Abstract
With an increase in the aging population, rotator cuff tears are becoming more common. High failure rates with shoulder rotator cuff repair surgery have been persistent, prompting the introduction of biologic methods to promote healing. The aim of the present technique is to deliver stem cells and growth factors to the footprint of the repair site. A platelet-rich plasma (PRP) fibrin clot is used as a scaffold for the delivery of stem cells, by using PRP, to provide a source of growth factors, and platelet-poor plasma (PPP) as a source for fibrinogen for the matrix for the scaffold. In the second step, bone marrow is harvested from the proximal humerus, concentrated, and combined with the PRP and PPP. Using a customized device, we then activate the clotting process and transfer the scaffold containing the stem cells using a suture anchor onto the shoulder rotator cuff footprint. In an effort to promote healing, this technique reimplants autologous stem cells and growth factors into the operative site. This technique may serve as an alternative for typically used scaffolds such as collagen matrices or decellularized human dermis patches.
It has been proposed that biologic agents may augment healing after surgery by creating a more natural enthesis, leading to a more robust repair.1 The growing field of tissue engineering is an area that offers advancements for this type of augmentation. The current techniques call for a scaffold and a biologically active element, such as mesenchymal stem cells (MSCs). The scaffold should enhance cellular proliferation and integrate into the bone over time.2 Fibrin has been shown to be beneficial for the delivery of MSCs as it is an autologous 3-dimensional construct that facilitates migration and proliferation of these cells.3, 4 Platelet-rich plasma (PRP), which contains a variety of growth factors, is thought to aid in tissue repair owing to the release of alpha granules from the platelets. PRP has also been shown to stimulate MSC proliferation as a result of the abundance of growth factors.5, 6, 7
This surgical technique is designed to transport a fibrin scaffold between footprint and rotator cuff tendon containing PRP and concentrated bone marrow to aid in the proliferation and differentiation of MSCs. This study shows that a fibrin scaffold can maintain MSC viability for long periods of time (>4 weeks) and has mechanical properties appropriate for use in arthroscopic rotator cuff repair (Video 1).
Surgical Technique
The protocol for aspirating bone marrow has been approved by the institutional review board (IRB No. 06-577-2). All patients received detailed information about the technique before surgery. The indication for this additional fibrin scaffold compared with a normal rotator cuff repair consists of a failed primary rotator cuff repair. This surgical procedure is carried out similar to a standard rotator cuff repair with additional steps that will be detailed below. The repair was carried out with the patient in the beach chair position under general anesthesia. The biologic scaffold consists of PPP (platelet-poor plasma) as a source of fibrin for the scaffold matrix, PRP as a source for growth factors, and concentrated bone marrow aspirate (cBMA) as the source of MSCs.
Preparation of PRP and PPP
Platelet rich and poor plasma can be obtained with different techniques. Intraoperatively, a fully automated 3-sensor technology system based on flow cytometry and light absorption is used (Angel System; Arthrex, Naples, FL), as it gives a constant and reproducible sterile product (Fig 1). Prior to the skin incision, 60 mL of venous peripheral blood is drawn using a 60-mL syringe prefilled with 8 mL of acid citrate dextrose–anticoagulant (ACD-A). The blood is then transferred into the automated 3-sensor technology system. Using a hematocrit setting of 7% and a high-spinning centrifugal process, approximately 2 to 3 mL of PRP and 20 to 25 mL of PPP are obtained. It is important to start the PRP and PPP procedure right after the patient is anesthetized, as it takes 17 to 20 minutes for processing. The blood products are then passed onto the sterile field.
Fig 1.
(A) Aspiration of 17 mL bone marrow from the proximal humerus using a nonfenestrated trocar (*) with a 3 mL ACD-A prefilled 60-mL syringe. (B) Sterile autologous products used for scaffold forming (PPP, PRP, cBMA, and bovine thrombin) as well as heparin and ACD-A for instrument preparation. (C) Transferring autologous PPP, PRP, and cBMA from sterile cups by using a 1.0-mL tuberculin syringe into a sterile multiwell dish. (ACD-A, acid citrate dextrose–anticoagulant; cBMA, concentrated bone marrow aspirate; PPP, platelet-poor plasma; PRP, platelet-rich plasma.)
Bone Marrow Aspiration to Obtain MSCs
Bone marrow is obtained from the proximal humerus during arthroscopy, as this eliminates additional patient morbidity involved using a distant harvest site. A heparin-flushed (1,000 IU/mL) nonfenestrated bone marrow aspiration trocar (11 gauge) is inserted to a depth of 25 to 30 mm. The hole is strategically placed for use in subsequent medial row anchor placement during the rotator cuff repair. The core needle from the trocar is removed and a heparin-flushed (1,000 IU/mL) 60-mL syringe containing 3 mL of ACD-A is connected. Seventeen milliliters (total = 20 mL) of bone marrow aspirate (BMA) is then harvested. This process is repeated with subsequent syringes until a minimum volume of 60 mL of BMA is obtained. The BMA is then transferred to the automated system used for the PRP and PPP processing. A 15% hematocrit setting is used, giving the maximum concentration of stem cells. During the BMA processing (17 to 20 minutes), the surgeon proceeds without interruption to the mobilization of the rotator cuff tendon and additional surgical procedures. The cBMA is then passed onto the sterile field.
Fibrin Scaffold Formation
To enhance the RC healing process, a fibrin clot was used as a biologic scaffold to deliver MSCs and growth factors directly to the repair site. For this purpose, we use a combination of PPP and PRP taken from whole blood and cBMA. The cBMA provides a source of stem cells, PRP provides growth factors, and PPP serves as our matrix as it contains a high concentration of fibrinogen. Bovine thrombin is used to activate and obtain a stable clot. Presurgical investigations have shown that the amount of thrombin found in whole blood and BMA products (PPP, PRP, RBC, cBMA, BMA) is not high enough to activate the coagulation cascade for this technique as the concentrations are about 4,000-fold less than commercially available thrombin.
To create a biologically and mechanically stable clot, volumes of 0.1 mL of cBMA, 0.1 mL of PRP, 0.6 mL of PPP, and 0.2 mL of bovine thrombin (5,000 IU/mL) are combined for each clot.
These concentrations showed the best results in terms of cell viability (dead/alive assay) and mechanical resistance testing, using an arthroscopic shoulder simulator and cadaveric implantations.
All autologous products (PRP, PPP, and cBMA) are transferred into one well of a sterile multiwell plate (6 × 4) by using a tuberculin syringe, ready to be activated and implanted (Fig 1). Depending on the amount of cBMA obtained from the spinning process, multiple clots can be produced. During the clot preparation, the surgeon has mobilized the cuff tendon, placed the medial row suture anchors, and shuttled the medial row sutures through the lateral portal.
Scaffold Implantation
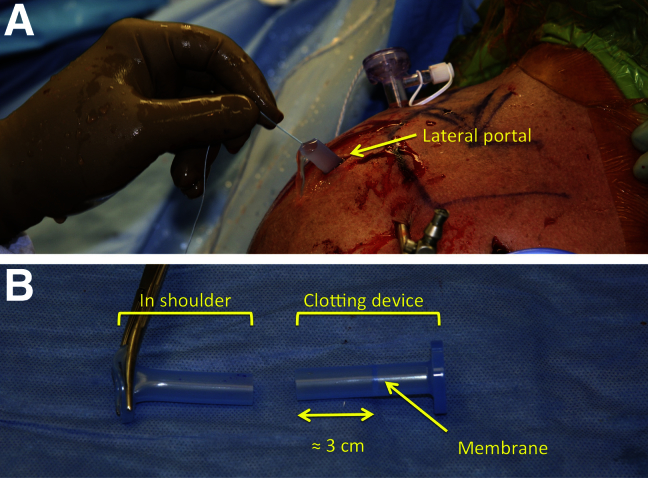
Arthroscopic cannulas are designed with a membrane to prevent loss of the arthroscopic fluid. Unfortunately, this membrane prevents the safe and reproducible implantation of the fibrin scaffold. Therefore, a modified cannula—one in which the membrane is cut away—is used (8 mm × 10 cm, PassPort; Arthrex). This cannula is inserted into the shoulder using a lateral portal, and the sutures (medial anchors) are shuttled through the cannula. After placement, the cannula is cut 3 cm below the membrane allowing direct access to the joint. It is important to cut the cannula 3 cm below the membrane, as the remainder of the cannula is used as a unique clotting device (Fig 2). The arthroscopic fluid flow is then turned off.
Fig 2.
(A) Customized PassPort cannula placed in a lateral portal to transfer the fibrin scaffold (clot) on the suture anchor used for rotator cuff repair. (B) The modified cannula and the customized clotting device. It is important to cut the cannula about 3 cm below the membrane. This allows for later clotting around the suture.
The free end of the suture from the medial anchor is placed into the center of the clotting device. A 1.0-mL tuberculin syringe is used to transfer 0.2 mL of bovine thrombin into the device followed by the transfer of the prefilled multiwells from the sterile table using a 1.0-mL syringe (filled with 0.8 mL of PPP, PRP, and cBMA). After 30 seconds, a clot is formed around the suture and the device can easily be removed. Because the clotting device has the same diameter as the cannula in the shoulder, it is easy to shuttle the clot into the joint by using an arthroscopic knot pusher (Fig 3). This process can be repeated until all the cBMA has been transferred onto the footprint.
Fig 3.
(A) Transferring the prepared mixture of PPP, PRP, and cBMA from the multiwell plate into the clotting device (*, prefilled with bovine thrombin) by using a 1.0-mL tuberculin syringe. Once the mixture is added, the clotting process is active and takes about 30 seconds. (B) By removing the clotting device distally, the clot remains on the suture. (C) The clot can now easily be transferred into the joint by using an arthroscopic knot pusher. (D) Arthroscopic view of clot at the bottom of a suture anchor placed onto the rotator cuff footprint. (cBMA, concentrated bone marrow aspirate; FP, footprint; PPP, platelet-poor plasma; PRP, platelet-rich plasma; SSP, supraspinatus tendon.)
After the fibrin scaffold transportation onto the rotator cuff footprint, the medial suture anchors are then passed through the rotator cuff, and the rotator cuff repair is finalized by adding the lateral row anchors in the usual fashion. This sequence of steps is important to ensure that the scaffold lies under the rotator cuff directly on the footprint. Arthroscopic glenohumeral visualization of the repair site is then carried out to document the flush repaired tendon and to check for scaffold loosening.
Discussion
This technique provides stem cells and growth factors to the surgical site without interfering in the mechanical repair of the tendon to the bony footprint. Recent literature has shown that the use of BMA can help to reduce the failure rate after arthroscopic rotator cuff surgery.8 To promote healing, this scaffold is designed to be resorbable by the biologic processes within the human body, leaving stem cells and growth factors at the site of injury. Bovine thrombin is used to activate platelet growth factors and produce a clot that acts as a repair-site scaffold for the MSCs. Combined, these factors may enhance the healing process and augment the rotator cuff repair.
Despite the fast clotting and simultaneous preparation, this technique adds about 25 to 35 minutes to a normal rotator cuff repair. The advantages, limitations, and risks are represented in in Table 1.
Table 1.
Advantages, Limitations, and Risks of Arthroscopic Fibrin Scaffold Augmentation in Revision Rotator Cuff Repair
| Advantages | Limitation | Risks |
|---|---|---|
| - Autologous products to enhance healing (except bovine thrombin). - Self-dismantling clot, which is simulating natural healing. - High concentration of growth factors and stem cells on the footprint. - No additional extraneous material such as sutures or nondegradable matrixes - All-in-one procedure: PPP, PRP, and cBMA can be produced with a single processing set with high cost-effectiveness. |
- Clot implantation adds time to surgical procedure. - The number of clots is limited to the amount of cBMA. - An additional person is needed in the operation theater to prepare the blood products. - The fibrin scaffold cannot be used to bridge gaps in rotator cuff repairs, and therefore the remaining rotator cuff must be fully mobilized and brought back onto the footprint. - This technique was only used in revision rotator cuff repairs and might be used in primary repairs as well. |
- This technique does not add any surgical risks besides the known risks for arthroscopic rotator cuff repairs. - An intolerance reaction to the bovine thrombin cannot be excluded. |
cBMA, concentrated bone marrow aspirate; PPP, platelet-poor plasma; PRP, platelet-rich plasma.
Conclusions
In conclusion, this technique may provide the biologic elements necessary to promote healing after rotator cuff surgery using autologous PRP, PPP, and cBMA.
Footnotes
The authors report the following potential conflict of interest or source of funding: The University of Connecticut Health Center / UConn Musculoskeletal Institute has received direct funding and material support for this study from Arthrex (Naples, FL). The company had no influence on study design, data collection or interpretation of the results, or the final manuscript. A.B.I. receives support from International Society of Arthroscopy, Knee Surgery, and Orthopaedic Sports Medicine; AGA (German-speaking Association for Arthroscopy); DGOU (German Association for Orthopaedics and Traumatology); DGOOC (German Association for Orthopaedics and Orthopaedic Surgery); Arthrex; Arthrosurface; medi-bayreuth; Springer and Thieme; Archives of Orthopaedic and Trauma Surgery; Arthroskopie, Journal of Shoulder and Elbow Surgery, Knee Surgery, Sports Traumatology, Arthroscopy; and Operative Orthopädie und Traumatologie. A.D.M. receives support from Arthrex.
Supplementary Data
The first part of the video describes the manufacturing of the platelet-rich plasma and platelet-poor plasma by using patient's peripheral whole blood, drawn prior to surgery. Both blood products are prepared under sterile conditions by using a fully automated 3-sensor technology system based on flow cytometry and light absorption (Angel System; Arthrex, Naples, FL). The patient is then brought to a beach chair position, and diagnostic arthroscopy is performed using a posterior standard portal and an anterosuperior portal. The second part of the video shows a technical instruction how to create a fibrin scaffold seeded with mesenchymal stem cells and growth factors and how to arthroscopically implant this scaffold onto the rotator cuff footprint in addition to the repair.
References
- 1.Bunker D.L., Ilie V., Ilie V., Nicklin S. Tendon to bone healing and its implications for surgery. Muscles Ligaments Tendons J. 2014;4:343–350. [PMC free article] [PubMed] [Google Scholar]
- 2.Apostolakos J., Durant T.J., Dwyer C.R. The enthesis: A review of the tendon-to-bone insertion. Muscles Ligaments Tendons J. 2014;4:333–342. [PMC free article] [PubMed] [Google Scholar]
- 3.Rohringer S., Hofbauer P., Schneider K.H. Mechanisms of vasculogenesis in 3D fibrin matrices mediated by the interaction of adipose-derived stem cells and endothelial cells. Angiogenesis. 2014;17:921–933. doi: 10.1007/s10456-014-9439-0. [DOI] [PubMed] [Google Scholar]
- 4.Xiong Q., Hill K.L., Li Q. A fibrin patch-based enhanced delivery of human embryonic stem cell-derived vascular cell transplantation in a porcine model of postinfarction left ventricular remodeling. Stem Cells. 2011;29:367–375. doi: 10.1002/stem.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amable P.R., Carias R.B., Teixeira M.V. Platelet-rich plasma preparation for regenerative medicine: Optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 2013;4:67. doi: 10.1186/scrt218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boswell S.G., Cole B.J., Sundman E.A., Karas V., Fortier L.A. Platelet-rich plasma: A milieu of bioactive factors. Arthroscopy. 2012;28:429–439. doi: 10.1016/j.arthro.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Magalon J., Bausset O., Serratrice N. Characterization and comparison of 5 platelet-rich plasma preparations in a single-donor model. Arthroscopy. 2014;30:629–638. doi: 10.1016/j.arthro.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 8.Hernigou P., Flouzat Lachaniette C.H., Delambre J. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: A case-controlled study. Int Orthop. 2014;38:1811–1818. doi: 10.1007/s00264-014-2391-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The first part of the video describes the manufacturing of the platelet-rich plasma and platelet-poor plasma by using patient's peripheral whole blood, drawn prior to surgery. Both blood products are prepared under sterile conditions by using a fully automated 3-sensor technology system based on flow cytometry and light absorption (Angel System; Arthrex, Naples, FL). The patient is then brought to a beach chair position, and diagnostic arthroscopy is performed using a posterior standard portal and an anterosuperior portal. The second part of the video shows a technical instruction how to create a fibrin scaffold seeded with mesenchymal stem cells and growth factors and how to arthroscopically implant this scaffold onto the rotator cuff footprint in addition to the repair.