Abstract
Background
Treatment of testicular germ cell cancer constitutes a major success story in modern oncology. Today, the vast majority of patients are cured by a therapeutic strategy using one or more highly effective components including surgery (orchiectomy), radiotherapy and/or chemotherapy. However, the excellent cancer specific survival comes at considerable costs, as individuals with a history of germ cell cancer experience serious long-term complications, including markedly increased risk of cardiovascular morbidities and premature cardiovascular death. The factors responsible, as well as their mode of action, are not fully understood and there is a lack of knowledge concerning optimal evidence-based long-term follow-up strategies.
Results
Here, we present the growing body of evidence suggesting that germ cell cancer patients as a consequence of the different treatment components, are subjected to toxicities, which individually, and synergistically, can cause physiological impairments leading to sub-clinical or clinical cardiovascular disorders the ‘multiple-hit hypothesis’). Furthermore, we discuss the efficacy and utility of structured exercise training to ameliorate treatment-induced cardiovascular dysfunction to prevent premature onset of clinical cardiovascular disease in germ cell cancer survivors, with a view towards highlighting future directions of exercise-based survivorship research in the germ cell cancer setting.
Conclusion
Since exercise training may have the potential to ameliorate and/or reverse long-term cardiovascular disease sequelae in germ cell cancer survivors, a strong rationale exists for the promotion of exercise-oncology research in this setting, in order to provide exercise-recommendations for optimal germ cell cancer survivorship.
Keywords: germ cell cancer treatment, exercise, training, cardiovascular disease, rehabilitation, survivorship
INTRODUCTION
The treatment of testicular germ cell cancer (GCC) has been a major medical success story, since the introduction of platinum-based chemotherapy in the mid-1970’s [1]. Over the proceeding four decades, GCC prognosis has continuously improved with 5-year relative survival rate today surpassing 95% [2]. Thus, GCC largely constitutes the ‘best case scenario’ of modern oncology practice, as characterized by early detection and highly effective antineoplastic therapies. However curative GCC-treatment is a double-edged sword, as GCC survivors experience a wide range of short- and long-term toxicities, including markedly increased risk of cardio-vascular morbidities and mortality in comparison to age-matched individuals without a history of GCC [3;4]. Effective strategies to prevent or mitigate late occurring cardiovascular complications are highly warranted in order to improve GCC survivorship [5].
Structured exercise training, in the form of aerobic exercise (high volume-low force muscle contractions) and/or resistance exercise (low volume-high force muscle contractions), is a well-established strategy in the prevention and treatment of cardiovascular diseases [6]. Both exercise modalities cause acute stress onto the cardiovascular-, musculoskeletal- and central nervous system, which induce positive physiological adaptations protecting against cardiovascular dysfunction [7]. In the oncology setting, exercise has been shown to be safe, feasible, and efficacious in improving physical function and psycho-social endpoints during and after primary therapy [8]. Moreover, recent studies have shown that exercise training can modulate certain cardiovascular risk factors, including improved aerobic capacity (VO2peak) [9], reduced fat percentage [10], and changes in systemic biomarkers (e.g. C-Reactive Protein, insulin) in patients with cancer [11].
In the setting of GCC, there is a paucity of studies investigating the efficacy and utility of exercise training, and evidence-based GCC-specific guidelines are not available. Yet, the rationale for prescribing exercise in this setting seems evident, as patients experience a plethora of late-effects including elevated risk and/or premature onset of cardiovascular disorders, which may be mitigated by exercise training. Here, we describe GCC management and the putative mechanisms related to therapy-induced cardiovascular dysfunction, which individually, and synergistically, can cause increased risk of clinical cardiovascular diseases. Secondly, we discuss the potential of exercise training as a strategy to ameliorate treatment-induced cardiovascular complications, with a view towards research gaps and future directions.
GERM CELL CANCER MANAGEMENT
GCC incidence has doubled worldwide over the last 40 years, but remains a relatively rare disease comprising approximately 1% of all solid tumors in men. Nevertheless, it is the most common cancer in men aged 15-45 years, and given the relatively young age at diagnosis, the disease potentially accounts for a very high number of productive years of life lost [12].
GCC management has remained consistent over the last 20 year with the standard treatment consisting of surgery (orchiectomy) potentially followed by radiotherapy and/or combination chemotherapy [5]. GCC treatment is curative in the vast majority of cases, but is also associated with considerable toxicities, and risk of late-occurring disorders. Long-term complications from GCC treatment can range from premature onset of one or more cardiovascular disease risk factors, i.e. hypertension, central obesity, dyslipidemia, insulin resistance, characterizing the Metabolic Syndrome (MetS) [13], to increased incidence of clinical cardiovascular events (e.g. myocardial infarction, angina pectoris, coronary artery disease) [3]. In the following section, we present the therapeutic components of GCC management including orchiectomy, radiotherapy, chemotherapy, and antiemetic treatment, with regard to the evidence describing associations with late-occurring cardiovascular disorders and their potential candidate mechanisms.
Orchiectomy
Nearly all patients with histologically confirmed GCC are orchiectomized at the point of diagnosis, but the long-term implications of this surgery remain ambiguous. For example, Nuver et al. found the prevalence of MetS was approximately 4-fold increased among orchiectomized GCC patients compared with healthy controls [14], while a recent study found no difference in MetS prevalence between these GCC survivors and their healthy counterparts [15]. The diversity of these findings may be due to differences in diagnostic definitions of MetS and potential suboptimal dataset with national/regional differences in clinical follow-up proceedings. Recent findings, however, indicate that orchiectomy can cause sub-clinical hypogonadism in the form of disturbances of the pituitary-leydig cell axis. One study found that 57% of the patients had elevated LH/testosterone ratio one year after orchiectomy [16]. Thus, while increased prevalence of clinical hypogonadism (total plasma testosterone below 9.5 nmol/L) may not be detected, unfavorable changes in gonadal function can be present.
Mechanistically, there is a well-established link between male gonadal function and the risk of metabolic and cardiovascular dysfunction. Androgen deficiency can cause endothelial dysfunction, decreased activity of smooth muscle cells in the vessel wall, thickening of the intima and media of the vessels and increased synthesis of pro-inflammatory cytokines [17]. It is not established whether presence of sub-clinical hypogonadism can have similar negative impact of vascular function. Importantly, in young men without evidence of metabolic or cardiovascular diseases, systemic testosterone levels, across the full physiological concentration spectrum, was inversely associated with concentrations of pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin (IL)-6 [18]. Thus, gonadal dysfunction, even if it is not clinically detectable, potentially predisposes to onset of chronic low-grade inflammation, which is strongly associated with cardiovascular disease risk [17]. In a recent study, GCC patients with disseminated disease displayed increased systemic TNF-α (1.3-fold) and IL-6 (2-fold) concentrations in comparison with age-matched healthy controls [19]. This elevation of pro-inflammatory cytokines was evident both before chemotherapy was initiated and persisting for the full evaluation period 3 months after chemotherapy was terminated. Based on this persistent low-grade inflammation across the treatment trajectory, it was hypothesized that orchiectomy was the primary cause of these systemic changes.
The current trend in GCC management is that stage 1 patients are orchiectomized and subsequently enrolled in active surveillance programs as opposed to receiving adjuvant therapies [5]. Recent findings indicate that orchiectomy-induced gonadal dysfunction may facilitate increases in systemic inflammatory markers, which subsequently might be associated with metabolic and cardiovascular dysfunction, but this remains to be explored in detail.
Radiotherapy
Radiotherapy has traditionally been widely used in the treatment of GCC, and remains a useful therapeutic option in certain sub-groups (e.g. stage IIa-b seminoma with <3cm retroperitoneal lymph-node metastasis) [5], but the evidence describing long-term complications following this therapy is inconclusive. For example, a British study of 992 GCC patients (median age 35, range 19-82 years) reported that infradiaphragmatic irradiation was associated with a 2.4-fold increased risk of cardiovascular disease [20], and an American study of 477 GCC stage I or II patients, showed that treatment with radiotherapy was associated with a 1.8-fold increased risk of cardiac death compared with patients in active surveillance [21]. A recent report, however, does not support this association; a comprehensive analysis using a Danish national database of 4697 GCC patients diagnosed and treated from 1984 to 2007 (median follow up of 15.3 years [IQR 9.8-21.2 years]) showed no radiotherapy-associated increases in cardiovascular disease-risk [22]. There was, however, a 1.7-fold increase in the incidence of diabetes which subsequently can predispose to cardiovascular complications [22].
The candidate mechanisms involved in long-term complications following radiotherapy for GCC are not clear. Ionizing radiation can cause a rise in reactive oxygen species (ROS) triggering lipid oxidation, damage of the endothelium and activation of nuclear factor (NF)-κB, a transcriptional factor involved in the local inflammatory responses. Accordingly, GCC survivors treated with radiotherapy have been found to have elevated systemic levels of inflammatory markers up to 20 years after treatment, compared to patients treated with surgery alone and/or chemotherapy [23]. Also, exposure of the pancreas during irradiation of retroperitoneal lymph-node metastasis may lead to deficiency of pancreatic endocrine cells, most importantly β-cell apoptosis and/or dysfunction, which can contribute to the onset of both autoimmune type 1 and type 2 diabetes.
According to a recent ‘European Consensus Germ Cell Cancer Conference’ statement, a majority of clinicians prefer the use of chemotherapy to radiotherapy in most adjuvant settings, and the use of radiotherapy in GCC may therefore decrease in the coming years [5]. Due to this shift in therapeutic strategies, and the fact that recent evidence does not indicate that radiotherapy causes cardiovascular dysfunction [22], it is currently uncertain whether GCC patients following this treatment should be considered candidates for specific follow-up programs. However, pancreatic co-irradiation and/or radiation-derived elevation of inflammatory cytokines may comprise cardiovascular risk factors, but the clinical implications of these late-effects remains to be further explored.
Chemotherapy
Chemotherapy consisting of bleomycin-etoposide-cisplatin (BEP) has been standard treatment for more than 30 years in patients with disseminated disease. BEP-treatment is curative in the majority of cases, but also associated with long-term adverse effects include considerable elevated risk of cardiovascular problems. A Dutch study found that 16.5% of all patients treated for GCC developed cardiovascular disease within 25 years of treatment cessation [24], with BEP being independently associated with a 1.5-fold increase in cardiovascular disease risk. In accordance, the study of the Danish cohort of 4697 GCC patients showed a 1.5-fold increase in cardiovascular disease incidence, and a 1.8-fold increased cardiovascular mortality risk [22].
Despite the wealth of evidence outlining the long-term cardiovascular complications associated with BEP therapy, there is little available data describing the factors responsible and their mode of action. Nuver et al. found acute increase in intima-media thickness of the common carotid artery and increased plasma concentration of von Willebrand factor in GCC patients immediately after completion of BEP [25]. Also, evidence of microalbuminuria, a proposed marker of systemic vascular injury, was found in up to 22% of long-term GCC survivors after cisplatin treatment [26]. In support of this, 3 cycles of BEP was associated with 5-10%-reduction in capillary-per-myofiber ratio in muscle biopsies taken from m. vastus lateralis [27], indicating that BEP may cause vascular damage in both central and peripheral blood vessels. In addition to the impact on vascular function, 3 cycles of BEP was associated with negative effects on muscular function, as characterized by ~7% reduction in muscle fiber cross sectional area, ~6% increased proportion of glycolytic type IIx fibers, ~2.5 kg reduction of lean mass and ~10% lower isometric muscle strength [27]. The mechanisms responsible for these muscular alterations are not clear, but studies in mice have found that cisplatin cause induction of muscle atrophy-related genes, proteosomal proteolysis and inflammation [28]. Also, cisplatin is associated with strong neurotoxic actions which can induce muscle atrophy signals and a switch towards more glycolytic muscle fiber phenotypes.
With increasing GCC incidence rates and current trend towards the use of chemotherapy rather than radiotherapy in adjuvant settings, a more comprehensive knowledge of BEP-induced toxicities in the cardiovascular- and muscular system, as well as their long-term clinical implications is highly warranted.
Antiemetic Treatment
GCC patients undergoing BEP-therapy receive high doses glucocorticoids (e.g. prednisolone) as antiemetic treatment to prevent nausea and vomiting. To our knowledge, there is no available data describing to what extent, prednisolone-treatment may impact post-treatment cardiovascular disease risk in GCC survivors. Supra-physiological concentrations of glucocorticoids display strong immune-suppressive and anti-inflammatory actions, and the use of these agents has traditionally been hampered by considerable metabolic side-effects [29]. Studies have shown that 8 days of prednisolone use in healthy young men causes significant insulin resistance, in parallel with increased myofibrillar protein breakdown, blunted myofibrillar protein synthesis and inhibition of insulin-signaling [30]. Long-term side-effects of chronic glucocorticoid steroid administration include central adiposity, hepatic steatosis, dyslipidemia, muscular breakdown, insulin resistance and glucose intolerance [29].
The use of antiemetic treatment has greatly reduced the level of side-effects and improved BEP-therapy tolerance in GCC patients thus prednisolone-use is unlikely to decrease in the years to come. The impact of antiemetic drugs on post-treatment metabolic profile has not been explored in GCC patients, and whether this may predispose long-term cardiovascular dysfunction in GCC survivors is unknown.
The “Multiple-Hit” Hypothesis
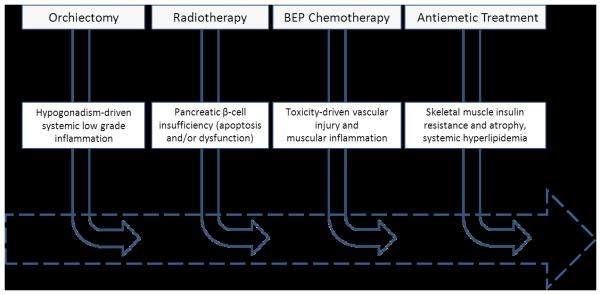
Through the course of GCC management, patients are subjected to several toxicities, which individually, and synergistically, can cause physiological impairments leading to clinical or sub-clinical cardiovascular diseases (figure 1). This phenomenon has been labeled the ‘multiple-hit hypothesis’ [31]. Attention, particularly in breast cancer, towards describing the interacting mechanisms of different anticancer therapies on cardiovascular injury as well as exploring effective countermeasures is emerging [32]. Here, we propose that the ‘multiple-hit hypothesis’ is applicable to GCC, although there is currently limited data supporting (or refuting) this notion. Based on the evidence reviewed here, we propose that orchiectomy-derived sub-clinical hypogonadism, and BEP-induced vascular injury and/or disturbance of metabolic homeostasis constitute primary candidates for the causes of late-occurring cardiovascular disease in GCC survivors. Importantly, the physiological impairments involved in this sequelae are, under normal circumstances, modifiable by structured exercise training, constituting a strong rationale for exploring the utility of tailored exercise-interventions to ameliorate cardiovascular dysfunction and potentially prevent onset of cardiovascular diseases in GCC survivorship.
Figure 1. The ‘Multiple-Hit Hypothesis”.
The ’multiple-hit hypothesis’ applied to the germ cell cancer setting – adapted from Jones et al. 2007, J Am Coll Cardiol [31]. As patients move along the trajectory of germ cell cancer treatment they will be subjected to one or several of the treatment components associated with various toxicities. Individually, and synergistically, these toxicities can significantly impair cardiovascular function, which leaves the patient more susceptible to cardiovascular injuries, and ultimately higher risk of premature cardiovascular morbidities and mortality.
EXERCISE TRAINING IN GERM CELL CANCER SURVIVORSHIP
Exercise training is currently emerging as a promising intervention in the oncology setting, and a growing body of evidence has outlined the beneficial potential of exercise to improve a broad range of physical/physiological (e.g. cardiorespiratory capacity, muscle strength, lean mass) and psycho-social (e.g. physical functioning, emotional wellbeing, and fatigue) endpoints [8]. The current body of evidence in exercise-oncology research however is based on trials carried out in relatively few diagnoses (predominantly breast and prostate cancer [8]), and studies in patients with a history of GCC are virtually non-existing. In the following section, we describe how structured exercise training may constitute an effective strategy in the prevention of cardiovascular disorders of particular relevance in GCC survivorship. We focus on two major adaptive effects in response to acute and long-term exercise training: regulation of metabolic and endocrine homeostasis; and improvements of cardiovascular- and muscular function.
Regulation of Metabolic and Endocrine Homeostasis
Regulation of whole-body homeostasis is highly complex and involves interaction between numerous organs, including the central nervous system, intestine, pancreas, liver, muscle and adipose tissues. Through different pathways, exercise can strongly influence systemic homeostasis, specifically via large increases in substrate uptake from the blood stream to the working muscles. A unique metabolic feature of exercise is insulin-independent contraction-induced muscle glucose uptake, driven by elevation in intracellular Ca++ concentration and low intracellular energy-status [33]. After cessation of exercise, basal metabolic rate remains elevated for up to 24hrs in order to recover myocellular homeostasis and to drive post-exercise cellular adaptations. Notably, an increase in fatty acid oxidation occurs during the recovery period to accommodate a high metabolic priority for muscle glycogen resynthesis, which is believed to play a key role in exercise-mediated effects on whole-body lipid metabolism [34]. Another immediate systemic regulatory effect of exercise constitutes skeletal muscle’s capacity to synthesize and secrete humoral factors, known as myokines (e.g. IL-6, IL-15). These factors have been shown to have multiple endocrine and autocrine regulatory functions including modification of systemic cytokine production and regulation of glucose metabolism [35].
Long-term effects of chronic exercise training include improved muscular insulin sensitivity, increased muscle mass, in parallel with reduction in fat mass. Collectively, these adaptations can improve regulation of whole-body homeostasis by increasing the basal metabolic rate, and lowering plasma concentrations of glucose, insulin, lipids and inflammatory cytokines [36]. The reduction in adiposity and exercise-induced secretion of anti-inflammatory myokines can also counteract chronic low-grade inflammation associated with metabolic diseases [35].
Improvement of Cardiovascular and Muscular Function
The capacity of exercise training to improve cardiovascular and skeletal muscle function is well-established and considered to be an essential part of exercise-protection against chronic diseases. A universal feature of exercise is the rapid increase in muscular oxygen-demand, which causes immediate stress on the cardiovascular system to increase blood supply to the working muscles. As a result of increased heart rate, stroke volume and vascular dilation, a dramatic (>30-fold) increase in muscular blood flow and oxygen consumption cause the muscles to rapidly increase energy production and substrate utilization. In response to the specific exercise modality, i.e. resistance or aerobic, these homeostatic perturbations induce down-stream signaling cascades promoting specific muscle gene-expression and muscle protein turnover [34].
Chronic exercise has great modulating effects on the structure and function of the cardiovascular system and skeletal musculature [7;34]. Cardiac function is improved by several changes including increased left ventricular relaxation and systolic function, and cardiac hypertrophy causing increased stroke volume, which in turn leads to lower resting – and submaximal work-load – heart rate [7]. Vascular effects of exercise include improvements in endothelium-mediated vasodilatation in larger resistance arteries, myogenic control, and metabolic vasodilatation in small resistance arteries. These effects facilitate reduction in aortic and arterial stiffness and improved compliance, improved endothelial function and capillary vessel formation [7]. Muscular adaptions to exercise training involve improved contractile strength and/or fatigue resistance, driven by mitochondrial biogenesis, oxidative and glycolytic enzyme activity, a switch towards an oxidative fiber phenotype-composition (more type I / IIa fibers, less type IIx fibers), and myofibrillar hypertrophy [34].
Efficacy of Exercise Training in Germ Cell Cancer Patients
Evidence describing the effects of exercise in patients with GCC during and after treatment is limited, and until recently, the only available data came from trials across multiple diagnoses [37;38], table 1. For example, in 278 cancer patients amongst whom 20 were diagnosed with GCC, Adamsen et al. reported that 6-weeks of high intensity combined fitness- and strength training during chemotherapy induced improvements of ~30% in 1-RM muscle strength, and ~10% in fitness compared with a waiting-list control group [37]. In a randomized controlled feasibility study of 30 GCC patients, structured, hospital-based resistance training was found to be safe and feasible during BEP. Moreover, resistance training significantly improved 1-RM muscle strength by ~30% and rescued a proportion of the muscular breakdown observed in a usual care control group, as characterized by clinically relevant differences in muscle fiber area (+600 um2) and lean body mass (+1.4 kg) [27]. This indicates that muscle function is modifiable by resistance training despite concurrent toxic impact of BEP. In contrast, no impact of resistance training was found on concentration levels of plasma total- and LDL-cholesterol and pro-inflammatory cytokines (IL-6, TNF-α) [19]. These toxic effects of BEP-chemotherapy were not reversible by resistance training, and improvements in muscle function did not translate into improved metabolic or inflammatory profile.
Table 1.
Available Randomized Controlled Exercise Trials with GCC Patients Included.
| N (% GCC of all patients) | Exercise prescription | Cardiovascular effects | Muscular effects | Metabolic effects | Endocrine effects | |
|---|---|---|---|---|---|---|
|
During chemotherapy
Adamsen et al. 2009 reference [37] |
16 (6%) |
Combined RT and AT, 3 sessions per week for 6 weeks |
Between-group estimates: +0.16 ml O2/min (p<0.001) In-group improvements: +10% fitness (estimated VO2max) in exercise group (p<0.001) |
Between-group estimates: +30 kg in 1-RM leg press muscle strength (p<0.001) In-group improvements: 30% improvement in 1-RM leg press muscle strength |
NA |
NA |
| Christensen et al. 2014a reference [27] |
30 (100%) | RT, 3 sessions per week for 9 weeks |
NA | Between group estimates: +600μm2 in myofiber cross sectional area (p=0.14) + 1.4 kg in lean mass (p=0.07) In-group improvements: +43 kg in 1-RM leg press (p<0.001) |
Between-group estimates: No differences in fat percentage, P-Total cholesterol, P-HDL cholesterol, P-LDL cholesterol, P-triglyceride P-glucose, P-insulin |
NA |
| Christensen et al. 2014b reference [19] |
30 (100%) | RT, 3 sessions per week for 9 weeks |
NA | NA | NA | Between-group estimates: No differences in systemic concentrations of TNF-α, IL- 6, IL-8, IL-10, IFN-γ |
|
After chemotherapy
Thorsen et al. 2005 reference [38] |
20 (18%) |
Mixed exercise, 2 sessions per week for 14 weeks |
In-group improvements: +23% in fitness (estimated VO2max) in the exercise group |
NA |
NA |
NA |
NOTE: Christensen 2014a and Christensen 2014b are reports from the same trial. Thorsen et al. 2005 and Adamsen et al. 2009 included multiple diagnoses, but did not report effect sizes stratified by diagnosis; therefore between-group estimates and in-group improvements are reported for the entire study group.
Abbreviations: AT, aerobic training; BEP, bleomycin-etoposide-cisplatin, GCC, germ cell cancer; IFN, interferon; IL, interleukin; NA; not assessed; P-, plasma; RM, repetition maximum; RT, resistance training; TNF, tumor necrosis factor.
In summary, clinical evidence and molecular research strongly support exercise training as an effective countermeasure of cardiovascular diseases; however, it remains unknown whether exercise is associated with such protective effects in cancer survivors, and including individuals with a history of GCC. Given the anti-neoplastic efficacy of modern GCC management, there is a growing need for optimal evidence-based follow-up strategies, and in particular whether structured exercise training should be part of such strategies.
FUTURE DIRECTIONS
For more than 20 years, GCC has been considered a model for a curable neoplasm [39], and with the steadily increasing number of long-term survivors, GCC may now be considered a model for cancer survivorship. Increased risk of clinical or sub-clinical cardiovascular diseases is among the most important challenges facing GCC patients after cessation of treatment, and there is a need for better understanding of the factors responsible and their mode of action. Longitudinal prospective investigations with repeated evaluations of known risk factors and potential candidates of prognostic biomarkers, preferably with measurements before and after the individual therapeutic components, are urgently warranted.
Exercise-oncology research has come a long way over the last 25 years, highlighting the potential of structured exercise to protect against and/or reverse anticancer-therapy induced physiological deterioration. Yet, despite initial promising findings, it remains unresolved whether exercise can effectively protect against onset of cardiovascular disorders in post-treatment survivors, which may be of special interest in the GCC patients due to their young age at diagnosis and high efficacy of treatment. Specifically, elucidating the long-term implications and underlining mechanisms of tailored exercise-training in patients displaying subclinical hypogonadism, and/or undergoing BEP-therapy should be prioritized, as these patients present with a significant cardiovascular risk-profile. Thus evidence describing which exercise-prescriptions (modality, intensity, volume and progression) may be most appropriate after orchiectomy, as well as during and after BEP-therapy, to reverse acute physiological side-effects, is required. In relation to long-term survivorship, there are no studies describing the effect of structured exercise on late-occurring disorders, and such interventions will likely need to be of longer duration (+6 months) and designed with appropriate progression in the exercise-prescription. There is likely a need for multicenter setups given the relative low incidence of GCC, and also there is likely a need for home- and/or community-based interventions for optimal compliance in GCC survivors, as these patients may return to work and normal daily life as soon as possible after cessation of treatment.
CONCLUSION
GCC is highly curable in modern oncology practice, thus the steadily increasing incidence means that an increasing number of individuals will undergo treatment in the future, and in turn be subjected to toxicities leading to increased risk of cardiovascular disease. Many of these complications involve negative changes in biological mechanisms, which, under normal circumstances, can be positively moderated by structured exercise. Therefore, a strong rationale exists for the promotion of exercise-oncology research in the GCC-setting, in order to provide exercise-recommendations based on high-quality evidence, and ultimately improve GCC survivorship.
Acknowledgments
JFC is supported by Research Grants from TRYGFONDEN, the Novo Nordic Foundation, The Danish Cancer Society and the Beckett Foundation. We thank Dr. Gedske Daugaard, and Dr. Mikael Rørth for their excellent assistance in the preparation of this manuscript.
Abbreviations
- BEP
bleomycin-etoposide-cisplatin.
Footnotes
Conflicts of Interest: No conflicts of interest to declare.
References
- 1.Einhorn LH, Donohue J. Cis-diamminedichloroplatinum, vinblastine, and bleomycin combination chemotherapy in disseminated testicular cancer. Ann Intern Med. 1977 Sep;87(3):293–8. doi: 10.7326/0003-4819-87-3-293. [DOI] [PubMed] [Google Scholar]
- 2.Verdecchia A, Francisci S, Brenner H, Gatta G, Micheli A, Mangone L, et al. Recent cancer survival in Europe: a 2000-02 period analysis of EUROCARE-4 data. Lancet Oncol. 2007 Sep;8(9):784–96. doi: 10.1016/S1470-2045(07)70246-2. [DOI] [PubMed] [Google Scholar]
- 3.Haugnes HS, Wethal T, Aass N, Dahl O, Klepp O, Langberg CW, et al. Cardiovascular risk factors and morbidity in long-term survivors of testicular cancer: a 20-year follow-up study. J Clin Oncol. 2010 Oct 20;28(30):4649–57. doi: 10.1200/JCO.2010.29.9362. [DOI] [PubMed] [Google Scholar]
- 4.Fossa SD, Gilbert E, Dores GM, Chen J, McGlynn KA, Schonfeld S, et al. Noncancer causes of death in survivors of testicular cancer. J Natl Cancer Inst. 2007 Apr 4;99(7):533–44. doi: 10.1093/jnci/djk111. [DOI] [PubMed] [Google Scholar]
- 5.Beyer J, Albers P, Altena R, Aparicio J, Bokemeyer C, Busch J, et al. Maintaining success, reducing treatment burden, focusing on survivorship: highlights from the third European consensus conference on diagnosis and treatment of germ-cell cancer. Ann Oncol. 2013 Apr;24(4):878–88. doi: 10.1093/annonc/mds579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003 Jun 24;107(24):3109–16. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 7.Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training: molecular mechanisms. Circulation. 2010 Sep 21;122(12):1221–38. doi: 10.1161/CIRCULATIONAHA.110.939959. [DOI] [PubMed] [Google Scholar]
- 8.Buffart LM, Galvao DA, Brug J, Chinapaw MJ, Newton RU. Evidence-based physical activity guidelines for cancer survivors: current guidelines, knowledge gaps and future research directions. Cancer Treat Rev. 2014 Mar;40(2):327–40. doi: 10.1016/j.ctrv.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Jones LW, Liang Y, Pituskin EN, Battaglini CL, Scott JM, Hornsby WE, et al. Effect of exercise training on peak oxygen consumption in patients with cancer: a meta-analysis. Oncologist. 2011;16(1):112–20. doi: 10.1634/theoncologist.2010-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irwin ML, Alvarez-Reeves M, Cadmus L, Mierzejewski E, Mayne ST, Yu H, et al. Exercise improves body fat, lean mass, and bone mass in breast cancer survivors. Obesity (Silver Spring) 2009 Aug;17(8):1534–41. doi: 10.1038/oby.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pekmezi DW, Demark-Wahnefried W. Updated evidence in support of diet and exercise interventions in cancer survivors. Acta Oncol. 2011 Feb;50(2):167–78. doi: 10.3109/0284186X.2010.529822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huyghe E, Plante P, Thonneau PF. Testicular cancer variations in time and space in Europe. Eur Urol. 2007 Mar;51(3):621–8. doi: 10.1016/j.eururo.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Haugnes HS, Aass N, Fossa SD, Dahl O, Klepp O, Wist EA, et al. Components of the metabolic syndrome in long-term survivors of testicular cancer. Ann Oncol. 2007 Feb;18(2):241–8. doi: 10.1093/annonc/mdl372. [DOI] [PubMed] [Google Scholar]
- 14.Nuver J, Smit AJ, Wolffenbuttel BH, Sluiter WJ, Hoekstra HJ, Sleijfer DT, et al. The metabolic syndrome and disturbances in hormone levels in long-term survivors of disseminated testicular cancer. J Clin Oncol. 2005 Jun 1;23(16):3718–25. doi: 10.1200/JCO.2005.02.176. [DOI] [PubMed] [Google Scholar]
- 15.Willemse PM, Burggraaf J, Hamdy NA, Weijl NI, Vossen CY, van WL, et al. Prevalence of the metabolic syndrome and cardiovascular disease risk in chemotherapy-treated testicular germ cell tumour survivors. Br J Cancer. 2013 Jul 9;109(1):60–7. doi: 10.1038/bjc.2013.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bandak M, Aksglaede L, Juul A, Rorth M, Daugaard G. The pituitary-Leydig cell axis before and after orchiectomy in patients with stage I testicular cancer. Eur J Cancer. 2011 Nov;47(17):2585–91. doi: 10.1016/j.ejca.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 17.Traish AM, Saad F, Feeley RJ, Guay A. The dark side of testosterone deficiency: III. Cardiovascular disease. J Androl. 2009 Sep;30(5):477–94. doi: 10.2164/jandrol.108.007245. [DOI] [PubMed] [Google Scholar]
- 18.Bobjer J, Katrinaki M, Tsatsanis C, Lundberg GY, Giwercman A. Negative association between testosterone concentration and inflammatory markers in young men: a nested cross-sectional study. PLoS One. 2013;8(4):e61466. doi: 10.1371/journal.pone.0061466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christensen JF, Tolver A, Andersen JL, Rorth M, Daugaard G, Hojman P. Resistance training does not protect against increases in plasma cytokine levels among germ cell cancer patients during and after chemotherapy. J Clin Endocrinol Metab. 2014 Aug;99(8):2967–76. doi: 10.1210/jc.2013-4495. [DOI] [PubMed] [Google Scholar]
- 20.Huddart RA, Norman A, Shahidi M, Horwich A, Coward D, Nicholls J, et al. Cardiovascular disease as a long-term complication of treatment for testicular cancer. J Clin Oncol. 2003 Apr 15;21(8):1513–23. doi: 10.1200/JCO.2003.04.173. [DOI] [PubMed] [Google Scholar]
- 21.Zagars GK, Ballo MT, Lee AK, Strom SS. Mortality after cure of testicular seminoma. J Clin Oncol. 2004 Feb 15;22(4):640–7. doi: 10.1200/JCO.2004.05.205. [DOI] [PubMed] [Google Scholar]
- 22.Lauritsen J. Risk of cardiovascular disease, diabetes and death in germ-cell cancer survivors in a large cohort. European Journal of Cancer. 2013 Sep;49(Supplement 2) [Google Scholar]
- 23.Wethal T, Kjekshus J, Roislien J, Ueland T, Andreassen AK, Wergeland R, et al. Treatment-related differences in cardiovascular risk factors in long-term survivors of testicular cancer. J Cancer Surviv. 2007 Mar;1(1):8–16. doi: 10.1007/s11764-007-0012-3. [DOI] [PubMed] [Google Scholar]
- 24.van den Belt-Dusebout AW, Nuver J, De WR, Gietema JA, ten Bokkel Huinink WW, Rodrigus PT, et al. Long-term risk of cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol. 2006 Jan 20;24(3):467–75. doi: 10.1200/JCO.2005.02.7193. [DOI] [PubMed] [Google Scholar]
- 25.Nuver J, Smit AJ, van der MJ, van den Berg MP, van der Graaf WT, Meinardi MT, et al. Acute chemotherapy-induced cardiovascular changes in patients with testicular cancer. J Clin Oncol. 2005 Dec 20;23(36):9130–7. doi: 10.1200/JCO.2005.01.4092. [DOI] [PubMed] [Google Scholar]
- 26.Meinardi MT, Gietema JA, van der Graaf WT, van Veldhuisen DJ, Runne MA, Sluiter WJ, et al. Cardiovascular morbidity in long-term survivors of metastatic testicular cancer. J Clin Oncol. 2000 Apr;18(8):1725–32. doi: 10.1200/JCO.2000.18.8.1725. [DOI] [PubMed] [Google Scholar]
- 27.Christensen JF, Jones LW, Tolver A, Jorgensen LW, Andersen JL, Adamsen L, et al. Safety and efficacy of resistance training in germ cell cancer patients undergoing chemotherapy: a randomized controlled trial. Br J Cancer. 2014 Jul 8;111(1):8–16. doi: 10.1038/bjc.2014.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hojman P, Fjelbye J, Zerahn B, Christensen JF, Dethlefsen C, Lonkvist CK, et al. Voluntary Exercise Prevents Cisplatin-Induced Muscle Wasting during Chemotherapy in Mice. PLoS One. 2014;9(9):e109030. doi: 10.1371/journal.pone.0109030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Raalte DH, Ouwens DM, Diamant M. Novel insights into glucocorticoid-mediated diabetogenic effects: towards expansion of therapeutic options? Eur J Clin Invest. 2009 Feb;39(2):81–93. doi: 10.1111/j.1365-2362.2008.02067.x. [DOI] [PubMed] [Google Scholar]
- 30.Short KR, Bigelow ML, Nair KS. Short-term prednisone use antagonizes insulin's anabolic effect on muscle protein and glucose metabolism in young healthy people. Am J Physiol Endocrinol Metab. 2009 Dec;297(6):E1260–E1268. doi: 10.1152/ajpendo.00345.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007 Oct 9;50(15):1435–41. doi: 10.1016/j.jacc.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 32.Koelwyn GJ, Khouri M, Mackey JR, Douglas PS, Jones LW. Running on empty: cardiovascular reserve capacity and late effects of therapy in cancer survivorship. J Clin Oncol. 2012 Dec 20;30(36):4458–61. doi: 10.1200/JCO.2012.44.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rose AJ, Richter EA. Skeletal muscle glucose uptake during exercise: how is it regulated? Physiology (Bethesda ) 2005 Aug;20:260–70. doi: 10.1152/physiol.00012.2005. [DOI] [PubMed] [Google Scholar]
- 34.Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013 Feb 5;17(2):162–84. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Pedersen BK. Exercise-induced myokines and their role in chronic diseases. Brain Behav Immun. 2011 Jul;25(5):811–6. doi: 10.1016/j.bbi.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 36.Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports. 2006 Feb;16(Suppl 1):3–63. doi: 10.1111/j.1600-0838.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 37.Adamsen L, Quist M, Andersen C, Moller T, Herrstedt J, Kronborg D, et al. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: randomised controlled trial. BMJ. 2009;339:b3410. doi: 10.1136/bmj.b3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thorsen L, Skovlund E, Stromme SB, Hornslien K, Dahl AA, Fossa SD. Effectiveness of physical activity on cardiorespiratory fitness and health-related quality of life in young and middle-aged cancer patients shortly after chemotherapy. J Clin Oncol. 2005 Apr 1;23(10):2378–88. doi: 10.1200/JCO.2005.04.106. [DOI] [PubMed] [Google Scholar]
- 39.Einhorn LH. Treatment of testicular cancer: a new and improved model. J Clin Oncol. 1990 Nov;8(11):1777–81. doi: 10.1200/JCO.1990.8.11.1777. [DOI] [PubMed] [Google Scholar]