Abstract
Background
Reliable quantification of mosquito host—seeking behaviours is required to determine the efficacy of vector control methods. For malaria, the gold standard approach remains the risky human landing catch (HLC). Here compare the performance of an improved prototype of the mosquito electrocuting grid trap (MET) as a safer alternative with HLC for measuring malaria vector behaviour in Dar es Salaam, Tanzania.
Methods
Mosquito trapping was conducted at three sites within Dar es Salaam representing a range of urbanicity over a 7-month period (December 2012–July 2013, 168 sampling nights). At each site, sampling was conducted in a block of four houses, with two houses being allocated to HLC and the other to MET on each night of study. Sampling was conducted both indoors and outdoors (from 19:00 to 06:00 each night) at all houses, with trapping method (HLC and MET) being exchanged between pairs of houses at each site using a crossover design.
Results
The MET caught significantly more Anopheles gambiae sensu lato than the HLC, both indoors (RR [95 % confidence interval (CI)]) = 1.47 [1.23–1.76], P < 0.0001 and outdoors = 1.38 [1.14–1.67], P < 0.0001). The sensitivity of MET compared with HLC did not detectably change over the course of night for either An. gambiae s.l. (OR [CI]) = 1.01 [0.94–1.02], P = 0.27) or Culex spp. (OR [CI]) = 0.99 [0.99–1.0], P = 0.17) indoors and declined only slightly outdoors: An. gambiae s.l. (OR [CI]) = 0.92 [0.86–0.99], P = 0.04), and Culex spp. (OR [CI]) = 0.99 [0.98–0.99], P = 0.03). MET-based estimates of the proportions of mosquitoes caught indoors (Pi) or during sleeping hours (Pfl), as well as the proportion of human exposure to bites that would otherwise occurs indoors (πi), were statistically indistinguishable from those based on HLC for An. gambiae s.l. (P = 0.43, 0.07 and 0.48, respectively) and Culex spp. (P = 0.76, 0.24 and 0.55, respectively).
Conclusions
This improved MET prototype is highly sensitive tool that accurately quantifies epidemiologically-relevant metrics of mosquito biting densities, behaviours and human exposure distribution.
Electronic supplementary material
The online version of this article (doi:10.1186/s12936-016-1513-1) contains supplementary material, which is available to authorized users.
Keywords: Malaria vector, Anopheles gambiae, Culex, Mosquito, Behaviour, Exposure-free, TRAPS, Africa
Background
Mosquito-biting behaviour plays an essential role in determining not only where and when vector-borne disease transmission occurs, but also in assessing the level of impact that can be reasonably expected of specific vector control interventions [1, 2]. Malaria vector species exhibit diverse feeding behaviours: some feed predominantly indoors and late at night while others bite mostly outdoors in the evening and early morning [3–12]. While behaviour characterization of Culex spp, especially the abundant populations of Culex quinquefasciatus that proliferate and transmit lymphatic filariasis in urban settings [13, 14], is rarely documented, this mosquito species may also exhibit diverse biting behaviour [15].
Measuring the timing and location of human exposure to mosquito bites is therefore essential for designing and selecting appropriate vector control strategies [2, 9, 16–19]. For example, the use of indoor-based control methods, such as long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS) are likely to have maximum effect against vectors that feed and rest indoors, such as Anopheles gambiae [17, 18, 20, 21], but will be less likely to reduce transmission by vectors such as Anopheles balabacensis that primarily feed outdoors in the evening hours before people go to bed [22]. Biting indoors, late at night when people are asleep is therefore the mosquito behaviour that is targeted by the use of LLINs [23], while IRS targets mosquitoes when they rest indoors [24]. Indeed this is why these interventions have drastically impacted malaria transmission across sub-Saharan Africa where the most important vectors exhibit both of these behaviours [25, 26]. These interventions have also contributed to the massive reduction of lymphatic filariasis [27, 28]. The wide-scale use of interventions that selectively target vectors with specific feeding behaviours (e.g., indoor, late night biting with LLINs) is thought to be responsible for shifts in species composition and distribution of biting behaviours. For example, shifts from endophagic (indoor biting) to exophagic (outdoor biting), late to evening biting and changes in species composition that have been observed in some African settings [29–33] and beyond [8, 34, 35]. Indeed the persecution pressure exerted by LLIN and IRS have also been hypothesized to drive selection within individual vector species for heritably altered behaviours [33], such as the changes observed within Anopheles funestus [4, 9], which are difficult to explain on the basis of phenotypic plasticity alone [36].
This potential for vector control methods to drive ecological and evolutionary changes in mosquito vector behaviour could undermine strategies that are currently very effective [18, 33, 35]. Thus, there is an urgent need to develop robust sampling tools that can monitor long-term trends in mosquito behaviour and how they respond to interventions.
Currently there are several sampling tools available for monitoring the host-seeking biting densities and associated infection rates of malaria vectors, which include the Centers for Disease Control and Prevention miniature light trap (CDC-LT) [37, 38], Ifakara Tent Trap [39–42] and Mbita trap [43, 44]. While these parameters play an essential role in understanding variations in human exposure hazard, reliable and consistent measurement of other key epidemiologically relevant, malaria vector behaviours (e.g., distribution of bites across different times of the night, or indoor versus outdoor locations) [9, 17, 18, 21, 45, 46] remains only possible with the human landing catch (HLC) gold standard method [38, 47, 48]. For example, even CDC-LT which are widely used for monitoring malaria vector mosquito biting densities, species composition and transmission intensity inside houses vectors across malaria endemic settings [37, 47, 49, 50], studies of the efficacy of CDC-LT for catching malaria vectors outdoors is limited to only few places in Africa [51–53], and our experience of east African settings indicates they catch very few mosquitoes when placed outdoors.
Although the HLC is widely viewed as providing the best representation of human exposure to mosquito bites [9, 17, 18, 21, 45, 46], this method is not without limitations. The number of mosquitoes caught with this method can vary significantly between collectors, likely as a result of variation in their skill and degree of alertness [37, 54–56]. An additional concern is the ethical dilemma arising from the requirement of the HLC to expose collectors to potentially infected mosquito bites [47, 48, 57]. Whilst these risks can be minimized by providing collectors with anti-malarial chemoprophylaxis, in which case participants may be safer from malaria than they would normally be [58], concerns with respect to other vector-borne pathogens such as lymphatic filariasis, dengue fever, and other arboviruses remain [14, 59–62].
Alternative methods which do not require human exposure to mosquito bites, and are sufficiently sensitive and accurate to measure key mosquito-biting behaviour metrics, which determine the choice and impact of vector controls are currently lacking but urgently needed. For example, the proportion of human exposure occurring indoors (πi) is an invaluable indicator of how much exposure an LLIN or mosquito-proofed housing may be realistically expected to prevent, as well the extent to which these measures may suppress mosquito human feeding frequency, survival, density and transmission capacity at population level [2, 9, 17–19, 63–65]. Indeed personal estimates of this behavioural metric for individual humans, based on questionnaire surveys of when they went indoors for the evening and left the house in the morning combined with local HLC surveys of mosquito activity, have recently been confirmed as strong epidemiological predictors of malaria infection risk in an urban African settings [65]. It is also noteworthy that it was Garret-Jones himself, who first coined the term epidemiological entomology [66], who first began adjusting biting exposure estimates to allow for changing distributions of humans across indoor and outdoor environments in the same way that the proportion of human exposure occurring indoors is calculated today [21].
A series of sequential prototype mosquito electrocuting grid traps, specifically designed to measure these specific metrics of mosquito human-feeding behaviour have therefore been developed and evaluated in previous proof-of-principle studies in Tanzania [67, 68]. In principle, these operate in a similar fashion to HLC by placing electrocuting grids around a human bait host to kill mosquitoes attempting to attack, whereas in HLC they are manually aspirated when they actually land on exposed limbs of the volunteer. While the first evaluation using a commercially available insect-zapping device [67] demonstrated malaria vectors could be captured with reasonable sensitivity, mosquito specimens obtained were often damaged and difficult to identify morphologically [67]. Also the sensitivity of this earlier version, relative to HLC, dropped over the course of the night for a variety of possible technical reasons, limiting their accuracy for measuring patterns of mosquito activity and human exposure because both were consequently skewed to exaggerate biting rates in the early evening [67]. Subsequent studies [68] expanded on these early experiences by developing a custom-engineered mosquito electrocuting trap (MET) that uses a novel, electrical output system, specifically designed to kill mosquitoes without burning the specimens, so that they remain intact for morphological and molecular identification. This MET prototype proved to have encouraging levels of sensitivity relative to HLC, especially when the trap was placed outdoors, and all specimens proved suitable for morphological identification and molecular analysis [68]. However, a number of technical problems with electrical delivery system and durability were reported for this prototype, which limited its ability to consistently reproduce HLC-derived estimates for the proportion of mosquito caught when most humans are indoors (Pfl) and the proportion of human biting exposure occurring indoors, (πi) [68].
Based on difficulties reported for this initial MET prototype, this article report the first full field evaluation of the performance of an improved MET prototype, which was redesigned based on lessons learned from these earlier iterations [68]. This improved MET prototype was evaluated, in terms of: (1) its ability to consistently reproduce HLC-derived estimates for key metrics of human-biting behaviours of malaria vectors, and (2) improved catch sensitivity relative to HLC.
Methods
Study area and experimental sites
The study was conducted in Dar es Salaam, the biggest city and commercial hub in Tanzania with population of 4.36 million people [69]. Historically, malaria transmission in Dar es Salaam has been stable but at a low level, with an entomological inoculation rates of just over one infectious bites per person per year [5, 65, 70]. However, by the time of this study, at the end of 2012 and the beginning of 2013, malaria transmission rates in Dar es Salaam had been reduced to entomological inoculation rates of fewer than 0.1 infectious bites per person per year as a results of high coverage with LLINs [71], house window screening, sealed eaves or ceilings [72], and regular application of biological larvicides [65]. Spending even 1 or 2 extra hours outdoors in the evening was predictive of malaria risk in Dar es Salaam, presumably due to an appreciable degree of outdoor and early evening biting [5, 63] exhibited by vector populations in the city than is typical for African vectors [17, 18]. Detailed descriptions of the area are available elsewhere [65, 73, 74]. Malaria vectors from the An. gambiae sensu latu species complex (consisting of An. gambiae s.s, Anopheles arabiensis, Anopheles merus) and An. funestus are present in Dar es Salaam with An. gambiae s.s. being responsible for the majority of transmission [5]. Anopheles arabiensis in particular tend to bite outdoor at dusk, so at least half of the biting exposure to this species occurs outdoors [63]. Although the peak-biting times of An. gambiae s.s. remain approximately consistent with those of classical reports [75], it does prefer to feed outdoors [5, 63]. Culex spp., especially Culex quinquefasciatus, are far more abundant, accounting for more than 95 % of all mosquitoes in Dar es Salaam [5, 42, 76, 77]. In addition to transmitting lymphatic filariasis and a variety of other pathogens, these species cause significant nuisance in Dar es Salaam and elsewhere [13, 78, 79]. Previous surveys of Culex spp. behaviours in Dar es Salaam reveal a strong preference for feeding outdoors, with an activity period that spans the entire night, much of which occurring when people sleep, so that slightly more than half of human exposure occurs indoors in the absence of protective bed nets or mosquito-proofed housing [67].
Within Dar es Salaam, three areas representing different levels of urbanization (Kigogo Mkwajuni (urban), Mbagala Bughudad (semi-urban) and Pemba Mnazi Buyuni (rural) (Fig. 1) with detectable levels of An. gambiae s.l. were selected as study location. Factors used in the classification of these selected sites included: geographical overview of the area, population density, land use type, socio-economic status, and based on people’s experience. Site selection was also guided by the necessity for sufficient malaria vector mosquito densities, so that it was possible to catch sufficient numbers to measure their biting behaviour. While Kigogo Mkwajuni (urban) and Mbagala Bughudadi (semi-urban) are both densely populated, these are informal, unplanned settlements, bordering rivers that regularly flood during the rainy season. While Mbagala Bughudadi is at the southern edge of the city along the Mbagala river, Kigogo Mkwajuni is located very centrally at the edge of the Msimbazi river valley, the largest flood plain in the city. Pemba Mnazi, although administratively part of the Dar es Salaam city region, is very rural in character, with only a few, small, scattered houses, some of them with thatched roofs (Fig. 1). It is approximately 70 km southeast of Dar es Salaam, where fishing with some agriculture are the main income-generating activities.
Fig. 1.
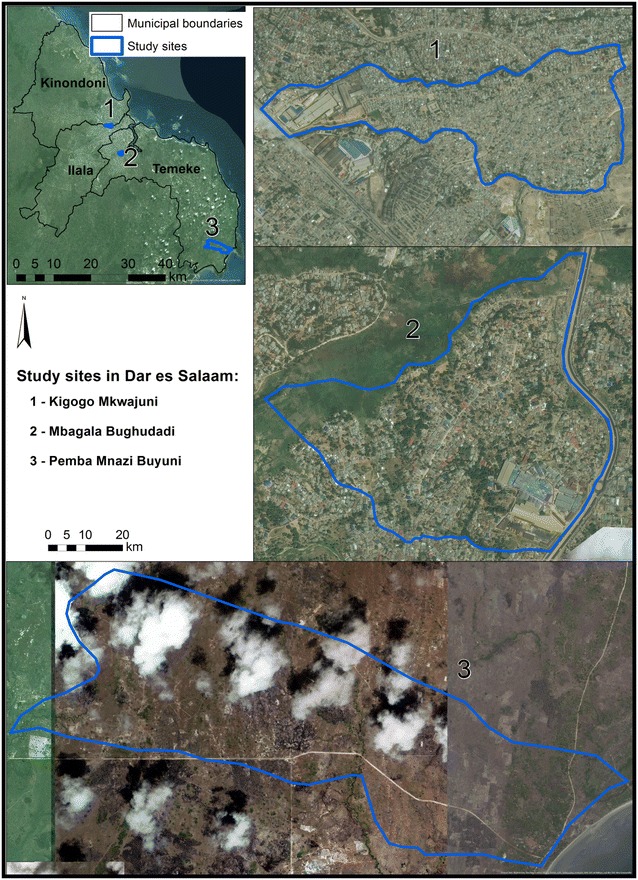
Study area and administrative units in Dar es Salaam. Administratively, Dar es Salaam consists of three municipalities: Kinondoni, Ilala and Temeke. The map highlights three study sites (Kigogo Mkwajuni (urban), Mbagala Bughudag (peri-urban) and Pemba Mnazi Buyuni (rural) where sampling of mosquitoes were carried out
Mosquito trapping methods
Mosquito electrocuting grids (MET)
The MET is composed of four wooden panel frames measuring 35 × 35 cm, arranged to form a square cavity into which human volunteers’ legs are placed (Fig. 2c). The panels hold sets of vertical parallel stainless steel wires spaced 5 mm apart, which are electrically connected to a 24 V battery-powered stable direct current (DC) power source, thereby creating an electric potential between the wires, which is sufficient enough to kill mosquitoes trying to pass through the wires, but without destroying the specimen, as observed with previous prototypes [68]. The power is supplied at low output, which is sufficient to kill mosquitoes on contact but poses no harm if accidentally contacted by volunteer. This combination of voltage with current setting was identified through pilot laboratory experiments using insectary-reared An. gambiae and An. arabiensis specimens with an a prior minimum kill probability threshold of 80 % [80]. The MET prototype used was modified to improve upon shortcomings reported in an earlier version which included the tendency to short circuit and weak physical stability [68]. Specific changes were: (1) introduction of hinges to secure the four angles of the main frame (Fig. 2a), and (2) better alignment of grid wires into the frame using grooves which minimized the possibility of opposing wires contacting each other and short circuiting. During mosquito trapping, each MET unit was placed on a 2 m × 2 m wooden frame platform placed on a white sheet (Fig. 2c) which made it easier for collectors to see the electrocuted mosquitoes that dropped on the floor. The four legs of the platform were placed in water bowls to create a barrier that prevented ants from crawling onto it and consuming dead mosquito samples. During mosquito collection, a volunteer sits with their lower limbs placed inside the square trapping box (Fig. 2c) to act as attractive bait. Mosquitoes were captured by a single adult male per location using a MET over a 12-h period on each night of experiments (18:00–06:00 h). Sampling was conducted for 45 min of each hour, followed by a 15-min break period during which the trap was turned off, and mosquitoes collected either from the floor of the platform where they had fallen after electrocution, or from the grid panel surfaces using forceps. This 15-min break also allowed for exchange of collectors between matched indoor and outdoor stations at each house after each hour.
Fig. 2.

Step-wise setting and improvement made to the mosquito electrocuting trap (MET). a Locking together of hinges connecting individual panels with bolts; b locking of assembled panels into the main, outer frame; c fully assembled MET in use by a human participant wearing protective clothes except for on his feet, which are placed within the MET frame
Human landing catch (HLC)
To do a HLC, a single male adult volunteer exposed his legs and collected mosquitoes upon landing on his legs with a mouth aspirator as previously described [38, 54, 55, 81]. Similar to MET, sampling here was also conducted at each sampling location for 45 min of each hour, from 18:00 to 06:00, allowing 15-min breaks for rest and refreshment, and for exchange of collectors between matched indoor and outdoor location at each house.
Experimental design
Within each of the three study sites described above, and in Fig. 1, a block (site) of four houses with open eaves, all of which were all at least 50 m from each other, were purposively selected for entomological survey by HLC and MET. At each house, a corresponding outdoor-catching station was established approximately 5 m outside the assigned house with a raised platform and plastic sheeting roof to protect against rain, exactly as previously described [67]. In all houses, the indoor mosquito-capture stations were set up within the living room. On each night of experiments, mosquitoes were sampled both indoors and outdoors at all four houses at a given site. On the first night of sampling, two of the four houses were randomly allocated for sampling using HLC, and the remaining two allocated to MET collection. On the second night of experimentation, the two capture methods were exchanged between houses (e.g., MET sampling was conducted at houses where HLC had been done previously and vice versa) such that all methods were used at all four houses in a two-day period (thus completing one replicate) of crossover design. A specific pair of volunteers was assigned to each household (one for indoor and outdoor sampling, respectively) and remained there for the two-night replicate to ensure that only sampling techniques and not volunteers were exchanged between houses. However, each pair of volunteers were swapped between the indoor and outdoor catching stations after each hour to minimize systematic bias due to differential attractiveness and collection skill of collectors. After each two-night survey replicate at a single site, the experiment moved to the next site for implementation of another replicate with the same crossover design. These two-night survey replicates were rotated through all three sites over a total of six nights of sampling within a single working week to complete one full round of experimental replication. This weekly replication cycle of experimentation was conducted from 17 December, 2012 to 4 July, 2013, over a total of 168 nights of sampling and 28 replication weeks.
Processing of samples
Mosquito samples from all catches were first sorted, counted and morphologically identified as either An. gambiae s.l., An. funestus [75, 82] or Culex spp. with the aid of a stereomicroscope. All An. gambiae s.l. were stored in 1.5-mL tubes containing desiccated silica gel under cotton wool for subsequent polymerase chain reaction (PCR) assay [83] to determine sibling species within the complex and enzyme-linked immunosorbent assay (ELISA) [84, 85] for sporozoite infection identification.
Data analysis
Only An. gambiae s.l. and Culex spp. were collected in appreciable numbers by this study. Although An. funestus is an important malaria vector in Africa, it is now rare in Dar es Salaam and only three specimens were collected in this study, so no detailed analysis of this species was possible. Given the low numbers of An. gambiae s.l. specimens caught, and the high proportion of specimens whose DNA failed to amplify in PCR analysis, separate statistical analyses for each sibling species was not possible. While the problem of low DNA amplification rates was consistent across trapping methods by Chi square test, the underlying reasons for poor amplification is suspected to be linked with elevated air temperature in the laboratory. The laboratory air conditioner was out of order at the time when this PCR analyses was conducted. Analysis was, therefore, conducted on An. gambiae s.l. as a single taxon, based on counts and derived proportions of mosquitoes identified to complex level using morphological criteria. The other major mosquitoes taxon of interest, as vectors of lymphatic filariasis and other pathogens and as the major cause of biting nuisance in Dar es Salaam and many other African urban centres, were Culex spp, which were identified to genus only and correspondingly analysed as a single taxon. Generalized linear mixed effects model (GLMM), allowing for important sources of variance that are not of direct interest were used for all analyses, using R open source statistical software (version Rx 64 2.15.2) augmented with the lme4 package.
Catching sensitivity in alternative trap relative to human landing catch
To evaluate the relative sensitivity of the MET, the total of either An. gambiae s.l. or Culex spp. catch per night was treated as the dependent variable, with trapping method (MET versus HLC) treated as a fixed, categorical variable, with house, participant nested within site, and night of sampling fit as random effects. Since the observations were count data and were not normally distributed, models were fitted using a Poisson distribution. The effect estimates were obtained by exponential transformations of the parameter estimates obtained with this logarithmic link function. Initially, indoor and outdoor catches were analysed separately. Thereafter a similar model was constructed combining both indoor and outdoor data. Additionally, a model that included an interaction term to test for and quantify the effect of any interaction between trap and location (indoor versus outdoor) was fitted so as to check whether the sampling sensitivity of MET relative to HLC is influenced by trap location.
Effect of hour of night (time) on sampling efficiency of MET
To test whether the sensitivity of MET declined with time over the course of 12 h of collection each night, data were first aggregated to obtain total catches of each mosquito taxon from MET, and from MET plus HLC combined for each site and house on each night, separately calculated for each hour (h) in the nightly survey sequence (h = 1 for 18:00–19:00, h = 2 for 19:00–20:00, h = 3 for 20:00–21:00, h = 4 for 21:00–22:00, h = 5 for 22:00–23:00, h = 6 for 23:00–24:00, h = 7 for 24:00–01:00, h = 8 for 01:00–02:00, h = 9 for 02:00–03:00, h = 10 for 03:00–04:00, h = 11 for 04:00–05:00 and h = 12 for 05:00–06:00). Indoor and outdoor collections were analysed separately. The proportion of mosquitoes that were captured with the MET (PMET = MET/(MET + HLC) was treated as the dependent variable with a binomial distribution and logit link function in a GLMM with the sequence hour (h) included as a continuous independent variable, and house nested within site as well as sampling night treated as random effects.
Density dependence
Two mosquito traps are said to exhibit density-dependence (DD) if their relative sampling sensitivity varies with mosquito density, and density-independence (DI) if their relative sampling sensitivity is constant. Graphically, DI can be represented as a linear correlation between the two traps in catches taken across differing densities, and DD as a deviation from linear correlation. Mathematically, two traps show DI if E(xi) = αE(yi), where xi and yi are the ith of n paired mosquito catches from traps X and Y, respectively, E(xi) and E(yi) are the expected counts of xi and yi, and α is a scaling constant. DD can be modelled as following a power law, E(xi) = αE(yi)β, where the exponent β governs the degree of non-linearity and therefore the degree of DD [53, 86]. DI is therefore a special case of DD where β = 1, so the extend of DD can be assessed as deviation of an estimate of β from 1. Estimation of β by regression of y on x, or vice versa, would give biased results when neither trap is an error-free measure of mosquito density. Instead we modelled the n paired catches as reflecting variation in underlying mosquito density, zi, which was taken to be a log-normally distributed latent variable, with . The expected values of xi and yi were modelled as and . By solving both equations for zi it can be shown that and , so that and , giving a power law relationship between the expected densities of traps X and Y with exponent β, so that E(xi) ∝ E(yi)β and E(yi) ∝ E(xi)1/β. The observed counts, xi and yi, were assumed to be drawn from a negative binomial distribution such that xi ∼ NB(λxi, θ) and yi ∼ NB(λyi, θ), using the parameterisation of the negative binomial with mean λ and variance λ + λ2/θ. The dispersion parameter θ is inversely related to trap reliability, lower values of θ corresponding to higher levels of over-dispersion in the X and Y catches, and consequently weaker correlation. This method differs from existing methods [53, 86] by incorporating symmetry between the traps and by modelling overdispersion. It can be shown that the DI model is equivalent to a negative binomial GLMM with an indicator for trap type centred on zero fitted as a fixed effect and log(zi) being a normally distributed random effect. This GLMM can be extended to DD by allowing the X:Y ratio of random effect standard deviations, which is β, to differ from 1. The DD GLMM is therefore the DI GLMM extended to include random slopes, with the inter-trap random effects correlation set to 1. It should, therefore, be possible to fit the DI and DD models using standard maximum likelihood methods for GLMMs. However, we used MCMC in the program JAGS [87, 88] because of the ease of obtaining credible intervals (CI) for the model parameters. The extent of deviation from DI was gauged by estimating β from the DD model, while the strength of linear correlation between the X and Y catches was calculated from the log-scale variance components estimated from the DI model as , where , and Var(y) was calculated by replacing αx with αy in the formula for Var(x). ψ(1)(θ) approximates the variance from the gamma component of the negative binomial distribution, where ψ(1) represents the trigamma function [89], and approximates the variance from the Poisson component appendix 1 of [90]. Because ψ(1)(θ) ≈ 1/θ, higher values of θ, which correspond to lower levels of overdispersion, will also correspond with higher values of rxy, in line with intuition. This method was used to assess density dependence between MET (taken to be Y) and HLC (taken to be X). Estimates and 95 % CIs for β and r were calculated as mean and 2.5 and 97.5 % centiles from 5 × 105 MCMC samples from the posterior distribution following 105 burn-in iterations. The effective MCMC sample size for all parameters was >2000. Prior distributions for log(αx), log(αy) and log(β) were normal with means of zero and variances of 104, and the prior distributions for log(θ) and log(σ2z) were uniform from −10 to 10. Note, because multiple traps of the same type were used simultaneously each night, mosquito catches were first aggregated by trap types, night of collection and by hour. This analysis was followed by plotting catches between the two methods.
Estimating epidemiologically relevant metrics of mosquito behaviours and human exposure patterns
Two key metrics of the behavioural preferences of mosquitoes, as well as another metric of the distribution of human exposure to mosquito bites, were estimated as previously described [17, 21, 67, 68] from the entomological data collected as described above, and combined with questionnaire survey data describing when residents of Dar es Salaam spend their time indoors and outdoors [5]: (1) the proportion of mosquitoes caught indoors (Pi), which is obtained by dividing the total number of mosquitoes that were caught indoors by the total caught indoors and outdoors (I18:00→06:00 h)/(I18:00→06:00 h + O18:00→06:00 h): where I and O represent mosquitoes collected indoors and outdoors, respectively, and subscripts indicate the start and end time of collection period; (2) the proportion of mosquitoes that are caught between the first (f) and last (l) hours when most (at least 50 %) people were asleep and indoors (Pfl), obtained by dividing the total number of mosquitoes caught between 22.00 and 05.00 [5] by the total number of mosquitoes caught over the entire night (I22:00→05:00 h + O22:00→05:00 h)/(I18:00→06:00 h + O18:00→06:00 h); (3) the proportion of human exposure to mosquito bites that would occur indoors in the absence of personal or household physical protection (πi), and that can therefore be directly prevented by using a bed net, obtained by dividing the number of mosquitoes that were collected indoors during sleeping hours from 22:00 to 05:00 by itself plus the number collected outdoors outside of sleeping hours from 18:00 to 22:00 plus from 05:00 to 06:00 (I22:00→05:00 h)/(I22:00→05:00 h + O05:00→22:00 h). Calculation of Pi,Pfl and πi have been previously described elsewhere [17, 18, 21]. To estimate these metrics of mosquito behaviours, the proportions of mosquitoes caught or the proportions of human exposure to mosquito bites from each taxon were each treated as dependent variables with a binomial distribution and a logit link function in GLMMs [91]. Trap type (MET versus HLC) was fitted as a fixed categorical factor, with participant nested within house and then house nested within site, as well as night of experimentation as random effects, to account for the substantial variance that is typically associated with these nuisance variables in mosquito capture experiment [92–94]. Because multiple traps of the same type were used simultaneously in each experimental night, data were aggregated by sampling night, house, hour, location (indoor versus outdoor), site and trap type. These estimate proportions of Pi, Pfl, and πi were derived from count data as previously described [67].
Results
A total of 62,202 female mosquitoes were sampled from all three collection sites, of which 96 % (59,814) were Culex spp. Of 1373 female anopheline mosquitoes collected, 86 % (1184) were An. gambiae s.l., 0.2 % (3) An. funestus, and 13.5 % (186) Anopheles tenebrosus. Table 1 summarizes the number of mosquitoes from different groups that were collected from each site by the two sampling methods. Because, An. funestus and An. tenebrosus were collected in very low numbers, they were excluded from further GLMM analysis.
Table 1.
Number of mosquitoes caught from different sites by two methods and crude estimates of sensitivity of mosquito electrocuting trap (MET) relative to human landing catch (HLC)
| Collection sites | Catch per method | Total catch | Relative sensitivity | |
|---|---|---|---|---|
| MET | HLC | |||
| Anopheles gambiae s.l. | ||||
| Kigogo Mkwajuni (urban) | 102 | 129 | 231 | 0.78 |
| Bughudad (semi-urban) | 492 | 236 | 728 | 2.08 |
| Pemba Mnazi (rural) | 127 | 98 | 225 | 1.30 |
| Overall catch | 721 | 463 | 1184 | 1.56 |
| Anopheles funestus | ||||
| Kigogo Mkwajuni (urban) | 0 | 0 | 0 | NA |
| Bughudad (semi-urban) | 2 | 1 | 3 | 2 |
| Pemba Mnazi (rural) | 0 | 0 | 0 | NA |
| Overall catch | 2 | 1 | 3 | NA |
| Anopheles tenebrosus | ||||
| Kigogo Mkwajuni (urban) | 5 | 3 | 8 | 1.67 |
| Bughudad (semi-urban) | 47 | 64 | 111 | 0.73 |
| Pemba Mnazi (rural) | 24 | 43 | 67 | 0.58 |
| Overall catch | 76 | 110 | 186 | 0.69 |
| Culex spp. | ||||
| Kigogo Mkwajuni (urban) | 10,172 | 10,986 | 21,156 | 0.93 |
| Bughudad (semi-urban) | 10,418 | 11,327 | 21,745 | 0.92 |
| Pemba Mnazi (rural) | 8338 | 8573 | 16,911 | 0.97 |
| Overall catch | 28,928 | 30,886 | 59,814 | 0.94 |
| Mansonia sp. | ||||
| Kigogo Mkwajuni (urban) | 36 | 28 | 64 | 1.29 |
| Bughudad (semi-urban) | 315 | 558 | 873 | 0.56 |
| Pemba Mnazi (rural) | 32 | 26 | 58 | 1.23 |
| Overall catch | 384 | 612 | 995 | 0.63 |
| Aedes aegypti | ||||
| Kigogo Mkwajuni (urban) | 0 | 0 | 0 | NA |
| Bughudad (semi-urban) | 0 | 0 | 0 | NA |
| Pemba Mnazi (rural) | 20 | 0 | 0 | NA |
| Overall catch | 20 | 0 | 0 | NA |
Of the 1172 An. gambiae s.l. that were subjected to PCR analysis, only 427 (36 %) were successfully amplified and identified as An. gambiae s.s. (136, 31.8 %), An. arabiensis (258, 60.4 %) and An. merus (33, 8 %). Although amplification success was generally poor, it was consistent for specimens caught with either methods [39 % (236/598) for MET versus 33 % (191/574) for HLC, , df = 1, P = 0.116]. The MET consistently caught at least a third more An. gambiae s.l. than the reference HLC gold standard method but caught slightly less Culex spp. (Table 2). No significant difference between indoor versus outdoor locations was detected for the relative capture efficacy of MET compared to HLC, for either An. gambiae s.l. (RR = 0.98, P = 0.86) or Culex spp. (RR = 0.97, P = 0.15).
Table 2.
Comparisons of numbers of female Anopheles gambiae complex and Culex sp. caught between indoors and outdoors by alternative mosquito electrocuting grid (MET) relative to reference human landing catch (HLC), pooling data from each sites and analysed by generalized linear mixed effect model (GLMM)
| Collection methods | Trap nights | Total catch | Mean catch | RR [95 % CI] | P |
|---|---|---|---|---|---|
| Anopheles gambiae s.l. | |||||
| Indoors | |||||
| HLC | 308 | 226 | 0.73 | 1a | NA |
| MET | 308 | 348 | 1.13 | 1.47 [1.23–1.76] | <0.0001 |
| Outdoors | |||||
| HLC | 308 | 237 | 0.77 | 1a | |
| MET | 308 | 373 | 1.12 | 1.38 [1.14–1.67] | <0.0001 |
| Indoors and outdoors combined | |||||
| HLC | 616 | 463 | 0.75 | 1a | |
| MET | 616 | 721 | 1.17 | 1.42 [1.24–3.48] | <0.0001 |
| Culex spp. | |||||
| Indoors | |||||
| HLC | 308 | 13,613 | 44.19 | 1a | |
| MET | 308 | 12,576 | 40.83 | 0.93 [0.90–0.95] | <0.0001 |
| Outdoors | |||||
| HLC | 308 | 17,273 | 56.08 | 1a | NA |
| MET | 308 | 16,352 | 53.09 | 0.95 [0.93–0.97] | <0.0001 |
| Indoors and outdoors combined | |||||
| HLC | 616 | 30,886 | 50.14 | 1a | |
| MET | 616 | 28,928 | 46.96 | 0.94 [0.92–0.95] | <0.0001 |
NA not applicable, RR relative rate, CI confidence interval
aReference group
The MET also exhibited strong sampling consistency over the course of the night relative to the HLC (Fig. 3). Its relative sampling efficacy did not detectably change with time over the course of the entire night for both An. gambiae s.l. (OR [95 % CI] = 1.01 [0.94–1.02], P = 0.27) and Culex spp. (OR [CI] = 0.99 [0.99–1.0], P = 0.17) in indoor environment, but with significant decline in outdoor environment (An. gambiae s.l. OR [CI] = 0.92 [0.86–0.99], P = 0.04), and Culex spp. (OR [CI] = 0.99 [0.98–0.99], P = 0.03). The size of the effect for such decline was however, not big enough to affect human-vectors interactions behavioural outcome.
Fig. 3.
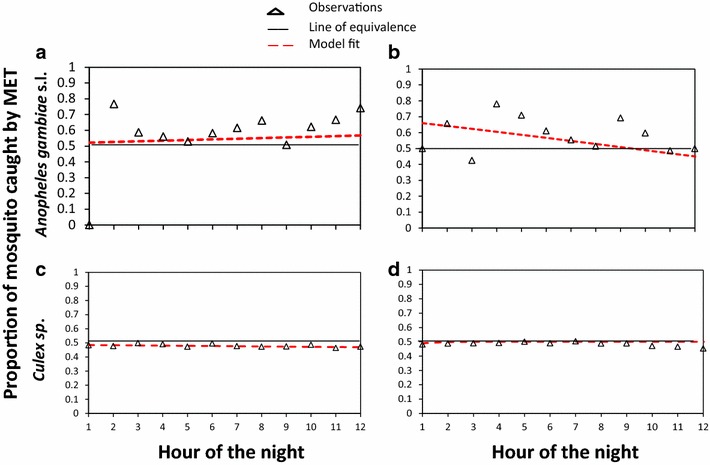
Sensitivity of electrocuting grids trap (MET) relative to human landing catch through the entire time of the night. Relative sensitivity of MET to catch An. gambiae s.l. indoor (a), An. gambiae s.l. outdoor (b), Culex spp. indoors (c) and Culex spp. outdoors (d) compared to HLC. Diamond shows the actual observations, discontinued lines the model fit for the observed values and solid line assumes equivalence of sensitivity between MET and HLC overnight
Significant density dependence between MET and HLC was detected only for An. gambiae s.l. indoors (Fig. 4a). The 95 % CI for the density dependence exponent, β, was entirely below one, suggestion that MET sampling sensitivity increases relative to HLC at higher densities ( [95 % CI] = 0.73 [0.52, 0.95]). There was no evidence of density dependence outdoors ( [95 % CI] = 0.94 [0.65, 1.26]). However, both data sets contained outlier observations where the MET catch was anomalously high (1 indoors and 2 outdoors with catch >40, Fig. 4a, b). We tested the sensitivity of the An. gambiae s.l. results to these outliers by removing them and re-estimating (Additional file 1: Figure S1). The finding of density dependence in An. gambiae s.l. indoors proved to be sensitive to the MET outlier ( [95 % CI] = 0.79 [0.57, 1.04]), while the non-detection of density dependence in the An. gambiae s.l. outdoors analysis was unchanged ( [95 % CI] = 1.17 [0.77, 1.58]). There was no evidence of density dependence in Culex spp. indoor ( [95 % CI] = 0.94 [0.75, 1.14]) or outdoor ( [95 % CI] = 0.91 [0.76, 1.06]). The wider 95 % CIs in An. gambiae s.l. relative to Culex spp. suggest that sensitivity to detect density dependence was lower in the former species, probably due to the lower catch numbers. The greater noisiness of the An. gambiae s.l. catches was also reflected in lower estimates of linear correlation between the two methods (Fig. 4). Anopheles gambiae s.l. gave the lowest correlation estimates ( [95 % CI] = 0.59 [0.46, 0.71] indoors, [95 % CI] = 0.58 [0.43, 0.71] outdoors, which were not substantially changed by removing outliers (Additional file 1: Figure S1), while Culex spp., where mean catches were considerably higher (Table 2), showed rather higher correlations ( [95 % CI] = 0.64 [0.53, 0.74] indoors, [95 % CI] = 0.74 [0.66, 0.82] outdoors). No statistical difference between MET and HLC was apparent in terms of the estimates of proportion of mosquitoes caught indoors (Pi), the proportion caught during sleeping hours spent indoors (Pfl) or the proportion of human exposure to mosquito bites that occurs indoors (πi) for either An. gambiae s.l. or Culex spp. (Table 3). These observed behavioural patterns of mosquito-biting activity linked with human behaviour also appeared descriptively very similar for MET and HLC (Fig. 5). Both An. gambiae s.l. and Culex spp. show a clear tendency to prefer feeding during sleeping hours of the night (Pfl ≫ 0.5) (Fig. 5b), so that the associated proportions of human exposure occurring indoors were also high (πi ≫ 0.5) (Fig. 5c). Although Culex spp. showed a preference for feeding after 22:00 when most people were likely to be indoors, they also exhibit exophagic behaviour (Pi = 0.43, and 0.44, respectively, as measured by MET and HLC) (Fig. 5a). Unlike Culex spp., An. gambiae s.l. can be explained as neither endophagic nor exophagic because it exhibits no strong preference for feeding indoors or outdoors (Pi = 0.48 and 0.49, respectively for MET and HLC) (Fig. 5a).
Fig. 4.
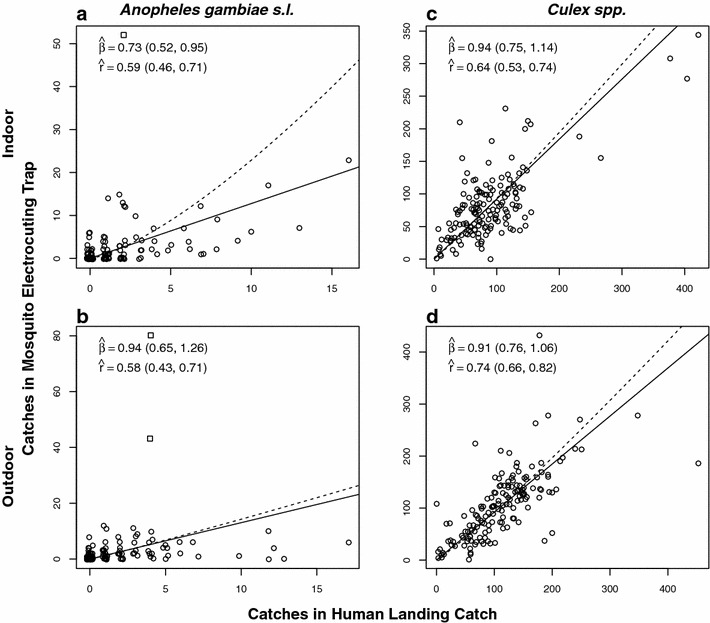
Panels illustrate density-dependence by plotting the mosquito catches in MET against those in HLC. a, b represent An. gambiae s.l. catches indoors and outdoors, respectively, while c, d represent Culex spp. catches indoors and outdoors, respectively. Data points are open circles, except for three data points, depicted with open squares, which represent high outlier MET catches or low outlier HLC catches (see text for details). Estimates and 95 % CIs are given for the density dependence exponent, (), and the linear correlation coefficient (). Model-predicted relationships are shown between the MET and HLC catches as estimated with either the linear density independence model (solid line) and the non-linear density dependence model (dashed line)
Table 3.
Comparison between an alternative mosquito electrocuting grid trap (MET) and human landing catch (HLC) methods in estimating three epidemiologically relevant mosquito behaviours of both female Anopheles gambiae complex and Culex spp. as analysed using binomial logistic generalized linear mixed effect model (GLMM)
| Method | Proportion caught indoors (Pi) | Proportion caught during sleeping hours (Pfl) | Proportion of human exposure occurring indoors (πi) | |||
|---|---|---|---|---|---|---|
| OR [95 % CI] | P | OR [95 % CI] | P | OR [95 % CI] | P | |
| Anopheles gambiae | ||||||
| HLC | 1a | NA | NA | 1a | NA | |
| MET | 1.12 [0.84–1.51] | 0.43 | 0.76 [0.56–1.03] | 0.07 | 0.84 [0.51–1.37] | 0.48 |
| Culex spp. | ||||||
| HLC | 1a | NA | NA | NA | ||
| MET | 0.99 [0.96–1.03] | 0.76 | 1.02 [0.99–1.06] | 0.24 | 1.02 [0.96–1.07] | 0.55 |
aReference group
Fig. 5.
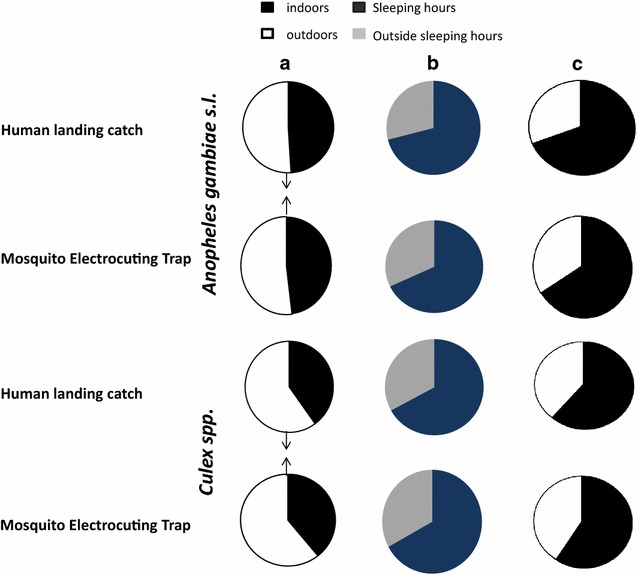
Pie charts illustrating the proportions of mosquitoes caught. Indoors and outdoors (a), during sleeping and outside sleeping hours (b), human exposure to mosquitoes bites occurring indoors and outdoors (c) as estimated by the HLC and MET. The light areas represent proportion of mosquitoes caught outdoors and the outdoors human exposure, the dark areas represent proportion caught indoors and indoors human exposure, the dark blue and grey areas represent proportion of mosquitoes caught during sleeping and outside sleeping hours, respectively
Discussion
This improved MET represents the first evaluated sampling device that captures Afrotropical malaria and lymphatic filariasis vectors with efficacy that is comparable to the HLC gold standard method. Similar estimates of mosquito abundance were obtained by the MET and HLC, both indoors and outdoors locations, as well as over the course of the night even in the rain. On account of the MET’s ability to accurately reproduce estimates of mosquito abundance and hourly biting profiles, this trapping method generated estimates of epidemiologically relevant metrics of mosquito behaviour (Pi, Pfl, πi) [17, 21, 67, 68] that were indistinguishable from those obtained by HLC. It is encouraging that this MET performed similarly well for two different mosquito taxa: the An. gambiae s.l. and the group of Culex spp. that mediate transmission of lymphatic filariasis. These two groups have differing ecological characteristics, with the An. gambiae s.l. complex being relatively sparse but efficient vectors of residual malaria transmission [5], while the sundry Culex spp. reach very high densities and mediate transmission of lymphatic filariasis in Dar es Salaam [95]. Sampling stability over the course of the night together with consistency sampling efficacy between indoors and outdoors appear to be essential requirements of any trap used to measure distributions of mosquito-biting activity across time of night, so that interactions with human behaviour can be accurately calculated [67]. Even with the prototype described here, there was a decline in sampling efficacy for both mosquito taxa over the course of the night in outdoor environment, these declines were quantitatively modest and appear to have had negligible influence on the estimates for the epidemiologically relevant metrics of interactions between humans and vectors that were measured. The unprecedented stability and consistency of sampling efficacy observed in this study is probably the result of specific modifications made to the trap design, especially the introduction of grooves which prevented the possibility of contact between the two adjacent wires which often caused short circuits in previous prototypes [68]. Also, the introduction of hinges increased the physical stability of trap against buffeting by wind and enabled rapid fixing of the device whenever a defect occurred in one panel during an active sampling experiment. Unlike the previous study [68], here the current flow across traps were closely monitored before the start of sampling and after every 3 h of the night. However, there was one occasion when one MET was found to be operating inadequately noting voltage fluctuation at the voltage amplifier unit, so that the defective panel was identified and replaced immediately with the spare one. While previous evaluations of commercially available electric grids anecdotally attributed declining sensitivity over the course of the night to declining battery charge and the accumulation of burnt mosquito cadavers [67], both of these design concerns were addressed by stabilizing the power supply and modifying the electrical configuration of the MET used here. Another important feature that may have contributed to the MET reproducing HLC-derived estimates for metrics of mosquito behaviour is the fact that the trap operated in a similar way to HLC by capturing mosquitoes exactly when they attack a seated human subject. Like evaluations of previous prototypes of electrocuting traps, the MET here also exhibited some differential capture efficacy with respect to different mosquito taxa. The relative sensitivity of MET was consistently higher for An. gambiae s.l. than Culex spp.. In the earlier study in rural Kilombero Valley, the relative capture efficacy of that MET was consistently higher for An. funestus s.l. than for An. gambiae s.l. [68]. Similarly, in a preceding study in urban Dar es Salaam, using commercially available electrocuting grids, the sensitivity was 39, 26 and 32 % for An. gambiae s.s., An. arabiensis and Culex spp., respectively [67]. While such differential sensitivity may be a common property of this sampling device, the relative sensitivity observed in this study was consistently high, being as good as or better than HLC for both An. gambiae s.l. and Culex spp. regardless of being indoors or outdoors.
Another positive result with respect to MET is the association between numbers of mosquitoes caught by the MET method and the HLC method did not show strong evidence for deviation from linearity. In other words, MET tended to exhibit constant sampling efficiency regardless of density. An exception to this tendency was An. gambiae s.l. captured indoors, for which the deviation from linearity was detected, with the sampling efficiency of MET being higher relative to HLC at higher densities. However, the evidence for density dependence in An. gambiae s.l. indoors was unreliable, being contingent on a single outlying observation. The lack of strong evidence for DD is also consistent with a previous evaluation of a preceding prototype, in a rural Tanzanian setting where high malaria vector densities provided far greater statistical power [68]. It should also be noted that, the correlation coefficients of these two capturing methods were relatively lower in An. gambiae s.l. than in Culex spp. (Fig. 4), in line with the noisier Culex scatter plots, but nevertheless not close to zero, suggesting that both MET and HLC are sensing substantial variation in underlying An. gambiae s.l. density. In addition, given that low-rate count data is intrinsically noisy, it seems likely that a substantial amount of the scatter on the plots is caused by noise intrinsic to both methods when densities are low, rather than simply due to unreliability of MET.
While this prototype version can representatively estimate the three key mosquito host-seeking behavioural metrics mentioned above, this MET design could be enlarged in size to accommodate the whole body of a person or a calf and thus allow measuring of another important epidemiologically relevant indicator of vector-borne disease transmission [96, 97], such as host preference of mosquitoes [98–100]. While this indicator is often measured by examining the blood meal origin of wild mosquitoes, usually collected while resting [98, 99, 101, 102], the derived estimates for the proportion of blood meals obtained from each host type is also depend on the abundance and acceptability of each host species [98, 102–104], rather than just host preference. Estimate of host preference based upon blood meal identification are largely driven by the sampling location and methodology. For example blood meals of mosquitoes collected in houses tend to be biased towards humans [105]. Direct competitive-choice experiments [98] may therefore provide complementary direct estimates of actual host preferences rather than the ultimate outcomes of behavioural processes in the field.
Despite all the advantages listed above, this MET design also has some drawbacks which merit attention during further development: while the mosquito-borne diseases it has been designed for predominantly occur in poorly resourced countries of the tropics, the trap requires batteries that need to be recharged at least every 2 days. However, this limitation could be readily overcome with solar recharging technology, even in isolated African rural settings, similar to recent applications of CDC-light traps [49]. Although the four panels of the trap are interconnected by pre-fixed hinges, so it takes less than 5 min to set-up or disassemble this prototype MET, there is clearly room for further improvements with respect to convenience, integrity and robustness. For example, the design may also benefit from improving the frame materials from wooden to lightweight durable materials, such as polyvinyl chloride, so that it is easy to carry, set up and transport. Note also that while the sample collected from MET are intact and can be identified both morphologically [68] and with molecular genetic methods [67, 68], they may be unsuitable for age determination by dissection [106–108] because they tend to dry up relatively fast. This could perhaps be overcome by using other methods of age determination such as near-infrared spectroscopy, which may work with dry samples [109–111]. Also, the material costs alone for this prototype are of $200 per set, so clearly needs to undergo further modification for mass production and large-scale use.
Conclusions
This improved MET prototype matches the performance of the gold standard HLC method for measuring mosquito abundance and behaviour in Dar es Salaam. This device appears capable of accurately quantifying not only the level of human transmission exposure occurring indoors and outdoors, but also of other underlying behavioural characteristics of mosquito vector populations that determine the degree to which transmission of malaria [4, 18, 35, 112] and other mosquito-borne diseases, such as lymphatic filariasis [13] are vulnerable to targeting with specific vector control measures. This is the first time that an alternative exposure-free mosquito sampling method to potentially risky HLC has been shown to representatively measure these important metrics of mosquito behaviour and human exposure distribution. So while considerable further optimization and validation across a wider variety of settings and mosquito populations remains to be done, these results are encouraging. Furthermore, this device could have broader applications in a range of insect surveillance and control applications.
Authors’ contributions
NJG designed and implemented mosquito sampling protocol, supervised the data collection, performed statistical data analysis, interpreted the results, and drafted the manuscript with assistance from GFK and HMF. AT and JP implemented all field activities with assistance from NJG. DFM, NJG, HMF and NM contributed to the revised design of the trap. PJ contributed to the statistical analysis and commented on the manuscript. HMF and GFK both contributed to the study design and reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank the residents of Kigogo Mkwajuni, Mbagala Bughudad and Pemba Mnazi Buyuni in Dar es Salaam for their hospitality and cooperation during this study. We extend our thanks to all volunteers for their commitment and hard work throughout the study. We also thank Ms Victoria Mwakalinga for preparing the map presented in Fig. 1 and to Mr Thomas Wallace from Bioelectronic Unit College of Medicicine, Veterinary and Life Sciences, University of Glasgow for assisting with construction of the trap.
Competing interests
NJG, DFM and HMF have filed a patent (with Easy Access IP) for the MET prototype described here on behalf of University of Glasgow and the Ifakara Health Institute. AT, JP, PJ, and GFK declares that they have no competing interests.
Availability of data and materials
Access and use of data supporting this article will have to comply with the Ifakara Health Institute data sharing policy. If data are requested and no competing interest is apparent, the requested data will made be made available under defined conditions expressed in writing through an exchange of letters between parties stipulating those conditions and any agreed limits to use of data.
Consent for publication
Written informed consent was obtained from volunteers for participation in the study and for publication of this report and any accompanying images. Consent and approval for publication was also obtained from the Medical Research Coordination Committee of the National Institute of Medical Research in Tanzania.
Ethics approval and consent to participate
Prior to any fieldwork, permission was obtained from the Institutional Review Board of the Ifakara Health Institute (IHI/IRB/A 50 and IHI/IRB/AMM/19-2010) and the Medical Research Coordination Committee of the National Institute of Medical Research in Tanzania (NIMR/HQ/R.8c/Vol.II/125). Each participant (someone involved by carrying out HLC or being a bait in a MET), together with house owners where the catches took place, provided written and oral informed consent prior the study, during which they were advised of their right to leave the study at any time. Adult male volunteers were provided with anti-malarial drug prophylaxis atovaquone-proguanil (Malarone®, GlaxoSmithKline) taken daily before, during and after mosquito collection and were screened weekly for malaria parasites by rapid diagnostic test. Consistent with recent evidence of the effectiveness of providing drug prophylaxis to volunteers in mosquito collection experiments [58], none was diagnosed positive. Had any volunteer tested positive for malaria during the study, they would have been treated with the standard front-line treatment in Tanzania, artemisinin-lumefantrine (Co-artem®, Novartis) free of charge as per protocol.
Funding
This work was funded by the European Union Seventh Framework Programme (FP7/2007-2013) through the African Vector Control: New tools project (Award number 265660, coordinated by Prof. Hilary Ranson at Liverpool School of Tropical Medicine) and by the Wellcome Trust through Research Training Fellowship number 102368/Z/13/Z awarded to NJG.
Additional file
10.1186/s12936-016-1513-1 Catches in human landing catch.
Contributor Information
Nicodem J. Govella, Phone: +255686997298, Email: govella@ihi.or.tz
Deodatus F. Maliti, Email: deodatusmalit@yahoo.co.uk
Amos T. Mlwale, Email: athomas@ihi.or.tz
John P. Masallu, Email: jpaliga@ihi.or.tz
Nosrat Mirzai, Email: Nosrat.mirzai@glasgow.ac.uk.
Paul C. D. Johnson, Email: paul.johnson@glasgow.ac.uk
Heather M. Ferguson, Email: Heather.Ferguson@glasgow.ac.uk
Gerry F. Killeen, Email: gkilleen@ihi.or.tz
References
- 1.Briet OJT, Chitnis N. Effects of changing mosquito host searching behaviours on the cost effectiveness of a mass distribution of long lasting, insecticidal nets: a modelling study. Malar J. 2013;12:215. doi: 10.1186/1475-2875-12-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Killeen GF, Seyoum A, Gimnig JE, Stevenson JC, Drakeley CJ, Chitnis N. Made-to measure malaria vector control strategies: rational design based on insecticide properties and coverage of blood resources for mosquitoes. Malar J. 2014;13:146. doi: 10.1186/1475-2875-13-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferguson HM, Dornhaus A, Beeche A, Borgemeister C, Gottlieb M, Mulla MS, et al. Ecology: a prerequisite for malaria elimination and eradication. PLoS Med. 2010;7:e1000303. doi: 10.1371/journal.pmed.1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sougoufara S, Diedhiou SM, Doucoure S, Diagne N, Sembene PM, Harry M, et al. Biting by Anopheles funestus in broad daytime after use of long-lasting insecticidal nets: a new challenge to malaria control. Malar J. 2014;13:125. doi: 10.1186/1475-2875-13-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geissbühler Y, Chaki P, Emidi B, Govella NJ, Shirima R, Mayagaya V, et al. Interdependence of domestic malaria prevention measures and mosquito-human interactions in urban Dar es Salaam, Tanzania. Malar J. 2007;6:126. doi: 10.1186/1475-2875-6-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pates H, Curtis C. Mosquito behavior and vector control. Ann Rev Entomol. 2005;50:53–70. doi: 10.1146/annurev.ento.50.071803.130439. [DOI] [PubMed] [Google Scholar]
- 7.Taylor B. Changes in feeding behaviour on malaria vector, Anopheles farauti Lav., following use of DDT a residual spray in houses in the British Solomon Island Protectorate. Trans R Entomol Soc. 1975;127:277–292. doi: 10.1111/j.1365-2311.1975.tb00576.x. [DOI] [Google Scholar]
- 8.Bugoro H, Cooper RD, Butafa C, Iro’ofa C, Mackenzie DO, Chen C, et al. Bionomics of the malaria vector Anopheles farauti in Temotu Province, Solomon Islands: issues for malaria elimination. Malar J. 2011;10:133. doi: 10.1186/1475-2875-10-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moiroux N, Damien GB, Egrot M, Djenontin A, Chandre F, Corbel V, et al. Human exposure to early morning Anopheles funestus biting behavior and personal protection provided by long-lasting insecticidal nets. PLoS One. 2014;9:e104967. doi: 10.1371/journal.pone.0104967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trung HD, Bortel WV, Sochantha T, Keokenchanh K, Briet OJT. Behavioural heterogeneity of Anopheles species in ecologically different localities in southeast Asia: a challenge for vector control. Trop Med Int Health. 2005;10:251–262. doi: 10.1111/j.1365-3156.2004.01378.x. [DOI] [PubMed] [Google Scholar]
- 11.Rubio-Palis Y, Curtis CF. Biting and resting behaviour of Anophelines in western Venezuela and implications for control of malaria transmission. Med Vet Entomol. 1992;6:325–334. doi: 10.1111/j.1365-2915.1992.tb00628.x. [DOI] [PubMed] [Google Scholar]
- 12.Ahumada ML, Pareja PX, Buitrago LS, Quinones ML. Biting behaviours of Anopheles darling Root, 1926 (Diptera: Culicidae) and its association with malaria transmission in Villavicencio (Meta, Colombia) Biomedica. 2013;33:241–250. [PubMed] [Google Scholar]
- 13.Bockarie MJ, Pedersen EM, White GB, Michael E. Role of vector control in the global program to eradicate lymphatic filariasis. Ann Rev Entomol. 2009;54:469–487. doi: 10.1146/annurev.ento.54.110807.090626. [DOI] [PubMed] [Google Scholar]
- 14.Simonsen PE, Pedersen EM, Rwegoshora RT, Malecela MN, Derua YA, Magesa SM. Lymphatic filariasis control in Tanzania: effect of repeated mass drug administration with ivermectin and albendazole on infection and transmission. PLoS Negl Trop Dis. 2010;4:e696. doi: 10.1371/journal.pntd.0000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uttah EC, Wokem GN, Okonofua C. The abundance and biting patterns of Culex quinquefasciatus Say (Culicidae) in the Coast region of Nigeria. ISRN Zool. 2013;2013:1–7. doi: 10.1155/2013/640691. [DOI] [Google Scholar]
- 16.Govella NJ, Chaki PP, Killeen GF. Entomological surveillance of behavioural resilience and resistance in residual malaria vector populations. Malar J. 2013;12:124. doi: 10.1186/1475-2875-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huho B, Briet OJT, Seyoum A, Sikala C, Bayoh N, Gimnig JE, et al. Consistently high estimates for proportion of human exposure to malaria vector populations occurring indoors in rural Africa. Int J Epidemiol. 2013;42:235–247. doi: 10.1093/ije/dys214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Killeen GF. Characterizing, controlling and eliminating residual malaria transmission. Malar J. 2014;13:330. doi: 10.1186/1475-2875-13-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindblade KA. Does a mosquito bite when no one is around to hear it. Int J Epidemiol. 2013;42:247–249. doi: 10.1093/ije/dyt004. [DOI] [PubMed] [Google Scholar]
- 20.Killeen GF, Kihonda J, Lyimo E, Oketch FR, Kotas ME, Mathenge E, et al. Quantifying behavioural interactions between humans and mosquitoes: evaluating the protective efficacy of insecticidal nets against malaria transmission in rural Tanzania. BMC Infect Dis. 2006;6:161. doi: 10.1186/1471-2334-6-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seyoum A, Sikala C, Chanda J, Chinula D, Ntamatungiro AJ, Hawela M, et al. Human exposure to Anopheline mosquitoes occurs primarily indoors, even for users of insecticide-treated nets in Luangwa valley, South-east Zambia. Parasit Vectors. 2012;5:101. doi: 10.1186/1756-3305-5-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong ML, Chua TH, Leong CS, Khaw LT, Fornace K, Wan-Sulaiman WY, et al. Seasonal and spatial dynamics of the primary vector of Plasmodium Knowlesi within major transmission focus in Sabah, Malaysia. PLoS Negl Trop Dis. 2015;9:e0004135. doi: 10.1371/journal.pntd.0004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lengeler C. Insecticide-treated nets for malaria control: real gains. Bull World Health Organ. 2004;82:84. [PMC free article] [PubMed] [Google Scholar]
- 24.Pluess B, Tanser FC, Lengeler C, Sharp BL. Indoor residual spraying for preventing malaria. Cochrane Database Syst Rev. 2010;4:CD006657. doi: 10.1002/14651858.CD006657.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.UNICEF, WHO . Achieving the malaria MDG target: reversing the incidence of malaria 2000–2015. Geneva: World Health Organization and the United Nations Children’s Fund; 2015. pp. 1–32. [Google Scholar]
- 27.Simonsen PE, Malecela MN, Michael E, Mackenzie CD. Lymphatic filariasis research and control in Eastern and Southern Africa. Frederiksberg: DBL-Centre for Health Research and Development; 2008. [Google Scholar]
- 28.WHO . Global programme to eliminate lymphatic filariasis. Lymphatic filariasis: practical entomology. Geneva: World Health Organization; 2013. [Google Scholar]
- 29.Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10:80. doi: 10.1186/1475-2875-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Derua YA, Alifrangis M, Hosea KM, Meyrowitsch DW, Magessa SM, Pedersen EM, et al. Change in composition of Anopheles gambiae complex and its possible implications for the transmission of malaria and lymphatic filariasis in north-eastern Tanzania. Malar J. 2012;11:188. doi: 10.1186/1475-2875-11-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in Western Nyanza Province, Kenya. Malar J. 2010;9:62. doi: 10.1186/1475-2875-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mwangangi JM, Mbogo CM, Orindi BO, Muturi EJ, Midega JT, Nzovu J, et al. Shifts in malaria vector species composition and transmission dynamics along the Kenyan coast over the past 20 years. Malar J. 2013;12:13. doi: 10.1186/1475-2875-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gatton ML, Chitnis N, Churcher T, Donnelly MJ, Ghani AC, Godfray HC, et al. The importance of mosquito behavioural adaptations to malaria control in Africa. Evolution. 2013;67:1218–1230. doi: 10.1111/evo.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russell TL, Beebe NW, Cooper RD, Lobo NF, Burkot TR. Successful malaria elimination strategies require interventions that target changing vector behaviours. Malar J. 2013;12:56. doi: 10.1186/1475-2875-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Durnez L, Mao S, Denis L, Roelants P, Sochantha T, Coosemans M. Outdoor malaria transmission in forested villages of Cambodia. Malar J. 2013;12:329. doi: 10.1186/1475-2875-12-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Killeen GF, Chitnis N. Potential causes and consequences of behavioural resilience and resistance in malaria vector populations: a mathematical modelling analysis. Malar J. 2014;13:97. doi: 10.1186/1475-2875-13-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Briet OJT, Huho B, Gimnig JE, Bayoh N, Seyoum A, Sikaala C, et al. Applications and limitations of Centers for Disease Control and Prevention miniature light traps for measuring biting densities of African malaria vector populations: a pooled analysis of 13 comparisons with human landing catches. Malar J. 2015;14:247. doi: 10.1186/s12936-015-0761-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mboera LEG. Sampling techniques for adult Afrotropical malaria vectors and their reliability in the estimation of entomological inoculation rates. Tanzania Health Res Bull. 2005;7:117–124. doi: 10.4314/thrb.v7i3.14248. [DOI] [PubMed] [Google Scholar]
- 39.Govella NJ, Chaki PP, Geissbühler Y, Kannady K, Okumu FO, Charlwood JD, et al. A new tent trap for sampling exophagic and endophagic members of the Anopheles gambiae complex. Malar J. 2009;8:157. doi: 10.1186/1475-2875-8-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Govella NJ, Chaki PP, Mpangile J, Killeen GF. Monitoring mosquitoes in urban Dar es Salaam: evaluation of resting boxes, window exit traps, CDC light traps, Ifakara tent traps and human landing catches. Parasit Vectors. 2011;4:40. doi: 10.1186/1756-3305-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Govella NJ, Moore J, Killeen GF. An exposure free tool for monitoring adult malaria mosquito populations. Am J Trop Med Hyg. 2010;83:596–600. doi: 10.4269/ajtmh.2010.09-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaki PP, Mlacha Y, Msellem D, Muhili A, Malishee AD, Mtema ZJ, et al. An affordable, quality-assured community-based system for high-resolution entomological surveillance of vector mosquitoes that reflects human malaria infection risk pattern. Malar J. 2012;11:172. doi: 10.1186/1475-2875-11-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathenge EM, Misiani GO, Oulo DO, Irungu LW, Ndegwa P, Smith TA, et al. Comparative performance of the Mbita trap, CDC light trap and the human landing catch in the sampling of Anopheles arabiensis, An. funestus and culicine species in a rice irrigation scheme in western Kenya. Malar J. 2005;4:7. doi: 10.1186/1475-2875-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mathenge EM, Omweri GO, Irungu LW, Ndegwa PN, Walczak E, Smith TA, et al. Comparative field evaluation of the Mbita trap, the Centers for Disease Control light trap, and the human landing catch for sampling of malaria vectors in western Kenya. Am J Trop Med Hyg. 2004;70:33–37. [PubMed] [Google Scholar]
- 45.Bradley J, Lines J, Fuseini G, Schwabe C, Monti F, Slotman M, et al. Outdoor biting by Anopheles mosquitoes on Bioko Island does not currently impact on malaria control. Malar J. 2015;14:170. doi: 10.1186/s12936-015-0679-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reddy MR, Overgaard HJ, Abaga S, Reddy VP, Caccone A, Kiszewski AE, et al. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malar J. 2011;10:184. doi: 10.1186/1475-2875-10-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lima JBP, Rosa-Freitas MG, Rodovalho CM, Santos F, Lourenco-de-Oliveira R. Is there an efficient trap or collection method for sampling Anopheles darlingi and other malaria vectors that can describe the essential parameters affecting transmission dynamics as effectively as human landing catches? A review. Mem Inst Oswaldo Cruz. 2014;109:685–705. doi: 10.1590/0074-0276140134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Achee NL, Youngblood L, Bangs MJ, Lavery JV, James S. Considerations for the use of human participants in vector biology research. Vector Borne Zoonotic Dis. 2015;15:89–102. doi: 10.1089/vbz.2014.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sikaala C, Killeen GF, Chanda J, Chinula D, Miller JM, Russell TL, et al. Evaluation of alternative mosquito sampling methods for malaria vectors in lowland south east Zambia. Parasit Vectors. 2013;6:91. doi: 10.1186/1756-3305-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sadanandane C, Jambulingam P, Subramanian S. Role of modified CDC miniature light-traps as an alternative method for sampling adult Anophelines (Diptera: Culicidae) in the National Mosquito Surveillance Programme in India. Bull Entomol Res. 2013;94:55–64. doi: 10.1079/ber2003281. [DOI] [PubMed] [Google Scholar]
- 51.Costantini C, Sagnon NF, Sanogo E, Merzagora L, Colluzi M. Relationship to human biting collections and influence of light and bednets in CDC light-trap catches of West African malaria vectors. Bull Entomol Res. 1998;88:503–511. doi: 10.1017/S000748530002602X. [DOI] [Google Scholar]
- 52.Faye O, Diallo S, Gaye O, Ndir O. Efficacité comparée de l’utilisation des pièges lumineux du type CDC et des sujets humains pour l’échantillonnage des populations anophéliennes. Résultats obtenus dans la zone de Bignona. Bull Soc Pathol Exot. 1992;85:185–189. [PubMed] [Google Scholar]
- 53.Overgaard HJ, Saebo S, Reddy MR, Reddy VP, Abaga S, Matias A, et al. Light traps fail to estimate reliable malaria mosquito biting rates on Bioko Island, Equatorial Guinea. Malar J. 2012;11:56. doi: 10.1186/1475-2875-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Service MW. Mosquito ecology-field sampling methods. New York: John Wiley and Sons; 1977. [Google Scholar]
- 55.Service MW. A critical review of procedures for sampling populations of adult mosquitoes. Bull Entomol Res. 1977;67:343–382. doi: 10.1017/S0007485300011184. [DOI] [Google Scholar]
- 56.WHO . Malaria entomology and vector control. Learner’s guide. 3. Geneva: World Health Organization; 2002. [Google Scholar]
- 57.Ndebele P, Musesengwa R. View Point: ethical dilemmas in malaria vector research in Africa: Making the difficult choice between mosquito, science and humans. Malawi Med J. 2012;24:65–68. [PMC free article] [PubMed] [Google Scholar]
- 58.Gimnig JE, Walker ED, Otieno P, Kosgei J, Olang G, Omboki M, et al. Incidence of malaria among mosquito collectors conducting human landing catches in western Kenya. Am J Trop Med Hyg. 2013;88:301–308. doi: 10.4269/ajtmh.2012.12-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moi ML, Takasaki T, Kotaki A, Tajima S, Lim C, Sakamoto M, et al. Importation of dengue virus type 3 to Japan from Tanzania and Cote d’Ivoire. Emerg Infect Dis. 2010;16:1770–1772. doi: 10.3201/eid1611.101061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chretien J, Anyamba A, Bedno S, Breiman SF, Sang R, Sergon K, et al. Drought associated Ckikungunya emergency along coastal East Africa. Am J Trop Med Hyg. 2007;76:405–407. [PubMed] [Google Scholar]
- 61.Faye O, Freire CM, Lamarino A, Faye O, de Oliveira JVC, Diallo M. Molecular evolution of Zika virus during its emergence in the 20th Century. PLoS Negl Trop Dis. 2014;8:e2634. doi: 10.1371/journal.pntd.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Musso D, Cao-Lormeau VM, Gubler DJ. Zika virus: following the path of dengue and chikungunya? Lancet. 2015;386:243–244. doi: 10.1016/S0140-6736(15)61273-9. [DOI] [PubMed] [Google Scholar]
- 63.Govella NJ, Okumu FO, Killeen GF. Insecticide-treated nets can reduce malaria transmission by mosquitoes which feed outdoors. Am J Trop Med Hyg. 2010;82:415–419. doi: 10.4269/ajtmh.2010.09-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cook MK, Kahindi SC, Oriango RM, Owaga C, Ayoma E, Mabuka D, et al. A bite before bed’: exposure to malaria vectors outside the times of net use in the highlands of western Kenya. Malar J. 2015;14:259. doi: 10.1186/s12936-015-0766-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Msellemu D, Namango HI, Mwakalinga VM, Ntamatungiro AJ, Mlacha Y, Mtema ZJ, et al. The epidemiology of residual Plasmodium falciparum malaria transmission and infection burden in an African city with high coverage of multiple vector control measures. Malar J. 2016;15:288. doi: 10.1186/s12936-016-1340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garrett-Jones C. Epidemiological entomology and its application-to malaria. Geneva: World Health Organization; 1968. [Google Scholar]
- 67.Majambere S, Masue D, Mlacha Y, Govella NJ, Magesa SM, Killeen GF. Advantages and limitations of commercially available electrocuting grids for studying mosquito behaviour. Parasit Vectors. 2013;6:53. doi: 10.1186/1756-3305-6-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maliti DF, Govella NJ, Killeen GF, Mirzai N, Johnson PCD, Kreppel K, et al. Development and evaluation of mosquito-electrocuting traps as alternatives to the human landing catch technique for sampling host-seeking malaria vectors. Malar J. 2015;14:502. doi: 10.1186/s12936-015-1025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.NBS . Population and housing census: population distribution by 2013. Nigeria: National Bureau of Statistics; 2012. [Google Scholar]
- 70.Geissbühler Y, Kannady K, Chaki PP, Emidi B, Govella NJ, Mayagaya V, et al. Microbial larvicide application by a large-scale, community-based program reduces malaria infection prevalence in Urban Dar Es Salaam, Tanzania. PLoS One. 2009;4:e5107. doi: 10.1371/journal.pone.0005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.NBS . Tanzania HIV/AIDS and Malaria Indicator Survey 2011–2012. Nigeria: National Bureau of Statistics; 2013. pp. 1–229. [Google Scholar]
- 72.Ogoma SB, Kannady K, Sikulu M, Chaki PP, Govella NJ, Mukabana WR, et al. Window screening, ceilings and closed eaves as sustainable ways to control malaria in Dar es Salaam, Tanzania. Malar J. 2009;8:221. doi: 10.1186/1475-2875-8-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Castro MC, Tsuruta A, Kanamori S, Kannady K, Mkude S. Community-based environmental management for malaria control: evidence from a small-scale intervention in Dar es Salaam, Tanzania. Malar J. 2009;8:57. doi: 10.1186/1475-2875-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Castro MC, Kanamori S, Kannady K, Mkude S, Killeen GF, Fillinger U. The importance of drains for the larval development of lymphatic filariasis and malaria vectors in Dar es salaam, United Republic of Tanzania. PLoS Negl Trop Dis. 2010;4:e693. doi: 10.1371/journal.pntd.0000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gillies MT, DeMeillon B. The Anophelinae of Africa south of the Sahara (Ethiopian zoogeographical region) Johannesburg: South African Institute for Medical Research; 1968. [Google Scholar]
- 76.Chavasse DC, Lines JD, Ichimori K, Marijani J. Mosquito control in Dar es Salaam. I. Assessment of Culex quinquefasciatus breeding sites prior to intervention. Med Vet Entomol. 1995;9:141–146. doi: 10.1111/j.1365-2915.1995.tb00170.x. [DOI] [PubMed] [Google Scholar]
- 77.Chavasse DC, Lines JD, Ichimori K, Majala AR, Minjas JN, Marijani J. Mosquito control in Dar es Salaam. II. Impact of expanded polystyrene beads and pyriproxyfen treatment of breeding sites on Culex quinquefasciatus densities. Med Vet Entomol. 1995;9:147–154. doi: 10.1111/j.1365-2915.1995.tb00171.x. [DOI] [PubMed] [Google Scholar]
- 78.Stephens C, Masamu ET, Kiama MG, Keto AJ, Kinenekejo M, Ichimori K, et al. Knowledge of mosquitos in relation to public and domestic control activities in the cities of Dar es Salaam and Tanga. Bull World Health Organ. 1995;73:97–104. [PMC free article] [PubMed] [Google Scholar]
- 79.Malecela MN, Lazarus W, Mwingira U, Mwakitalu E, Makene C, Kabali C, et al. A progress report from Tanzania. J Lymphoedema. 2009;4:10–12. [Google Scholar]
- 80.Maliti DF. Ecological and genetic determinants of malaria vectors feeding and resting behaviours. Glasgow: University of Glasgow, Institute of Biodiversity, Animal Health and Comparative Medicine, School of Life Sciences, College of Medical, Veterinary and Life Sciences; 2015. [Google Scholar]
- 81.WHO. Manual on practical entomology. Part 2. Methods and techniques. Vol 13. Geneva: World Health Organization; 1975.
- 82.Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara (Afrotropical region) Johannesburg: South African Medical Research Institute; 1987. [Google Scholar]
- 83.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of Anopheles gambiae complex by polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 84.Wirtz RAF, Zavala Y, Charoenvit GH, Campbell TR, Burkot I, Schmeider KM, et al. Comperative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. Bull World Health Organ. 1987;65:39–45. [PMC free article] [PubMed] [Google Scholar]
- 85.Wirtz RA, Duncan JF, Njelesani EK, Schneider I, Brown AE, Oster CN, et al. ELISA method for detecting Plasmodium falciparum circumsporozoite antibody. Bull World Health Org. 1989;67:535–542. [PMC free article] [PubMed] [Google Scholar]
- 86.Hii JLK, Smith T, Mai A, Ibam E, Alpers MP. Comparison between anopheline mosquitoes (Diptera: Culicidae) caught using different methods in a malaria endemic area of Papua New Guinea. Bull Entomol Res. 2000;90:211–219. doi: 10.1017/S000748530000033X. [DOI] [PubMed] [Google Scholar]
- 87.Plummer M. JAGS: a Program for Analysis of Bayesian Graphical Models Using Gibbs Sampling. In: Proceedings of the 3rd international workshop on distributed statistical computing (DSC 2003). Vienna; 2003.
- 88.R2jags: using R to Run ‘JAGS’. R package version 0.5–7. http://www.cran.r-project.org/package=R2jags. Accessed 23 Aug 2015.
- 89.Matos CA, Thomas DL, Gianola D, Tempelman RJ, Young LD. Genetic analysis of discrete reproductive traits in sheep using linear and nonlinear models: I. Estimation of genetic parameters. J Anim Sci. 1997;75:76–87. doi: 10.2527/1997.75176x. [DOI] [PubMed] [Google Scholar]
- 90.Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol. 2013;4:133–142. doi: 10.1111/j.2041-210x.2012.00261.x. [DOI] [Google Scholar]
- 91.Crawrey MJ. The R book. Int Stat Rev. 2007;75:425–426. doi: 10.1111/j.1751-5823.2007.00030_16.x. [DOI] [Google Scholar]
- 92.Smith T, Charlwood JD, Takken W, Tanner M, Spiegelhalter DJ. Mapping densities of malaria vectors within a single village. Acta Trop. 1995;59:1–18. doi: 10.1016/0001-706X(94)00082-C. [DOI] [PubMed] [Google Scholar]
- 93.Magbity EB, Lines JD, Marbiah MT, David K, Peterson E. How reliable are light traps in estimating biting rates of adult Anopheles gambiae s.l. (Diptera: Culicidae) in the presence of treated bed nets? Bull Entomol Res. 2002;92:71–76. doi: 10.1079/BER2002200. [DOI] [PubMed] [Google Scholar]
- 94.Lindsay SW, Schellenberg JRMA, Zeiler HA, Daly RJ, Salum FM, Wilkins HA. Exposure of Gambian children to Anopheles gambiae vectors in an irrigated rice production area. Med Vet Entomol. 1995;9:50–58. doi: 10.1111/j.1365-2915.1995.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 95.Mwakitalu ME, Malecela MN, Pedersen EM, Mosha FW, Simonsen PE. Urban lymphatic filariasis in the metropolis of Dar es Salaam, Tanzania. Parasit Vectors. 2013;6:286. doi: 10.1186/1756-3305-6-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.MacDonald G. The epidemiology and control of malaria. London: Oxford University Press; 1957. [Google Scholar]
- 97.Garrett-Jones C, Shidrawi GR. Malaria vectorial capacity of a population of Anopheles gambiae. Bull World Health Organ. 1969;40:531–545. [PMC free article] [PubMed] [Google Scholar]
- 98.Takken W, Verhulst NO. Host preferences of blood-feeding mosquitoes. Annu Rev Entomol. 2013;58:433–453. doi: 10.1146/annurev-ento-120811-153618. [DOI] [PubMed] [Google Scholar]
- 99.Lyimo IN, Ferguson HM. Ecological and evolutionary determinants of host species choice in mosquito vectors. Trends Parasitol. 2009;25:189–196. doi: 10.1016/j.pt.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 100.Lefevre T, Gouagna LC, Dabire KR, Elguero E, Fontenille D, Renaud F, et al. Beyond nature and nurture: phenotypic plasticity in blood-feeding behavior of Anopheles gambiae s.s. when humans are not readily accessible. Am J Trop Med Hyg. 2009;81:1023–1029. doi: 10.4269/ajtmh.2009.09-0124. [DOI] [PubMed] [Google Scholar]
- 101.Kiszewski A, Mellinger A, Spielman A, Malaney P, Sachs SE, Sachs J, et al. A global index representing the stability of malaria transmission. Am J Trop Med Hyg. 2004;70:486–498. [PubMed] [Google Scholar]
- 102.Mayagaya VS, Nkwengulila G, Lyimo I, Kihonda J, Mtambala H. The impact of livestock on the abundance, resting behaviour and sporozoite rate of malaria vectors in southern Tanzania. Malar J. 2015;14:17. doi: 10.1186/s12936-014-0536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kay BH, Boreham PFL, Edman JD. Application of the “feeding index” concept to studies of mosquito host-feeding patterns. Mosq News. 1979;39:68–73. [Google Scholar]
- 104.Killeen GF, McKenzie FE, Foy BD, Bogh C, Beier JC. The availability of potential hosts as a determinant of feeding behaviours and malaria transmission by mosquito populations. Trans R SocTrop Med Hyg. 2001;95:469–476. doi: 10.1016/S0035-9203(01)90005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Garrett-Jones C. The human blood index of malarial vectors in relationship to epidemiological assessment. Bull World Health Organ. 1964;30:241–261. [PMC free article] [PubMed] [Google Scholar]
- 106.Detinova TS, Gillies MT. Observations on the determination of the age composition and epidemiological importance of populations of Anopheles gambiae Giles and Anopheles funestus Giles in Tanganyika. Bull World Health Org. 1964;30:23–28. [PMC free article] [PubMed] [Google Scholar]
- 107.Detinova TS. Age-grouping methods in diptera of medical importance, with special reference to some vectors of malaria. Geneva: World Health Organization; 1962. [PubMed] [Google Scholar]
- 108.Charlwood JD, Wilkes TJ. Studies on the age composition of sample of Anopheles darlingi Root (Diptera: culicidae) in Brazil. Bull Entomol Res. 1979;69:337–342. doi: 10.1017/S0007485300017818. [DOI] [Google Scholar]
- 109.Mayagaya VS, Michel K, Benedict MQ, Killeen GF, Wirtz RA, Ferguson HM, et al. Non-destructive determination of age and species of Anopheles gambiae s.l. using near-infrared spectroscopy. Am J Trop Med Hyg. 2009;81:622–630. doi: 10.4269/ajtmh.2009.09-0192. [DOI] [PubMed] [Google Scholar]
- 110.Sikulu M, Killeen GF, Hugo LE, Ryan PA, Dowell KM, Wirtz RA, et al. Near-infrared spectroscopy as a complementary age grading and species identification tool for African malaria vectors. Parasit Vectors. 2010;3:49. doi: 10.1186/1756-3305-3-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dowell FE, Noutcha AEM, Michel K. The effect of preservation methods on predicting mosquito age by near infrared spectroscopy. Am J Trop Med Hyg. 2011;85:1092–1096. doi: 10.4269/ajtmh.2011.11-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yohannes M, Boelee E. Early biting rhythm in the Afro-tropical vector of malaria, Anopheles arabiensis and challenges for its control in Ethiopia. Med Vet Entomol. 2012;26:103–105. doi: 10.1111/j.1365-2915.2011.00955.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Access and use of data supporting this article will have to comply with the Ifakara Health Institute data sharing policy. If data are requested and no competing interest is apparent, the requested data will made be made available under defined conditions expressed in writing through an exchange of letters between parties stipulating those conditions and any agreed limits to use of data.


