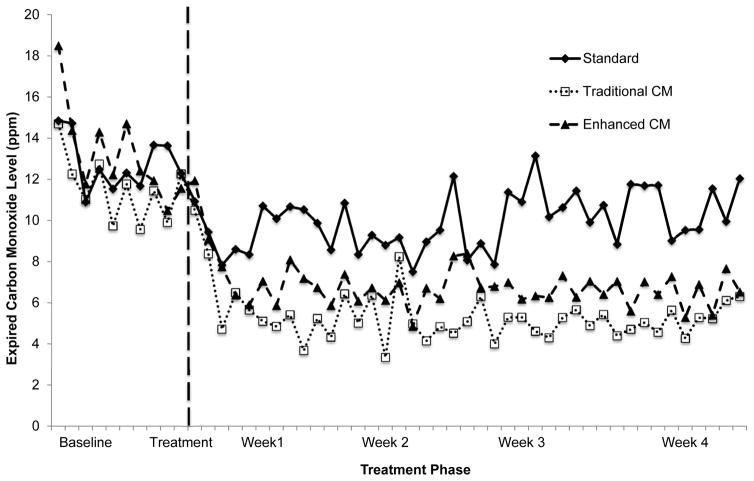
Baseline CO levels were similar between CM and SC (Figure 1). Linear mixed model analysis revealed a significant time X condition interaction, F(50,2860.37) = 2.71, p < .001, with CM participants reducing CO levels over time relative to SC.
Figure 1.
Average twice-daily carbon monoxide levels (parts per million) for combined CM conditions showed significant reductions relative to baseline and relative to Standard treatment (p < .05). Traditional and Enhanced CM conditions did not differ from each other. (Note: Traditional and Enhanced conditions are presented, but the combined CM condition is not, to enhance the readability of the graph).
CM-treated participants demonstrated significantly greater LDA than SC patients (p < .05; Table 1), with a medium effect size (d = .50). Percentage of negative CO tests was also greater with CM than SC (p < .01; Table 1).
Participants attended an average (SD) of 87.8%(16.0%) of baseline and 70.3% (28.5%) of treatment sessions, without differences between conditions (Table 1).
Month 2 and 6 follow-up rates were 85% and 77%, respectively, without significant differences between treatment conditions (ps >.10). CM and SC did not differ on PPA at 2-month (6.3% vs. 0%; χ2(1, n = 81) = 1.12 p = .29) or 6-month follow-up (6.3% vs. 5.9%; χ2(1, n = 81) = .00, p = .96).
3.3. Traditional versus Early-Enhanced CM
Linear mixed model analysis comparing TCM to ECM revealed a time main effect (F(50,2193.12) = 25.77, p < .001), with CO levels decreasing over time regardless of condition. Neither the condition main effect nor time X condition interaction was significant (p > .05; Figure 1).
TCM and ECM conditions did not differ significantly on LDA, percentage of CO-negative test or attendance (Table 1), nor PPA at 2-months (10.7% vs. 2.8%; χ2(1, n = 64) = 1.69 p = .19) or 6-month follow-up (10.7% vs. 2.8%; χ2(1, n = 64) = 1.69 p = .19).
3.4. Prize Earnings
Approximately 81% of CM participants earned prizes (median = $120.56). Differences in earnings with TCM and ECM during week-1 or across the four weeks were nonsignificant (Table 1).
4. DISCUSSION
Our findings demonstrate that prize CM is an efficacious smoking abstinence intervention, consistent with research on money/voucher CM for smoking (Ledgerwood 2008), prize CM for illicit substances (Petry et al., 2000; Petry et al., 2012), and Alessi et al’s (2008) pilot study on prize CM for smoking. We found no significant differences in outcomes between TCM and ECM. We thought that providing a schedule of increased chances of winning prizes early in treatment would result in more abstinence initiation and subsequent abstinence based on findings that higher magnitude of reinforcement results in better outcomes (Petry et al., 2004). However, our data are more consistent with studies revealing that start-up bonuses may not enhance CM effectiveness (Silverman et al., 1998). Indeed, TCM LDA and percent negative were somewhat (but nonsignificantly) higher than ECM, but CO levels during the week-1 enhanced period were comparable between conditions. Furthermore, although the TCM and ECM were scheduled to have different initial magnitudes, Table 1 demonstrates that in reality the early magnitude of reinforcement actually received did not differ between groups.
Although CM facilitated greater smoking reductions during treatment, differences were not apparent at follow-up. CM studies for smoking have primarily revealed no longer-term benefits of CM (Ledgerwood, 2008). However, it is notable that high relapse rates are also found for other efficacious smoking cessation interventions (Piasecki et al., 2002).
The present study has limitations. The sample size was relatively small. More importantly, the treatment required an intense in-person visit schedule that would be difficult to sustain in many populations (e.g., rural populations, low socioeconomic status).
In summary, prize CM is efficacious in promoting initial smoking abstinence among nicotine-dependent smokers. Research on methods to improve response rates and maintain abstinence is needed.
Supplementary Material
Acknowledgments
We thank Ken Bates, Lisa Sulkowski, Melissa Williams, Joi Moore, Caren Steinmiller, Debra Kish, Katie Linehan, Bojana Knezevic, Ashley Wiedemann, Veeral Patel and the staff of the Psychiatric & Addiction Research Center for their assistance on this project.
Footnotes
Clinical Trial Identifier: NCT00865254
Contributors: Dr. Ledgerwood was the study principal investigator and contributed to all aspects of study design, data collection and analysis, and manuscript preparation. Dr. Arfken assisted Dr. Ledgerwood in conducting data analysis and manuscript writing. Drs. Petry and Alessi contributed to developing the study design, assisted with data analysis and assisted in preparing this manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest: None.
Role of Funding Source: This study was funded by NIH grant R21 DA021839 and by Joe Young Sr. funding through the State of Michigan. Neither funder had any further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
References
- Alessi SM, Petry NM, Urso J. Contingency management promotes smoking reductions in residential substance abuse patients. J Appl Behav Anal. 2008;41:617–622. doi: 10.1901/jaba.2008.41-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Smoking-attributable mortality, years of potential life lost, and productivity losses – United States, 2000–2004. Morbidity and Mortality Weekly Report. 2008;57(45):1226–1228. [PubMed] [Google Scholar]
- Corby EA, Roll JM, Ledgerwood DM, Schuster CR. Contingency management interventions for treating the substance abuse of adolescents: A feasibility study. Exp Clin Psychopharmacol. 2000;8:371–376. doi: 10.1037//1064-1297.8.3.371. [DOI] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psycosocial interventions for substance use disorders. Am J Psychiatry. 2008;165:179–187. doi: 10.1176/appi.ajp.2007.06111851. [DOI] [PubMed] [Google Scholar]
- Fagerstrom K-O. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. The Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- Gerstein D, Murphy S, Toce M, Hoffmann J, Palmer A, Johnson R, et al. Gambling impact and behavior study. Chicago: University of Chicago; 1999. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Dumeer AM, Thomas CS, Solomon LJ, Bernstein IM. Smoking status in the initial weeks of quitting as a predictor of smoking-cessation outcomes in pregnant women. Drug Alcohol Depend. 2006;85:138–41. doi: 10.1016/j.drugalcdep.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Heil SH, Solomon LJ, Bernstein IM, Lussieur JP, Abel RL, Lynch E, Badger GJ. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine Tob Res. 2004;6:1015–1020. doi: 10.1080/14622200412331324910. [DOI] [PubMed] [Google Scholar]
- Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation. Who will quit with and without the nicotine patch. JAMA. 1994;271:589–94. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Morral AR, Kirby KC, Iguchi MY, Galbicka G. Shaping smoking cessation using percentile schedules. Drug Alcohol Depend. 2004;76:247–259. doi: 10.1016/j.drugalcdep.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Ledgerwood DM. Contingency management for smoking cessation: Where do we go from here? Curr. Drug Abuse Rev. 2008;1:340–349. doi: 10.2174/1874473710801030340. [DOI] [PubMed] [Google Scholar]
- Peirce JM, Petry NM, Stitzer ML, Blaine J, Kellogg S, Satterfield F, Schwartz M, Krasnansky J, Pencer E, Silva-Vazquez L, Kirby KC, Royer-Malvestuto C, Roll JM, Cohen A, Copersino M, Kolodner K, Li R. Lower-cost incentives increase stimulant abstinence in methadone maintenance treatment: A National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiatry. 2006;63:201–208. doi: 10.1001/archpsyc.63.2.201. [DOI] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Ledgerwood DM. A randomized trial of contingency management delivered by community therapists. J Consult Clin Psychol. 2012;80:286–298. doi: 10.1037/a0026826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Martin B. Low-cost contingency management for treating cocaine- and opioid-abusing methadone patients. J Consult Clin Psychol. 2002;70:398–405. doi: 10.1037//0022-006x.70.2.398. [DOI] [PubMed] [Google Scholar]
- Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes and they will come: Contingency management for treatment of alcohol dependence. J Consult Clin Psychol. 2000;68:250–257. doi: 10.1037//0022-006x.68.2.250. [DOI] [PubMed] [Google Scholar]
- Petry NM, Tedford J, Austin M, Nich C, Carroll KM, Rounsaville BJ. Prize reinforcement contingency management for treating cocaine abusers: How low can we go, and with whom? Addiction. 2004;99:349–360. doi: 10.1111/j.1360-0443.2003.00642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, Fiore MC, McCarthy DE, Baker TB. Have we lost our way? The need for dynamic formulations of smoking relapse proneness. Addiction. 2002;97:1093–1108. doi: 10.1046/j.1360-0443.2002.00216.x. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll JM. Contingency management for treatment of substance use disorders: A meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Silverman K, Wong CJ, Umbricht-Schneiter A, Montoya ID, Schuster CR, Preston KL. Broad beneficial effects of cocaine abstinence reinforcement among methadone patients. J Consult Clin Psychol. 1998;66:811–824. doi: 10.1037//0022-006x.66.5.811. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.